Abstract
OBJECTIVES:
To evaluate vascular protection treatment patterns and attainment of the 2003 Canadian Diabetes Association’s recommended targets in ambulatory patients with type 2 diabetes.
METHODS:
Between 2005 and 2006, 3002 outpatients with type 2 diabetes were enrolled by 229 primary health care settings across Canada. Baseline characteristics, therapeutic regimens and treatment success – defined as the achievement of a blood pressure (BP) of 130/80 mmHg or lower, glycosylated hemoglobin (A1C) of 7% or lower, low-density lipoprotein cholesterol (LDL-C) lower than 2.5 mmol/L and total cholesterol/high-density lipoprotein cholesterol ratio lower than 4.0 – are reported.
RESULTS:
Overall, 46% of individuals had a BP that was above the Canadian Diabetes Association’s recommended target. Of these, 11% were untreated, 28% were receiving monotherapy, 38% were not receiving an angiotensin-converting enzyme inhibitor and 16% were not receiving either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker. Optimal A1C levels were achieved in 53% of patients. Of those who did not attain A1C targets, 3% were not on glucose-lowering pharmacotherapy and 27% were receiving monotherapy. A total of 74% of patients were treated with statins. Overall, 64% and 62%, respectively, met the target LDL-C and the target total cholesterol/high-density lipoprotein cholesterol ratio. Statins were not prescribed to 43% of patients with LDL-C above target. Antiplatelet therapy was implemented in 81% of patients. In total, 21% achieved the combined targets for BP, A1C and LDL-C.
INTERPRETATION:
A substantial proportion of patients did not achieve guideline-recommended targets and were not receiving evidence-based therapy for vascular protection two years after publication of the Canadian guidelines. More research is warranted, and novel and effective strategies must be tested and implemented to correct this ongoing treatment gap.
Keywords: Cardiovascular protection, Clinical practice guidelines, Diabetes, Target achievement, Treatment
Abstract
OBJECTIFS :
Évaluer les modèles de traitement pour la protection vasculaire et l’atteinte des cibles recommandées par l’Association canadienne du diabète en 2003 chez des patients ambulatoires atteints du diabète de type 2.
MÉTHODOLOGIE :
En 2005 et 2006, 3 002 patients ambulatoires atteints du diabète de type 2 ont participé à l’étude dans 229 établissements de soins primaires au Canada. Sont transmis les caractéristiques de départ, les schémas thérapeutiques et la réussite du traitement, définie comme l’atteinte d’une tension artérielle (TA) maximale de 130/80 mmHg, d’une hémoglobine glycosylée (A1c) maximale de 7 %, d’un cholestérol à lipoprotéines de basse densité (C-LDL) inférieur à 2,5 mmol/L et d’un ratio entre le cholestérol total et le cholestérol à lipoprotéines de haute densité inférieur à 4,0.
RÉSULTATS :
Dans l’ensemble, 46 % des participants avaient une TA supérieure à la cible recommandée par l’Association canadienne du diabète. De ce nombre, 11 % n’étaient pas traités, 28 % recevaient une monothérapie, 38 % ne recevaient pas d’inhibiteur des enzymes de conversion de l’angiotensine et 16 %, d’inhibiteur de l’enzyme de conversion de l’angiotensine ou d’antagoniste de réception de l’angiotensine. Cinquante-trois pour cent des patients ont atteint un taux d’A1c optimal. Chez ceux qui ne l’ont pas atteint, 3 % ne prenaient pas de pharmacothérapie pour abaisser leur glycémie et 27 % recevaient une monothérapie. Au total, 74 % étaient traités au moyen de statines. Dans l’ensemble, 64 % et 62 %, respectivement, respectaient le C-LDL ciblé et le ratio entre le cholestérol total et le cholestérol à lipoprotéines de haute densité. Quarante-trois pour cent des patients dont le C-LDL était supérieur à la cible n’avaient pas de prescription de statines. Chez 81 % des patients, un traitement antiplaquettaire était amorcé. Au total, 21 % ont atteint les cibles combinées de TA, d’A1c et de C-LDL.
INTERPRÉTATION :
Une forte proportion de patients n’atteignaient pas les cibles recommandées par les lignes directrices et ne recevaient pas de traitement probant pour assurer leur protection vasculaire deux ans après la publication des lignes directrices canadiennes. D’autres recherches s’imposent, et il faudra mettre à l’essai et mettre en œuvre des stratégies nouvelles et efficaces pour corriger cette lacune thérapeutique continue.
Cardiovascular disease remains the leading cause of morbidity and mortality in patients with type 2 diabetes, and is an enormous economic burden (1,2). The available literature indicates that microvascular and cardiovascular complications (3) as well as mortality in extended follow-up (4) are appreciably reduced in patients with type 2 diabetes when a multifactorial, intensive lifestyle modification coupled with pharmacotherapy intervention that targets hypertension, dyslipidemia and hyperglycemia is implemented. Accordingly, evidence-based clinical practice guidelines (CPGs) have been published in several jurisdictions, including Canada, the United States and Europe (1,5,6), with specific recommendations for the intensive management of risk factors in patients with type 2 diabetes.
