Abstract
Study Design
Prospective cohort study.
Objective
Estimate the prevalence of spondylolisthesis and determine the factors associated with higher or lower prevalence among men aged 65 years or older.
Summary of Background Data
Spondylolisthesis prevalence is reported to increase with age and to be higher among women than men. Among women aged ≥65 years, prevalence was estimated to be 29%, but no estimates among men of this age have been reported.
Methods
Lateral lumbar spine radiographs were obtained at baseline and a follow-up visit in the Osteoporotic Fractures in Men (MrOS) study, a cohort of community dwelling men ages ≥ 65 years. Average time between radiographs was 4.6 (±0.4) years. For the present study, 300 men were sampled at random at baseline. Of these, 295 had a usable baseline radiograph; 190 surviving participants had a follow-up radiograph. Spondylolisthesis was defined as a forward slip ≥ 5%. Progression was defined as a 5% increase in slip severity on the follow-up radiograph. Associations of spondylolisthesis prevalence with baseline characteristics were estimated with age-adjusted prevalence ratios and 95% confidence intervals from log binomial regression models.
Results
The mean (sd) age of the men studied was 74 (±6) years. Prevalence of lumbar spondylolisthesis was 31%. Spondylolisthesis was observed at the L3/4, L4/5, and L5/S1 levels. In 96% with spondylolisthesis, only one vertebral level was involved. The degree of slip ranged from 5%–28%, and nearly all listhesis was classified as Meyerding grade I. During follow-up, 12% of men with prevalent spondylolisthesis had progression; 12% without baseline spondylolisthesis had new onset. Prevalence did not vary by height, BMI, smoking history, diabetes, or heart disease. However, men with spondylolisthesis more often reported higher levels of physical activity or walking daily for exercise than men without spondylolisthesis.
Conclusions
Spondylolisthesis may be more common among older men than previously recognized.
Introduction
Spondylolisthesis is defined as the anterior migration, or slip, of one vertebra in relation to the next caudad vertebra. Spondylolisthesis is considered to have two main etiologies, spondylolytic and degenerative.1 Spondylolytic spondylolisthesis is distinguished by chronic fracture of the pars interartucularis and is observed primarily during childhood.2, 3 Degenerative spondylolisthesis refers to anterior slip “without an associated defect or disruption in the vertebral ring.” 4 It is considered to be a classic example of spinal instability resulting from progressive degeneration of the facet joints and the intervertebral discs with aging.5 Over 300,000 lumbar spine fusions are performed in the United States each year and the number is steadily increasing.6–8 Many of these fusions are performed to correct the perceived instability resulting from this disorder.9–12
Despite the considerable amount of surgery performed for spondylolisthesis, the basic epidemiology of this condition is not well documented. Most studies have focused on the anatomic features associated with degenerative spondylolisthesis among symptomatic patients.5, 13–17 Data from non-clinical, community-based populations are limited.18–22
It is widely accepted that women are about three times more likely to be affected by spondylolisthesis than men.4 Prevalence estimates among women range from 6% in Taiwan22 to 8% in Denmark20 to 20%–25% in the US,18, 19, 21 whereas among men estimates range from 3% in Taiwan22 and Denmark20 to 4%–8% in the US.19, 21 Although the prevalence of spondylolisthesis increases with age,18–21 few studies have focused specifically on the elderly.18, 19 Moreover, estimates among men from two studies were based on just 8 men classified with spondylolisthesis and are therefore likely to be imprecise.19, 21 Thus, additional data on spondylolisthesis prevalence of among men would be informative, particularly among elderly men for whom precise estimates are lacking.
Knowledge about the progression or onset of spondylolisthesis is limited.23, 24 Further, characteristics that distinguish those with spondylolisthesis from those without have not been studied in detail,18–20 although some have reported a role for occupational factors.22, 25 Thus, a clearer understanding of the epidemiology of spondylolisthesis is needed to inform discussions with patients and to formulate evidence-based treatment plans.
To address the specific need for information on spondylolisthesis among elderly men, we conducted a prospective study using spine radiographs and comprehensive data obtained in the Osteoporotic Fractures in Men Study (MrOS), a cohort of community-dwelling US men age 65–100 years.26 The primary objectives of this study were to 1) estimate the prevalence, incidence and progression of spondylolisthesis among older men, and 2) determine whether certain demographic factors, lifestyle characteristics, or medical conditions are associated with the prevalence of this condition. A comprehensive assessment of prevalent spondylolisthesis, back pain and functional limitations with will be addressed in detail elsewhere.
