Abstract
Tumor-reactive T cells can be primed in vivo, then activated in vitro to provide numerical expansion and uniform acquisition of effector phenotype and function. Adoptive transfer of effector T cells mediates complete regression of established tumors in animal models. Some experimental models indicate that extensive in vitro proliferation of T cells inhibits efficacy and that TCM provide greater activity than TEM. Clinical studies also demonstrate that persistence of adoptively transferred T cells is associated with therapeutic response, thus identifying conditions to maximize effector cell numbers yet retain memory function are important. Here, we demonstrate that adoptive transfer of in vitro activated effector CD4+ T cells into tumor-free congenic mice mediates rejection of tumor challenge nine months later, at which time T cells re-express activation markers and undergo rapid proliferation at tumor sites. Analysis of the phenotype of memory cells in lymphoid tissues following adoptive transfer shows high CD44 expression with heterogeneous expression of CD62L, indicating a mixture of TEM and TCM phenotypes. Memory cells were sorted into two subsets based on CD62L expression levels and then activated in vitro. Although TEM cells proliferated more rapidly, both TEM and TCM cells acquired effector phenotype and function. These data indicate that controlled in vitro expansion of tumor-reactive T cells for adoptive immunotherapy also provides a competent memory response.
Introduction
T cell adoptive immunotherapy of cancer has shown promise but has not achieved its full potential (1,2). This is, in part, because of difficulty in achieving long-term persistence of cells that have previously been activated and numerically expanded in vitro, thereby acquiring effector properties. Clinical studies have shown an association between persistence of transferred T cells and therapeutic response, so this is a critical parameter to optimize (3–6). Many factors influence survival of adoptively transferred T cells including; the stage of differentiation, conditions used during in vitro activation, host lymphodepletion, and exogenous cytokine support. The typical conditions of in vitro activation are designed to induce rapid proliferation and are accompanied by acquisition of phenotypic characteristics of effector cells. To maintain a highly activated functional state and to protect against apoptosis from cytokine withdrawal several protocols involve host lymphodepletion and high-dose IL-2 administration. However, strategies to induce rapid in vitro growth or cytolytic function might not optimally permit differentiation to long-term memory cells. In a search for controllable parameters that could improve survival of adoptively transferred T cells several recent reports compared adoptive transfer of in vitro activated CD8+ CTL at various stages of differentiation and concluded that TCM provide superior persistence (7) and anti-tumor function vis-à-vis TEM (8). It is important to note that in one experimental model, the source of CMV-specific CD8+ T cells was from primate hosts with chronic latent CMV infections (7). The other model was dependent on in vivo restimulation of transferred T cells with fowlpox vector which occurred in lymphoid tissues thus favoring TCM with preferential lymphoid trafficking (8). Because cancer patients have chronic exposure to tumor antigens and dysfunctional CD8+ cells may require augmentation with tumor vaccines, such studies identify TCM as an important subset of T cells to activate. Particular details of various experimental models, such as the previous activation history of T cells or requirements for homing to lymph organs are critical because the interactions that maintain homeostasis of immune cells are complex and differ between CD4+ and CD8+ T cells as well as between naïve and memory cells (9,10).
We and others have been interested in using experimental models to analyze the roles that CD4+ T cells play in the immune response against tumors (11–13). CD4+ T cells provide help for CD8+ CTL during antigen priming. Moreover, establishment and maintenance of CD8+ memory cells is facilitated by CD4+ T cells (14,15). Our recent studies indicated that tumor-reactive CD4+ T cells provide synergistic effector responses with CD8+ CTL. Intriguingly, CD4+ effector T cells, without CD8+ cells, can even mediate regression of tumors that do not express MHC-II molecules implicating indirect antigen presentation by tumor-associated APC (11,16). For our experiments, we use subcutaneous inoculation of a weakly immunogenic tumor into naïve hosts to sensitize T cells in tumor-draining lymph nodes (TDLN). This generates a primary immune response and among the changes associated with antigen sensitization, it is well established that responding T cells down-regulate CD62L expression (17). LN resident naive T lymphocytes and TCM that are unresponsive to specific tumor antigens retain high expression of CD62L. Therefore, it is possible to segregate the tumor-sensitized T cells from irrelevant cells by enrichment of the CD62Llow subset. Experiments have confirmed that the CD62Llow subset of TDLN contains cells with anti-tumor efficacy whereas the reciprocal CD62Lhigh subset is devoid of activity (18,19). Consistent with features of the primary immune response and similarly to other investigators, we have been able to activate tumor-primed T cells in vitro under conditions that induce rapid proliferation and even after 108-fold numerical expansion such cells retain potent effector function (11,20). To extend our previous studies and determine the long-term potential of such cells to mediate anti-tumor responses, we transferred effector T cells to naïve hosts and then challenged with tumor after an extended period. Our results indicate that adoptively transferred effector T cells can persist and acquire the phenotype and functions of TEM and TCM without ongoing exposure to antigen or exogenous cytokine support.
Materials and methods
Animals and cell lines
Female C57BL/6N (B6, Thy1.2) mice were purchased from the biologic Testing Branch, Frederick Caner Research and Development Center, National Cancer Institute (Frederick MD), and female B6.PL.Thy1.1 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in a specific pathogen-free environment according to National Institute of Health guidelines using an Institutional Animal Care and Use Committee approved protocol. The 3-methylcholanthrene-induced fibrosarcomas MCA 205 and MCA 207 originally derived in B6 mice have been maintained in vivo by serial s.c. transplantation in syngeneic mice as described previously (21). Single-cell tumor suspensions were prepared from solid tumors by enzymatic digestion (11).
Isolation and in vitro activation of tumor-sensitized C62Llow LN T cells
Mice were immunized by s.c. inoculation of 1.5×106 MCA205 or MCA 207 tumor cells in the flank region bilaterally. A single cell suspension was prepared from draining LNs 12 days later. Anti-mouse CD62L, CD4, CD8 or anti-PE microbeads, purchased from Miltenyi Biotec (Auburn, CA) were used for negative selection of CD62Llow cells. CD62Llow/CD4+ cells were subsequently purified by depletion of CD8+ cells followed by positive selection with CD4 MACS beads. Alternatively, CD62Llow/CD8+ cells were purified by depletion of CD4+ cells followed by positive selection of CD8+ cells as previously described (11). CD62Llow/CD4+ or CD62Llow/CD8+ T cells were suspended in complete medium (CM) at 2×106 /ml and activated with plate-bound anti-CD3 mAb (145-2C11, ATCC, Rockville MD)) for 48 hrs, then were cultured in CM supplemented with a mixture of 4U/ml of IL-2 plus 10ng/ml of rmIL-7 or 10ng/ml of rmIL-7 plus rmIL-23 (2 ng/mL) (each from R&D Systems, Minneapolis, MN). For long-term expansion, cultures were re-stimulated with anti-CD3 mAb for 14 hours on day 21 and CD4+ cells were re-purified.