Previous studies investigating the success of guidelines on the management of diabetes have shown that treatment goals are often not met in ‘real-life’ practice, and clinical implementation of vascular protection strategies remains suboptimal in patients with type 2 diabetes (7–9). In 2003, the Canadian Diabetes Association (CDA) published new CPGs for the management of diabetes (1). A major focus of these CPGs was the importance of a comprehensive, multifactorial vascular protective strategy similar to that used in the Steno-2 trial (3). Consequently, an extensive program was launched to implement the CPGs.
The objective of the present study was to evaluate vascular protection treatment patterns and attainment of the recommended targets of the CDA CPGs in a practice-based registry of Canadian ambulatory patients with type 2 diabetes two years after the publication of these guidelines. Additionally, we aimed to qualitatively assess whether the guideline-to-clinical practice gap is narrowing over time.
METHODS
Study design
The present study was a cross-sectional analysis of the prospective Diabetes Registry to Improve Vascular Events (DRIVE) cohort. It was a practice-based registry designed to examine current management of patients with diabetes and to identify the degree to which CPGs are implemented into patient care in primary care offices across Canada. The registry also sought to determine whether the outcomes observed in randomized clinical trials were applicable to the ‘real-life’ setting and to evaluate the impact of physician educational intervention on diabetes management.
Physicians were recruited by direct mail or fax campaigns, continuing medical education (CME) events, and from participation in previous or ongoing registries with the coordinating centre – the Canadian Heart Research Centre (Toronto, Ontario). Physicians participating in any other diabetes study involving physician education were excluded. In total, 229 primary care physicians from 10 provinces across Canada participated in the DRIVE study, with patient enrollment occurring between March 2005 and March 2006. Ethics approval for the investigation was received from an independent central ethics review board (Optimum Clinical Research Inc Ethics Review Board, Oshawa, Ontario) and provincial Colleges of Physicians and Surgeons review committees where appropriate. Participation in the registry was voluntary and all enrolled patients provided written informed consent.
Patient population
Physicians were instructed to enroll consecutive patients with type 2 diabetes from their practices. Type 2 diabetes was defined as treatment with an oral hypoglycemic agent or insulin, a fasting plasma glucose of 7 mmol/L or greater, a plasma glucose 2 h after a 75 g glucose challenge of 11.1 mmol/L or greater, or symptoms of diabetes (including fatigue, polyuria, polydipsia and unexplained weight loss) plus a random/casual plasma glucose of 11.1 mmol/L or greater. The diagnosis of type 2 (as opposed to type 1) diabetes was made based on clinical criteria that included all of the following: diagnosis after 30 years of age, lack of a history of ketoacidosis and lack of requirement for insulin therapy within the first six months of the diagnosis of diabetes. A history of coronary artery disease was defined as previous coronary artery bypass surgery or percutaneous coronary intervention, previous myocardial infarction or unstable angina, or stable angina with a positive stress test or more than 50% stenosis of at least one major coronary artery on angiography. The presence of peripheral vascular disease was established if any of the following was documented: intermittent claudication; decreased peripheral pulses or femoral artery bruit with an ankle-brachial index lower than 0.90; or abnormal duplex ultrasound findings (greater than 50% stenosis of one or more major artery). Cerebrovascular disease was defined as a history of previous stroke or transient ischemic attack. Hypertension was defined as a systolic blood pressure (BP) of 130 mmHg or greater and/or a diastolic BP of 80 mmHg or greater and/or previous use of antihypertensive medications. In total, 3017 patients with diabetes were recruited. To limit the analysis to adults with type 2 diabetes, 15 patients who did not have all of the clinical criteria for type 2 diabetes (listed above) were excluded; the resultant study population consisted of 3002 patients.
Before enrollment, physicians attended CME programs, where they were given clear guidelines and goals for therapy that were based on the 2003 CDA CPGs (1). Briefly, according to these guidelines, a comprehensive, multifaceted strategy aimed at all cardiovascular risk factors should be implemented in the care of the vast majority of patients with type 2 diabetes, including lifestyle modification, use of angiotensin-converting enzyme (ACE) inhibitors, lipid-lowering agents (primarily statins) and antiplatelet agents with the following goals: BP 130/80 mmHg or lower; glycosylated hemoglobin (A1C) 7% or lower (6% or lower if it can be safely achieved); low-density lipoprotein cholesterol (LDL-C) lower than 2.5 mmol/L; and total cholesterol/high-density lipoprotein cholesterol ratio (TC/HDL-C) lower than 4.0.
Data collection
For each patient enrolled, participating physicians were asked to complete case report forms that detailed their management strategies. Patient demographics, medication use and presence of cardiovascular risk factors, as well as micro- and macrovascular complications were collected. Information on physical measurements (height, weight, BP and waist circumference) and most recent laboratory results were recorded on standardized case report forms. If the BP was measured more than once at the most recent clinic visit, physicians were instructed to provide the average BP measurement. All case report forms were returned to the coordinating centre and scanned into an electronic database (TELEform, version 7; Cardiff Software Inc, USA).
Data analysis
Numerical variables are summarized as medians with interquartile ranges. Categorical variables are presented as percentages. All analyses were performed using SAS software version 9.1 (SAS Institute Inc, USA).