Materials and Methods
Parent Cohort
The MrOS Study enrolled 5,995 participants from March 2000 through April 2002 as described elsewhere.26, 27 Briefly, recruitment occurred at six US academic medical centers in Birmingham AL, Minneapolis MN, Palo Alto CA, Pittsburgh PA, Portland OR, and San Diego CA. Eligible participants were at least 65 years of age, able to walk unassisted by another person, and had at least on natural hip for femoral bone density measurement. The recruitment criteria were established so that the study results from the cohort would be applicable to a broad population of similarly aged community-dwelling men.26 All participants in the MrOS cohort provided written informed consent, completed the baseline self-administered questionnaire, and attended the baseline visit at their local site at which lumbar spine films were obtained primarily for assessment of vertebral fractures. From March 2005 through April 2006, surviving participants returned for a second study visit at which the baseline measures and assessments, including the spine films, were repeated. The average time between visits was 4.6 (±0.4) years.
Spine radiographs were obtained at each study visit using the same standardized protocol. Participants were placed on their left side in the lateral position with legs flexed and both arms at right angles to the body. The long axis of the spine was set parallel to the table and the mid-axillary (coronal) plane of the body was aligned to the table midline. Images were obtained from T12 to S1. All films were sent to the MrOS San Francisco Coordinating Center for central quality review, digitization and archiving.
Selection of the Study Sample
To establish initial data on spinal conditions other than vertebral fracture in the MrOS cohort, 300 participants were randomly sampled at baseline using a computer generated random number. Available baseline and visit 2 films for this sample were transferred to Oregon Health & Science University for analysis. Baseline films that were unreadable from L1 through S1 because of spine fusion surgery or other reasons were eliminated, leaving a study sample of 295.
By the second visit, 12% in the sample had died (32) or voluntarily withdrawn (2) from MrOS and this proportion was comparable to that (11%) in the entire cohort. Of the 261 in our sample eligible for visit 2, 2% (5) refused and 20% (53) completed only the questionnaires; in the entire cohort 2% refused and 13% completed only the questionnaires. Among the 203 in the sample who attended the visit, 5 refused the radiograph and 8 had films that were missing in the central archive, leaving 190 in the sample with both a baseline and follow-up radiograph.
Assessment of Spondylolisthesis
The presence of spondylolisthesis was assessed from L1 to S1. The magnitude of listhesis was measured by dividing the slip distance by the caudad body width.28, 29 The Meyerding Grading Scale was used to categorize the magnitude of slip with Grade 0: no slip, Grade I: 1–25%, Grade II: 26–50%, Grade III: 51–75%, Grade IV: 76–100%, and Grade V: complete slippage.28 Measurements of equal to or greater than 5% slip percentage were considered spondylolisthesis. Baseline and follow-up images were analyzed separately, so that radiographs of the same participant were not observed consecutively. Progression of the listhesis at follow-up was defined as a 5% increase in the slip severity.23
Inter- and intra-rater reproducibility was assessed by having both raters (PD, JY) independently evaluate spondylolisthesis on 35 randomly chosen images. The kappa statistic was computed as the measure of agreement. Kappa values demonstrated good inter-rater agreement at L3/4 (0.65) and L5/S1 (0.65) and excellent agreement at L4/5 (1.0). Intra-rater agreement was excellent with Kappa values being 0.84 at L4/5, 1.0 at L3/4, and 1.0 and L5/S1. Agreement was excellent regarding the presence of any lumbar spondylolisthesis, with Kappa values of 0.89 observed for both inter- and intra- rater agreement.
Other Baseline Measures
Comprehensive information regarding medical history, anthropometric, and lifestyle characteristics was obtained at baseline. Self-reported information on stroke, hypertension, cancer, myocardial infarction, and diabetes was evaluated in the study. History of cigarette smoking was classified for analysis as never, past, or current. The Physical Activity Scale for the Elderly (PASE) provides a total physical activity score calculated from sub-scores for leisure, occupational and household activities.30 Men also reported whether they walked daily for exercise. Height (cm) was measured using a Harpenden stadiometer. Participants were weighed (kg) on balance beam or digital scales while wearing light indoor clothing and no shoes. Body mass index (BMI) (kg/m2) was calculated from the height and weight measures and categorized as normal (18–24.9), overweight (25–29.9), and obese (≥30).31
Statistical Methods
Spondylolisthesis prevalence was estimated as the proportion with any listhesis in the L1 to S1 region divided by the number (295) in the study sample. Prevalence at each vertebral level was also estimated. Proportions of men with progression or with onset of spondylolisthesis were computed among 190 men with radiographs at both visits. Progression was estimated among men with baseline spondylolisthesis. New onset of spondylolisthesis was estimated among men without this condition at baseline, and was further described as occurring at a vertebral level previously without baseline anterolisthesis.