FACS analysis and intracellular IFN-γ detection
FITC, CyChrome or PE-conjugated anti-CD8, anti-TCR, anti-IFN-γ, anti-CD4, anti-CD62L, anti-CD69, anti-CD25, anti-CD44, Thy1.1, anti-Thy1.2 and isotype mAbs were purchased from BD Biosciences (San Jose, CA). PE-conjugated anti-CD127 (IL-7 receptor α) was purchased from eBioscience (San Diego, CA). Cell surface phenotypes were measured by direct immnofluorescence staining with conjugated mAbs, and stained cells analyzed using the CellQuest Software (Becton Dickinson, San Jose,, CA). In vitro activated T cells were incubated with a single cell tumor digest at a 1:1 cell ratio, stained for intracellular IFN-γ and analyzed by FACS as previously described (11)
Tumor challenge and in vivo expansion assay
Culture-activated CD62Llow/CD4+ or CD62Llow/CD8+ T cells derived from MCA 205 or MCA 207 tumor-draining LN cells were adoptively transferred into 5 Gy irradiated or non-irradiated B6 hosts. Mice surviving for more than 75 days received 3×105 MCA 205 or 3×105 MCA 207 tumor cells by tail vein injection. Mice were sacrificed on days 19–21 after tumor challenge, and the lungs were insufflated with India ink and the number of tumor nodules on the surface was enumerated.
In some experiments, Thy1.1+/CD62Llow/CD4+ T cells were injected i.v into irradiated B6 (Thy1.2+) hosts. At the indicated number of days after adoptive transfer, the hosts received MCA 205 or MCA 207 tumor challenge. At the designed time points following intraveneous tumor injection, mice were anesthetized prior to removal of 0.5 ml blood then euthanized for removal of spleen, lung, and lymph nodes. A single cell suspension was prepared by mechanical separation of spleen or LNs, or enzymatic digestion for lungs. Small lymphoid cells in the lungs were isolated with Percoll (Pharmacia) density gradient centrifugation as described (22). Cells were stained with the indicated FITC, CyChrome or PE-conjugated antibodies and phenotypes were analyzed by FACS. The percentage of donor Thy1.1+ T cells in the lungs, spleen, LNs and blood was determined by FACS assay, the number of memory CD4+ T cells was also calculated by multiplying the total lymphoid cell count by their percentage.
Isolation and culture activation of CD4+ TCM or CD4+ TEM T cells
Spleens were removed from recipients of Thy1.1+/CD62Llow/CD4+ cells at the indicated time points. A single cell suspension was incubated with Thy1.2 microbeads and applied to MACS columns (Milteyi Biotec) to delete host (Thy1.2) cells. The flow-through fraction was incubated with anti-CD62L microbeads to positively isolate CD62Lhigh cells (TCM) and the flow through fraction contained CD62Llow T cell subset (TEM). After anti-CD3 activation for 48 hrs, Thy 1.1+ cells were positively selected on a MACS column. Thy1.1+ CD62Llow or CD62Lhigh subsets were cultured IL-2 plus IL-7 as described above. Thy1.1 purification in each subset was repeated on day 7 and day 14, and CD8+ T cells were depleted on day 14. After 2 cycles of stimulation with anti-CD3/IL-2/IL-7, expanded Thy1.1+ memory T cell subsets were used for adoptive immunotherapy.
Adoptive immunotherapy
Pulmonary metastases were established by i.v inoculation of 3×105 MCA 205 tumor cells suspended in 1.0 ml of HBSS. Three days later, mice were treated with the indicated number of purified CD4+ TCM or CD4+ TEM cells by i.v injection. Mice were sacrificed on days 19–21 after tumor inoculation, and the lungs were harvested and tumor nodules on the surface were enumerated as described above.
Statistical analysis
The significance of differences between groups was analyzed by two-tailed Student’s t test, or by the Wilcoxon rank-sum test. A two-tailed p value of <0.05 was considered significant.
Results
Adoptive transfer of tumor-reactive effector T cells into a tumor-free host establishes memory
We have previously demonstrated that lymph nodes draining subcutaneous tumors contain tumor-sensitized T cells and that this response peaks 9–12 days after tumor inoculation. The kinetics of this response in previously tumor-naïve animals coupled with the phenotype of the sensitized T cells (CD62Llow) are consistent with recently antigen stimulated T cells. We harvested MCA 205 tumor-draining lymph nodes (TDLN) 12 days after inoculation and depleted CD62Lhigh cells, obtaining 14% of the original cell number. The CD4+ or CD8+ subsets were additionally purified by depletion of the reciprocal subset followed by positive selection. Although the multiple selection steps decreased the final yield of CD8+ cells to only 4% of the initial CD62Llow subset, and the CD4+ subset to 14% of initial cells, the purity was high. The CD4+ or CD8+ T cell subsets were each separately activated with anti-CD3 mAb for 48 hrs and again overnight on day 21. T cells were cultured in medium supplemented with IL-2 plus IL-7 or alternatively IL-7 plus IL-23 for a total of 29 days. The CD4+ cultures required an additional depletion of residual CD8+ T cells because the CD8+ cells are capable of more rapid proliferation (23). However, at the time of adoptive transfer the CD4+ or CD8+ cultures were greater than 95% pure with less than 1% of the reciprocal population and had proliferated greater than 1000-fold. Table 1 shows the overall proliferation of each T cell culture and their phenotype. In addition the T cells were tested for IFN-γ production when stimulated with a single cell digest of tumors which contains numerous infiltrating MHC-II+ APC that can activate CD4+ cells. As previously observed, CD4+ or CD8+ cells cultured with IL-7 plus IL-23 displayed greater production of IFN-γ than the T cells cultured in IL-2 plus IL-7 (20). Similar to our previous experiments, the cultured T cells did not produce IFN-γ spontaneously nor in response to a distinct tumor cell line MCA 207 but were highly polarized to produce IFN-γ in response to anti-CD3 stimulation.
Table 1.
