Abstract
It is accepted that the aldehyde-based fixation of cells can affect the immunodetection of antigens; however, the effects of tissue processing on immunodetection have not been analyzed systematically. We therefore investigated the effects of aldehyde-based fixation and the individual steps of tissue processing on immunohistochemical detection of specific antigens. DU145 (prostate) and SKOV3 (ovarian) cancer cell lines were cultured as monolayers on microscope slides. The immunohistochemical detection of Ki67/MIB-1 and PCNA was evaluated after various times of fixation in 10% neutral-buffered formalin (NBF) plus after each of the individual cumulative steps of tissue processing. The effect of antigen retrieval (AR) was evaluated concomitantly as an additional variable. Our results indicate that, in addition to fixation, each of the different steps in tissue processing has effects on immunorecognition of the epitopes recognized by these antibodies. The extensive dehydration through ethanols to absolute ethanol had only modest effects except for the detection of Ki67/MIB-1 in SKOV-3 cells where the effect was stronger. In general, however, the establishment of a hydrophobic environment by xylene resulted in the greatest decrease in immunorecognition. Antigen retrieval was able to compensate for most, but not all of the losses in staining following fixation and exposure to xylene; however, AR gave very consistent results for most steps of tissue processing, suggesting that AR should also be used in staining for PCNA. The cellular variations that were noted indicate that the effects of fixation and other steps of tissue processing may depend upon how antigens are packaged by specific cells.
Keywords: Antigen retrieval, epitope masking, formalin fixation, immunohistochemistry, tissue processing, Ki67, PCNA
INTRODUCTION
Immunohistochemistry is one of the more frequently used methods in the characterization of the molecular features of invasive and preinvasive neoplastic lesions, e.g., ductal carcinoma of the breast and ductal carcinoma in situ (DCIS). Immunohistochemical detection of biomarkers is now used to assist in early detection and diagnosis and in determining prognosis and risk assessment. More recently, immunohistochemical detection has been expanded to the analysis of biomarkers that are used as surrogate endpoints in evaluating therapies (e.g., gene therapy) as well as in predicting clinical responses to various therapies (predictive). Thus, immunohistochemistry has important roles both in medicine and in research. While the performance of immunohistochemical methods is considered routine, the outcome of immunohistochemical analysis is affected by multiple interdependent variables including the quality and avidity of the primary antibodies and amplification of the secondary detection systems (Grizzle et al. 1998). Also, immunohistochemistry is strongly affected by fixation, especially aldehyde-based fixation, and by potential interactions of fixation with the cumulative steps of tissue processing and antigen retrieval (AR) (Grizzle et al. 2001, 2008).
Formalin fixation has been implicated in the loss or masking of epitopes (antigenic determinants) during immunohistochemistry. Formalin fixation causes denaturation of most molecules (Dapson 2007); it either causes changes in the conformation of the proteins together with their epitopes, modifies the epitopes directly by binding to them (Bogen et al. 2008), or blocks access of antibodies or detection systems to these epitopes (O’Leary et al. 2008). Such changes prevent the antibody from recognizing or binding to altered or hidden epitopes (Hayat 2001). Also, aldehyde fixation may affect the secondary detection system by similar changes. The major effects of fixation with aldehydes have been considered to be crosslinking of the antigens, which acts to change the configuration of proteins or peptides and render the epitopes undetectable or unreachable by specific antibodies. Currently, however, most laboratories use a relatively short time of fixation (< 12 hr.) which may be too short even for optimal histochemical staining (Dapson 2007). Fixation for this period of time may not result in extensive crosslinking and many changes of aldehyde fixation attributed currently to crosslinking are more likely due to the addition of reactive hydroxymethyl groups (i.e., -CH2OH) (Eltoum et al. 2001a., Eltoum et al. 2001b., Grizzle et al. 2008, Bogen et al. 2008, O’Leary et al. 2008).
Loss of immunorecognition in paraffin processed tissue has been well documented (Arnold et al. 1996, Eltoum et al. 2001a, 2001b; Grizzle et al. 2001, 2008; Namimatsu et al. 2005; Rait et al. 2004a; Rait et al. 2004b); while this has usually been attributed to fixation in 10% neutral buffered formalin, the contributions of tissue processing to loss of immunorecognition have not been studied adequately. In addition to fixation, the steps involved in tissue processing include dehydration in alcohols, the establishment of a hydrophobic environment which is produced by infiltration with a hydrophobic solvent, such as xylene, and impregnation of tissue in the hydrophobic environment with paraffin (for review see Grizzle et al. 2008; Spencer and Bancroft 2008). It is evident that during one or more of these steps of tissue processing some epitopes may be altered (see Dapson, 2007, for a review). Also, the formation of large compact protein complexes or the refolding of the protein may hinder the binding of the antibody and mask the epitope (Hayat 2001, see Guest Editorial, Grizzle 2008, this issue). In addition, chemical modification secondary to dehydration plus establishing a hydrophobic environment may contribute to irreversible alteration of the epitope and inhibit binding of the primary or secondary antibody or detection system. Although, both fixation and tissue processing may influence the quality of immunostains, the effects of each of the steps of fixation and tissue processing on the quality of immunostains have not been evaluated adequately.
We therefore undertook an analysis of the effects of fixation, tissue processing, and AR on the outcome of immunohistochemical staining. For these studies, we used a model of fresh tissue in which cells were cultured as monolayers on microscope slides maintained in media in Petri dishes. After rinsing, the cells were subjected to fixation for different periods of time in combination with the individual cumulative steps of tissue processing prior to immunohistochemical staining with and without AR. Two cancer cell lines, DU145 (prostate cancer) and SKOV3 (ovarian cancer) were used in the studies. The effects on detection of proliferating cell nuclear antigen (PCNA) and Ki67/MIB-1 were compared. PCNA and Ki67 are both nuclear antibodies used to monitor the extent of cellular proliferation, but each exhibits a different pattern of epitope masking upon fixation and processing to paraffin. Staining for PCNA is sometimes performed without AR; whereas, detection of the MIB-1 epitope of Ki67 in formalin-fixed, paraffin-embedded tissues requires AR (Grizzle et al. 2001). The results of this study, indicate that the cumulative steps in tissue processing greatly affect immunorecognition and that the effects differ with cell type; however, the establishment of the hydrophobic environment was usually more detrimental to immunorecognition than was dehydration; in addition, the effects of establishing a hydrophobic environment blocked recognition of Ki67/MIB-1 in DU145 cells even when the cells were not fixed in an aldehyde based fixative. In contrast, Ki67/MIB-1 staining is not blocked when frozen sections are fixed for 24 hours in 10% NBF prior to immunostaining (Stockard et al. unpublished).
MATERIALS AND METHODS
Cells and Antibodies
The DU145 and SKOV3 cells were obtained from the ATCC (Manassas, VA) and maintained in culture according to the guidelines recommended by the ATCC. The primary antibodies used were monoclonal anti-Ki67/MIB-1 (clone MIB-1, Biogenex, San Ramon, CA), which was used at a dilution of 1:120 in phosphate-buffered saline with 1% bovine serum albumin, 1 mM ethylene diamine tetra-acetic acid (EDTA), and 1.5 mM sodium azide, pH 7.6 (PBE) and anti-PCNA (clone PC10; Santa Cruz Biotechnology, Santa Cruz, CA), which was used at a dilution of 1:18,000 in PBE (pH 7.6). Goat serum was obtained from Sigma, Aldrich, Inc., St. Louis, Mo. The dilutions were chosen specifically to result in “light” immunostaining to ensure changes in staining could be detected, i.e., staining was not saturated.
Culture of cells on microscope slides
To gain a clear insight into the effects of the duration of fixation on epitope masking, DU145 and SKOV3 cells grown on microscope slides were used as models of fresh tissues. Pre-cleaned microscope slides (#00299, Surgipath, Richmond, IL) were immersed in a staining dish containing 10% poly-lysine (Sigma-Aldrich, Inc., St. Louis, MO) for 5 min., removed and then left to dry overnight at room temperature. The coated slides were transferred into Petri dishes (Pyrex, 14 cm diameter) and autoclaved. Preparation of the slides using this protocol reduced the variations in cell staining observed in pilot studies in which the slides were prepared using commercially available slides without coating with poly-lysine. The cells were harvested from flasks by trypsinization and the cell-suspension seeded into the autoclaved Petri-dishes that contained the sterile microscope slides coated with poly-lysine in the media recommended by ATCC. After incubation in a water-jacketed incubator (Forma Scientific, Inc.) with a 5% CO2 atmosphere at 37°C overnight, the microscope slides were rinsed gently with PBS and the attached cells were then fixed and processed.
Fixation and Tissue Processing
The cells were fixed in 10% NBF (Richard Allan Scientific, Kalamazoo, MI) at 4°C for the indicated periods of time (no fixation, 5 min., 6 hr., 24 hr., 48 hr. and 72 hr.). Tissue processing was carried out manually to mimic the individual cumulative steps of automated processing. Each cumulative step of tissue processing was analyzed as to the effects of various times of fixation on immunorecognition in individual experiments which modeled each of the cumulative steps of tissue processing.
In a separate experiment, a fixation time of 24 hr. was selected and the effects on immunorecognition of the various steps in tissue processing were analyzed in one experiment. This permitted the effects on immunorecognition of individual steps of tissue processing to be compared statistically.
Immunodetection
Prior to immunostaining, the microscope slides with cells were rinsed in Tris buffer (pH 7.6) for 10 min., and the cells permeabilized using 0.5% Triton X-100 (Sigma Aldrich, Inc., St. Louis, MO) in PBS for 3 min. PAP pens were used to identify the boarder of the microscope slides so that drainage of reagents and edge effects on staining were minimized. The slides were immunostained on a DAKO Autostainer (DAKO, Carpinteria, CA) programmed to the standard protocol used in the laboratory. The program was as follows: 5 min. exposure to 3% aqueous H2O2 to quench endogenous peroxidase; 1 hr. incubation with 3% goat serum to reduce nonspecific staining; 1 hr. incubation with each primary antibody. All slides were rinsed with Tris buffer pH 7.6 and incubated for 10min.in Multispecies Link (Signet, Dedham, MA) washed, incubated for 5 min. in Streptavidin Label (Signet) and washed before incubation for 7 min. with diaminobenzedine (DAB) (Biogenex). Finally, the slides were rinsed with deionized water, counter-stained with Harris’ hematoxylin for 1 min. 15 sec., blued in tap water, and dehydrated in 70%, 90% and absolute ethanol, before clearing in three changes in xylene (Fisher Scientific, Fair Lawn, NJ) (5 min. each from dehydration to clearing). Coverslips were mounted on the slides using Permount. The slides were stored at room temperature until evaluated.
Antigen Retrieval
For antigen retrieval, the slides were submerged in plastic Coplin jars filled with 0.01M EDTA (pH 8.0) in an electric pressure cooker (Cook’s Essentials, model CEPC 800) containing approximately 500 ml of deionized water for 5 min. at high pressure, 121°C.
Analysis of immunostaining
Five fields of the stained slides were selected at random and photographed at a magnification of X400 using a Nikon model 14.0 microscope that was equipped with a SPOT digital camera linked to a computer loaded with Hitachi SuperScan Pro 800 software. The pictures of each field were printed separately using a Hewlett-Packard Desk Jet 990CSE Professional Series printer. Each cell in the photograph was assessed using a scoring system developed in our laboratory (Arnold et al. 1996). In this scoring system the intensity of nuclear staining is determined by assigning the staining of the nuclei a value from 0.0 (for no nuclear staining) up to a maximum of 4.0 (for darkest staining) in 0.5 increments. The tallies were entered into a Microsoft Excel spreadsheet template designed to calculate the total number of cells from all the five fields per treatment by staining intensity. From the template, the percentages of cells staining at each intensity level were obtained and used to determine the pattern of immunostaining and the total immunostaining score. Each immunostaining score is a product of the percentage of cells staining and the staining intensity. The total immunostaining score of a treatment or slide is obtained by determining the sum of all individual intensity immunostaining scores, divided by 100.
In the separate experiment (24 hour fixation) to determine the effects on immunorecognition of the various steps in tissue processing, the immunostaining was were analyzed similarly to permit the immunostaining scores of the individual steps of tissue processing to be compared statistically.
