Abstract
BACKGROUND:
The Inuit are commonly portrayed to be somehow protected from cardiovascular diseases (CVDs) through their traditional lifestyle and diet. However, actual sociocultural transition and related major, modifiable risk factors have scarcely been quantified in the Inuit population. Such knowledge is extremely valuable in terms of public health intervention.
METHODS:
A total of 887 Inuit residents from Nunavik, Quebec, participated in a cohort study. The estimates presented were derived from anthropometric and biological measurements gathered at the time of recruitment and enhanced by information collected in the medical file of each participant. All estimates were corrected for a complex sampling strategy and bootstrapped to ensure the representativeness of the general Nunavik population.
RESULTS:
Overall, 19% of Inuit had a disease of the circulatory system according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision. Among all disorders, peripheral circulatory system disease was the most prevalent (9%). Prevalences of ischemic heart disease and cerebrovascular disease were of similar magnitude (2.5%). No significant difference in disease prevalence was noted between sexes. The major modifiable CVD risk factors were smoking (84%), obesity (49%) (body mass index of greater than 30 kg/m2) and elevated blood pressure (130/85 mmHg or greater) (18%). Prevalences were globally higher among women.
CONCLUSION:
The current belief that the Inuit are protected from CVD is seriously questioned by the results of the present study. Considering the extremely high prevalence of CVD risk factors, a population-based intervention reinforced for women is urgently needed to reduce their risk.
Keywords: Epidemiology, Health outcomes, Hypertension, Ischemia, Morbidity, Myocardial infarction, Obesity, Prevalence, Risk factors, Stroke
Abstract
HISTORIQUE :
Les Inuits sont souvent dépeints comme une population quelque peu protégée des maladies cardiovasculaires (MCV) en raison de leur mode de vie traditionnel et de leur régime alimentaire. Cependant, la transition socioculturelle en cours et les facteurs de risque modifiables connexes ont rarement été quantifiés au sein de la population inuite. Ces connaissances sont très précieuses pour les interventions en santé publique.
MÉTHODOLOGIE :
Au total, 887 Inuits qui habitent le Nunavik, au Québec, ont participé à une étude de cohorte. Les évaluations présentées sont dérivées de mesures anthropométriques et biologiques colligées au moment du recrutement et améliorées par l’information recueillie dans le dossier médical de chaque participant. Toutes les évaluations ont été corrigées pour tenir compte d’une stratégie d’échantillonnage complexe et dotées d’un amorçage afin d’assurer la représentativité de la population générale du Nunavik.
RÉSULTATS :
Dans l’ensemble, 19 % des Inuits avaient une maladie du système respiratoire selon la Classification statistique internationale des maladies et problèmes de santé connexe, 10e révision. Parmi toutes les pathologies, la maladie du système circulatoire périphérique était la plus prévalente (9 %). La prévalence des cardiopathies ischémiques et des accidents vasculaires cérébraux avait une magnitude similaire (2,5 %). On n’a remarqué aucune différence significative entre les sexes pour ce qui est de la prévalence de la maladie. Les principaux facteurs de risque de MCV modifiables étaient le tabagisme (84 %), l’obésité (49 %) (indice de masse corporelle supérieur à 30 kg/m2) et une tension artérielle élevée (130/85 mmHg ou plus) (18 %). En général, les prévalences étaient plus élevées chez les femmes.
CONCLUSION :
La croyance selon laquelle les Inuits sont protégés des MCV est sérieusement remise en question par les résultats de la présente étude. Étant donné la prévalence extrêmement élevée de facteurs de risque de MCV, il est urgent d’instaurer une intervention renforcée pour les femmes afin de réduire leur risque.
Studies undertaken during the 1960s and 1970s suggested that the Inuit were protected from some cardiovascular diseases (CVDs) such as coronary artery disease (CAD) and ischemic heart disease (IHD) (1,2). Moreover, Arctic populations seemed to be spared by the diabetes epidemic experienced by many North American-Indian groups (3–7). The traditional Inuit lifestyle involved vigorous physical activities, and the consumption of products from fishing, hunting and berry picking is believed to support good health status, particularly at the cardiovascular level (8–11).