RESULTS
The demographics and clinical characteristics of the 3002 patients included in the present report are summarized in Table 1, while the most recent laboratory data available before patient enrollment are presented in Table 2. The distribution of the number of patients enrolled by each of the 229 participating physicians was relatively homogeneous: more than 90% of primary case providers enrolled 10 to 15 patients. The median age of the study population was 64 years, and the majority of the patients were male and Caucasian. Most (99.5%) of the DRIVE population had either established macro- or microvascular complications, or cardiovascular risk factors (81% of the patients had at least one complication, while of those without any established complication, 97% had at least one additional cardiovascular risk factor and 73% had two or more cardiovascular risk factors).
TABLE 1.
Demographics and clinical characteristics (n=3002)
|
Demographics | |
| Age, years | 64 (56–72) |
| Age at diagnosis, years | 55 (47–63) |
| Time since diagnosis, years | 6 (3–11) |
| Male sex, % | 59 |
| Caucasians, % | 84 |
|
Physical | |
| Blood pressure, mmHg | |
| Systolic | 130 (120–140) |
| Diastolic | 78 (70–80) |
| Body mass index, kg/m2 | 30 (27–35) |
| Waist circumference, cm | |
| Men | 105 (97–116) |
| Women | 102 (92–111) |
|
CVD risk factors, % | |
| Current smoker | 13 |
| Family history of premature CAD | 25 |
| Hypertension | 76 |
| Dyslipidemia | 95 |
|
Complications, % | |
| Established CVD | 31 |
| CAD | 23 |
| PVD | 9 |
| Cerebrovascular disease | 8 |
| Heart failure | 5 |
| Microvascular complication | 40 |
| Nephropathy* | 33 |
| Retinopathy | 8 |
| Neuropathy | 14 |
| Nontraumatic amputation | 1 |
| Foot/leg ulcer | 2 |
| Erectile dysfunction (n=1765) | 34 |
Data presented as median (interquartile range) unless otherwise indicated.
History of micro- or macroalbuminuria reported by participating physicians. CAD Coronary artery disease; CVD Cardiovascular disease; PVD Peripheral vascular disease
TABLE 2.
Laboratory data (n=3002)
| Biochemical measurements | Median (interquartile range) |
|---|---|
| Fasting plasma glucose, mmol/L | 7.5 (6.5–9.0) |
| Glycosylated hemoglobin, % | 6.9 (6.3–7.8) |
| Urinary albumin/creatinine ratio, mg/mmol | |
| Men | 1.4 (0.6–3.6) |
| Women | 1.3 (0.7–3.4) |
| Serum creatinine, μmol | 84.0 (71.0–99.0) |
| Total cholesterol, mmol/L | 4.3 (3.7–5.0) |
| LDL cholesterol, mmol/L | 2.2 (1.8–2.8) |
| HDL cholesterol, mmol/L | 1.2 (1.0–1.4) |
| Total cholesterol/HDL cholesterol ratio | 3.6 (3.0–4.4) |
| Triglycerides, mmol/L | 1.7 (1.2–2.3) |
HDL High-density lipoprotein; LDL Low-density lipoprotein
Within the patient population, 15% had a body mass index (BMI) of lower than 25 kg/m2, 33% were overweight (BMI 25 kg/m2 to 29.9 kg/m2) and 52% were obese (BMI 30 kg/m2 or greater). The waist circumferences of 31% of the study patients were below the recommended targets for Caucasian subjects (102 cm for men, 88 cm for women). In total, 11% of the patients had BMI values lower than 25 kg/m2 and a waist circumference that was lower than these thresholds.
BP control and management
The BP readings of 1374 patients (46%) were above the recommended target of 130/80 mmHg or lower. Of those, 149 (11%) were not receiving any pharmacotherapy for hypertension, 391 (28%) were receiving monotherapy, 374 (27%) were receiving dual therapy and 460 (33%) were receiving three or more antihypertensive medications. Of the patients who had not attained target BP levels, 528 (38%) were not receiving an ACE inhibitor and 214 (16%) were not receiving either an ACE inhibitor or an angiotensin receptor blocker. Overall, 60% of the patients were prescribed an ACE inhibitor and 27% were receiving ramipril 10 mg/day or perindopril 8 mg/day – the doses that were used in the clinical trials (10,11) that demonstrated the vascular protective benefit of these regimens.
Glycemic control and management
A1C results obtained within the year before enrollment were available for 97% of the patients. As per glycemic targets defined in the 2003 CDA CPGs, 432 patients (15%) had an A1C of 6% or lower and 1524 patients (53%) had an A1C of 7% or lower. Suboptimal control was noted in 1376 patients (47%) and this included 1111 patients (38%) with an A1C between 7% and 9%, and 265 patients (9%) with an A1C of 9% or greater.
Overall, 91% of the patients were prescribed at least one antihyperglycemic agent, with 2277 patients (76%) maintained only on oral agents (1105 [48.5%] on monotherapy, 832 [36.5%] on dual therapy and 340 [15%] on three or more agents). Metformin was the most commonly prescribed oral agent (n=2163, 72%) in monotherapy as well as in combination therapy. Insulin monotherapy was reported in 151 patients (5%), while insulin in combination with oral agents was documented in 292 patients (10%).
Of the 1376 patients (47%) who did not attain target A1C levels (A1C of greater than 7%), 40 (3%) were not receiving any antihyperglycemic pharmacological therapy, while an additional 366 (27%) were receiving monotherapy.