Statistical analysis was done using the proc CATMOD procedure in SAS for categorical data analysis (SAS Institute Inc., 1999). Counting of the number of cells and scoring (categorization) of the intensity of nuclear staining was done manually by the same person twice and there was no significant difference between the two counts (p<0.73, χ2=0.11, df=1). The scoring was very consistent so most conditions studied were statistically different (p<0.05); however, in many cases only a small difference was detected. Small differences in immunostaining have no clinical or practical meaning. In such cases, we describe a difference as a statistically significant, but not a practical difference.
RESULTS
Immunodetection of Ki67/MIB-1
In this first series of experiments, we determined the effects of each of the cumulative steps of tissue processing after exposure to fixative for different periods of time. Staining at each condition was evaluated with or without antigen retrieval. To ensure that the staining was comparable and the times of tissue fixation could be compared statistically, all of the slides representing a particular cumulative stage of tissue processing were stained at the same time. The purpose of this step was to evaluate the pure effects of fixation without tissue processing on immunodetection.
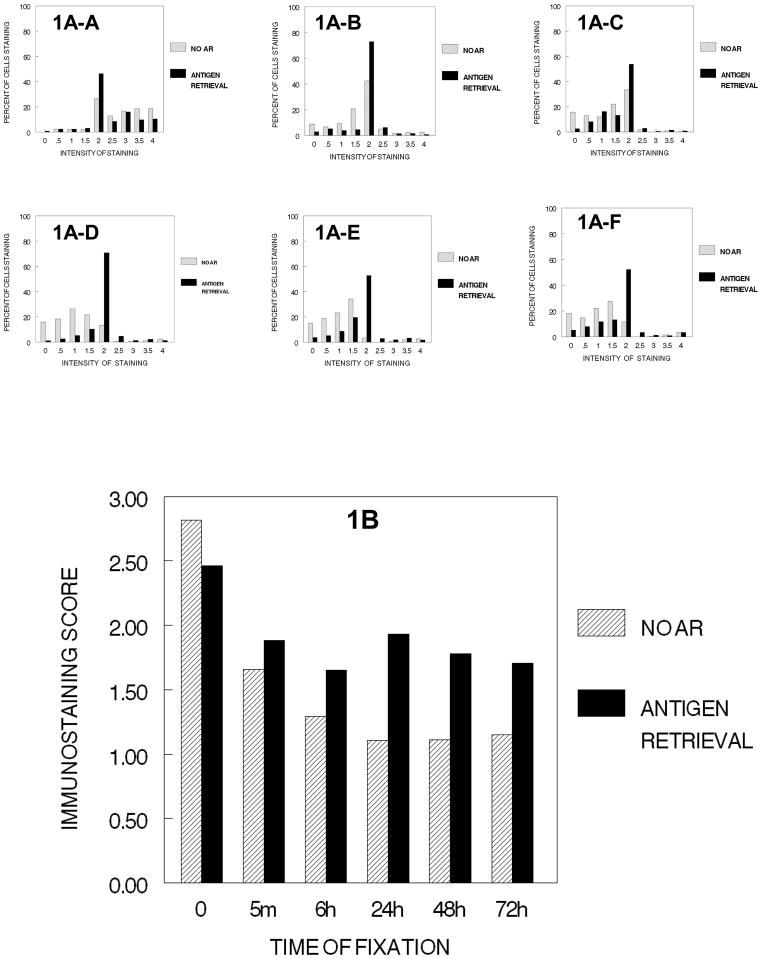
We found that Ki67 staining was localized mostly within the nucleus, although observable amounts of oxidized and precipitated DAB were found in the cytoplasm in the close proximity of the nucleus after fixation for 5 min. In general, mitotic cells including the chromosomes in all phases of mitosis were stained strongly for Ki67 as has been described previously (Hayat, 2001). The effects of fixation on immunodetection of Ki67 in DU145 cells are shown in Figure 1. We found that fixation was not required to demonstrate Ki67 by immunohistochemistry; in fact, the most intense immunostaining was obtained using cells that were neither fixed (0 min.) nor subject to tissue processing. Figure 1A-A demonstrates the distribution of cell staining for Ki67 with no fixation and no processing. As soon as there is even minimal fixation (5 min.), there is a practical reduction in the immunostaining score (p<0.001) and a change in the distribution; subsequently, the distributions of cell staining continues relatively unchanged until 24 hr. of fixation (1A–D); from 24 hr. to 72 hr., the distribution of staining remains relatively constant (1A–D to 1A–F). Figure 1B demonstrates that the immunostaining score decreases from no fixation to 24 hr. of fixation in 10% NBF. Each immunostaining change 0 min. to 5 min. to 6 hr. to 24 hr. is statistically significant (p<0.002) and of practical importance. In addition, we found that exposure to fixative for time periods of 24 hr. or longer did not result in a greater reduction in staining than that observed after fixation for 24 hr.
Figure 1.
DUI45 cells were either not fixed (0 min.) (1A-A) or fixed in 10% NBF at 4°C for 5 min. (1A–B), 6 hr. (1A–C), 24 hr. (1A–D), 48 hr. (1A–E) or 72 hr. (1A–F), washed, subjected to no antigen retrieval or to antigen retrieval, and then immunostained to detect the Ki67 antigen. Panel A demonstrates the percentage of cells stained at each intensity (1A-A to 1A–F). Panel B demonstrates the immunostaining scores calculated based on the intensity of staining and the proportion of cells staining at each intensity.
When antigen retrieval (AR) is used, the highest value occurs with no fixation. (p<0.001) and there is no practical difference in immunostaining as a result of time of fixation after 5 min. to 72 hr. As would be expected, AR did not enhance the intensity of staining of cells that were not fixed. For Ki67, the highest value of staining following AR was with no fixation (Fig 1B). Comparison of the immunostaining values for cell preparations that had been fixed for various time periods and then stained following AR, indicated that AR significantly improved (p<0.001) immunostaining for each time of fixation and improved the consistency of staining as can be noted in the staining distributions of Figures 1A-A to 1A–F. However, even after antigen retrieval, the staining intensity of Ki67 in fixed cells was never as high as that observed in cells that had not been fixed (p<0.001). Similarly, the immunostaining following AR was never as high in fixed specimens (5 min., 6 hr., 24 hr., 48 hr., 72 hr.) as it was for the unfixed specimen (p<0.001, practical difference). Thus, immunorecognition following AR was never complete.
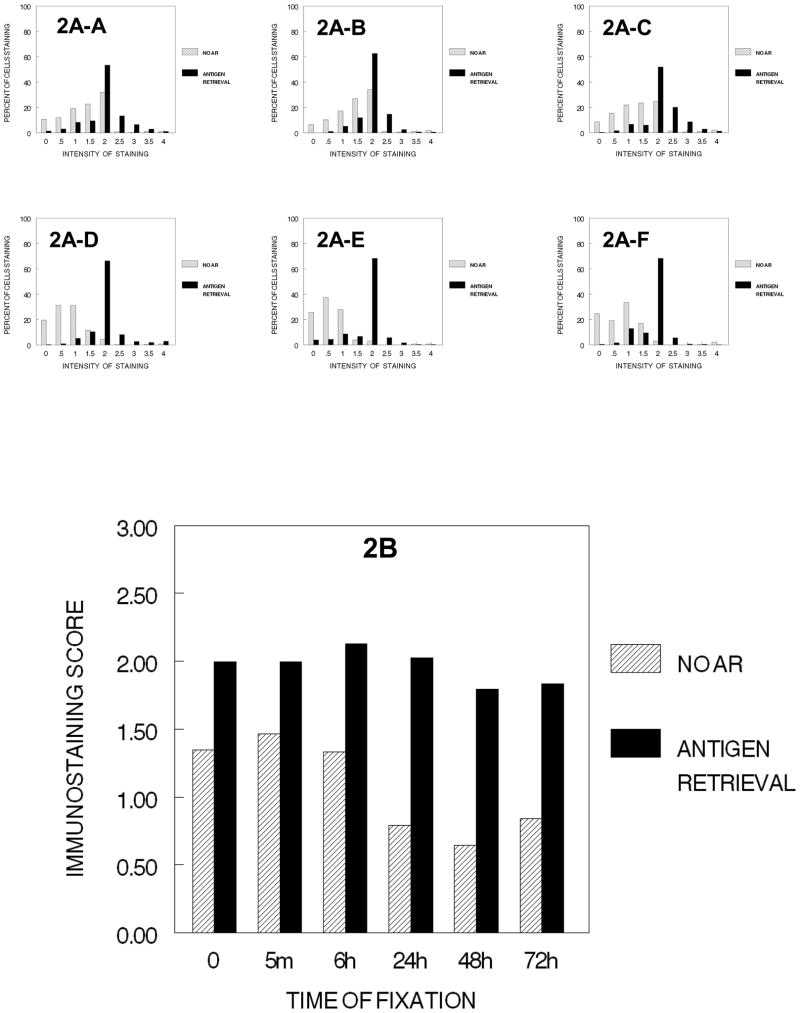
We next analyzed the effects of fixation in combination with the usual second step of tissue processing after formalin, i.e., exposure to 70% ethanol for 1 hr. (Figure 2). The immunostaining of cells that had not been exposed to 10% NBF was reduced on exposure to 70% ethanol but immunostaining could be recovered partially with AR (Fig. 2B), suggesting that an effect of fixation by ethanol had occurred and that ethanol fixation reduced immunorecognition and that this reduction also could be reversed partially by AR. Treatment with 70% ethanol for 1 hr. following fixation for ≥24 hr. resulted in a reduction in the percentage of cells stained and a statistically significant (p<0.001) and practical reduction in immunostaining values as compared to treatment with 70% ethanol following fixation for 6 hr. or less. AR was successful in recovering a large part of the immunodetection in all treatment conditions (p>0.001). As the results shown in Figures 1 and 2 were obtained in different experiments, the values cannot be compared statistically; however, the results suggest that the immunostaining values obtained after AR were never as high as those observed when the cells were not subjected to fixation plus ethanol treatment (Figure 1). This would suggest that not all immunorecognition lost on fixation with either formaldehyde or ethanol is recovered by AR. Of interest is the consistency of staining following AR as is noted in 2A-A through 2A–F and 2B. This supports the importance of using antigen retrieval in most conditions of immunohistochemical staining.
Figure 2.
DUI45 cells were either not fixed (0 min.) (2A-A) or fixed in 10% NBF at 4°C for 5 min. (2A–B), 6 hr. (2A–C), 24 hr. (2A–D), 48 hr. (2A–E) or 72 hr. (2A–F), washed, then all were immersed in 70% ethanol at room temperature for 1 hr. The cells were then subjected to antigen retrieval or no antigen retrieval, and then immunostained to detect the Ki67 antigen, Panel A. The percentage of cells stained at each intensity, Panel B. The immunostaining score was calculated as described.