Proliferation, phenotype and IFN-γ response of activated CD4+ or CD8+ T cells
| T cell subset and cytokines |
Prolif. X-fold |
FACS (%) | IFN-γ (%) positive among CD4+ or CD8+ cells |
||||
|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | no-stim | MCA205 | MCA207 | Anti-CD3 | ||
| CD4+, IL-7/IL-23 | 1,123 | 97 | 0.3 | 0 | 31 | 0.8 | 91 |
| CD8+, IL-7/IL-23 | 2,673 | 0.3 | 96 | 0.2 | 32 | 0.3 | 97 |
| CD4+, IL-2/IL-7 | 3,447 | 99 | 0.2 | 0 | 5 | 0.1 | 70 |
| CD8+, IL-2/IL-7 | 12,174 | 0.2 | 95 | 0.2 | 13 | 0.5 | 93 |
CD62Llow T cells were isolated from tumor-draining lymph nodes and CD4+ or CD8+ subsets were additionally purified and activated with anti-CD3 mAb on day 0–2 and day 23 and were maintained in IL-2 + IL-7 or in IL-7 + IL-23. The total proliferation over the 29 day culture period was calculated. The phenotype of each culture was determined by FACS staining. The IFN-γ production in response to incubation with the MCA 205 tumor used for priming of LNs or an antigenically distinct tumor MCA 207 or anti-CD3 mAb was determined.
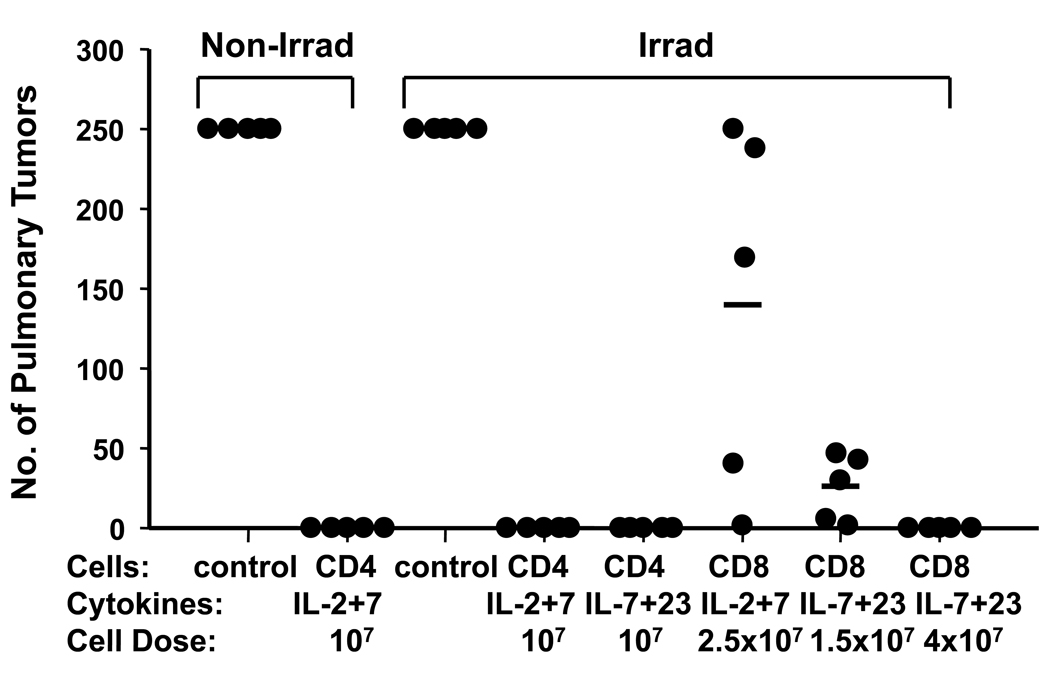
The T cells were adoptively transferred to naïve hosts, conditioned with 5 Gy of TBI to achieve lymphodepletion as well as non-irradiated hosts. Notably, recipient mice were not treated with exogenous cytokines. The mice were challenged 75 days later with intravenous MCA 205 tumor cells which embolize in the pulmonary vasculature and induce lung metastases. Recipients were sacrificed 20 days later and the number of lung metastases was determined. As demonstrated in Figure 1, a dose of 107 CD4+ cells, cultured with IL-2 plus IL-7 cytokine support in vitro, protected hosts from tumor challenge. Host lymphodepletion was not required because protection was effective in non-irradiated as well as irradiated hosts. This is interesting because many T cell adoptive immunotherapy strategies are postulated to require both profound lymphodepletion as well as exogenous IL-2 to support lymphocyte viability (24–26). Figure 1 also demonstrates that CD8+ cells cultured with IL-2 plus IL-7 were less effective “per-cell” than similarly prepared CD4+ cells. IL-23 supplementation during in vitro culture resulted in a higher percentage of T cells that produced IFN-γ specifically in response to tumor (Table 1). Similarly, CD8+ cells cultured with IL-7 plus IL-23 displayed greater “per-cell” efficacy in resisting tumor challenge than cultures stimulated with IL-2 plus IL-7. Another experiment of identical design also showed complete protection against tumor challenge 75 days after adoptive transfer of 107 CD4 cells cultured in IL-2 plus IL-7 or alternatively IL-7 plus IL-23. We also performed subcutaneous tumor challenge in TBI conditioned hosts with or without transfer of 107 CD4 cells cultured in IL-2 plus IL-7. We observed transient tumor growth for 7 days followed by complete tumor regression measured up to 100 days post-challenge. By contrast, subcutaneous tumors grew progressively in irradiated only hosts requiring sacrifice on day 28. In each of these experiments, the T cells were also used to treat hosts with 10-day established lung metastases and were curative indicating that they had effector function at the time of adoptive transfer (data not shown).
Figure 1. Adoptively transferred CD4+ or CD8+ effector T cells establish memory.
Purified CD62Llow CD4+ or CD8+ T cells derived from MCA 205 tumor-draining LNs were culture-activated with anti-CD3/IL-2/IL-7 or anti-CD3/IL-7/IL-23 for a total of 29 days then adoptively transferred into irradiated or non-irradiated tumor-free mice. Mice were challenged with MCA 205 tumor 75 days later. Recipients of effector CD4+ or CD8+ T cells resisted tumor challenge (p<0.001 vs. controls).