Lipid control and management
Overall, 78% of patients were receiving at least one lipid-lowering agent. A total of 2233 patients (74%) were treated with statins (93% on statin monotherapy, 4% on statin plus fibrates, 2% on statins plus ezetimibe and 1% on statin plus other agents). A further 96 patients (3%) were receiving fibrate alone, while use of niacin was minimal (less than 1% of the patients).
LDL-C levels were recorded for 2862 patients (95%); of these, TC/HDL-C was available for 2791 (93%). A total of 1837 patients (64%) had an LDL-C level below the CDA CPG recommended target (at the time of the DRIVE study) of less than 2.5 mmol/L and 1732 (62%) had a TC/HDL-C ratio below the target of 4.0. Overall, 1386 patients (50%) attained both of the recommended lipid targets.
Among patients who did not achieve the recommended LDL-C target, 43% were not prescribed statins, 37% were not receiving any lipid-lowering therapy and 2% were being maintained on a statin in combination with ezetimibe or colestipol.
Antiplatelet therapy
Overall, 2444 patients (81%) were receiving an antiplatelet agent. Acetylsalicylic acid was the most commonly prescribed pharmacotherapy (n=2366, 79%). Of the 929 patients with established atherosclerotic disease (coronary artery disease, peripheral vascular disease and/or cerebrovascular disease), 835 (90%) were receiving an anti-platelet agent.
Nephropathy screening/follow-up and management
Data on albumin/creatinine ratios a year before enrollment were unavailable for 30% of the patients. Of the 977 patients with diabetic nephropathy, defined as a history of micro- or macroalbuminuria reported by participating physicians, 101 (10%) were not on a recommended ACE inhibitor or angiotensin receptor blocker therapy.
Achieving treatment targets and combined control of risk factors
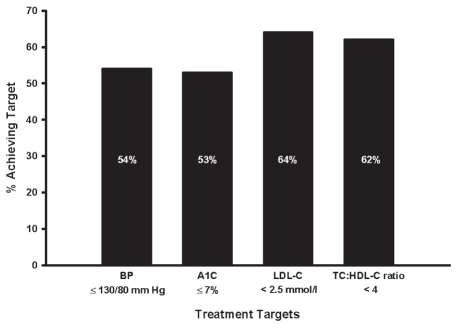
The percentage of patients who achieved targets as per the 2003 CDA CPG recommendations are presented in Figure 1. Overall, 21% of the 2741 (91%) patients with available BP, LDL-C and A1C data met the combined targets for all three parameters.
Figure 1).
Achievement of treatment targets. A1C Glycosylated hemoglobin; BP Blood pressure; HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; TC Total cholesterol
DISCUSSION
The present cross-sectional study of more than 3000 ambulatory Canadian subjects with type 2 diabetes showed that a substantial proportion of the patients failed to achieve the targets listed within the CDA CPGs two years after their publication. In particular, despite well-established published guidelines and goals for therapy through CME events and other instructive opportunities, the combined targets for BP, A1C and LDL-C were not adequately managed with evidence-based medical therapies. These observations highlight the necessity for more aggressive public health efforts to manage cardiovascular risk factors among individuals with type 2 diabetes.
Approximately one-half of our study subjects attained both systolic and diastolic BP targets – an achievement that was relatively more successful than those reported in two studies from the United States (8,9), in which only 30% of the patients reached the recommended targets. In the present study, the poor BP control observed in the remaining patients can be largely attributed to inadequate treatment, given that a significant proportion of this group was either prescribed no antihypertensive medications or was receiving monotherapy. Our findings support and extend earlier reports that over-reliance on monotherapy likely constitutes an important contributor to the persistently low rates of hypertension control (12). This is consistent with data from several clinical trials (13,14), which demonstrated that patients with diabetes typically require three to four antihypertensive medications to achieve BP targets.
We found that ACE inhibitors and angiotensin receptor blockers were significantly underused in our cohort of patients. These results, therefore, suggest that the concept of diabetes alone as an indication for treatment with ACE inhibitors or angiotensin receptor blockers was ostensibly not a widely accepted practice among the participating physicians (10,15).
Optimal glycemic control with a recommended A1C goal of 7% or lower was only achieved in approximately one-half of the study patients. This less than satisfactory outcome is not dissimilar to that of a previous Canadian national chart audit (16) that comprised data collected between September 2002 and February 2003. Taken together, the findings suggest that the publication and implementation strategies of the 2003 CDA CPGs have had a disappointingly minor impact on clinical practice in Canada. Hoerger et al (17) recently reported that a similar proportion (43%) of the National Health and Nutrition Examination Survey (NHANES) participants demonstrated A1C values of more than 7% in the latest (2003/2004) assessment. Interestingly, however, the available data indicate that the population averages for A1C values of less than 7% in the United States are improving (37%, 49% and 57% in 1999/2000, 2001/2002 and 2003/2004, respectively).
Notably, a considerable percentage of the patients who failed to reach adequate glycemic targets in the present study were either prescribed only one oral agent or were not treated with any antihyperglycemic agents. Furthermore, only a small number of patients were receiving insulin therapy, either alone or in combination with oral agents. These observations were somewhat unexpected because type 2 diabetes is a progressive disease and glycemic control erodes over time, often necessitating combination therapies to achieve ideal A1C levels. Results from the UK Prospective Diabetes Study (18) previously indicated that most newly diagnosed type 2 diabetes patients required multiple therapies to meet glycemic targets as the study progressed. This previous report found that less than 55% of patients at three years and less than 28% of patients at nine years randomly assigned to any monotherapy could maintain A1C levels below 7%. Given the long duration of diabetes in the patients in the study, it would have been expected that a combination of oral agents with or without the addition of insulin should have been prescribed much earlier.