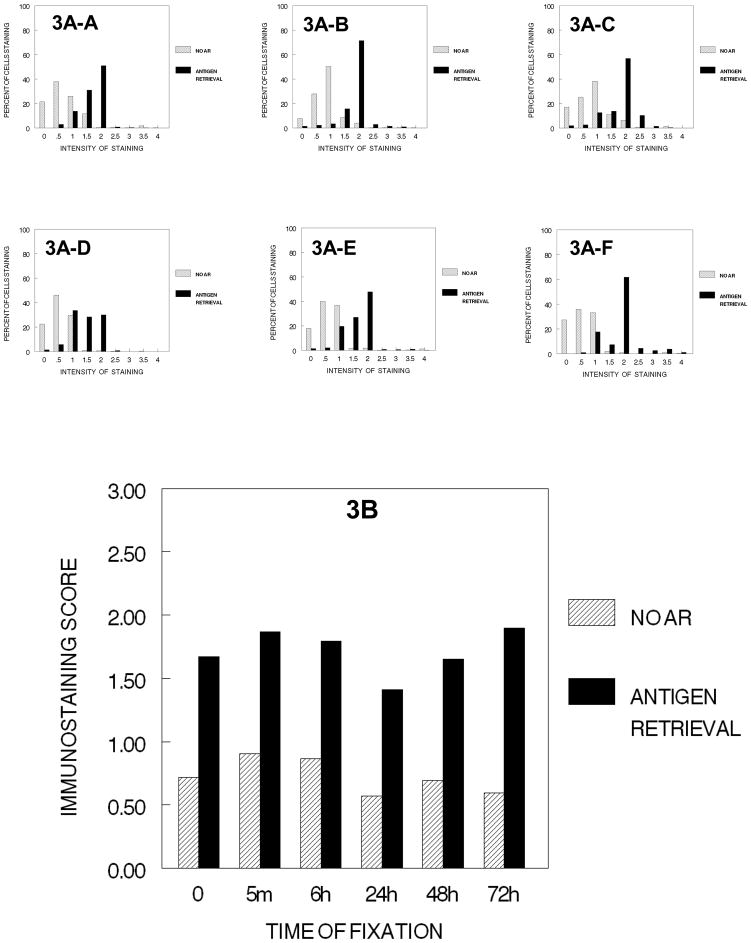
After the fixed cells were subjected to the three initial dehydration steps of tissue processing (i.e., 70% ethanol for 1 hr. + 80% ethanol for 1 hr. + 90% ethanol for 1 hr.) there was no practical difference in immunostaining even though values were slightly lower after fixation in 10% NBF for 24 hr. or more, similar to the results after step 1, 70% ethanol for 1 hour (Figure 2). The distribution of values were also very similar (3A-A to 3A–F) regardless of the length of time the cells had been fixed in 10% NBF (Fig. 3). Antigen retrieval resulted in significant recovery of immunorecognition of the Ki67 antigen to the levels attained with any amount of fixation (p<0.001).
Figure 3.
DUI45 cells were either not fixed (0 min.) (3A-A) or fixed in 10% NBF at 4°C for 5 min. (3A–B), 6 hr. (3A–C), 24 hr. (3A–D), 48 hr. (3A–E)or 72 hr. (3A–F), washed, then all were immersed in 70% ethanol for 1 hr.,80% ethanol for 1 hr., and 90% ethanol for 1 hr. at room temperature. The cells were then subjected antigen retrieval or no antigen retrieval and immunostained to detect the Ki67 antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B demonstrates the immunostaining score that was calculated as described.
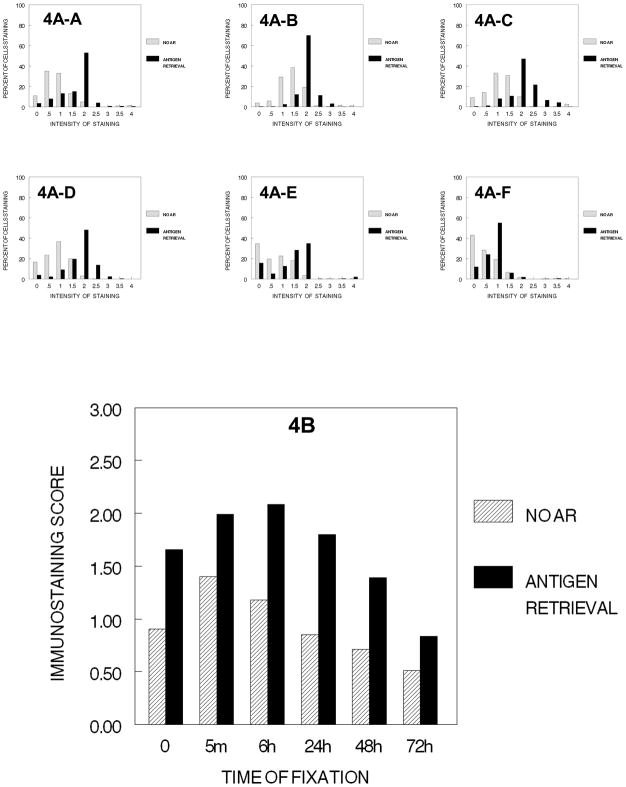
On exposure of the cells to the cumulative steps of tissue processing from 70% ethanol through absolute ethanol, we found that complete dehydration did not result in a practical reduction in staining of Ki67 (Fig. 4B). After 6 hr. of fixation, there was a practical and statistically significant reduction in immunostaining, 6 hr. > 24 hr. > 48 hr. > 72 hr. (p<0.001). Antigen retrieval significantly improved the immunostaining of cells that had been dehydrated after fixation for a short time (5 min. to 6 hr.); however, it was less effective for cells fixed for 24 hr. to 72 hr. This decrease in immunostaining after 6 hr. of fixation and AR was of practical importance and of statistical significance (p<0.001). Thus, there were strong interactive effects on immunostaining between the state of dehydration and each of the specific times of fixation in 10% NBF.
Figure 4.
DUI45 cells were either not fixed (0 min.) (4A-A)or fixed in 10% NBF at 4°C for 5 min. (4A–B), 6 hr. (4A–C), 24 hr. (4A–D), 48 hr. (4A–E) or 72 hr. (4A–F), washed, then all were immersed in 70% ethanol for 1 hr., 80% ethanol for 1 hr., 90% ethanol for 1 hr., then absolute ethanol for 3 hr. at room temperature. There are four typical sequential steps on a tissue processor and this figure demonstrates the combined effects of these sequential steps. The cells were then subjected to antigen retrieval or no antigen retrieval and immunostained to detect the Ki67 antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
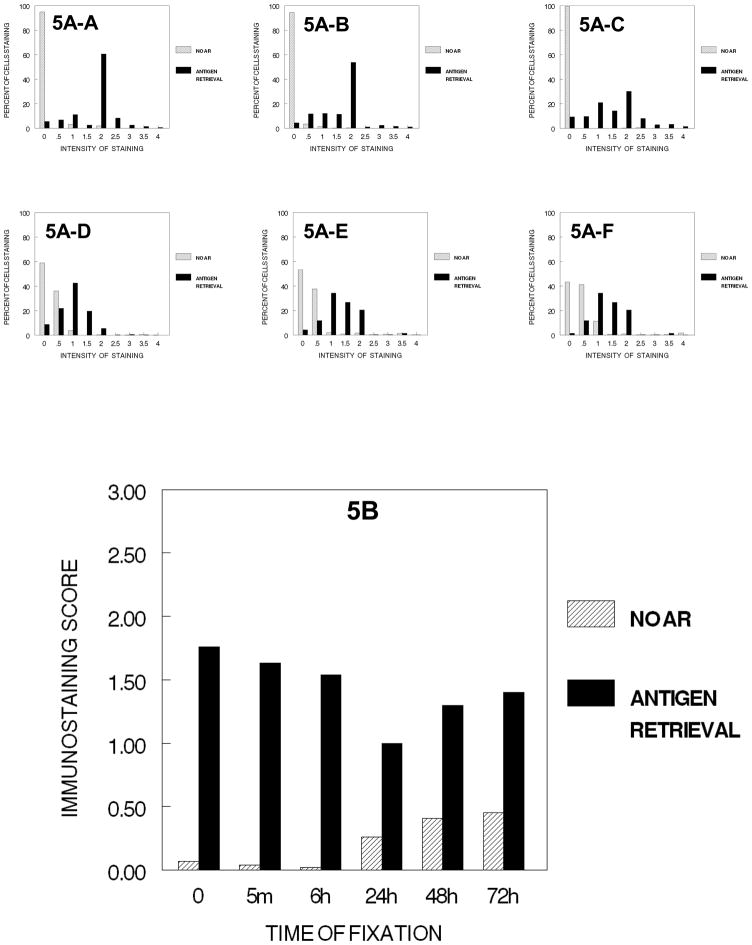
In the next step of tissue processing, the cells after dehydration in all ethanol steps were exposed to xylene and the hydrophobic environment that it establishes; thus, these cells were fixed for various times and processed through 70%, 80%, 90% and 100% ethanol followed by 2 hours of xylene before staining. Notably, this resulted in a practical reduction in immunostaining for all times of fixation including no fixation (Fig. 5). There was an almost complete loss of immunorecognition of Ki67 in cells that had not been fixed or had been fixed for shorter periods of time, and only very slight inconsequential staining was observed in cells that had been fixed for 24 hr. to 72 hr. The overall results (Fig. 5B) is emphasized in the distribution of staining (5A-A to 5A–C) and similarly consistent (5A–D, 5A–F) for 24 to 72 hr. of fixation.
Figure 5.
DUI45 cells were either not fixed (0 min.) (5A-A) or fixed in 10% NBF at 4°C for 5 min. (5A–B), 6 hr. (5A–C), 24 hr. (5A–D), 48 hr. (5A–E) or 72 hr. (5A–F), washed, then all were immersed in 70% ethanol for 1 hr., 80% ethanol for 1 hr., 90% ethanol for 1 hr., absolute ethanol for 3 hr., and then xylene for 2 hr. at room temperature. The cells were then subjected to antigen retrieval or no antigen retrieval and immunostained to detect the Ki67 antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
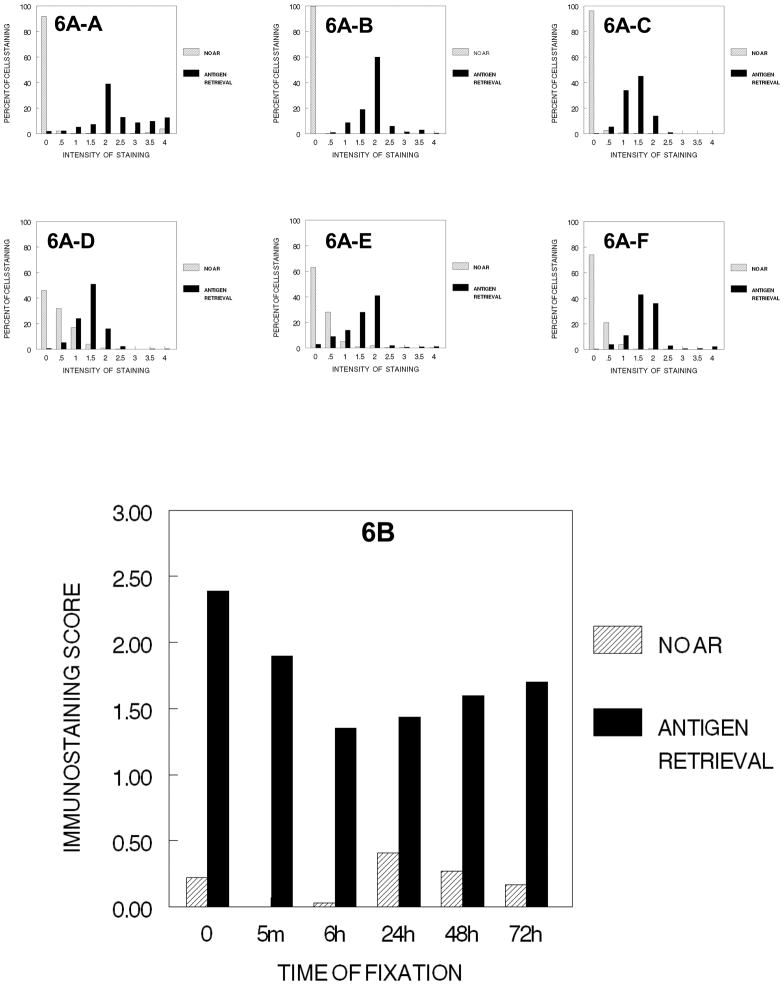
Following AR, however, the values of immunostaining were enhanced significantly. Comparison of these values with those obtained in the earlier experiments indicated that AR restored the immunostaining values to levels comparable to those obtained after each of the previous steps of tissue processing. Similar effects were observed when the cells were taken through the entire tissue processing protocol and infiltrated with paraffin (Fig. 6). Note the similar distributions, 6A to 6F to 5A to 5F and similar immunostaining scores, 5B to 6B.
Figure 6.