Because of rapid social transition subsequent to improvements in communications and transportation to and from southern regions, and with the settlement of Inuit populations into permanent communities, a progressive shift away from their traditional lifestyles and diet has been observed (10,12–16). This epidemiological transition among circumpolar Inuit populations has been associated with an increased prevalence of CVD risk factors such as obesity, high blood pressure (HBP), elevated blood lipid levels (3–7,17,18) and diabetes (19–22).
Despite the unfavourable findings mentioned above, mortality rates from CVD, especially IHD, are still lower in the Inuit compared with southern populations, probably reflecting protective lifestyle factors still prevailing to some degree (23–25) and, possibly, lower genetic susceptibility (19,20,26–29).
In 1992, a health survey of the Inuit population of Nunavik, Quebec, was performed. Regarding CVDs and their risk factors, the main conclusions were that blood pressure (BP) (30) and lipid profile (25) were better in the Inuit than in southern populations of the province of Quebec, despite a 19% prevalence of obesity (body mass index [BMI] 30 kg/m2 or greater) and a high rate of tobacco consumption (74%) (31).
The recent Nunavik Health Survey entitled “Qanuippitaa? – How are we?”, was conducted to update information on the global health status of the Inuit population. Most participants (97%) of the population-based study agreed to join its cohort component. An overview of the CVDs recorded and their related risk factors is presented in the current report.
METHODS
Population
The Nunavik Health Survey entitled “Qanuippitaa? – How are we?”, of Inuit adults (age 18 years and older) in Nunavik was undertaken.
Data were collected from August 27 to October 1, 2004, in the 14 villages of Nunavik on the Canadian research icebreaker (Canadian Coast Guard Ship Amundsen) (Figure 1). Sampling strategies for the present population-based study are described in detail elsewhere (32).
Figure 1).
Map of Nunavik (Quebec) and 14 communities visited during the study
A total of 887 individuals were accepted to be enrolled in the longitudinal follow-up component of the study. This component involved permission to follow the study participants in the future as well as permission to obtain individual medical file data.
The survey was approved by the ethics committees of Laval University (Quebec City, Quebec) and Institut national de santé publique du Québec. Written informed consent was provided by all participants after watching a video describing the study.
Anthropometric and BP data
Height was quantified by stadiometer. Body weight and composition were measured with a bioelectrical impedance analyzer (Tanita TBF-300, Tanita, Canada). Waist circumference (WC) was with a tape measure graduated in cm, at the narrowest circumference of the trunk, at the end of a normal expiration. Hip circumference was quantified by placing the tape around the pubic symphysis and the most prominent part of the buttocks. Anthropometric data were transformed into BMI (kg/m2) and internationally recommended cut-off points were used (33). The designation of abdominal obesity was based on WC (102 cm or greater in men, and 88 cm or greater in women), according to cut-off levels proposed by the National Cholesterol Education Program – Adult Treatment Panel III (NCEP-ATP III) (34).
BP was measured according to the Canadian Coalition for High Blood Pressure technique (35) with mercury sphygmomanometers, 15-inch stethoscopes and cuffs appropriately sized to the subjects’ arms (32).
Biological parameters
Complete lipid profile, glucose and insulin concentrations were quantitated at the Centre Hospitalier Universitaire de Québec. Insulin sensitivity was evaluated by homeostasis model assessment – insulin resistance, defined as the product of fasting insulin (μU/mL) and glucose concentration (mmol/mL), divided by 22.5 (36).
Definition of CVD
CVDs were classified according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10), 2007 version: Diseases of the circulatory system [I00 to I99]. CVD prevalence was assessed by individual medical file review. While hypertension was reported as a CVD in the ICD-10, based on current knowledge (37,38), it was decided that it would be presented as a risk factor.
Definition of diabetes
Type 2 diabetes (T2D) and impaired glucose tolerance were diagnosed according to the Canadian Diabetes Association’s cut-off levels (39). T2D prevalence was obtained by combining data from the medical files and fasting glucose data. Participants were required to fast at least 8 h before venipuncture to provide unbiased information on basal biological parameters. Some of them did not conform to this condition and were, therefore, excluded from further analysis (n=97) such as descriptive parameters of fasting glucose, insulin and triacylglycerol (TAG).