Effector T cells progressively decrease in frequency and acquire the phenotype of memory T cells
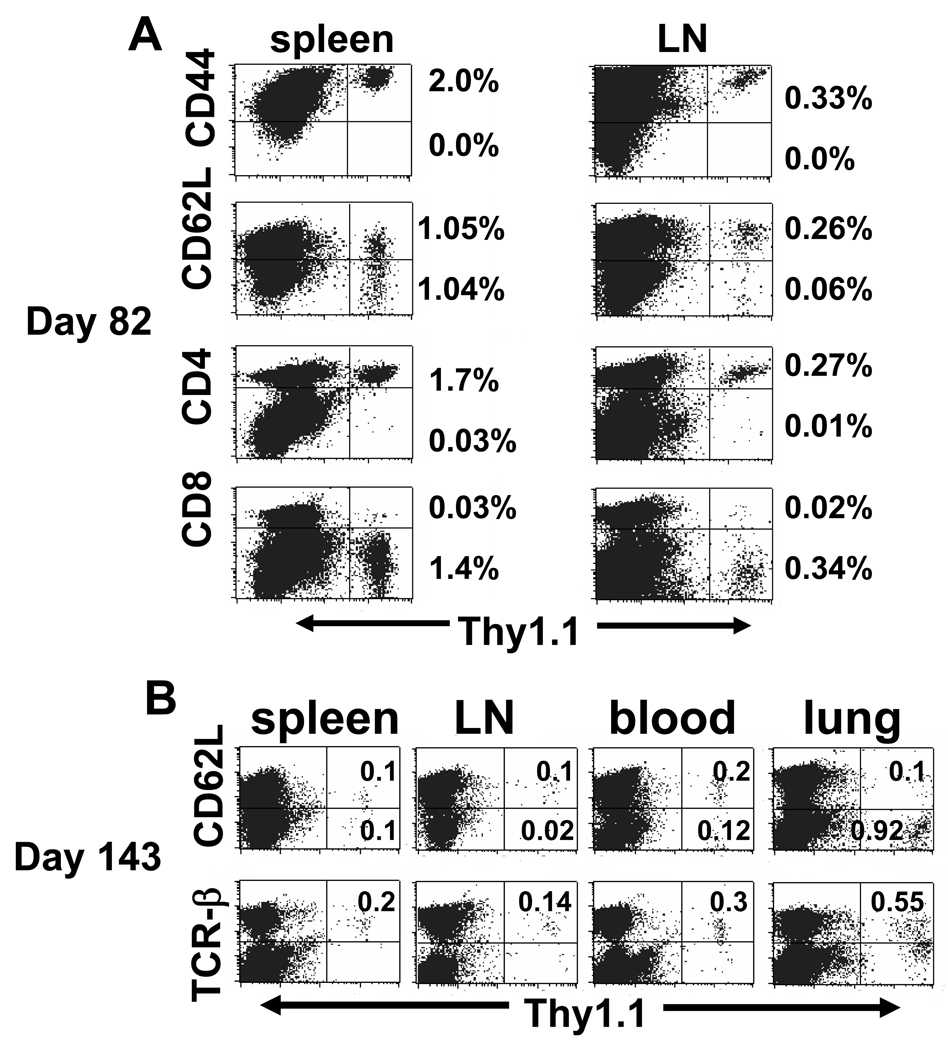
We prepared MCA 205 TDLN in Thy 1.1+ hosts and, after 29 day in vitro stimulation, 4×107 activated CD4+ T cells were transferred to congenic Thy 1.2+ hosts to track their frequency and phenotype in lymph tissues. We observed that after 82 days spleen contained 2% Thy 1.1+ cells that were CD4+, CD44high and which had heterogeneous expression of CD62L with approximately 50% of cells expressing high levels (Figure 2A). In lymph nodes the frequency of transferred T cells was lower at 0.3%, however 80% of these cells were CD44high, CD62Lhigh. By day 143 (Figure 2B), the frequency of Thy 1.1+ cells had declined to 0.2% in spleen, 0.12% in lymph nodes, and 0.3% in blood, but the relative frequency of CD62Lhigh cells was similar with 50% in spleen, 80% in lymph nodes, and 66% in blood. Interestingly, lung tissue contained far fewer T lymphocytes but 1% of them were the transferred Thy 1.1+ cells, among which 90% were CD62Llow in contrast to lymph tissues. These findings are consistent with a preferential distribution of cells with TEM in peripheral tissues distinct from TCM in lymph nodes with spleen containing a mixed population reflecting contributions from white pulp and red pulp compartments.
Figure 2. CD62Llow/CD4+ effector T cells differentiate into TEM and TCM in vivo.
Purified Thy1.1+/CD62Llow/CD4+ T cells were activated in vitro and 4×107 cells were adoptively transferred to irradiated tumor-free Thy 1.2+ congenic mice. (A) Day 82 post transfer, the percentage of Thy 1.1+ cells in the spleen and LNs was determined. Among donor T cells, 100% were CD44high and >95% were CD4+ with high expression of CD62L on 50.2% in spleen or 81.3% in LN. (B) Day 143 post transfer, Thy 1.1+ T cells were analyzed for CD62L expression in spleen, LNs, blood, and lung. The numbers represented the percentage of donor cells in the dot-plot quadrants. Each experiment included 2 mice.