DUI45 cells were either not fixed (0 min.) (6A-A) or fixed in 10% NBF at 4°C for 5 min. (6A–B), 6 hr. (6A–C), 24 hr. (6A–D), 48 hr. (6A–E) or 72 hr. (6A–F), washed, then all were immersed in 70% ethanol for 1 hr., 80% ethanol for 1 hr., 90% ethanol for 1 hr., absolute ethanol for 3 hr., and xylene for 2hr. The cells were then immersed in paraffin for 2 hr., deparaffinized, and rehydrated prior to antigen retrieval or no antigen retrieval and immunostaining to detect the Ki67 antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
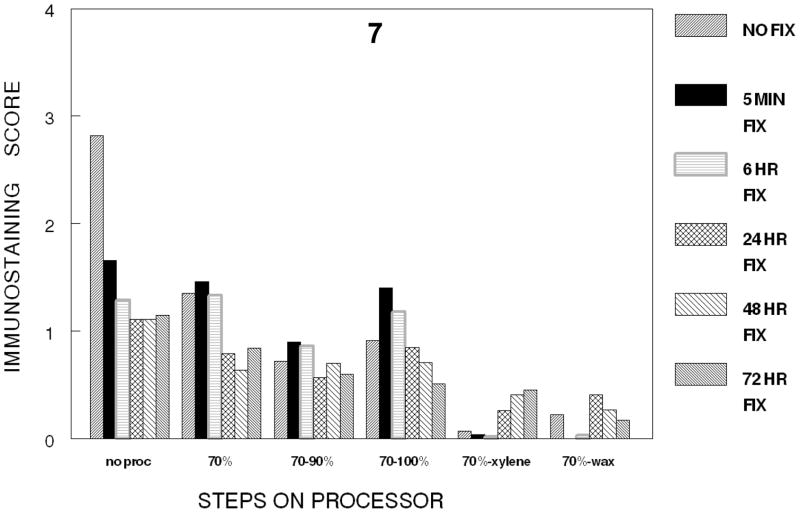
In the first series of experiments, each of the steps in tissue processing was analyzed in a separate experiment of one step in tissue processing in which the various times of fixation were compared. Therefore, one cannot statistically compare the various steps of tissue processing with each other because the staining steps were performed at different times; however, general trends may be observed when comparing the steps in tissue processing which we think are valid due to our careful attention to standard operating procedures of our immunostaining and our use of automated staining procedures. Based on Figure 7 in which the various stages in tissue processing are plotted at the various times of fixation, there is the suggestion that most of the immunorecognition is lost following any time of fixation in 10% NBF and processing to xylene. Also immunorecognition is almost completely lost even without fixation when the cells were processed to xylene or subsequently infiltrated with paraffin (See Figures 5B, 6B and 7).
Figure 7.
Figure 7 summarizes the conditions studied for the immunostaining of Ki67/MIB-1. The figure demonstrates a trend to decreasing immunostaining as tissues are processed to a hydrophobic environment produced by infiltration with xylene.
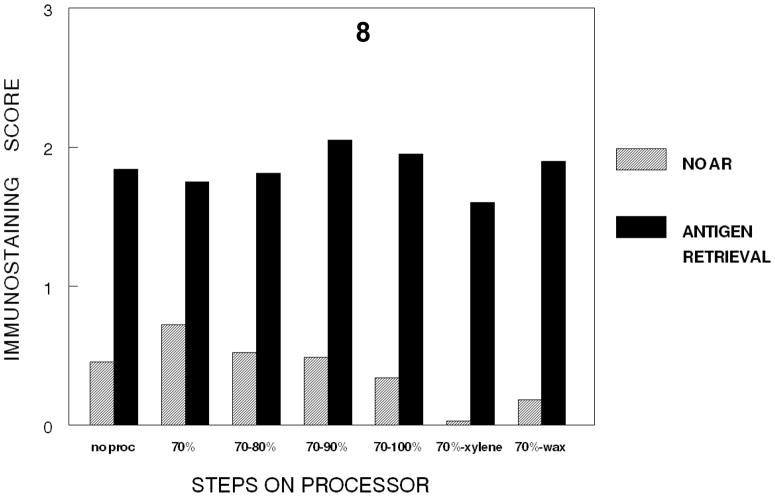
In a separate experiment, a fixation time of 24 hr. was selected and the effects on immunorecognition of the various steps in tissue processing were analyzed in one experiment. This permitted the effects on immunorecognition of individual steps of tissue processing to be compared statistically. Figure 8 demonstrates that there is a statistically significant (p<0.001) and practical decrease in immunorecognition when the hydrophobic environment was established i.e., either when the tissue is exposed to xylene or infiltrated with paraffin.
Figure 8.
Figure 8 provides a direct comparison of the cumulative steps in tissue processing for tissues stained for 24 hr. in 10% NBF. While this indicates some slight decrease in immunostaining for Ki67 with the cumulative steps of dehydration, the major practical and statistically significant (p<0.001) decrease in immunostaining for Ki67 occurred with infiltration by xylene to establish a hydrophobic environment.
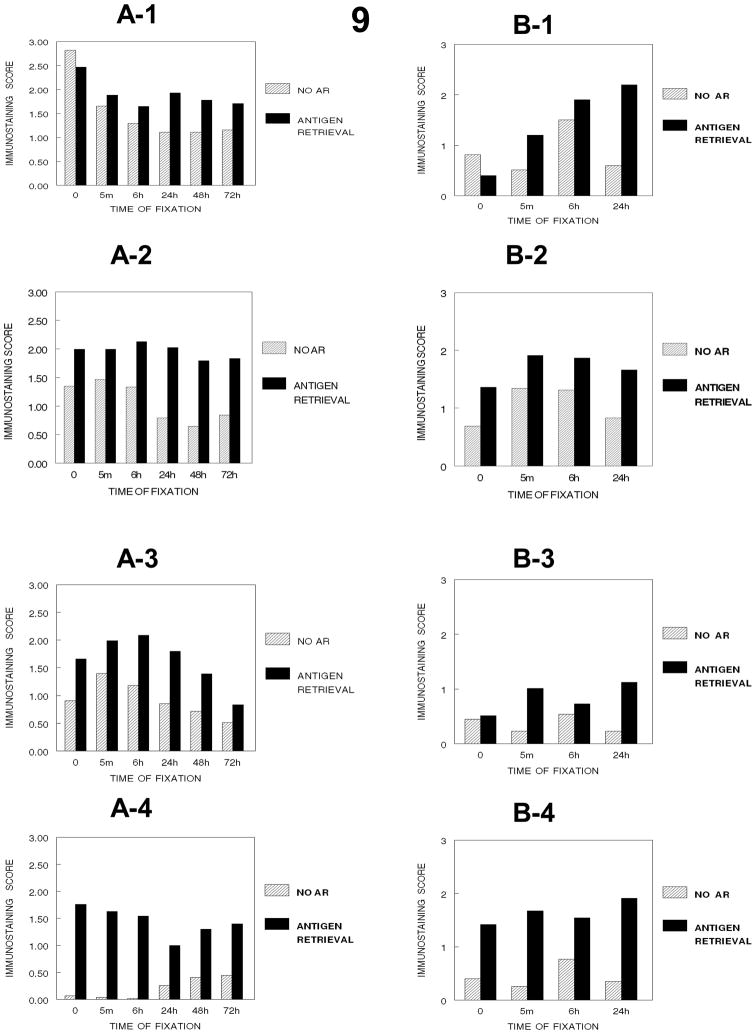
Comparison of the effects of fixation in 10% NBF on immunostaining of Ki67 in SKOV3 cells over a 24-hour period (9B1–9B4, 10B) indicated some differences (Figs. 9 and 10) compared to DU145 cells (9A1–9A4, 10A). The results of prior experiments demonstrated that the immunostaining of unfixed DU145 cells was stronger than that observed in formalin-fixed DU145 cells (Panel 9-A-1). In contrast, fixation of the SKOV3 cells enhanced the immunostaining for Ki67 optimally at 6 hr. of fixation (Panel 9-B-1) with a subsequent statistically significant (p<0.001) and practical decrease in immunostaining at 24 hr. Similarly, to DU145 cells AR was not as effective at enhancing the staining of the formalin-fixed SKOV3 cells until fixation at 24 hr. (Figure 9). Fixation in 10% NBF followed by processing from 70% ethanol to xylene caused an almost complete loss of immunostaining of Ki67 in DU145 cells, as had been observed and discussed previously, as well as in the SKOV3 cells (Fig. 10). However, in contrast with DU145 cells, SKOV-3 cells were sensitive to complete dehydration via processing through absolute ethanol with most immunorecognition lost with dehydration through absolute ethanol (Panel 9-B-3) and this loss was maintained when the cells were exposed to the hydrophobic environment established by xylene. Also antigen retrieval was not as effective after dehydration. AR, however, resulted in almost complete recovery of immunorecognition in the xylene-treated SKOV3 cells. This was similar to the effects of AR on immunostaining of Ki67 in the DU145 cells, except that in the SKOV3 cells, AR improved the immunostaining scores for those cells that had been treated with xylene after fixation for 24 hr. whereas AR was less effective for DU145 cells that had been treated with xylene after fixation for 24 hr. (Fig. 8, p<0.001, not practical difference).
Figure 9.
Figure 9 demonstrates how DU145 cells and SKOV-3 cells compare as to immunostaining scores in their response to the cumulative steps of tissue processing (A-1, no processing), (A-2, processing to 70% ethanol), (A-3, processing to absolute ethanol), (A-4, processing to xylene). Note that establishing a hydrophobic environment has the greatest effect of immunorecognition of Ki67. In contrast, in SKOV-3 cells, dehydration (B-3) or dehydration followed by establishing a hydrophobic environment (B-4) has the major effects on immunorecognition of Ki67 (B-1, no processing), (B-2, processing to 70% ethanol).
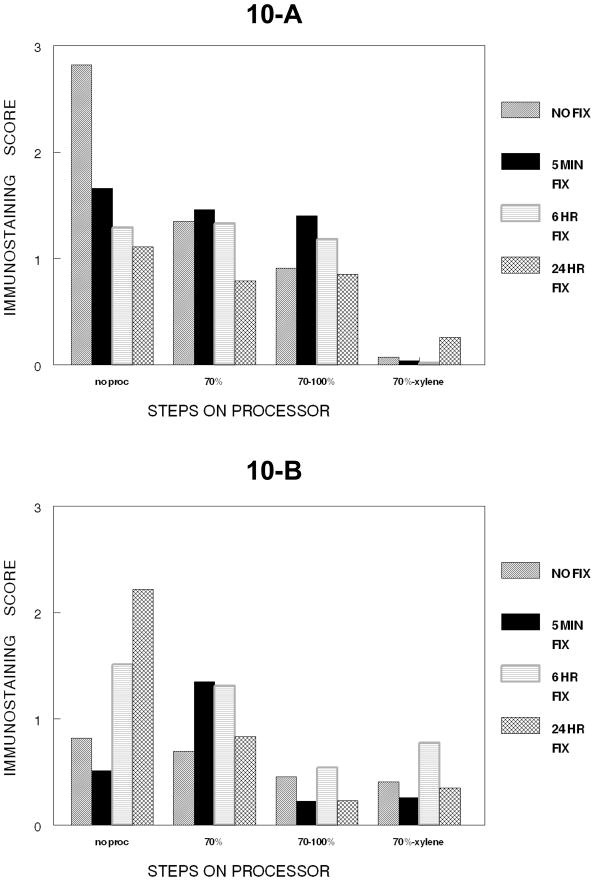
Figure 10.
Figure 10 is a summary of the comparison of how DU145 cells (Panel 10A) and SKOV-3 cells (Panel 10B) respond to the major cumulative steps of tissue processing. SKOV-3 cells were not tested beyond 24 hr.
Overall for Ki67, there was a statically significant difference between each of the steps in tissue processing (i.e., fixation alone versus fixation plus a combination of the tissue processing steps) (p<0.0001, χ2=3901.83, df=5). There also was a significant difference in each of the combined time-treatments (i.e., time of fixation alone and a combination of fixation plus the tissue processing steps) effect (p<0.0001, χ2=2353.49, df=25). In most cases, these statistical differences were inconsequential in that the changes were too small to make a difference in interpretation of the immunostaining results, referred to as a change not being a “practical change.”