Distributions of baseline characteristics among men with and without prevalent spondylolisthesis were first compared with chi-square tests for categorical variables or t-tests for continuous variables. The association of spondylolisthesis prevalence with baseline characteristics was estimated with age-adjusted prevalence ratios (PR) and 95% confidence intervals (CI) from log binomial regression models.32, 33 Age-adjusted PR are presented because none of the other baseline variables were observed to confound any of the estimated associations. Statistical analyses were conducted using SAS software (SAS Institute, Cary NC, USA).
Results
Baseline Characteristics
Distributions of baseline characteristics in the study sample and in the entire cohort were comparable (Table 1). Men in the sample were on average 74 years of age, 174 cm tall, with BMI of 28 kg/m2. Most were Caucasian. Over half had at least a college degree, 25% were obese, nearly 60% reported a history of smoking, and most reported their health as either excellent or good. Diabetes and heart disease were common.
Table 1.
Distributions of baseline characteristics among men in the study sample and in the entire MrOS cohort.
| Study Sample | MrOS Cohort | |
|---|---|---|
| Number | 295 | 5,995 |
| Characteristic | Mean ±sd | Mean ±sd |
| Age (years) | 74±6 | 74±6 |
| Height (cm) | 174±7 | 174±7 |
| BMI (kg/m2) | 28±4 | 27±4 |
| Physical Activity Score * | 150±66 | 147±68 |
| Number (%) | Number (%) | |
| Race | ||
| Caucasian | 265 (90%) | 5362 (89%) |
| African American | 13 (4%) | 244 (4%) |
| Asian | 8 (3%) | 191 (3%) |
| Hispanic | 5 (2%) | 127 (2%) |
| Other | 4 (1%) | 71 (1%) |
| Education | ||
| Less than high school | 24 (8%) | 393 (7%) |
| High school | 106 (36%) | 2413 (40%) |
| College | 85 (29%) | 1727 (29%) |
| Graduate School | 80 (27%) | 1462 (24%) |
| BMI (kg/m2) | ||
| Normal: 18–24 | 46 (19%) | 1329 (22%) |
| Overweight: 25–29 | 141 (55%) | 3169 (53%) |
| Obese: 30+ | 84 (26%) | 1495 (25%) |
| Smoking | ||
| Never | 125 (42%) | 2249 (38%) |
| Past | 164 (56%) | 3539 (59%) |
| Current | 6 (2%) | 206 (3%) |
| Self Rated Health | ||
| Excellent | 92 (31%) | 2017 (34%) |
| Good | 153 (52%) | 3119 (52%) |
| Fair | 44 (15%) | 760 (13%) |
| Poor/Very Poor | 6 (2%) | 97 (2%) |
| Diabetes | 36 (12%) | 653 (11%) |
| Angina | 51 (17%) | 856 (14%) |
| High Blood Pressure | 146 (49%) | 2581 (43%) |
| Myocardial Infarction | 53 (18%) | 835 (14%) |
From the Physical Activity Scale for the Elderly(PASE)30
Spondylolisthesis Prevalence, Progression and Incidence
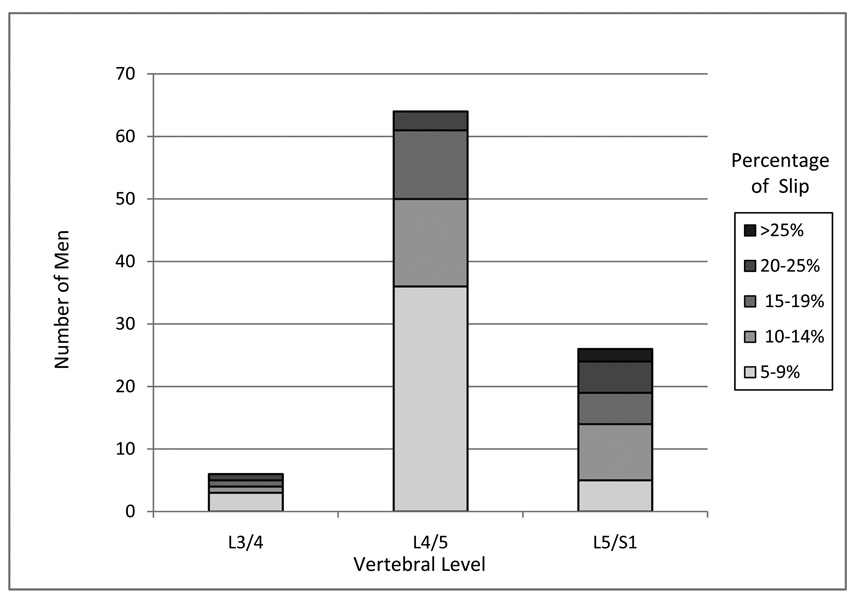
The prevalence of spondylolisthesis at any level was 31% (95% CI: 26%–36%) (Table 2). Spondylolisthesis was observed at L3/4, L4/5 and L5/S1, with the greatest prevalence at L4/5. Only one vertebral level was involved 96% of time (data not shown). Among those with spondylolisthesis, the percent of slip ranged from 5% –28%, with 99% being Meyerding grade I slip (Figure 1).