Metabolic syndrome (MetS) and Framingham risk scores based on the NCEP-ATP III definition (40) were calculated to adequately describe CVD risk in the population.
Statistical analyses
The analyses provided in the present report are descriptive, and the parameters presented are accompanied by their corresponding standard errors (SEs). The number of participants is included for information purposes only. All data were analyzed by the bootstrapping technique to take into account the complex sampling strategy used and to correct related sampling errors. Analyses were also weighted to achieve population representativeness. Weights were adapted to the nonresponse rate of each measurement instrument. Age-standardized rates were obtained when computation was possible by the direct method, with the 2001 population of Canada used as the standard (41).
Arithmetic means were calculated for normally distributed variables, and geometric means were considered for variables with a log-normal distribution. ANOVA allowed for the comparison of means or geometric means, and the χ2 test with correction for the design effect served to compare proportions. Statistical analyses were conducted at a threshold of alpha = 0.05. All analyses were performed using SAS version 9.1 (SAS Institute Inc, USA) and SUDAAN version 9.3 (RTI International, USA)
RESULTS
Prevalence of CVD
In the study population, 18.6% (SE 1.1%; n=166) of individuals had at least one cardiovascular event, according to the ICD-10 definition, during their lifespan. The age-standardized prevalence rate in this region was 19.6% (SE 1.5%). No sex differences in cardiovascular events were observed (P=0.37).
Compared with participants without already diagnosed CVD, individuals with a cardiovascular event were, on average, significantly older (46.0 years versus 31.7 years, P<0.0001). However, no sex differences were detected between people with and without CVD (P=0.078).
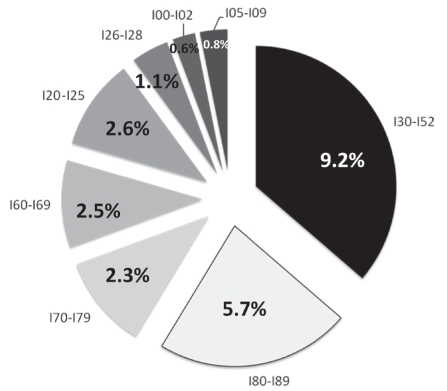
Figure 2 depicts the prevalence of CVD reported in the medical files of participants. Among IHD cases, the majority were angina pectoris (72.0%), with 18.0% being acute myocardial infarction.
Figure 2).
Prevalence (%) of cardiovascular disease among adult Nunavik (Quebec) Inuit according to disease codes from the International Statistical Classification of Diseases and Related Health Problems, 10th revision, by order of appearance: I30 to I52 Other forms of heart disease; I80 to I89 Diseases of the veins, lymphatic vessels and lymph nodes not classified elsewhere; I70 to I79 Diseases of the arteries, arterioles and capillaries; I60 to I69 Cerebrovascular diseases; I20 to I25 Ischemic heart diseases; I26 to I28 Pulmonary heart disease and diseases of the pulmonary circulation; I00 to I02 Acute rheumatic fever; I05 to I09 Chronic rheumatic heart diseases
Atherosclerosis (specified and unspecified) was the most prevalent disease (52%) in the ICD-10 – I70 to I79 category. Aortic and carotid aneurysms (11%), Raynaud’s syndrome (17%), embolisms and thromboses of lower extremity arteries (3.5%) as well as arterial strictures (14%) were also recorded.
Diseases of the veins, lymphatic vessels and lymph nodes not classified elsewhere (ICD-10 – I80 to I89) were observed in 5.6% (SE 0.7%) of participants. The majority were lymphangitis cases (52.0%). The rest were spread among varicose veins of the lower extremities (20.0%), phlebitis and thrombophlebitis (15.0%) as well as venous insufficiency (6.0%) and compression (2.0%).