Primary management of dyslipidemia in the patients in our study was relatively superior to that for BP and glycemic control. Nonetheless, a significant proportion of our study patients did not achieve LDL-C below the guideline-recommended target of lower than 2.5 mmol/L at the time of data collection (1). Our results do, however, demonstrate improvements compared with the treatment success rate reported in another Canadian-based study that evaluated dyslipidemia management in high-risk patients (19). It is additionally noteworthy that only 37% of our patients would have achieved the current national recommendation (LDL-C target of lower than 2.0 mmol/L) published in 2006 (20). The present study, therefore, provides a useful benchmark for evaluating the achievement of this LDL-C goal in the future. A remarkable 43% of our study population that was not at target LDL-C was not receiving any statin therapy, suggesting that insufficient implementation of statin regimens is a key cause for the suboptimal proportion of patients reaching recommended guideline LDL-C levels. This concurs with the findings of the international Analysis and Understanding of Diabetes and Dyslipidaemia: Improving Treatment (AUDIT) study (21), which found that despite being treated by specialists, many patients with diabetes, particularly those without any known vascular disease, did not achieve acceptable LDL-C levels. These results also concur with those of Saydah et al (9), which demonstrated that prescription rates for lipid lowering in patients with diabetes remain low, even in those with existing cardiovascular disease.
The greatest treatment gap noted in the present study was in the management of combined cardiovascular risk factors. Overall, only 21% of the study population was able to meet all three targets recommended by the CDA for the control of BP, glycemia and LDL-C. Compelling evidence suggests that tight control of multiple risk factors combined with a comprehensive vascular protection approach (including antiplatelet agents, ACE inhibitors and statins) yields substantial risk reduction in patients with diabetes (4). This is especially evident in diabetic subgroups with established complications or multiple cardiovascular risk factors (3) – features akin to the majority of the DRIVE population.
In contrast to the poor BP, glycemia and dyslipidemia management observed, antiplatelet agents were widely used in this population. This was reflected by a high proportion of patients being prescribed antiplatelet pharmacotherapy, especially when in the presence of established atherosclerotic disease. However, it is plausible that the low cost of acetylsalicylic acid may have contributed to the more widespread use of antiplatelet agents among these patients.
The majority of our study patients with nephropathy were prescribed inhibitors of the renin-angiotensin system, suggesting that the benefits of these agents in the management of diabetic nephropathy are well translated into clinical practice. Additionally, in contrast to a previous report (8), annual screening/follow-up for diabetic nephropathy was performed in most of our study patients, indicating an overall more concerted management of the disease.
Limitations
Our study had several limitations that should be considered in the interpretation. Although consecutive patient enrollment was encouraged, the nonrandom selection of physicians could have resulted in the inclusion of physicians who were more willing to follow treatment guidelines, resulting in a selection bias that would underestimate the treatment gap. Furthermore, we were unable to distinguish between patient nonadherence to medical therapies versus the prescription patterns of participating physicians. We also did not collect detailed data on lifestyle intervention, which is an important strategy in the management of patients at high risk for vascular events. Finally, we did not document whether BP was remeasured on a separate visit if it was found to be above target; this may have led to an increased prevalence of hypertension in our study.
Notwithstanding these limitations, the current study had several unique strengths. First, our results were derived from a large sample size with detailed information directly submitted by participating physicians. Second, a large number of primary care physicians from across Canada were involved in enrolling patients into this registry, thereby facilitating generalizability of the findings. Third, unlike in many previous studies, our primary care physicians were given clear guidelines and goals for therapy before enrollment via CME programs that were based on the 2003 CDA CPGs. Indeed, our study further emphasizes that care gaps cannot be simply attributed to physician’s lack of knowledge or awareness of established guidelines. Fourth, our data stem from a practice-based registry and provide important real-life insights (versus a clinical trial setting) into primary care delivery and management as well as treatment gaps in high-risk patients with type 2 diabetes.
SUMMARY
The present study highlights the fact that although some progress has been made in achieving specific guideline-recommended targets, a substantial proportion of patients with type 2 diabetes in Canada still fail to meet adequate goals of therapy. This trend is particularly evident in the combined control of the main cardiovascular risk factors. Our results underscore the necessity for more ‘real-life’ practice research to identify the barriers that contribute to the care gap (eg, physician’s guidelines knowledge, inertia to adopt new therapeutic strategies, patient resistance, nonadherence or intolerance, etc). These findings also emphasize that novel and more effective strategies must be tested and implemented to rectify this treatment gap in patients with type 2 diabetes. Possible approaches for consideration include increased aggressive strategies in initiating vascular protection measures in such patients as well as earlier initiation of combination therapies to achieve and maintain recommended targets. Innovative strategies could encompass greater patient involvement, more effective physician educational interventions, physician extenders such as case managers, information technology programs and, possibly, physician ‘pay-for-performance’ initiatives aimed at improving diabetes care. Based on the available evidence, appropriate execution of this multifactorial management approach will play a significant role in reducing the associated complications of diabetes, and its clinical and economic burdens.