Table 2.
Spondylolisthesis prevalence, progression and incidence according to vertebral level among men ages ≥ 65 years: the MrOS Study
| Prevalence* | Progression** | Incidence† | |
|---|---|---|---|
| Number of men examined | 295 | 57 | 133 |
| Vertebral level affected | Number (%) | Number (%) | Number (%) |
| Any level | 92 (31%) | 7 (12%) | 16 (12%) |
| L1/2 | 0 (0) | 0 (0) | 0 (0) |
| L2/3 | 0 (0) | 0 (0) | 0 (0) |
| L3/4 | 6 (2%) | 0 (0) | 2 (2%) |
| L4/5 | 64 (22%) | 5 (9%) | 14 (11%) |
| L5/S1 | 26 (9%) | 2 (4%) | 0 (0) |
Spondylolisthesis was classified as ≥ 5% slippage.
Progression was defined as ≥ 5% increase in slip percentage among men with baseline spondylolisthesis.
New onset of spondylolisthesis among men without spondylolisthesis at any vertebral level at baseline.
Figure 1.
Number of men in category of slip percentage at each lumbar vertebral level with spondylolisthesis: the MrOS study.
Among 190 men with films at both visits, 57 (30%) had prevalent spondylolisthesis, in agreement with the estimate from the entire sample (Table 2). Progression of spondylolisthesis was observed among 12% (95% CI: 4%–21%) with baseline spondylolisthesis. The increase in slip ranged from 5%–10%. Among 133 men without baseline spondylolisthesis, 12% (95% CI: 7%–18%) had a new onset. New occurrences were observed at L3/4 and L4/5. Three men with baseline spondylolisthesis experienced new onset at a different vertebral level (data not shown).
Correlates of Spondylolisthesis Prevalence
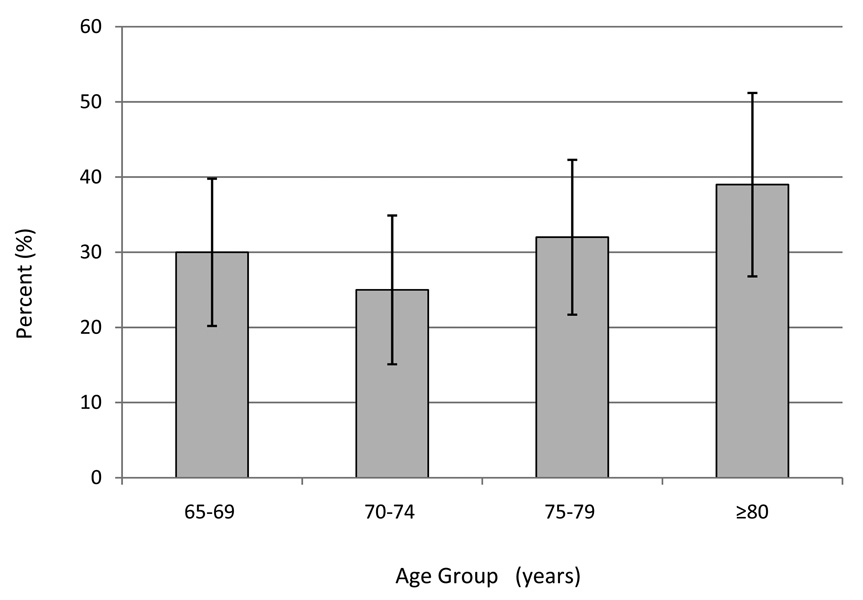
Spondylolisthesis prevalence increased with age (Figure 2). Among men aged ≥80 years, 39% were affected, compared with 30% among men aged 65–69. In age-adjusted analyses, spondylolisthesis prevalence did not vary significantly by height, BMI, smoking history, or medical history (Table 3). Prevalence was greater among men with higher PASE scores. Separation according to leisure time or household activities indicated that prevalence was greater among those with higher leisure activity (43% in the highest as compared to 31% in the lowest category, p for trend = 0.06). Consistent with these observations, spondylolisthesis prevalence was elevated among those who reported walking daily for exercise compared to those who did not. When we repeated the analysis with slip percentage in categories of ≥15% (n=28) compared with ≤14% (n=267), the prevalence ratios and 95% CI based on this classification were not materially different from those shown in Table 3 for each characteristic.