Immunodetection of PCNA
We used a similar protocol to determine the effects of fixation and tissue processing on the immunostaining of PCNA in DU145 and SKOV-3 cells. In contrast to Ki67, the PCNA staining was located primarily in the cytoplasm surrounding the chromatids of mitotic cells and the chromatids, themselves, did not stain. In the absence of AR, we found that immunostaining of PCNA was not detectable in cells that had not been fixed in 10% NBF. Because only a few cells remained on the slide after no fixation, this observation (no staining with PCNA without some type of fixation) was confirmed by a cytospin of DU145 and SKOV-3 cells that were unfixed, but treated with the anti-PCNA antibody. The lack of staining for PCNA without fixation of the section also was confirmed by frozen unfixed sections of tissue (Stockard et al. unpublished).
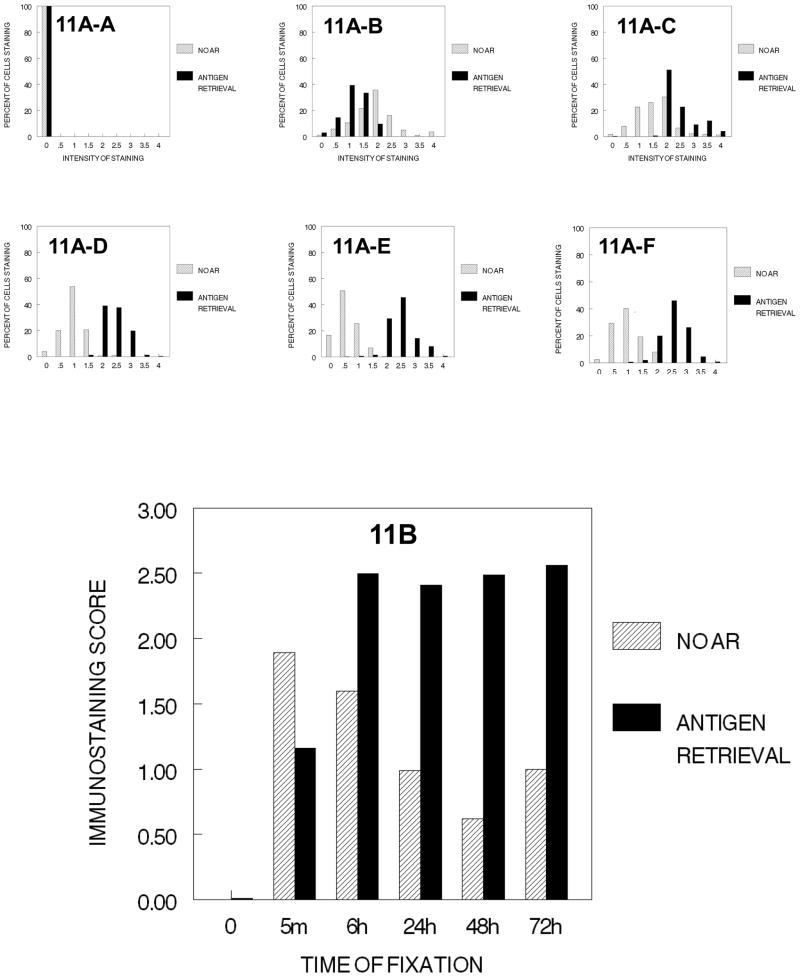
PCNA immunostaining was observed in cells that had been fixed in 10% NBF, with the highest values of staining being observed in those cells that had been fixed for 5 min. (Fig. 11). As the time of fixation in 10% NBF increased, practical and significant decreases (p<0.001, for each time of fixation) occurred with staining of 5 min. > 6 hr. > 24 hr. > 48 hr. < 72 hr. (Fig. 11B). Note that the frequency pattern was relatively consistent (11A–B to 11A–F). In those cells that were subjected to AR, fixation in 10% NBF for at least 6 hr. was required for maximal immunostaining of PCNA (Fig. 11). The immunostaining scores following AR varied little among the DU145 cells that were fixed in 10% NBF for longer than 6 hr. and the frequency/intensity patterns of staining were relatively consistent (11A–C to 11A–F). Thus, fixation is required for recognition of PCNA antigens by antibodies to PCNA and, in the absence of tissue processing, a certain minimal time of exposure to 10% NBF is required.
Figure 11.
DUI45 cells were either not fixed (0 min.) (11A-A) or fixed in 10% NBF at 4°C for 5 min. (11A–B), 6 hr. (11A–C), 24 hr. (11A–D), 48 hr. (11A–E) or 72 hr. (11A–F), washed, subjected to antigen retrieval or no antigen retrieval, and then immunostained to detect the PCNA antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
Analysis of the effects of exposure of the cells to 70% ethanol for 1 hr. indicated that in the absence of the antigen retrieval, the exposure to 70% ethanol for 1 hr. resulted in some staining of the PCNA antigen in cells that had not been fixed in 10% NBF (Fig. 12), which was most likely secondary to some degree of fixation by the 70% ethanol. The percentage of PCNA-positive DU145 cells (12A-A to 12A–B) was higher in those cells that had been fixed for even a short time in NBF (5 min.) prior to exposure to 70% ethanol for 1 hr., as compared to those cells that had not been fixed in 10% NBF prior to exposure to 70% ethanol for 1 hr. (Figure 12B, p<0.001, practical change). AR caused a significant (p<0.001, practical) enhancement of staining of DU145 cells that had been fixed for at least 6 hr. prior to exposure to 70% ethanol for 1 hr. as compared to similarly treated cells that were not subjected to AR (Figure 12B). Antigen retrieval did result in an increase in the immunostaining of cells that had been fixed for 6 hr. or longer in 10% NBF prior to exposure to 70% ethanol for 1 hr.
Figure 12.
DUI45 cells were either not fixed (0 min.) (12A-A) or fixed in 10% NBF at 4°C for 5 min. (12A–B), 6 hr. (12A–C), 24 hr. (12A–D), 48 hr. (12A–E) or 72 hr. (12A–F), washed, then all were immersed in 70% ethanol at room temperature for 1 hr. The cells were then subjected to antigen retrieval or no antigen retrieval, and then immunostained to detect the PCNA antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
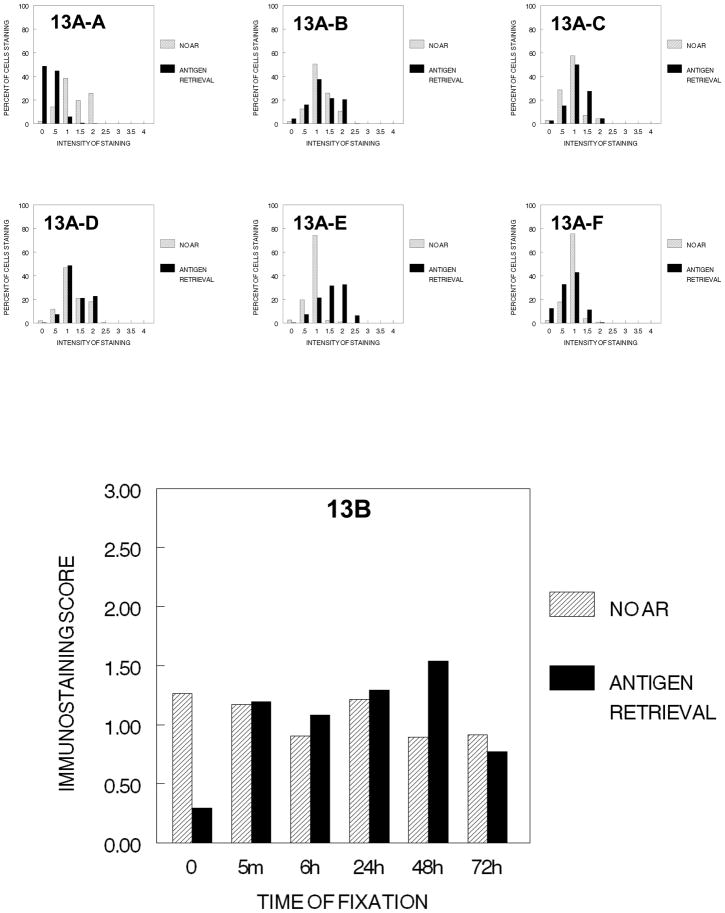
We next evaluated the effects of graded concentrations of ethanol (70%, 80% and 90% for 1 hr. at each concentration) on immunostaining of the PCNA antigen in DU145 cells (Fig. 13) not fixed or fixed for variable periods in 10% NBF. In the absence of AR, the immunostaining values for PCNA after exposure to the graded concentrations of ethanols were similar for all times of fixation. Although statistically significant differences were observed in the immunostaining values for cells that had been fixed for various different times prior to dehydration through the graded concentrations of ethanol, the magnitude of these difference would not be of any practical significance. When AR was used, little staining beyond that without AR was observed in cells that had been fixed in 10% NBF for 5 min. to 24 hr. and at 72 hr. prior to passage through the graded concentrations of ethanol. Only at fixation for 48 hr. did AR act to increase immunostaining. These results could be interpreted as being consistent with the observation of Fowler et al. 2008, that ethanol exposure inhibits the improvement of immunorecognition using AR.
Figure 13.
DUI45 cells were either not fixed (0 min.) (13A-A) or fixed in 10% NBF at 4°C for 5 min. (13A–B), 6 hr. (13A–C), 24 hr. (13A–D), 48 hr. (13A–E)or 72 hr. (13A–F), washed, then all were immersed in 70% ethanol for 1 hr., 80% ethanol for 1 hr., and then 90% ethanol for 1 hr. at room temperature. The cells were then subjected to antigen retrieval or no antigen retrieval and immunostained to detect the PCNA antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
As demonstrated in Figure 14, extension of the tissue processing steps to include exposure through absolute ethanol for 3 hr. resulted in immunostaining patterns that were similar to those observed when the cells had been exposed to 70% ethanol; specifically, less staining was observed in the absence of fixation; however, in contrast to exposure to 70% ethanol, there was slightly less staining in those cells that had been subjected to 72 hr. of fixation plus complete dehydration. Of note is the consistent frequencies of staining following some degree of fixation (5 min.) as is demonstrated in 14A–B to 14A–E. Similarly, in those cells that had been subjected to AR, the immunostaining patterns after processing through absolute ethanol for 3 hr. had a staining pattern that was similar to the pattern observed in cells that had been exposed to dehydration for 70%–80%–90% ethanol, except that the results were more consistent after use of AR than those obtained when AR was not used. The levels of staining observed after AR for cells that had not been fixed were lower after antigen retrieval than cells that had not been subjected to AR and the staining of cells with no fixation and antigen retrieval were lower than cells fixed for 5 min. or greater and stained following AR. The immunostaining values were practically the same after AR as those obtained without antigen retrieval for cells that had been fixed in 10% NBF for 5 min. to 48 hr. Thus for DU145 cells, immunorecognition was not lost because of dehydration; however, the results of using AR could be interpreted similar to the 70–90% ethanol step, i.e., that ethanol inhibits staining using AR (Fowler et al. 2008).
Figure 14.
DUI45 cells were either not fixed (0 min.) (14A-A) or fixed in 10% NBF at 4°C for 5 min. (14A–B), 6 hr. (14A–C), 24 hr. (14A–D), 48 hr. (14A–E) or 72 hr. (14A–F), washed, then all immersed in 70% ethanol for 1 hr., 80% ethanol for 1 hr., 90% ethanol for 1 hr., and then absolute ethanol for 3 hr. at room temperature. The cells were then subjected to antigen retrieval or no antigen retrieval and immunostained to detect the PCNA antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
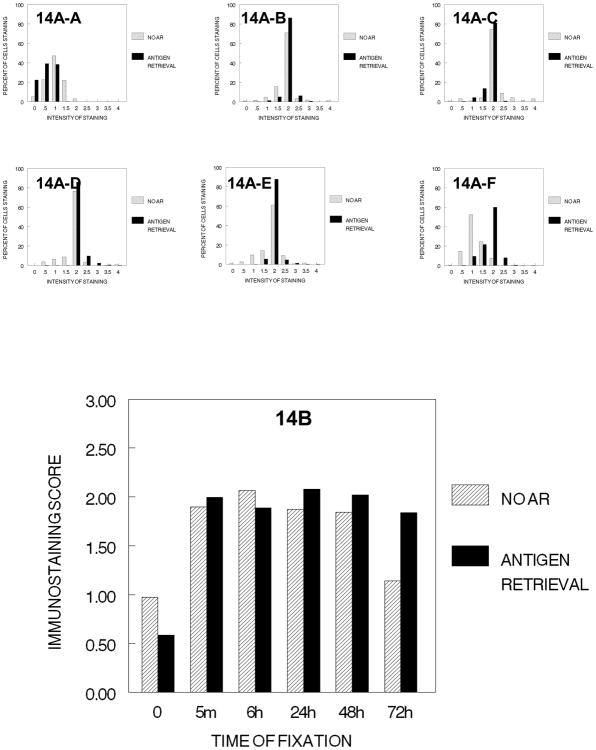
The inclusion of treatment with xylene for 2 hr., which would establish a hydrophobic environment, resulted in a major reduction in immunostaining of PCNA in DU145 cells that had been fixed for at least 5 min. in 10% NBF prior to processing (Fig. 15). This decrease in immunostaining was reversed partially by antigen retrieval for most conditions of fixation tested especially after fixation in 10% NBF for 24 hr. or more. Similar data were obtained following infiltration with paraffin (Figure 16).