Acknowledgments
The DRIVE project was conceived, designed and coordinated by the Canadian Heart Research Centre – a federally incorporated, not-for-profit, academic research organization.
APPENDIX A: Participating Diabetes Registry to Improve Vascular Events (DRIVE) Investigators:
Dale W Magnuson, Delta, British Columbia; Robert E Humber, Bay Roberts, Newfoundland and Labrador; Anne Kensole, Toronto, Ontario; Shahin Jaffer, Delta, British Columbia; Francois Nicholas Urfer, Hawkesbury, Ontario; Galina Gotesman, Willowdale, Ontario; Mohammed Vakani, Burlington, Ontario; Cristina Kiai, North Vancouver, British Columbia; Talaat Lotfallah, Kingston, Ontario; S John Weisnagel, Ste-Foy, Quebec; Darryl J Ableman, Coquitlam, British Columbia; Niloofer Baria, Vancouver, British Columbia; Oliver H Bereznay, Winnipeg, Manitoba; Ian M Bridger, Victoria, British Columbia; Ewan M Cadger, Sidney, British Columbia; Grant K Campbell, Edmonton, Alberta; Kenneth Cho, Vancouver, British Columbia; Ronald S Collette, Burnaby, British Columbia; David H Cram, Souris, Manitoba; Daniel Ezekiel, Vancouver, British Columbia; Christopher James, Sidney, British Columbia; Philip Kelly, Victoria, British Columbia; Edward C-H Luke, Vancouver, British Columbia; Ernest J Pauls, Abbotsford, British Columbia; Walter R Salmaniw, Victoria, British Columbia; Ronnald Schriemer, Kelowna, British Columbia; Abraham Vermeulen, Regina, Saskatchewan; Danny HK Wong, Richmond, British Columbia; David Haligowski, Winnipeg, Manitoba; Guy Chouinard, Charlesbourg, Quebec; Donald Delisle, Bromptonville, Quebec; Jacques Desroches, Saint-Pie, Quebec; Pierre Gailloux, Richelieu, Quebec; Gilles Gaudreau, Sorel-Tracy, Quebec; Munir Habra, Greenfield Park, Quebec; Andre Hudon, Charlesbourg, Quebec; Paul-Andre Hudon, Deux-Montagnes, Quebec; Robert Lagarde, Repentigny, Quebec; John Lawless, Donnacera, Quebec; Ghislain Levesque, Saint Pascal, Quebec; Michel Meunier, Sainte-Julie, Quebec; Alain Ouimet, Sainte-Adele, Quebec; Pierre Payer, L’Ile-Perrot, Quebec; Francois Perreault, Sainte-Anne-De-Bellevue, Quebec; Rodrigue Prud’homme, Gatineau, Quebec; Claude Roberge, St Stanislas Comte Champlain, Quebec; Lorraine Tessier, Montreal, Quebec; Sebastien Toussaint, Maria, Quebec; Jean Turcotte, Valcourt, Quebec; Rene Vallee, Sainte-Anne-De-Beaupre, Quebec; Martin Toussaint, L’Islet, Quebec; Robert Larocque, Ste-Agathe-Des-Monts, Quebec; Roger Emsley, Hawkesbury, Ontario; Shiva K Gaur, Scarborough, Ontario; Wing K Tse, Agincourt, Ontario; David YK Chan, Toronto, Ontario; Shiraz H Ismail, Toronto, Ontario; Tommy HT Tung, Keswick, Ontario; Robert I Ciomyk, Toronto, Ontario; Martin L Kates, Toronto, Ontario; Norman Abramson, Mississauga, Ontario; Ralph Epstein, Hamilton, Ontario; William R Awrey, Hamilton, Ontario; Gino Pannozzo, Waterloo, Ontario; Jay Baker, Cambridge, Ontario; Duncan W Sinclair, Aylmer West, Ontario; David T Fisher, Windsor, Ontario; Michael WJ Zajner, Windsor, Ontario; Rajendranath Ramgoolam, Winnipeg, Manitoba; Jean-Francois Goulard, Bathurst, New Brunswick; David A Wade, Bathurst, New Brunswick; Alan J McComiskey, Stephenville, Newfoundland and Labrador; Marek Smolarkiewicz, Stephenville, Newfoundland and Labrador; James G Casserly, Ottawa, Ontario; Ian K Shiozaki, Newboro, Ontario; Stephen Tat-Wah Wu, Whitby, Ontario; Upender K Mehan, Cambridge, Ontario; Majid Boozary, Richmond Hill, Ontario; Ramesh Tandon, Saskatoon, Saskatchewan; Terry R Babick, Winnipeg, Manitoba; Gary M Lindsay, Brandon, Manitoba; John PY Ng, Burnaby, British Columbia; Shelley Ross, Burnaby, British Columbia; Andrew Hosie, Sidney, British Columbia; Herbert Frank Wong, Surrey, British Columbia; Gunvant S Bhatt, Prescott, Ontario; Ruth Ellen Dubin, Kingston, Ontario; Hein J De Haan, Campbellford, Ontario; Chin K Chung, Willowdale, Ontario; Carlyle SHA Chow, Baddeck, Nova Scotia; Bruno Bernucci, St-Leonard, Quebec; Pierre Liboiron, Laval, Quebec; Real Racine, Plessisville, Quebec; Yvonne Nault, St Peter’s, Nova Scotia; Valdemar Martinho, Ottawa, Ontario; Natan Khotianov, North York, Ontario; Asiru Abu-Bakare, Thunder Bay, Ontario; Phyllis J Hierlihy, Ottawa, Ontario; Wendy Rosenthall, Mississauga, Ontario; Linda JE Sinnaeve, Chatham, Ontario; Boji