Figure 2.
Prevalence of spondylolisthesis according to age among men: the MrOS study. Numbers in groups are 26 (65–69 years), 20 (70–74 years), 26 (75–79 years) and 26 (≥80 years). Bars denote 95% confidence intervals for the proportion.
Table 3.
Spondylolisthesis prevalence according to demographic, lifestyle, and medical conditions among men age ≥ 65 years: the MrOS Study.
| Number (%) with Spondylolisthesis |
Age-adjusted PR |
95% CI | P-value* | |
|---|---|---|---|---|
| Height (cm) | ||||
| < 170 | 30 (38%) | 1.3 | 0.8–2.1 | 0.26 |
| 170–174 | 22 (30%) | 1.1 | 0.6–1.8 | |
| 175–179 | 22 (28%) | 1.0 | 0.6–1.7 | |
| ≥ 180 | 17 (27%) | Referent | ||
| BMI (kg/m2) | ||||
| Normal: 18–24 | 24 (33%) | Referent | ||
| Overweight: 25–29 | 51 (32%) | 1.0 | 0.7–1.4 | 0.52 |
| Obese: ≥ 30 | 17 (26%) | 0.8 | 0.5–1.4 | |
| Smoking | ||||
| Never | 42 (34%) | Referent | ||
| Ever | 50 (29%) | 0.9 | 0.6–1.2 | 0.44 |
| Medical Condition** | ||||
| Diabetes-No | 79 (31%) | Referent | ||
| Diabetes-Yes | 13 (36%) | 1.3 | 0.8–2.0 | 0.33 |
| Angina--No | 78 (32%) | Referent | ||
| Angina-Yes | 14 (27%) | 0.8 | 0.5–1.3 | 0.44 |
| Myocardial Infarction-No | 72 (30%) | Referent | ||
| Myocardial Infarction-Yes | 20 (38%) | 1.3 | 0.9–1.9 | 0.22 |
| Hypertension-No | 48 (32%) | Referent | ||
| Hypertension-Yes | 44 (30%) | 0.9 | 0. 7–1.3 | 0.73 |
| Physical Activity Score† | ||||
| < 100 | 20 (31%) | Referent | ||
| 100–140 | 16 (20%) | 0.7 | 0.4–1.2 | 0.11 |
| 141–180 | 30 (38%) | 1.2 | 0.8–2.0 | |
| > 180 | 26 (37%) | 1.3 | 0.8–2.2 | |
| Leisure Activity Score†† | ||||
| ≤10 | 23 (31%) | Referent | ||
| 11–24 | 14 (25%) | 0.8 | 0.5–1.4 | 0.06 |
| 25–50 | 21 (25%) | 0.8 | 0.5–1.3 | |
| >50 | 34 (43%) | 1.4 | 0.9–2.1 | |
| Household Activity Score†† | ||||
| 1–60 | 19 (31%) | Referent | ||
| 61–100 | 26 (29%) | 0.6 | 0.3–1.5 | 0.24 |
| 101–140 | 30 (29%) | 0.6 | 0.3–1.5 | |
| >140 | 17 (45%) | 1.5 | 0.9–2.5 | |
| Walk daily for exercise | ||||
| No | 40 (26%) | Referent | ||
| Yes | 52 (36%) | 1.4 | 1.0–1.9 | 0.07 |
PR=prevalence ratio, CI = confidence interval, BMI = body mass index
Wald chi-square p-value or p-value for a test of linear trend (height, BMI, and the physical activity variables).
Self-report of diagnosis made by a doctor or other health care professional.
From the Physical Activity Scale for the Elderly (PASE)26
Examples of leisure activities are: walking, light recreation like shuffleboard, moderate recreation like dancing, and strenuous sports like jogging, swimming etc.
Examples of household activities are: dusting, washing dishes, vacuuming, scrubbing floors, washing windows etc.