Figure 15.
DUI45 cells were either not fixed (0 min.) (15A-A) or fixed in 10% NBF at 4°C for 5 min. (15A–B), 6 hr. (15A–C), 24 hr. (15A–D), 48 hr. (15A–E) or 72 hr. (15A–F), washed, then all immersed in 70% ethanol for 1 hr., 80% ethanol for 1 hr., 90% ethanol for 1 hr., absolute ethanol for 3 hr., and then xylene for 2 hr. at room temperature. The cells were then subjected to antigen retrieval or no antigen retrieval and immunostained to detect the PCNA antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
Figure 16.
DUI45 cells were either not fixed (0 min.) (16A-A) or fixed in 10% NBF at 4°C for 5 min. (16A–B), 6 hr. (16A–C), 24 hr. (16A–D), 48 hr. (16A–E) or 72 hr. (16A–F), washed, then all immersed in 70% ethanol for 1 hr., 80% ethanol for 1 hr., 90% ethanol for 1 hr., absolute ethanol for 3 hr., xylene for 2hr., followed by immersing in paraffin for 2 hr. The slides were then deparaffinized and rehydrated prior to antigen retrieval or no antigen retrieval and immunostaining to detect the PCNA antigen. Panel A demonstrates the percentage of cells stained at each intensity. Panel B shows the immunostaining score which was calculated as described.
The cumulative effects on immunostaining for the PCNA antigen in DU145 cells at the standard variable times of fixation in 10% NBF plus processing through graded concentrations of ethanol to xylene and then to paraffin are summarized in Figure 17A. The results of immunostaining for PCNA following fixation, dehydration, exposure to the hydrophobic environment plus the infiltration with paraffin were similar to those for staining with Ki67/MIB-1 following fixation, dehydration plus exposure to the hydrophobic environment of xylene in the previous analysis of Ki67 except that there was more variability in results in staining for PCNA. There was a large reduction in immunostaining of cells that had been fixed for 6 hr. or longer and processed through xylene with some recovery after infiltration with paraffin. Antigen retrieval resulted in consistent enhancement of the immunostaining of cells that had been fixed for 24 hr. or longer (Fig. 17B). In contrast, AR did not enhance the staining of cells that were unfixed or had been fixed for short periods of time (5 min. or 6 hr.). These results may explain why antigen retrieval is not always used in staining to demonstrate PCNA because fixation currently is frequently 6 hr. or less.
Figure 17.
Panel A of Figure 17 summarizes the immunostaining of PCNA in DU145 cells over all the cumulative steps of tissue processing. Note how fixation interacts with each cumulative step and that the transition to a hydrophobic environment has the greatest loss of immunorecognition for PCNA (p<0.001). Panel B shows the staining of PCNA following antigen retrieval for each of the cumulative steps of tissue processing at each time of fixation. Note that fixation for 24 hr. or greater gives the most consistent results for immunostaining for PCNA after antigen recovery.
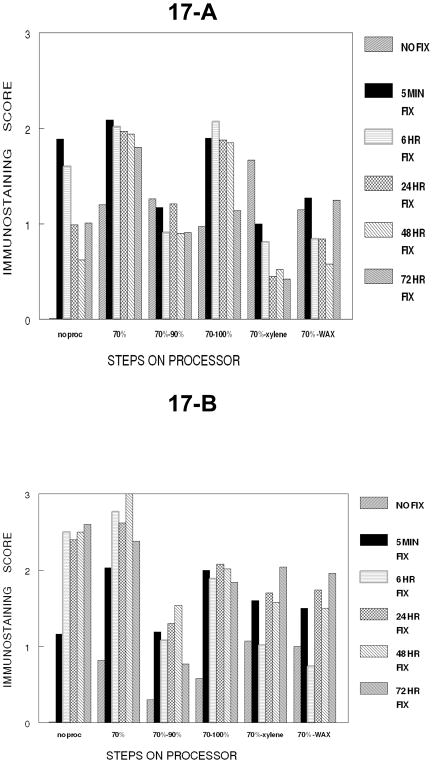
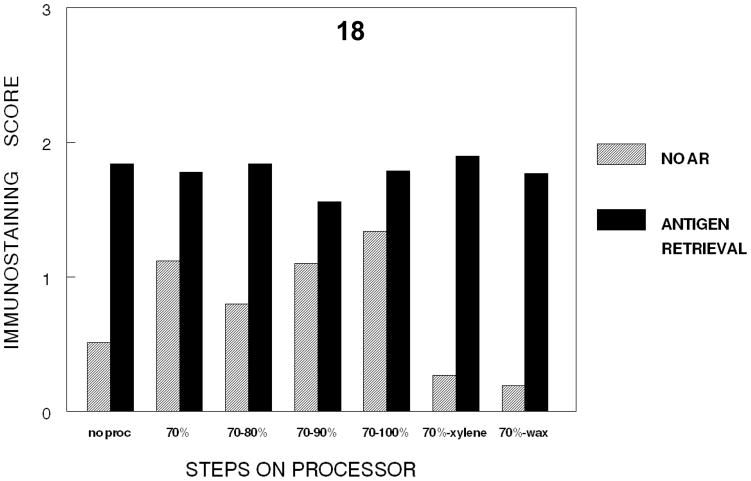
To permit actual comparison of the effects on immunodetection of the cumulative steps in tissue processing, the steps in tissue processing were compared on cells fixed at one time point, 24 hr. This permitted all the steps in processing as well as all immunostaining to be completed in one run in one day; thus a valid statistical comparison can be made. Figure 18 demonstrates that a 24 hour fixation with 10% NBF plus dehydration through absolute ethanol does not inhibit immunodetection. The results do indicate that 24 hr. of fixation without tissue processing results in a lower value than alcohol steps in tissue processing, a statistically significant p<0.001 and practical change. However, a large statistically significant (p<0.001) and practical reduction in immunorecognition after 24 hr. of fixation in 10% NBF occurred after cumulative processing through xylene infiltration (establishing a hydrophobic environment). Use of AR produced essentially uniform immunostaining either with no processing or complete processing through paraffin infiltration. The difference between no AR and AR for the later step of dehydration in tissue processing (70–90 and 70-absolute) was less than between no AR and AR in other steps. Rather than an inhibition of AR after exposure to ethanols at various concentrations, this is more consistent with AR producing a consistent value at all cumulative steps of processing.
Figure 18.
Figure 18 demonstrates the loss of immunorecognition of PCNA in DU145 cells at each cumulative step of tissue processing for tissues fixed for 24 hr. in 10% NBF. Note there is a practical and statistically significant (p<0.001) decrease in immunorecognition of PCNA when a hydrophobic environment is established via xylene infiltration.
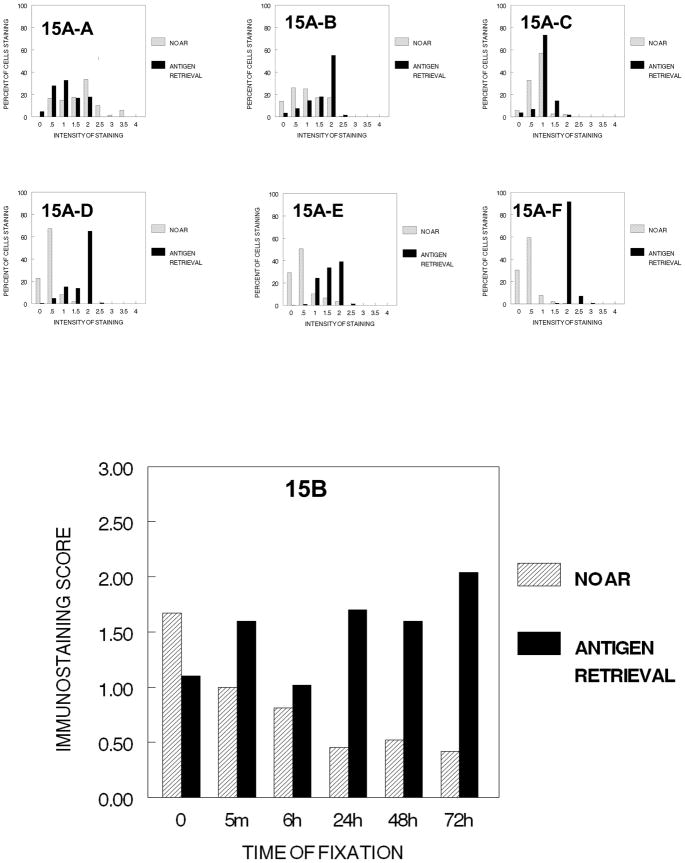
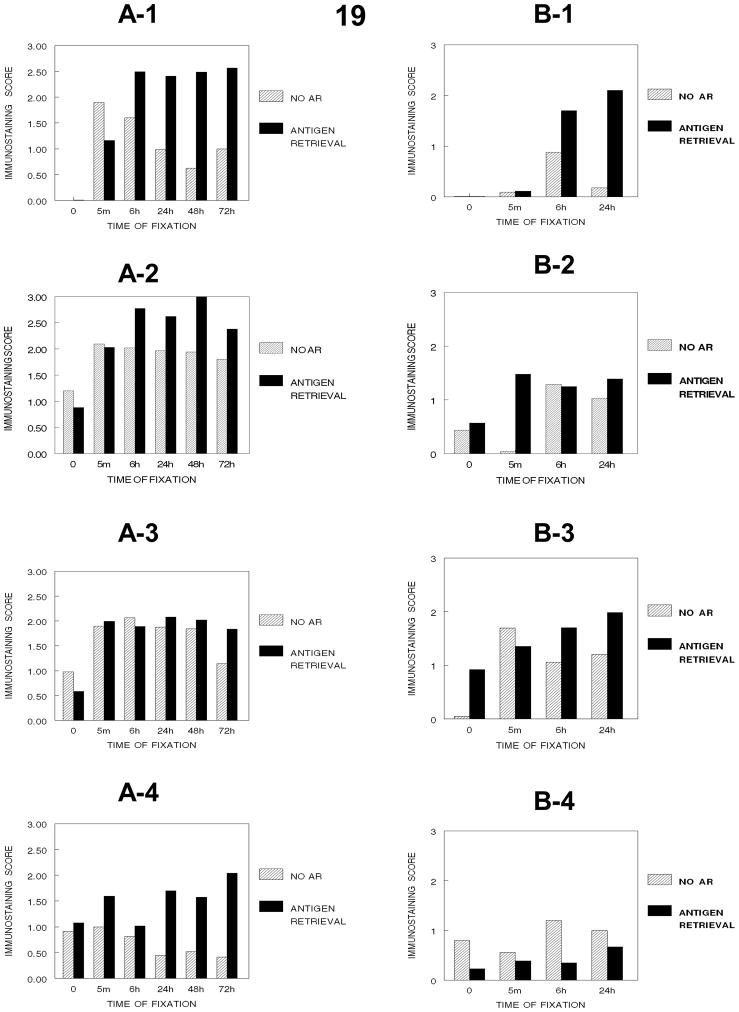
As shown in Figure 19, the comparison of the effects of fixation on the immunodetection of PCNA in DU145 or SKOV3 cells indicated that some fixation was required for the detection of PCNA in these cells. In DU145 cells, fixation in 10% NBF for 5 min. was sufficient for detection of PCNA by immunostaining and as the fixation time was increased statistically significant and practical reductions in staining were observed (Figure 19-A-1). In contrast, in SKOV3 cells, at least 6 hr. of fixation in 10% NBF was required for immunodetection of PCNA even when antigen retrieval was used, but 24 hr. of fixation decreased immunostaining compared to 6 hr. of fixation. These studies suggest that fixation in 10% NBF for at least 6 hr. is required to ensure immunodetection of PCNA and the effectiveness of antigen retrieval.