Among cerebrovascular diseases (CDs) (ICD-10 – I60 to I69), unspecified stroke was reported most often (37.0%), followed by cerebral infarction (20.0%), subarachnoid hemorrhage (8.33%), carotid artery occlusion and stenosis (4.2%), and cerebral artery occlusion and stenosis (9.0%) not resulting in cerebral infarction. Other CDs, such as cerebral aneurysm, nonruptured, nonpyogenic thrombosis of the intracranial venous system, acute cerebrovascular insufficiency and cerebral ischemia (chronic), were found in 17%.
Pulmonary heart and pulmonary circulation diseases (ICD-10 – I26 to I28) as well as rheumatic heart disease (ICD-10 – I00 to I02 and I05 to I09) were 1.1% (SE 0.4%) and less than 2.0%, respectively.
“Other forms of heart disease” (ICD-10 – I30 to I52) appeared as the main code with a prevalence of 9.2% (SE 0.1%) (Figure 2). Among them, 27.0% of cases were heart failure (mainly cardiomegaly) and 18.0% were other cardiac arrhythmias (mainly sick sinus syndrome [15.0%]) and tachycardia-bradycardia syndrome (3%). Thirteen per cent were nonrheumatic mitral valve disorders, 10.0% were other conduction disorders and 9.0% were nonrheumatic aortic valve disorders. Nonrheumatic tricuspid valve disorders (2.2%), pulmonary valve disorders (1.12%), paroxysmal tachycardia (5.0%), atrial fibrillation and flutter (2.4%) were also reported.
Prevalence of CVD risk factors
Because preliminary descriptive analysis highlighted some significant differences according to sex, a separate descriptive analysis was performed. Tables 1 and 2 present the mean of the main CVD risk factors by decade and sex of participants (n=887).
TABLE 1.
Cardiovascular disease risk factors stratified by age among the male Nunavik (Quebec) Inuit population
|
Age, years |
P* | MetS | Framingham risk category | |||||
|---|---|---|---|---|---|---|---|---|
| 20–39 (n=25) | 40–49 (n=224) | 50–59 (n=58) | 60–69 (n=44) | ≥70 (n=38) | ||||
| Body mass index, kg/m2 | 23.7 (0.9) | 26.3 (0.3) | 27.1 (0.7) | 28.3 (0.8) | 29.9 (1.0) | <0.0001 | 5.5 (1.2) | CAD or CAD risk equivalent (10-year risk <20%): 2% |
| Body fat, % | 15.01 (1.6) | 19.3 (0.4) | 21.8 (1.1) | 24.5 (1.1) | 29.0 (1.5) | <0.0001 | ||
| Waist circumference, cm | 81.9 (0.7) | 89.1 (2.4) | 91.3 (1.7) | 96.4 (1.7) | 102.6 (2.4) | <0.0001 | ||
| Systolic BP, mmHg | 121 (1) | 120 (2) | 122 (2) | 129 (2) | 131 (2) | <0.0001 | ||
| Diastolic BP, mmHg | 68 (2) | 76 (2) | 77 (1) | 79 (1) | 73 (1) | 0.043 |
Multiple risk factors and 10-year risk ≤20%: 10% |
|
| TC, mmol/L | 3.8 (0.1) | 4.7 (0.1) | 5.3 (0.1) | 5.4 (0.1) | 5.1 (0.1) | <0.0001 | ||
| TC/HDL-C ratio† | 2.8 (0.1) | 3.4 (0.1) | 3.9 (0.2) | 3.8 (0.2) | 3.2 (0.2) | 0.032 | ||
| HDL-C, mmol/L | 1.4 (0.05) | 1.4 (0.02) | 1.4 (0.05) | 1.5 (0.01) | 1.7 (0.1) | 0.002 | ||
| LDL-C, mmol/L | 2.0 (0.1) | 2.7 (0.001) | 3.2 (0.1) | 3.2 (0.1) | 2.8 (0.2) | <0.0001 | ||
| Triglycerides†, mmol/L | 0.73 (0.04) | 1.1 (0.03) | 1.1 (0.1) | 1.1 (0.1) | 0.9 (0.1) | 0.18 | ||
| HOMA-IR† (n=216) | 1.1 (0.1) | 1.2 (0.04) | 1.3 (0.1) | 1.6 (0.2) | 1.9 (0.3) | 0.03 |
0 to 1 risk factor: 88% |
|
| Fasting glucose†, mmol/L | 4.1 (0.01) | 4.3 (0.04) | 4.7 (0.1) | 4.8 (0.1) | 5.2 (0.2) | <0.0001 | ||
| Fasting insulin†, pmol/L | 43.4 (3.0) | 43.7 (1.4) | 43.7 (3.1) | 51.3 (4.7) | 58.4 (7.8) | 0.016 | ||
| Smoking (yes), % | 100 (–) | 80.3 (2.6) | 82.6 (4.6) | 55.2 (7.3) | 52.7 (7.9) | <0.0001 | ||
| Sedentary lifestyle, % | 40.6 (9.4) | 42.2 (3.7) | 56.2 (7.4) | 57.6 (7.6) | 63.5 (7.8) | 0.01 | ||
Data presented as arithmetic mean (standard error) unless otherwise indicated.