Discussion
New information about the epidemiology of spondylolisthesis among elderly community-dwelling men emerged from this study. First, spondylolisthesis prevalence was 31%, an estimate much greater than previously reported among men. Second, progression of existing spondylolisthesis over about 5 years time was observed in 12%, and new onset of spondylolisthesis occurred among 12% without the condition at baseline. Third, spondylolisthesis prevalence was moderately elevated among men who reported the highest levels of leisure time physical activity and among those who reported walking daily for exercise compared to counterparts with low activity or no daily walking. However, spondylolisthesis prevalence did not vary by height, obesity, history of smoking, diabetes or heart disease.
The spondylolisthesis prevalence of 31% observed in MrOS is nearly identical to the prevalence of 29% among women aged ≥ 65 years from the Study of Osteoporotic Fractures (SOF) cohort.18 Women in SOF and men in MrOS were recruited in a similar manner.34 The similarity of these estimates suggests that the spondylolisthesis prevalence among elderly men and women may not be as disparate as previously reported. However, the prevalence estimate from the present study is considerably higher than the 3%–8% that would be expected based on previous studies of men.19–22 Our estimate may be higher for a number of reasons. First, other studies may have lacked sufficiently large numbers of older men to provide age-specific prevalence estimates, because the study groups included ages spanning from 23–93 years20 or 40–80 years.21 Second, the estimate of 4% for older men from the Framingham cohort may have been spuriously low, because radiographs could not be obtained in half of the participants living at the time.19 Finally, prevalence may vary by birth cohort. MrOS participants enrolled in 2000–2002 may have been exposed to factors which could influence the likelihood of developing spondylolisthesis that men who were studied in the early 1990s19, 20 may not have encountered.
Despite differences in prevalence, our data are consistent with previous reports regarding the anatomic features of spondylolisthesis. Like others,5, 18–20, 23 we observed that the most common location for spondylolisthesis among adults was at the L4/5 level. The majority of spondylolisthesis observed in MrOS was Meyerding grade I and no slips higher than grade II were observed. Similarly, all slips20or the majority19 were Meyerding grade I among men in other cohorts. We observed that the degree of slip ranged from 5%–28%. Not all studies have consistently reported this information. Degree of slip spanned 9%–30% in the Framingham cohort but was not separated for men and women,19 and from 7% to ≥20% among elderly women.18
Spondylolisthesis progression has been minimally studied. Among 145 patients with symptomatic degenerative spondylolisthesis managed non-surgically who were followed on average for 16 years (range 10–18 years), progression defined as a 5% increase in slip severity occurred among 34%;24 but estimates were not reported separately for men and women. In the present study, progression of spondylolisthesis was observed in 12% of men during follow-up that averaged 4.6 years. On an annualized basis, results from both studies indicate that progression occurs at around 2% per year. However, neither study provides detail on the pattern of slip progression. Additional prospective studies are needed to elucidate whether progression occurs relatively constantly over time or whether a maximal slip distance is achieved after which progression ceases.
Smoking and obesity have been implicated in degeneration of the lumbar spine 35 and therefore may play a role in spondylolisthesis etiology. Others have suggested that diabetes and atherosclerotic disease may be associated with degenerative spondylolisthesis.36 However, we did not observe any association between prevalent spondylolisthesis and these factors. Our results are consistent with previous reports that body size and smoking were not associated with spondylolisthesis prevalence among men,20 or among men and women combined.19 Similarly, anterolisthesis prevalence among older women was unrelated to smoking status or diabetes history.18
Our results add to the accumulating data indicating that spondylolisthesis prevalence is higher among those reporting greater physical activity. Among Taiwanese taxi drivers, spondylolisthesis prevalence was 2.2-fold higher among those with a history of frequent strenuous exercise compared to those without.22 Among Italian adults aged ≥40 years, spondylolisthesis prevalence was over 7-fold greater among those who self-reported a heavy workload and among those who had engaged in competitive strenuous sports.25 In contrast, no association between spondylolisthesis and occupational exposure with repeated daily lifting was observed in the Copenhagen cohort.20 The cross-sectional study design precludes our ability to determine the temporal relation between spondylolisthesis and physical activity, making the clinical relevance of this observation uncertain. However, from a mechanical perspective, this association does have biologic plausibility. Physical activity places increased loads on the lumbar spine, which cumulatively could contribute to spondylolisthesis via degeneration of facet joints and or intervertebral discs.37 Prospective studies are needed to clarify the possible role of physical activity in spondylolisthesis etiology.