Figure 19.
Figure 19 demonstrates how immunorecognition of PCNA varies with the cumulative stages of tissue processing and the times of fixation in DU145 (A-1, no processing), (A-2, processing to 70% ethanol), (A-3, processing to absolute ethanol), (A-4, processing to xylene) and in SKOV-3 cells (B-1, no processing, B-2, processing to 70% ethanol, B-3, processing to absolute ethanol, B-4, processing to xylene). SKOV-3 cells were not evaluated beyond 24 hr. Note the major decrease in immunorecognition for PCNA occurs upon establishing a hydrophobic environment for DU145 cells (A-4) (p<0.001) and SKOV-3 cells (B-4) (p<0.001).
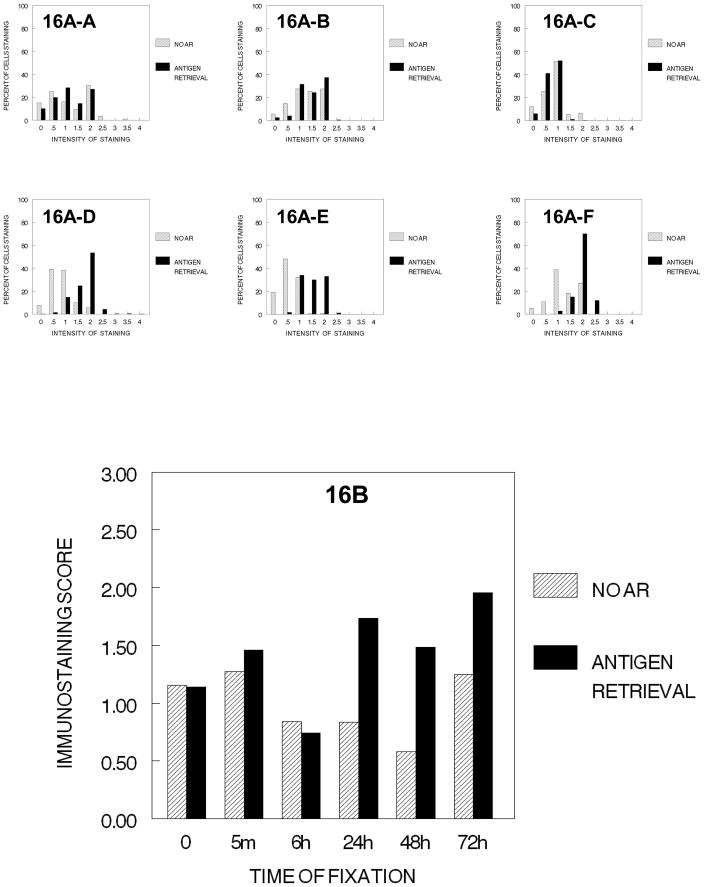
Comparison of the effects of fixation together with tissue processing through xylene in DU145 and SKOV3 cells indicated that in DU145 cells, fixation and processing to xylene results in a statistically significant (p<0.001) and a practical reduction in immunostaining of PCNA; similarly, in SKOV3 cells, a similar reduction occurs in cells that have been fixed in 10% NBF for longer than 5 min. prior to processing through xylene (Figure 19). In DU145 cells, AR of cells that had not been fixed prior to processing to xylene or to paraffin resulted in a reduced signal for PCNA (15B, 16B, and 19-A-4). In these cells, antigen retrieval of cells that had been fixed for greater than 6 hr. in 10% NBF prior to processing through xylene or paraffin resulted in more consistent staining for PCNA. In SKOV3 cells, AR of cells that had not been fixed prior to processing to xylene produced variable results but primarily a reduction in the PCNA signal, similar to that observed for DU145 cells (Figure 20). However, unlike the results obtained using DU145 cells, in SKOV3 cells, AR after fixation and processing to xylene resulted in very low staining (Figure 19-B-4). This complicated the overall evaluation of fixation as most of the staining was lost after AR (Figure 19-B-4).
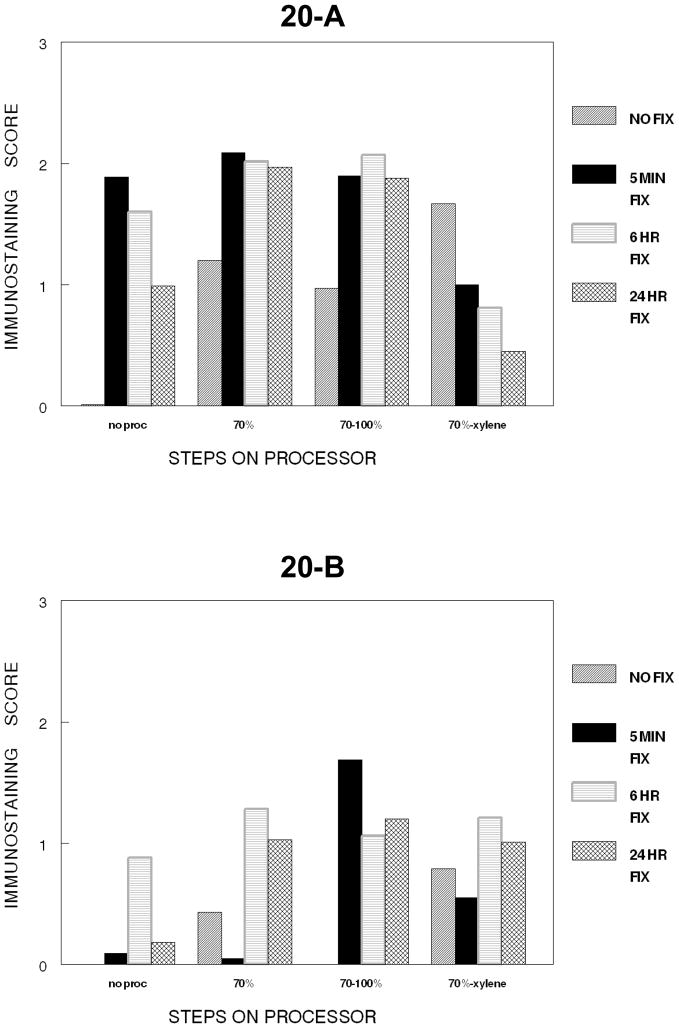
Figure 20.
Figure 20, summarizes the cumulative steps of tissue processing for DU145 cells (20-A) and SKOV-3 cells (20-B) as to the immunostaining for PCNA. A major decrease in staining for PCNA for both is caused by infiltration by xylene.
DISCUSSION
It has been reported previously that the longer tissues remain in an aldehyde-based fixative, the greater the reduction in immunostaining (Eltoum et al. 2001a; Eltoum et al. 2001b; Grizzle et al. 2001; Namimatsu et al. 2005; Rait et al. 2004a; Rait et al. 2004b; Dapson 2007). Also, because pathology laboratories have been pushed to rapidly prepare tissue specimens for histologic analysis (Grizzle et al. 2001, 2008), most pathology laboratories fix tissues for only 6 hr. to 24 hr. as this is considered to be a good compromise in terms of preserving tissue morphology on hematoxylin and eosin (H+E) and special histochemical staining while providing quick turn-around times. Note, the quality of H+E staining following such short times of fixation has been questioned by Dapson 2007. Based on the results of our study, in most cases staining for more than 6 hr. should yield good immunohistochemical staining which is further enhanced and made more consistent on AR.
Due to the scanty information on the combined effects of aldehyde fixation and tissue processing steps and reagents on immunohistochemical staining, we sought to explore any potential interactive effects of fixation and tissue processing using cells grown on microscope slides as a model for fresh tissue. Two cancer cell lines, DU145 (prostate) and SKOV3 (ovarian), were used for generalization.
Based on our results, we recommend the growth of cells on microscope slides as a model for carrying out fixation studies because the growth of the cells as a thin layer on the microscope slides permits uniform penetration of fixative and tissue processing reagents and thus “cleaner” preparations that can be readily evaluated. In addition, the model minimizes any false positives that could occur as a result of the passive uptake of serum proteins or diffusion of antigens. Such effects, which are the result of inadequate or delayed fixation, are quite common in the middle of tissue blocks that are too large. We also argue that the cell monolayer ensures uniform and sufficient fixation, avoiding the hardening and shrinkage effects of the fixative that may result in uneven penetration of the fixative within tissues and also may cause problems with the interpretation of staining of sections of tissue due to cracking and fracture of tissue sections on cutting. Thus, the use of the cell monolayers minimized barriers that could hinder rapid and uniform diffusion of the fixative. The use of cells also minimized possible sources of error, such as contamination in a water bath that may be introduced during the preparation of tissue sections or loss of the target on multiple sections from a block. If fixation effects vary with cell type, this model may be difficult to generalize to all tissues; however, other data using frozen sections suggests that there are some apparent generalizations (Stockard et al. unpublished). Our only difficulty was the small amount of cells that attached to slides without fixation and tissue processing and variable staining when slides were not coated with poly-lysine.
Immunohistochemistry involves many steps and variables in addition to those involved in the preparation of the tissues, and thus, it poses a challenge because any one factor within a step of the cumulative steps of tissue fixation, processing, and staining might affect the attainment of consistent immunohistochemical results with or without antigen retrieval. The variability in immunostaining continues to be a challenge in immunohistochemistry (Taylor, 2006). Suffice to say, that in order to have reproducible results, laboratories should rigorously follow standard operating procedures (SOPs) from the initial fixation through tissue processing to antigen retrieval; even the formulas and pH of buffers for dilutions, washing and AR should be scrupulously checked before use.
In this study, we compared the effects of fixation and tissue processing on immunodetection of Ki67 and PCNA. Both of these are nuclear antigens, but each demonstrates a different pattern of epitope masking upon fixation. While Ki67 detection in formalin-fixed paraffin embedded tissues requires antigen retrieval (Grizzle et al. 2001, Stockard et al. unpublished), PCNA is sometimes used without AR. Ki67 is a highly positively charged alkaline nonhistone protein. It is a bimolecular complex of two polypeptides with molecular weights of 345 and 395 kDa. The Ki67 monoclonal antibody used in these studies has been shown to detect polypeptides with these molecular weights in proliferating cells. Ki67 antigen is expressed by proliferating cells in all active phases of the cell cycle but primarily in G1, S, G2 and some in M phase). It is absent in G0. Its expression becomes apparent at the beginning of the G1 phase and it accumulates within the nucleus during the late G1 phase. The expression of this antigen increases as the cell cycle progresses, reaches a maximum concentration in the S phase, and declines markedly thereafter. In the G1 phase, Ki67 antigen is located predominantly in the nucleoli and is particularly evident in cells containing relatively large nucleoli (Hayat, 2001).
It has been reported previously that Ki67 cannot be detected by routine immunostaining of paraffin sections from tissues fixed in NBF and that immunodetection of Ki67 in paraffin tissue sections requires some type of antigen retrieval (Frost et al. 2000, Bogen et al. 2008). In contrast, we have noted that fixation of frozen sections in NBF does not have adverse effects on the immunodetection of Ki67 and frozen sections that were treated identically to the paraffin sections, with the same length of fixation and identical staining protocol, exhibited staining for Ki67 (Stockard et al. unpublished) while the paraffin sections did not. Indeed, some fixation of frozen section in NBF may be necessary to reduce background staining and to produce detectable signals for many antigens as demonstrated in this study (Grizzle et al. 2001, Grizzle et al. 1998, Grizzle et al. 2008).