P values correspond to a linear trend across age categories;
Data presented as geometric mean (standard error). BP Blood pressure; CAD Coronary artery disease; HDL-C High-density lipoprotein cholesterol; HOMA-IR Homeostasis model assessment – insulin resistance; LDL-C Low-density lipoprotein cholesterol; MetS Metabolic syndrome; TC Total cholesterol
TABLE 2.
Cardiovascular disease risk factors stratified by age among the female Nunavik (Quebec) Inuit population
|
Age, years |
P* | MetS | Framingham risk category | |||||
|---|---|---|---|---|---|---|---|---|
| 20–39 (n=27) | 40–49 (n=278) | 50–59 (n=91) | 60–69 (n=56) | ≥70 (n=46) | ||||
| Body mass index, kg/m2 | 24.6 (0.8) | 27.4 (0.3) | 27.4 (0.6) | 28.7 (0.8) | 31.4 (1.0) | <0.0001 | 9.9 (1.3) | Multiple risk factors and 10-year risk ≤20%: 0.5% |
| Body fat, % | 26.0 (1.5) | 31.3 (0.5) | 31.1 (1.0) | 31.1 (1.0) | 33.7 (1.1) | <0.0001 | ||
| Waist circumference, cm | 83.3 (2.2 | 89.4 (0.7) | 91.0 (1.4) | 96.1 (1.9) | 103.0 (202) | <0.0001 | ||
| Systolic BP, mmHg | 109 (1) | 111 (1) | 114 (1) | 122 (2) | 137 (3) | <0.0001 | ||
| Diastolic BP, mmHg | 72 (0) | 68 (1) | 72 (1) | 73 (1) | 74 (1) | <0.0001 | ||
| TC, mmol/L | 4.3 (0.2) | 4.9 (0.1) | 5.3 (0.1) | 5.7 (0.1) | 5.5 (0.1) | <0.0001 | ||
| TC/HDL-C ratio† | 2.7 (0.01) | 3.0 (0.1) | 3.0 (0.1) | 3.0 (0.1) | 3.0 (0.1) | <0.0001 | ||
| HDL-C, mmol/L | 1.6 (0.1) | 1.7 (0.02) | 1.8 (0.05) | 2.0 (0.1) | 1.9 (0.1) | <0.0001 |
0 to 1 risk factor: 99.5% |
|
| LDL-C, mmol/L | 2.2 (0.1) | 2.7 (0.01) | 2.8 (0.1) | 3.0 (0.1) | 2.9 (0.1) | <0.0001 | ||
| Triglycerides†, mmol/L | 0.9 (0.01) | 1.0 (0.03) | 1.1 (0.04) | 1.1 (0.1) | 1.2 (0.1) | 0.04 | ||
| HOMA-IR† (n=216) | 1.2 (0.1) | 1.4 (0.1) | 1.3 (0.1) | 1.6 (0.1) | 2.2 (0.2) | 0.003 | ||
| Fasting glucose†, mmol/L | 4.0 (0.1) | 4.2 (0.1) | 4.5 (0.1) | 5.0 (0.2) | 5.1 (0.2) | <0.0001 | ||
| Fasting insulin†, pmol/L | 48.8 (4.4) | 51.0 (1.6) | 46.0 (2.5) | 52.0 (3.5) | 67.3 (6.1) | 0.16 | ||
| Smoking (yes), % | 100 (–) | 86.4 (2.0) | 85.2 (3.6) | 60.0 (6.5) | 39.3 (7.4) | <0.0001 | ||
| Sedentary lifestyle, % | 50.8 (10.19) | 71.0 (3.0) | 53.3 (5.2) | 56.0 (7.2) | 71.3 (7.0) | 0.473 | ||
Data presented as arithmetic mean (standard error) unless otherwise indicated.