Memory T cells convert to an activated phenotype and proliferate upon tumor challenge
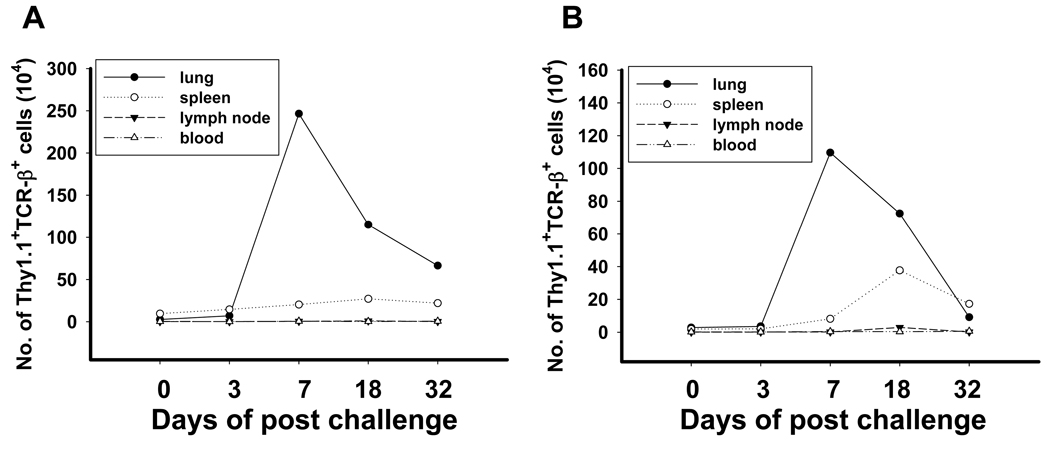
One of the hallmarks of memory T cells is their ability to undergo rapid proliferation upon reencounter with antigen. To monitor the fate of the transferred effector T cells, we harvested TDLN from Thy 1.1+ hosts inoculated with either MCA 205 or MCA 207 tumors 12 days earlier. The CD62Llow/CD4+ subset was activated in vitro for 29 days and 4×107 CD4+ effector T cells were adoptively transferred to congenic Thy 1.2+ hosts. Approximately nine months later (273 days), the mice were challenged with intravenous tumor cells to establish lung metastases and the number and phenotype of Thy 1.1+ cells was determined at several time points. As demonstrated in figure 3A, recipients had detectable, but very low numbers of Thy 1.1+ T cells in lung, spleen, lymph node, and blood prior to challenge. There was an increase in splenic Thy 1.1+ cells after tumor challenge, however there was a dramatic increase in the number of Thy 1.1+ cells isolated from lung on day 7, which subsequently declined but remained substantially above background levels 32 days after tumor challenge. A similar experiment using a distinct tumor (MCA 207) reproduced the pattern of rapid T cell expansion in lung tissue 7 days after tumor challenge (Figure 3B). In addition to their vigorous proliferation, the memory T cells also prevented the appearance of tumor at each time point indicating their effector function.
Figure 3. Tumor challenge induces rapid proliferation of long-term memory cells.
Thy1.1+/CD62Llow/CD4+ T cells were prepared from MCA 205 or MCA 207 tumor-draining LNs, activated in vitro for 29 days then adoptively transferred into tumor-free Thy1.2 hosts. (A) Day 273 for MCA 205 tumor model, or (B) day 277 for MCA 207 tumor model, the mice received tumor challenge by i.v injection. Lung, spleen and blood were harvested from two animals and pooled for processing. The number of Thy1.1+/TCRβ+ double T cells was enumerated. Similar results were obtained with Thy1.1/CD4 double staining. The Th1.1+ memory cells in the lung dramatically increased to the maximum at day 7 post tumor challenge, and peaking in the spleen at day 18.
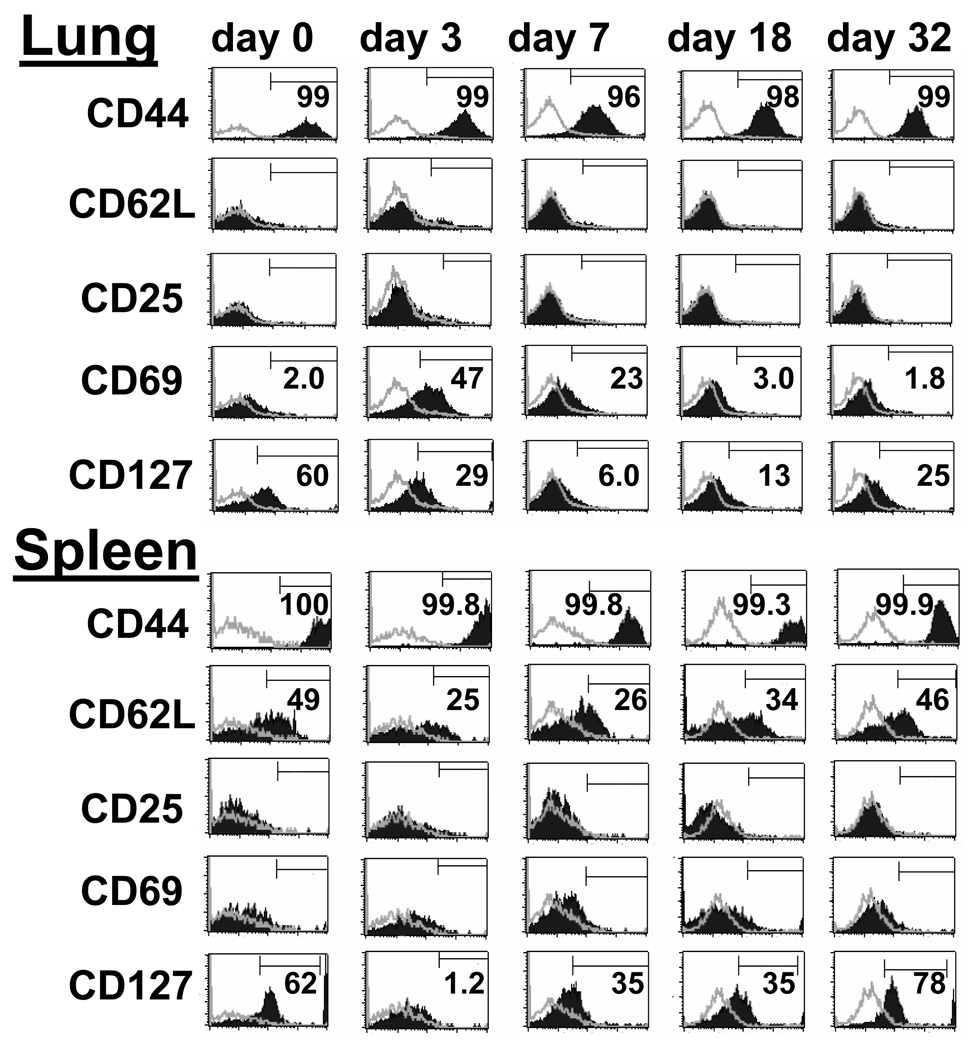
On day 273 following adoptive transfer and immediately prior to tumor challenge, the phenotype of the Thy 1.1+ T cells in the lung was exclusively CD44high, CD62Llow, CD127+, without expression of CD25 or CD69, consistent with a tissue resident TEM phenotype (Figure 4, Day 0). Three days after seeding of lung metastases, there was a dramatic increase in CD69 expression on Thy 1.1+ T cells. CD127 showed transient downregulation with different kinetics and maximal change on day 7 post tumor challenge. Expression of CD62L remained low on pulmonary T cells throughout the period of tumor rejection. By contrast, Thy 1.1+ T cells in the spleen prior to tumor challenge showed heterogeneous expression of CD62L in addition to high levels of expression of CD44 and CD127 and absence of activation markers such as CD25 or CD69. Interestingly, 50% of the T cells re-expressed high levels of CD62L consistent with a TCM phenotype. There was a different response among splenic T cells to tumor challenge with a decrease in the proportion of CD62Lhigh TCM between day 3 to 18 followed by a return to the basal level on day 32. Unlike pulmonary T cells, the splenic T cells displayed minimal expression of CD69 indicating that antigen stimulation was principally occurring in lung and bronchial lymph tissue.