In the current study, we found that the immunostaining was strongest in DU145 cells that had not been fixed. In fact, the intensities of staining obtained after exposure to 10% NBF and after each of the processing steps were never as high as those obtained when the DU145 cells had not been exposed to 10% NBF; only a few situations in which AR was used came close to this value. The effects of fixation on the immunodetection of Ki67 in SKOV-3 cells were somewhat different. In these cells, even fixation for 5 min. in 10% NBF resulted in only a very slight staining. As the time of fixation in 10% NBF increased, Ki67 staining increased in SKOV-3 cells until the strongest signal occurred at 6 hr. of fixation in 10% NBF. This suggests that the Ki67 antigens in the DU145 and SKOV3 cells react differently to fixation in 10% NBF and that these differences can affect the detection of the antigen. The reason for differences is unknown, but may relate to how antigens are packaged in different cells.
Although the length of time in 10% NBF fixative was identified as a factor that affected immunohistochemical staining for Ki67, we found that other steps in tissue processing also played a major role. In DU145 cells, the dehydration steps of tissue processing resulted in a loss of immunodetection of Ki67 on increased times of fixation; specifically, the combination of dehydration and greater than 6 hr. of fixation in 10% NBF resulted in reduced immunostaining (6 hr. > 24 hr. > 48 hr. > 72hr.) that was statistically significant and of practical importance.
The greatest effect on Ki67 detection at all times of fixation was observed at the establishment of a hydrophobic environment created by infiltration with xylene (Fig. 5B). In fact, with no fixation, just processing tissue through xylene completely abolished staining for Ki67 (Fig. 5B, Fig. 10). Although we noticed variations in responses to fixation plus tissue processing reagents between the two cell types as well as effects on staining patterns, it was at the xylene step of tissue processing that resulted in the greatest loss of immunorecognition of Ki67 and PCNA for DU145 cells; a similar loss of immunorecognition of Ki67 and PCNA occurred in xylene in SKOV-3 cells, but the loss of immunorecognition of Ki67 first occurred after dehydration. Our first observation in DU145 cells differs from some reports that have reported that, besides fixation, it is the dehydration step of tissue processing that causes the greatest loss of immunorecognition of epitopes thus preventing recognition of the antigen by the antibody during immunohistochemical staining (Cattoretti et al. 1993; Stein et al. 1985). We suspect that whether the cumulative steps of dehydration causes loss of immunorecognition or of the establishment of a hydrophobic environment depends upon the type of cell. The results of our study did confirm the previous reports that the Ki67 signal is not detectable in tissues processed through paraffin without AR. In our model, the detection of Ki67 without paraffin processing may reflect the ease of accessibility of the antibody to the monolayer of cells as well as the ease of observing and evaluating the immunostaining of cells and most especially those cells undergoing mitosis.
PCNA differs from Ki67 in that it is a 36-kDa, acid protein (Hayat, 2001). It has been identified as a polymerase-associated protein and is synthesized in the early G1 and S phases of the cell cycle. The expression of PCNA increases at the end of G1 phase immediately preceding DNA synthesis, reaches its maximum during the S phase, and declines through the G2 phase. It accumulates in larger intranuclear clumps in the S phase. In cells fixed with organic solvents, PCNA appears to be expressed more strongly in the nuclear regions where DNA synthesis occurs, whereas in cells fixed with aldehydes, the staining appears more diffuse and is apparent throughout all stages of the cell cycle. This difference in the staining pattern of PCNA is hypothesized to be due to the presence of two distinct forms of the PCNA protein in cells, a soluble form that is sensitive to organic fixation and not involved in replication, and a second form that is insoluble and is associated with ongoing DNA synthesis (Anonymous, World I, 2005).
In the current study, we found that some type fixation, either aldehyde or dehydrant, was required for immunodetection of PCNA. Specifically in DU145 cells, we found that immunodetection of PCNA required fixation in 10% NBF for at least 5 min. In SKOV3 cells, significant levels immunostaining required fixation in 10% NBF for more than 5 min. It is possible that the lack of detection of PCNA in the absence of fixation in 10% NBF is associated with leaching of this relatively small molecule from the nucleus. Alternatively, the recognition of the PCNA antigens by the antibody may require a conformational change for the antibody to gain access to the epitope of PCNA. Also, the lack of immunorecognition might be due to the form of PCNA that is being stained (Anonymous, World I, 2005). Nevertheless, we conclude that some type of fixation (aldehyde or dehydration) is required for detection of the signal of PCNA.
Application of AR after at least 6 hr. of fixation in 10% NBF resulted in an enhancement of detection of PCNA in both DU145 and SKOV-3 cells. As for immunodetection of Ki67, the dehydration steps in tissue processing did not prevent detection of the PCNA signal; however, at the establishment of a hydrophobic environment by infiltration by xylene, there was a dramatic reduction in detection of PCNA in both DU145 and SKOV-3 cells. We therefore conclude that it is the establishment of the hydrophobic environment during tissue processing that has the greatest effect on the detection of Ki67/MIB-1 as well as PCNA, even independent of fixation.
To the best of our knowledge, this is one of the few studies that attributes loss of antigen recognition on immunostaining to specific cumulative steps in tissue processing when all of the cumulative steps of tissue processing are evaluated concomitantly. The extension of these studies to analysis of additional cell lines and other antibody-antigen combinations will provide additional useful information regarding the potential generalizations of our observations as well as exceptions which other cell lines/antigens may present due to variations in antigen packaging of specific antigens among cell lines. Moreover, analysis of the effects of fixation for greater than 72 hr. and the cumulative steps of tissue processing in multiple cell lines would be informative.
This study indicates that at certain stages during fixation and tissue processing, the epitopes are no longer accessible for staining and thus these results suggest that either adjustments in tissue processing methods can be made to preserve immunostaining (Taylor, 2006) or that novel fixation and processing could be developed that would yield improved results. The finding that establishment of the hydrophobic environment has a major effect on the Ki67/MIB-1 epitope even without fixation could lead to development of improved approaches to antigen preservation. Notably, these studies pin-point the cumulative stages during tissue processing that may be targeted to enable analysis of potentially useful markers in diagnosis and prognosis in formalin-fixed, paraffin-embedded tissues.
In summary, our major observations were:
Immunorecognition of Ki67 and of PCNA decreases with increased duration of fixation in 10% NBF; most, but not all of this loss can be recovered with antigen retrieval.
In DU145 cells, fixation in 10% NBF plus tissue processing and most especially the establishment of a hydrophobic environment masks most of the expression of the Ki67 antigen and part of the expression of PCNA. This occurs even without fixation. In SKOV-3 cells, loss of immunorecognition of Ki67 occurs after dehydration and continues after a hydrophobic environment is established; PCNA expression is partially masked primarily following establishment of a hydrophobic environment.
PCNA requires some fixation with either 10% NBF or 70% ethanol for immunorecognition. Between 5 min. and 6 hr. of fixation are required.
Because there were some cell type-specific differences in the effects of fixation and processing on the immunorecognition of Ki67 and PCNA, antigens may be affected differentially because they have different packaging in different types of cells.
Acknowledgments
Supported in part by grants from the Susan G. Komen Foundation (BCTR0600484), the Early Detection Research Network (5U24CA086359-09) and the Cooperative Human Tissue Network (2U01CA044968-19) to William E. Grizzle, and the International Centre for Excellence in Research, Fogarty International Centre/NIH via Eric Chamot (5U2RTW006246-05) to Dennis Otali.
References
- Anonymous. World I (2005, 11-17-2006) PCNA immunohistochemical staining. Methods and Techniques for Histologists and Immunohistochemists. http://www.ihcworld.com/_protocols/antibody_protocols/pcna_santa_cruz.htm.
- Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem. 1996;71(5):224–230. doi: 10.3109/10520299609117164. [DOI] [PubMed] [Google Scholar]
- Bogen SA, Vani K, Sompuram SR. Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced crosslinks. Biotech Histochem. 2009 doi: 10.3109/10520290903039078. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Dapson RW. Macromolecular changes caused by formalin fixation and antigen retrieval. Biotech Histochem. 2007;82(3):133–140. doi: 10.1080/10520290701567916. [DOI] [PubMed] [Google Scholar]
- Eltoum I, Fredenburgh JL, Myers RB, Grizzle WE. Introduction to the theory and practice of fixation of tissues. J Histotechnology. 2001a;24:173–190. [Google Scholar]
- Eltoum I, Fredenburgh J, Grizzle WE. Advanced concepts in fixation:1. Effects of fixation on immunohistochemistry, reversibility of fixation and recovery of proteins, nucleic acids, and other molecules from fixed and processed tissues. 2. Developmental methods of fixation. J Histotechnology. 2001b;24(3):201–210. [Google Scholar]
- Fowler CB, O’Leary TJ, Mason JT. Modeling formalin fixation and histological processing with ribonuclease A: effects of ethanol dehydration on reversal of formaldehyde cross-links. Lab Invest. 2008;88:785–791. doi: 10.1038/labinvest.2008.43. [DOI] [PubMed] [Google Scholar]
- Frost AR, Sparks D, Grizzle WE. Methods of antigen recovery vary in their usefulness in unmasking specific antigens in immunohistochemistry. Applied Immunohistochemistry and Molecular Morphology. 2000;8(3):236–243. doi: 10.1097/00129039-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Grizzle WE, Myers RB, Manne U, Stockard CR, Harkins LE, Srivastava S. John Walker’s methods in molecular medicine - tumor marker protocols. Vol. 14. Totowa NJ: Humana Press Inc; 1998. [Google Scholar]
- Grizzle WE, Stockard C, Billings P. The effects of tissue processing variables other than fixation on histochemical staining and immunohistochemical detection of antigens. J Histotechnology. 2001;24(3):213–219. [Google Scholar]
- Grizzle WE, Fredenburgh JL, Myers RB. In: Chapter Four. Fixation of Tissues in Theory and Practice of Histological Techniques. 6. Bancroft JD, Gamble M, editors. Churchill Livingstone, Elsevier Limited; Philadelphia, PA: 2008. pp. 53–74. [Google Scholar]
- Hayat MA. Microscopy, immunohistochemistry, and antigen retrieval methods: Light and electron microscopy. New York, Boston, Dordrecht, London, Moscow: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- Namimatsu S, Ghazizadeh M, Sugisaki Y. Reversing the effects of formalin fixation with citraconic anhydride and heat: A universal antigen retrieval method. J Histochem Cytochem. 2005;53(1):3–11. doi: 10.1177/002215540505300102. [DOI] [PubMed] [Google Scholar]
- O’Leary TJ, Fowler CB, Evers DL, Mason JT. Protein fixation and antigen retrieval: Chemical studies. Biotech Histochem. 2009 doi: 10.3109/10520290903039086. in press. [DOI] [PubMed] [Google Scholar]
- Rait VK, O’Leary TJ, Mason JT. Modeling formalin fixation and antigen retrieval with bovine pancreatic ribonuclease a: I-structural and functional alterations. Lab Invest. 2004a;84(3):292–299. doi: 10.1038/labinvest.3700045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rait VK, Xu L, O’Leary TJ, Mason JT. Modeling formalin fixation and antigen retrieval with bovine pancreatic rnase a ii. Interrelationship of cross-linking, immunoreactivity, and heat treatment. Lab Invest. 2004b;84(3):300–306. doi: 10.1038/labinvest.3700041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. Sas/stat user’s guide (Version 8) Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Spencer LT, Bancroft JD. In: Chapter 6, Tissue Processing in Theory and Practice of Histological Techniques. 6. Bancroft JD, Gamble M, editors. Churchill Livingstone, Elsevier Limited; Philadelphia, PA: 2008. pp. 53–74. [Google Scholar]
- Stein H, Gatter K, Asbahr H, Mason DY. Use of freeze-dried paraffin-embedded sections for immunohistologic staining with monoclonal antibodies. Lab Invest. 1985;52(6):676–683. [PubMed] [Google Scholar]
- Stockard CR, Otali D, Grizzle WE. A hydrophobic environment is required for loss of immunorecognition for Ki67/MIB-1 during immunostaining - similar effects reduced immunorecognition for PCNA and Bcl-2. Unpublished 2008 [Google Scholar]
- Taylor CR. Standardization in immunohistochemistry: The role of antigen retrieval in molecular morphology. Biotech Histochem. 2006;81(1):3–12. doi: 10.1080/10520290600667866. [DOI] [PubMed] [Google Scholar]