P values correspond to a linear trend across age categories;
Data presented as geometric mean (standard error). BP Blood pressure; HDL-C High-density lipoprotein cholesterol; HOMA-IR Homeostasis model assessment – insulin resistance; LDL-C Low-density lipoprotein cholesterol; MetS Metabolic syndrome; TC Total cholesterol
Overall, modification of parameters was generally observed with increasing age (Tables 1 and 2). No linear trend was discernible for sedentary lifestyle among women, and TAG among men.
Although the BP parameters measured were within normal values (systolic BP 118 mmHg [SE 1 mmHg] and diastolic BP 73 mmHg [SE 1 mmHg]), in 12% of participants, a diagnosis of hypertension was found in their medical file. Moreover, among other participants, the prevalence of elevated BP (130/85 mmHg or greater) was 17.5% (SE 1.5%). This prevalence was higher among men (24% versus 10%, P<0.0001).
The smoking rate was extremely high (83.7%). An inverted gradient of tobacco consumption according to age decade was observed (P<0.0001); all individuals who were younger than 40 years of age reportedly smoked. The smoking rate reached 60.4% (SE 10.2%) for those who were 70 years of age and older.
Physical activity during leisure time and work was documented by questionnaire. Sedentary lifestyle (physically active less than once a week) was reported by 55% of individuals and reached 73% among women in the 40- to 50-year-old age group (Table 2).
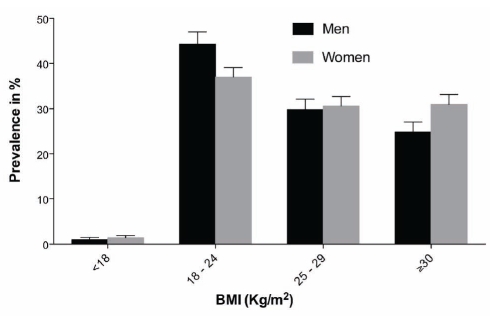
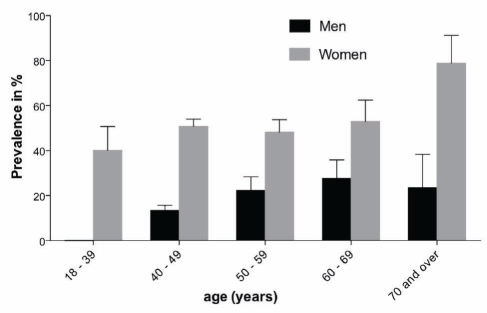
As illustrated in Figure 3, 29.4% (SE 1.8%) of the Inuit population were overweight and 22.3% (SE 1.7%) were obese according to BMI classification; the proportion was not different between sexes. The prevalence of abdominal obesity reached 31%. A significant sex difference was detected (Figure 4) in the prevalence of abdominal obesity. Women had higher rates of obesity (50% versus 15%, P<0.0001), consistently observed across age categories (Figure 4).
Figure 3).
Distribution of the Inuit population according to body mass index (BMI) strata: underweight (less than 18 kg/m2), normal (18 kg/m2 to 24 kg/m2), overweight (25 kg/m2 to 29 kg/m2) and obese (30 kg/m2 or greater), with the standard error, according to sex
Figure 4).