Varghese, Sudbury, Ontario; Edward Gee, Sherwood Park, Alberta; Yolanda Groenewoud, Toronto, Ontario; George Honos, Montreal, Quebec; Shajia Khan, Ottawa, Ontario; Paul DeYoung, Cornwall, Ontario; Hugues Beauregard, Montreal, Quebec; Alexander Skamene, Westmount, Quebec; David Grunbaum, Montreal, Quebec; Robert Zeman, Toronto, Ontario; Yun Chan, Niagara Falls, Ontario; Richard YY Chen, Mississauga, Ontario; Bach-Tuyet Dang, Toronto, Ontario; Shajahan Deen, Kitchener, Ontario; Alan Faiers, Toronto, Ontario; Andrew Godin, Quebec, Quebec; Lydia Hatcher, Mount Pearl, Newfoundland and Labrador; Helen Laporte, Joliette, Quebec; Robert G Luton, London, Ontario; David Chris Morgan, Victoria, British Columbia; Prafulchandra C Patel, Winnipeg, Manitoba; Jayshree Patel, Winnipeg, Manitoba; Laurindo Da Silva, Winnipeg, Manitoba; Kenneth J Greenwald, Toronto, Ontario; Arthur M Kushner, Etobicoke, Ontario; Heinrich D Mynhardt, Outlook, Saskatchewan; Pravinsagar Mehta, Winnipeg, Manitoba; Gilles Campeau, Drummondville, Quebec; Eitel ER Beduhn, Sault Ste Marie, Ontario; Victor John Figurado, Mississauga, Ontario; Roland J Genge, Baddeck, Nova Scotia; Heath Alsaffar, Ottawa, Ontario; Louis B Boucher, Meadow Lake, Saskatchewan; Gilles Brouillette, Lasalle, Quebec; Lily Huang, Oakville, Ontario; Yaw Twum-Barima, Oakville, Ontario; Jean Garon, Gatineau, Quebec; Kenneth W Deichert, Georgetown, Ontario; Iris R Noland, Colborne, Ontario; Leo P Quinn, Oshawa, Ontario; Danny McKinnon, Charlesbourg, Quebec; Debbie Singer, Thornhill, Ontario; Alice Yuk-Yan Cheng, Mississauga, Ontario; Bertrand Frenette, Coaticook, Quebec; Darcy Johnson, Winnipeg, Manitoba; Serge Lamontagne, Amos, Quebec; Pierre M Rheaume, Boucherville, Quebec; Suk Paul Chan, Toronto, Ontario; Christopher J Callery, Waterford, Ontario; Jacobus Kooy, Penticton, British Columbia; Theodore J Jablonski, Calgary, Alberta; Clement Lam, Etobicoke, Ontario; Gordon Yee, Lasalle, Quebec; Alan F Cook, Victoria, British Columbia; Ghislain Dube, Quebec, Quebec; Roger G Guy, Longueuil, Quebec; Teresa Cordoni, Port Coquitlam, British Columbia; Bernard Frenette, Coaticook, Quebec; Michael Nowlan, Grand Falls, New Brunswick; Arnold D Dlin, Vancouver, British Columbia; Bing Q Gore, Vancouver, British Columbia; Robert G Harrison, Maple Ridge, British Columbia; Gary M Koss, Richmond, British Columbia; Mona Lee, North Vancouver, British Columbia; Anthony E Nielsen, Victoria, British Columbia; Jacobus B Van Heerden, Regina, Saskatchewan; Amarjit S Nirwan, Victoria, British Columbia; Sy Lam, Calgary, Alberta; Michael R Lee, Calgary, Alberta; Luigia Bertolo, Lasalle, Quebec; Benoit Castonguay, Rimouski, Quebec; Marie-Jose Cucuzza, Montreal, Quebec; Frank T Lazzara, Lasalle, Quebec; Ghislaine Roederer, Montreal, Quebec; Jacques Auger, Princeville, Quebec; Jean-Simon Bouchard, Bedford, Quebec; Bernard Doyon, Beloeil, Quebec; Andrew Lam, Toronto, Ontario; Gerard Quinn, Brantford, Ontario; Roy A Harding, Digby, Nova Scotia; Daniel G Scott, Grand Bay-Westfield, New Brunswick; Byron B Barnhill, Bathurst, New Brunswick; Mark T Duerksen, Steinbach, Manitoba; Marc A Boileau, North Vancouver, British Columbia; Neil C Hudson, Kelowna, British Columbia; Pierre Juery, Ottawa, Ontario; Brent Edward Bukovy, Thunder Bay, Ontario; Brian Hartford, Thunder Bay, Ontario; Johanne Frenette, Donnacona, Quebec; Mario Lebel, Saint Pascal, Quebec; Denis Metivier, St Victor, Quebec; Denis Proulx, Beloeil, Quebec; Ian Blumer, Ajax, Ontario; Burton Knight, North York, Ontario; Ragbir S Kumar, Cambridge, Ontario; Nathalie MC Leung, Richmond Hill, Ontario; Kevin K Saunders, Winnipeg, Manitoba; Daniel Noiseux, St-Jean-Sur-Richelieu, Quebec; Malcolm Pike, Toronto, Ontario; Everton S Nicholas, Toronto, Ontario; John Axler, Toronto, Ontario; Gregory Baran, Kingston, Ontario; Noel J Browne, St John’s, Newfoundland and Labrador; Pierre Dauth, Cowansville, Quebec; Marianne PK Ho-Asjoe, Vancouver, British Columbia; Paul T Kwong, Brampton, Ontario; David Ross, Moncton, New Brunswick; Mario Lapointe, Chicoutimi, Quebec; Clint A Redhead, Mattawa, Ontario; Anand Sohla, Tillsonburg, Ontario; Brahm D Selhi, North Battleford, Saskatchewan; John R Mann, Vernon, British Columbia; John Andrew, Tillsonburg, Ontario; Narendra Makan, Calgary, Alberta; Ian A Hyams, Maple Ridge, British Columbia; Clement Lang, Winnipeg, Manitoba; Jit J Singh, Surrey, British Columbia; Naresh K Aggarwal, Brampton, Ontario; Roy Eappen, Montreal, Quebec; Brian Pasula, Sooke, British Columbia; Ka-Chee Lim, Timmins, Ontario; Mervin Johnson, Meadow Lake, Saskatchewan; Don Scaman, Abbotsford, British Columbia; Wojtek Ciszak, North Vancouver, British Columbia; Garabed Vartazarmian, Montreal, Quebec; William R Milligan, Tottenham, Ontario; Dean Joseph Zizzo, Hamilton, Ontario; Alcantro B Fernandez, Stoney Creek, Ontario; Stephen M Shore, Langley, British Columbia.