The use of recumbent lateral radiographs is a potential limitation in our study. One group reported that the magnitude of slip of the L5 vertebral body on the sacrum was at least 2mm greater when measured on standing radiographs than on recumbent radiographs for 13 of 50 patients with spondylolisthesis39 however, these authors failed to comment on slip among the remaining 37 patients for whom slip presumably did not change. In a more rigorous experiment, mean slip distances observed among 125 patients with spondylolysis were not significantly different on recumbent and standing radiographs, being 12.0mm (std=8.8) on recumbent and 12.3 (std=8.6) respectively.38 Flexion-extension radiographs may improve the detection of unstable spondylolisthesis;40 but the appropriate diagnostic imaging assessment remains unresolved.4 Use of recumbent lateral radiographs in this study may have underestimated slip distance, leading us to misclassify some men as being without spondylolisthesis or to assign a lower Meyerding grade to existing spondylolisthesis. Such misclassification would result in an underestimation of spondylolisthesis in the study sample and therefore is not a plausible explanation for the high prevalence of spondylolisthesis we observed.
Follow-up films were not available for all men in our sample. As expected in studies among the elderly, the main reasons for this were death before visit 2 and completion of only the questionnaire portion of the visit. If the likelihood of spondylolisthesis progression or onset differed among men for whom films were and were not available, then estimates of these events could be biased. However, the comparability of baseline spondylolisthesis prevalence (30%) among men with two radiographs to prevalence (31%) in the entire sample indicates that estimates of progression and onset were not biased. Therefore, the most likely consequence of attrition in the cohort is decreased precision of the progression and onset estimates due to fewer numbers of these events than might have been observed if the entire sample had a complete set of films.
This study has several strengths. We report on the prevalence and correlates of spondylolisthesis specifically in a well-characterized cohort of elderly men. The sample is larger and represents a broader geographic distribution than samples on which the previous prevalence estimates among US men have been made.19, 21 The availability of repeat radiographs afforded a unique opportunity to describe changes in spondylolisthesis among community dwelling men who were not pre-selected based on symptoms.
In summary, this study suggests that spondylolisthesis may occur more often among older men than previously reported. Study samples larger than ours are needed to identify factors associated with development and progression of spondylolisthesis in older men.
Funding Acknowledgement
The Osteoporotic Fractures in Men (MrOS) Study is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), and the National Center for Research Resources (NCRR) and the NIH Roadmap for Medical Research through grants U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140.
The study reported herein received Institutional Review Board approval at OHSU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiltse LL, Newman PH, Macnab I. Classification of spondylolisis and spondylolisthesis. Clin Orthop Relat Res. 1976:23–29. [PubMed] [Google Scholar]
- 2.Fredrickson BE, Baker D, McHolick WJ, et al. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am. 1984;66:699–707. [PubMed] [Google Scholar]
- 3.Beutler WJ, Fredrickson BE, Murtland A, et al. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine. 2003;28:1027–1035. doi: 10.1097/01.BRS.0000061992.98108.A0. [DOI] [PubMed] [Google Scholar]
- 4.North American Spine Society. Clinical Guidelines for Multidisciplinary Spine Care. Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis. Burr Ridge, IL: North American Spine Society; 2008. [Google Scholar]
- 5.Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57:467–474. [PubMed] [Google Scholar]
- 6.Weinstein JN, Lurie JD, Olson PR, et al. United States' trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31:2707–2714. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30:1441–1445. doi: 10.1097/01.brs.0000166503.37969.8a. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed May 9th, 2008];Healthcare Cost and Utilization Project, HCUPnet. Available at: http://www.ahrq.gov/data/hcup.