Figure 4. Activation of CD4+ TEM cells at site of tumor challenge.
Thy1.1+/CD62Llow/CD4+ T cells prepared from MCA 205 tumor-draining LN, activated in vitro then adoptively transferred into tumor-free Thy1.2 hosts. Day 273 later mice were challenged by i.v. injection of MCA 205 tumor cells. Lungs and spleen were harvested at the indicated time points post tumor challenge. Histograms are gated on Thy1.1+/CD4+ double positive cells. Open histograms indicate isotype control. On days 0 and 32 when CD4+ T cells were in a resting state, CD62L was expressed on approximately 50% of splenic T cells but not on lung-resident T cells. On days 3 and 7 following tumor challenge, CD69 expression was up-regulated, and CD127 expression was down-regulated on lung cells. The data are representative of two independently performed experiments.
TCM and TEM populations can be reactivated in vitro and convert to effector function
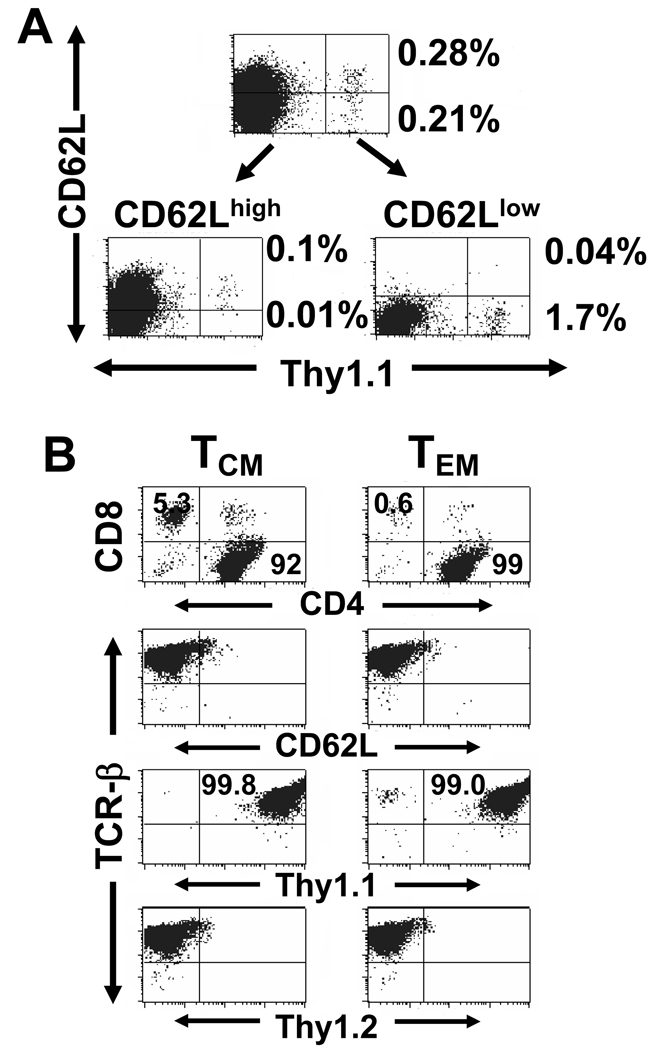
Despite activation and proliferation of Thy 1.1+ CD4+ T cells within lung tissue after tumor challenge it is possible that the principal function of adoptively transferred cells was to provide helper functions during sensitization of host Thy 1.2+ CD8+ T cells. In addition, the in vivo tumor challenge experiments did not identify whether the effector T cells for tumor rejection arose from TEM resident in the lung and circulation or recruitment of TCM from specialized lymph tissue. To explore the capability of TCM to differentiate again into secondary-response effector T cells, we separated spleen T cells based on CD62L expression into two populations on day 276 after adoptive transfer. As demonstrated in Figure 5A, this process effectively segregated the Thy 1.1+ cells into CD62Lhigh and CD62Llow fractions. For these experiments, it was necessary to deplete host Thy 1.2+ T cells two additional times to yield a pure population of Thy 1.1+ cells. This is because the in vitro activation utilizes anti-CD3 mAb which is an antigen-independent signal that can also stimulate host Thy 1.2+ T cells (Figure 5B). Notably, although the TCM subset was initially selected as CD62Lhigh these cells converted to a CD62Llow phenotype during in vitro activation. Two additional distinguishing features became apparent during culture activation. First, there was 1000-fold proliferation of the TEM subset in contrast to 190-fold proliferation of the TCM suggesting a more rapid entry into cell cycle following in vitro activation. Secondly, even though the initially transferred Thy 1.1+ T cells were <1% CD8+, there emerged a subset (5%) of CD8+ Thy 1.1+ T cells, but only in the TCM cultures.
Figure 5. Isolation and in vitro activation of Thy1.1+/CD4+ memory cells.
A. Spleens were harvested 276 days after adoptive transfer 4×107 Thy1.1+/CD62Llow/CD4+ effector T cells to tumor-free Thy1.2 mice. Thy1.2+ cells were depleted, and the negative fraction was separated into TCM (CD62Lhigh) and TEM (CD62Llow) subsets with MACS columns before culture activation. The percentage of Thy1.1+ TEM or TCM is indicated on the right of the dot-plot quadrants. B. On days 2, 7, 14 of culture, Thy1.1+ cells in each subset further were purified using MACS columns and on day 14, CD8+ T cells were depleted. At the completion of the in vitro activation with anti-CD3/IL-2/IL-7, cultures originally derived from TCM or TEM cells were analyzed for purity and phenotype.