Footnotes
FUNDING: This study was supported by sanofi-aventis Canada. However, the sponsor had no role in the collection, management, analysis or interpretation of the data; the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication and the target journal. MFB Braga is the former and H Teoh is the current sanofi-aventis Cardiometabolic Risk Fellow. LA Leiter has received research funding from, has provided CME on behalf of, and has acted as a consultant to AstraZeneca Canada, Boehringer Ingelheim (Canada) Ltd, Bristol-Myers Squibb Canada, Eli Lilly Canada Inc, GlaxoSmithKline Inc (Canada), Merck Frosst Canada Ltd, Novartis Pharmaceuticals Canada Inc, Novo Nordisk Canada Inc, Pfizer Canada, Roche Canada, sanofi-aventis Canada, Servier Canada Inc and Solvay Canada.
Appendix A provides a list of participating DRIVE Investigators
REFERENCES
- 1.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003;27(Suppl):S1–152. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 4.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 5.Standards of medical care in diabetes – 2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 6.Ryden L, Standl E, Bartnik M, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 7.Brown LC, Johnson JA, Majumdar SR, Tsuyuki RT, McAlister FA. Evidence of suboptimal management of cardiovascular risk in patients with type 2 diabetes mellitus and symptomatic atherosclerosis. CMAJ. 2004;171:1189–92. doi: 10.1503/cmaj.1031965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarlane SI, Jacober SJ, Winer N, et al. Control of cardiovascular risk factors in patients with diabetes and hypertension at urban academic medical centers. Diabetes Care. 2002;25:718–23. doi: 10.2337/diacare.25.4.718. [DOI] [PubMed] [Google Scholar]
- 9.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 10.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 11.Fox KM. EURopean trial On reduction of cardiac events wth Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: Randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 12.Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens (Greenwich) 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 13.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 14.Bakris GL. A practical approach to achieving recommended blood pressure goals in diabetic patients. Arch Intern Med. 2001;161:2661–7. doi: 10.1001/archinte.161.22.2661. [DOI] [PubMed] [Google Scholar]
- 15.Daly CA, Fox KM, Remme WJ, Bertrand ME, Ferrari R, Simoons ML. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: Results from the PERSUADE substudy. Eur Heart J. 2005;26:1369–78. doi: 10.1093/eurheartj/ehi225. [DOI] [PubMed] [Google Scholar]
- 16.Harris SB, Ekoe JM, Zdanowicz Y, Webster-Bogaert S. Glycemic control and morbidity in the Canadian primary care setting (results of the diabetes in Canada evaluation study) Diabetes Res Clin Pract. 2005;70:90–7. doi: 10.1016/j.diabres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31:81–6. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 18.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 19.Yan AT, Yan RT, Tan M, et al. Contemporary management of dyslipidemia in high-risk patients: Targets still not met. Am J Med. 2006;119:676–83. doi: 10.1016/j.amjmed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Dyslipidemia in adults with diabetes. Can J Diabetes. 2006;30:230–40. [Google Scholar]
- 21.Leiter LA, Betteridge DJ, Chacra AR, et al. AUDIT study. Evidence of global undertreatment of dyslipidaemia in patients with type 2 diabetes mellitus. Br J Diabetes Vasc Dis. 2006;6:31–40. [Google Scholar]