- 9.Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo award winner in clinical studies. degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726–733. doi: 10.1097/01.brs.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 11.Vibert BT, Sliva CD, Herkowitz HN. Treatment of instability and spondylolisthesis: Surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:222–227. doi: 10.1097/01.blo.0000200233.99436.ea. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinotti G, Postacchini F, Fassari F, et al. Predisposing factors in degenerative spondylolisthesis. A radiographic and CT study. Int Orthop. 1997;21:337–342. doi: 10.1007/s002640050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaosa Y, Kikuchi S, Hasue M, et al. Pathoanatomic mechanisms of degenerative spondylolisthesis. A radiographic study. Spine. 1998;23:1447–1451. doi: 10.1097/00007632-199807010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Love TW, Fagan AB, Fraser RD. Degenerative spondylolisthesis. Developmental or acquired? J Bone Joint Surg Br. 1999;81:670–674. doi: 10.1302/0301-620x.81b4.9682. [DOI] [PubMed] [Google Scholar]
- 16.Hammerberg KW. New concepts on the pathogenesis and classification of spondylolisthesis. Spine. 2005;30:S4–S11. doi: 10.1097/01.brs.0000155576.62159.1c. [DOI] [PubMed] [Google Scholar]
- 17.Iguchi T, Wakami T, Kurihara A, et al. Lumbar multilevel degenerative spondylolisthesis: Radiological evaluation and factors related to anterolisthesis and retrolisthesis. J Spinal Disord Tech. 2002;15:93–99. doi: 10.1097/00024720-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Vogt MT, Rubin D, Valentin RS, et al. Lumbar olisthesis and lower back symptoms in elderly white women. the Study of Osteoporotic Fractures. Spine. 1998;23:2640–2647. doi: 10.1097/00007632-199812010-00020. [DOI] [PubMed] [Google Scholar]
- 19.Kauppila LI, Eustace S, Kiel DP, et al. Degenerative displacement of lumbar vertebrae. A 25-year follow-up study in Framingham. Spine. 1998;23:1868–1873. doi: 10.1097/00007632-199809010-00014. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen S, Sonne-Holm S, Rovsing H, et al. Degenerative lumbar spondylolisthesis: An epidemiological perspective: The Copenhagen Osteoarthritis Study. Spine. 2007;32:120–125. doi: 10.1097/01.brs.0000250979.12398.96. [DOI] [PubMed] [Google Scholar]
- 21.Kalichman L, Kim DH, Li L, et al. Spondylolysis and spondylolisthesis: Prevalence and association with low back pain in the adult community-based population. Spine. 2009;34:199–205. doi: 10.1097/BRS.0b013e31818edcfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JC, Chan WP, Katz JN, et al. Occupational and personal factors associated with acquired lumbar spondylolisthesis of urban taxi drivers. Occup Environ Med. 2004;61:992–998. doi: 10.1136/oem.2003.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsunaga S, Sakou T, Morizono Y, et al. Natural history of degenerative spondylolisthesis. pathogenesis and natural course of the slippage. Spine. 1990;15:1204–1210. doi: 10.1097/00007632-199011010-00021. [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: A 10- to 18-year follow-up study. J Neurosurg. 2000;93:194–198. doi: 10.3171/spi.2000.93.2.0194. [DOI] [PubMed] [Google Scholar]
- 25.Mariconda M, Galasso O, Imbimbo L, et al. Relationship between alterations of the lumbar spine, visualized with magnetic resonance imaging, and occupational variables. Eur Spine J. 2007;16:255–266. doi: 10.1007/s00586-005-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Meyereding HW. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371–379. [Google Scholar]
- 29.Timon SJ, Gardner MJ, Wanich T, et al. Not all spondylolisthesis grading instruments are reliable. Clin Orthop Relat Res. 2005:157–162. doi: 10.1097/01.blo.0000154205.10944.72. [DOI] [PubMed] [Google Scholar]
- 30.Washburn RA, Smith KW, Jette AM, et al. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 31.Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 32.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 34.Cummings SR, Cawthon PM, Ensrud KE, et al. Orwoll ES. Osteoporotic Fractures in Men (MrOS) Research Groups. Study of Osteoporotic Fractures Research Groups. BMD and risk of hip and nonvertebral fractures in older men: A prospective study and comparison with older women. J Bone Miner Res. 2006;21:1550–1556. doi: 10.1359/jbmr.060708. [DOI] [PubMed] [Google Scholar]
- 35.Battie MC, Videman T, Gill K, et al. 1991 Volvo award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: An MRI study of identical twins. Spine. 1991;16:1015–1021. [PubMed] [Google Scholar]
- 36.Farfan HF. The pathological anatomy of degenerative spondylolisthesis. A cadaver study. Spine. 1980;5:412–418. doi: 10.1097/00007632-198009000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Troup JD. Mechanical factors in spondylolisthesis and spondylolysis. Clin Orthop Relat Res. 1976:59–67. [PubMed] [Google Scholar]
- 38.Saraste H, Brostrom LA, Aparisi T, et al. Radiographic measurement of the lumbar spine. A clinical and experimental study in man. Spine. 1985;10:236–241. doi: 10.1097/00007632-198504000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Lowe RW, Hayes TD, Kaye J, et al. Standing roentgenograms in spondylolisthesis. Clin Orthop Relat Res. 1976;10:80–84. [PubMed] [Google Scholar]
- 40.Mossaad MM. Degenerative lumbar spondylolisthesis with spinal stenosis: Natural history, diagnosis, clinical presentation, and nonoperative treatment. In: Herkowitz HN, Dvorak J, Bell G, Nordin M, Grob D, editors. The Lumbar Spine. Official Publication of the International Society for the Study of the Lumbar Spine. Third ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 514–523. [Google Scholar]