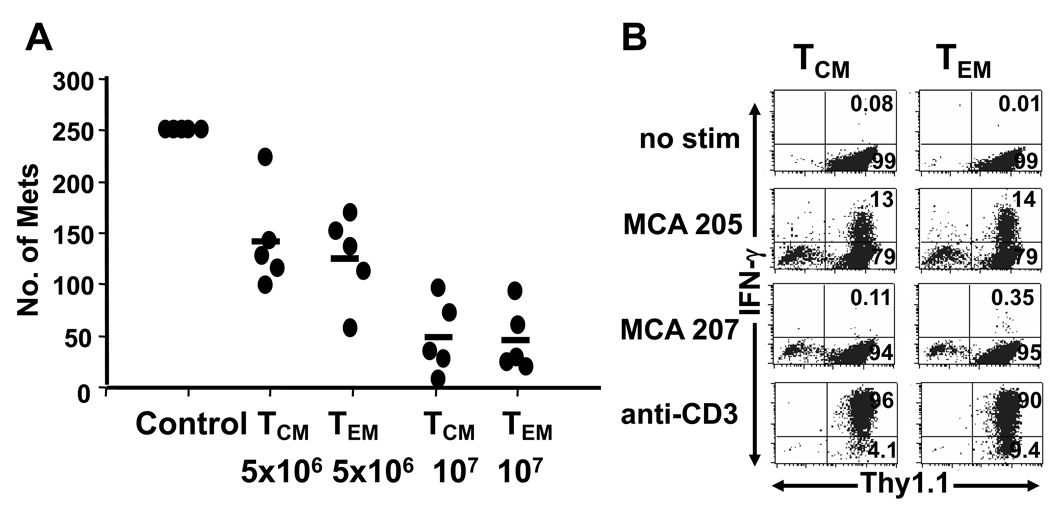
The TEM and TCM activated T cells were able to mediate regression of established pulmonary metastases upon adoptive transfer (Figure 6A). In addition, both populations displayed similar frequencies of T cells that could produce IFN-γ specifically in response to MCA205 tumor digest (Figure 6B). An independent experiment of similar design performed 285 days after the initial adoptive transfer of T cells also showed augmented proliferation of TEM compared with TCM as well as a small proportion of CD8+ cells arising in the TCM in vitro activated cells and similar proportion of IFN-γ producing cells in the TEM and TCM cultures. The therapeutic efficacy of the TCM cultures was moderately better than the TEM cultures in this second experiment but both TEM and TCM were fully competent to undergo reactivation and acquire secondary effector function.
Figure 6. Reactivated TEM and TCM CD4+ cells have similar therapeutic efficacy.
Thy1.1+ TCM or TEM cells isolated 276 days after adoptive transfer to tumor-free hosts were activated in vitro with anti-CD3/IL-2/IL-7. (A) The indicated number of cells initially derived from TCM or TEM was adoptively transferred to hosts bearing 3-day MCA 205 pulmonary metastases. Day 20 later, lungs were harvested and tumors were enumerated. Transfer of 5×106 TCM or TEM cells effectively eradicated pulmonary metastases (p<0.01 compared with control group). There was no significant difference between TCM vs. TEM groups at the two doses. (B) Culture-activated TCM or TEM cells were incubated alone (no stimulation), with a single-cell MCA 205 or MCA 207 tumor digest, or anti-CD3 and stained for intracellular IFN-γ. A similar percentage of TCM or TEM produced IFN-γ in response to MCA 205 stimulation (13% v.s 14%).
Discussion
Despite the theoretical appeal of harnessing the adaptive immune response for cancer therapy, there are significant barriers to its effective and routine clinical use. First, the extant immune response in patients with progressive metastatic disease is, by definition, inadequate. Recent elucidation of the process of tumor immunoediting, as well as the complex regulation of the immune response to self-antigens, suggest that high avidity TCR responses may be subverted during tumor development (27–29). Lower avidity T cells may receive inadequate stimulation during priming to generate a robust and sustained response. Moreover, chronic persistence of tumor antigens may also render tumor-reactive T cells in a state of exhaustion, much as in chronic viral infections (30). These features of the dampened immune response in cancer patients might account for the difficulty obtaining objective clinical responses with cancer vaccines, despite stimulating measurable immunologic responses and increases in precursor frequency.
Suboptimal conditions for active immunotherapy has rekindled interest in adoptive T cell transfer, which has advantages of in vitro generation of T cells with defined antigen reactivity and strict control over conditions of activation (31). Cell extrinsic regulatory mechanisms can thereby, be bypassed. Moreover, the strength and duration of TCR/CD3 stimulation as well as the composition and concentration of exogenous cytokines can be optimized for growth and long-term survival of effector T cells. Additionally, conditions for competitive survival of transferred T cells can be improved though host lymphodepletion or even myeloablation and hematopoetic stem cell rescue, combined with exogenous high-dose cytokine support (24,25). However, intensive concomitant treatments have their own inherent toxicity and limits on host tolerability may eventually outweigh gains to T cell survival. It would be advantageous to have a source of T cells that were activated under conditions that support effector function and preserve long-term persistence by virtue of intrinsic epigenetic and metabolic properties rather than extrinsic manipulations.
Our data indicate that tumor-reactive T cells can indeed be activated in vitro under conditions which promote their numerical expansion and acquisition of effector phenotype and function but which also preserve their capacity to subsequently differentiate into TEM and TCM. This linear differentiation of CD4+ effector cells to memory cells and their subsequent capacity to replicate and provide effector function is consistent with data from CD8+ effector cells (32). Interestingly, this differentiation did not require host lymphodepletion or extrinsic cytokine support after adoptive transfer. Consistent with well established models of CD4+ T cell differentiation, it also did not require antigen re-stimulation in vivo (33).
There are several aspects of our experimental model that likely contributed to the emergence of long-term memory cells from effector T cells. First, T cells were obtained from healthy naïve hosts at the peak of the priming response. At first pass, this does not seem applicable to clinical situations. However, it does suggest that MHC compatible normal donors, as used in this experimental model, might prove to be a superior source for tumor-reactive T cells. This is partly because the repertoire of tumor-reactive CD4+ and CD8+ T cell is intact, but more importantly, the T cells can be selected and manipulated during the primary immune response, before they have been functionally impaired by a suboptimal priming event. Using transgenic CD4+ T cells specific for a lymphocytic choriomeningitis virus (LCMV) epitope, it was recently demonstrated that diminished strength of the primary sensitization did not influence the extent of expansion or effector function but had a profound effect, preventing differentiation to memory cells (34). Interestingly transgenic CD4+ cells that were effectively primed by LCMV infection and became memory cells did not have a defective response during secondary stimulation. In our experiments, the strength of TCR/CD3 signaling can be controlled in vitro with the anti-CD3 mAb to provide full activation, which incidentally does not require preexisting knowledge of the antigen(s). Duration of TCR signaling is also important for programming the developmental pathways of CD4+ and CD8+ T cells and this is another parameter that can be controlled in vitro (35,36).
The provision of cytokines to T cell cultures during in vitro activation not only provided for numerical expansion but may also have contributed to development of long-term persistence after adoptive transfer. The initial proliferative signal was delivered with immobilized anti-CD3 mAb for 48 hrs at a high-density of T cells. Then T cells were removed from the stimulus, washed, and diluted to a low concentration with cytokine supplementation. T cells underwent rapid expansion over the subsequent 7 days in the presence of low concentrations of IL-2 (4 U/ml), IL-7 (10 ng/ml) or IL-23 (2 ng/ml). In CD4+ cell cultures, we have observed equivalent proliferation over the first 9 days in the presence of IL-7 with or without IL-2, suggesting a cell intrinsic source. IL-23 alone does not augment the intrinsic proliferative signal. Subsequently, CD4+ T cells in the absence of IL-7 decline in number. In contrast, when the cultures are maintained with IL-7 they convert to a low proliferative state and their viability is sustained until day 23 when they are again stimulated with anti-CD3 mAb and undergo another proliferative burst. It is possible that periodic strong TCR/CD3 stimulation with a subsequent period of quiescence after the proliferative phase is completed permits the acquisition of properties that enhance memory cell formation. Intriguing recent data using Bim−/− OT-I transgenic mice indicate that cell intrinsic factors, rather than competition for limited cytokine pools regulate the contraction phase of effector CD8+ T cells and that all effector T cells have the capacity to become fully functional memory cells (37). It appears that a shift in the metabolic state of T cells regulates conversion from effector to memory status. In particular modulation of the mTORC1 complex and change from an anabolic glycolytic metabolism to a catabolic metabolism underlies differentiation from effector to memory status (38). Although the proximal signals that regulate this conversion are not yet defined, it is anticipated that such signals could be optimized during in vitro culture activation. Provision of high-dose exogenous cytokines may in fact have a paradoxically negative effect on effector T survival following adoptive transfer if it hinders metabolic transition to a memory state.
Our experiments focused on CD4+ T cells because we have an interest in gaining a greater understanding of the mechanism by which they eliminate tumors that are MHC-II negative. Tumor antigens are indirectly presented to CD4+ T cells on MHC-II positive APC that infiltrate tumors and this provides sufficient re-stimulation to initiate a process of tumor rejection. Presumably tumor cell death occurs through cytokine release and activation of tumoricidal function in tumor-associated macrophages. Our focus on CD4+ T cells does not ignore the importance of CD8+ T cells and the data in Figure 1 indicate that they too were able to persist and provide memory function. Interestingly, the conditions of in vitro activation provide for more rapid proliferation of CD8+ T cells and even in the CD4+ cultures there were a small number of CD8+ T cells (<1%) that were adoptively transferred and established themselves within the long-term TCM pool as demonstrated in Figure 2. This is consistent with the stable persistence of TCM CD8+ T cells and declining persistence of CD4+ TCM specific to viral antigens (39). When CD8+ TCM were activated in vitro they again proliferated more rapidly than CD4+ T cells to become a 5% presence within cultured TCM cells. Although, we were not specifically examining the long-term response of the CD8+ TCM it is evident that they survived adequately in the presence of CD4+ TCM. Because tumor-specific CD4+ T cells can provide synergistic anti-tumor effects to CD8+ effector cells in the period shortly after adoptive transfer, adapting in vitro activation conditions to optimize CD4+ T cell growth is important. The current experiments indicate that effector CD4+ T cells transition to become memory cells and their capacity to provide persistent help to CD8+ memory cells should augment the overall efficacy of adoptive immunotherapy for cancer.
Acknowledgments
Grant Support: This work was supported in part by National Institutes of Health grant RO1CA120893 (GEP)
Reference List
- 1.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu. Rev. Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 3.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, Khalil M, Wu MF, Huls MH, Chang CC, Gresik MV, Gee AP, Brenner MK, Rooney CM, Heslop HE. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, Robbins PF. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J. Immunother. 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 10.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang LX, Shu S, Disis ML, Plautz GE. Adoptive transfer of tumor-primed, in vitro-activated, CD4+ T effector cells (TEs) combined with CD8+ TEs provides intratumoral TE proliferation and synergistic antitumor response. Blood. 2007;109:4865–4876. doi: 10.1182/blood-2006-09-045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr. Opin. Immunol. 2004;16:259–263. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plautz GE, Mukai S, Cohen PA, Shu S. Cross-presentation of tumor antigens to effector T cells is sufficient to mediate effective immunotherapy of established intracranial tumors. J. Immunol. 2000;165:3656–3662. doi: 10.4049/jimmunol.165.7.3656. [DOI] [PubMed] [Google Scholar]
- 17.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 18.Kagamu H, Touhalisky JE, Plautz GE, Krauss JC, Shu S. Isolation based on L-selectin expression of immune effector T cells derived from tumor-draining lymph nodes. Cancer Res. 1996;56:4338–4342. [PubMed] [Google Scholar]
- 19.Peng L, Kjaergaard J, Plautz GE, Awad M, Drazba JA, Shu S, Cohen PA. Tumor-induced L-selectinhigh suppressor T cells mediate potent effector T cell blockade and cause failure of otherwise curative adoptive immunotherapy. J. Immunol. 2002;169:4811–4821. doi: 10.4049/jimmunol.169.9.4811. [DOI] [PubMed] [Google Scholar]
- 20.Wang LX, Huang WX, Graor H, Cohen PA, Kim JA, Shu S, Plautz GE. Adoptive immunotherapy of cancer with polyclonal, 108-fold hyperexpanded, CD4+ and CD8+ T cells. J. Transl. Med. 2004;2:41. doi: 10.1186/1479-5876-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth RJ, Jr, Bock SN, Mule JJ, Rosenberg SA. Unique murine tumor-associated antigens identified by tumor infiltrating lymphocytes. J. Immunol. 1990;144:1531–1537. [PubMed] [Google Scholar]
- 22.Wang LX, Kjaergaard J, Cohen PA, Shu S, Plautz GE. Memory T cells originate from adoptively transferred effectors and reconstituting host cells after sequential lymphodepletion and adoptive immunotherapy. J. Immunol. 2004;172:3462–3468. doi: 10.4049/jimmunol.172.6.3462. [DOI] [PubMed] [Google Scholar]
- 23.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 24.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS. One. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 29.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 34.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J. Exp. Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J. Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 37.Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16689–16694. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]