Prevalence of abdominal obesity measured by waist circumference, with the standard error, according to sex and age
The prevalence of T2D in the Inuit population was 4.7% (SE 0.7%), and women experienced the disease more than men (women 6.6%, men 2.7%; P<0.001). Moreover, impaired fasting glucose (glycemia 6.1 mmol/L to 6.9 mmol/L) was found in 2.1% (SE 0.5%) of this population. The prevalence of hyperinsulinemia (90 pmol/L or greater) was higher in women (15.0%) than in men (9.2%), and the proportion was significantly different (P=0.01).
Women had a higher total cholesterol concentration and high-density lipoprotein cholesterol (HDL-C), and a lower total cholesterol/HDL-C ratio (all P<0.001) than men (Tables 1 and 2). Despite significant sex differences in blood lipid levels, few sex differences were observed in abnormal lipid prevalence (Table 3). The high prevalence of elevated low-density lipoprotein cholesterol among Inuit men was noteworthy.
TABLE 3.
Age-adjusted prevalence (%) of an abnormal lipid profile among the Nunavik (Quebec) Inuit population
|
Total* (n=887) |
Men (n=387) |
Women (n=498) |
P | ||||
|---|---|---|---|---|---|---|---|
| % | SE | % | SE | % | SE | ||
| Elevated TC levels, mmol/L | 11.5 | 1.6 | 11.1 | 1.5 | 12.0 | 1.3 | 0.64 |
| Elevated TC/HDL-C ratio | 6.8 | 0.8 | 10.2 | 1.4 | 3.4 | 0.8 | <0.001 |
| Low HDL-C levels, mmol/L | 10.2 | 1.0 | 8.3 | 1.5 | 12.7 | 1.5 | 0.03 |
| Elevated LDL-C levels, mmol/L | 22.0 | 1.4 | 25.1 | 2.0 | 18.5 | 1.7 | 0.01 |
| Hypertriglyceridemia, mmol/L† | 13.5 | 1.2 | 11.9 | 1.6 | 15.1 | 1.6 | 0.14 |
Adjusted for age and sex;
n=746: Elevated total cholesterol (TC) levels 6.2 mmol/L or greater; Elevated TC/high-density lipoprotein cholesterol (HDL-C) ratio 5.0 or greater; Low HDL-C levels lower than 1.03 mmol/L in men and lower than 1.29 mmol/L in women; Elevated low-density lipoprotein cholesterol (LDL-C) levels greater than 3.4 mmol/L; Hypertriglyceridemia 1.7 mmol/L or greater. SE Standard error
Finally, to get a better idea of the magnitude of burden of CVD risk factors in this population, Framingham risk score and MetS were computed (Tables 1 and 2). The mean absolute Framingham risk score was 3.1 (SE 0.9; range 1 to 30). Less than 1% of participants were in the highest risk categories (risk of CAD and CAD risk equivalent). A total of 99.5% of women were in the lowest Framingham risk categories, but the prevalence of MetS was higher in women (9.9% [SE 1.3%] versus 5.5% [SE 1.2%]).
DISCUSSION
Baseline findings from the circumpolar Inuit Health in Transition cohort study negate the belief that the Inuit are spared from CVD, and confirm that smoking, obesity and elevated BP are major, modifiable cardiovascular risk factors encountered in this population. However, the Nunavik Inuit still enjoy an exceptional plasma lipid profile (high HDL-C and low TAG), which influences their CVD risk.
Our study reveals that the prevalence of some CVDs among the Nunavik Inuit reached values recorded among other Canadians. For instance, in 2003, the prevalence of self-reported angina in Canadian men and women was 1.8% and 1.9%, respectively (42). In our investigation, we obtained similar results – age-standardized rates were 2.3% and 1.9%, respectively, among men and women.
We also compared our results with hospitalization separation rates after IHD and CD recorded in 1999 (43) in Canada (IHD 607.4/100,000 person-years, CD 221.9/100,000 person-years) and Quebec (IHD 646.4/100,000 person-years, CD 243.5/100,000 person-years). In Nunavik, the crude rate in 2004 was 883.3/100,000 person-years and 543.5/100,000 person-years for IHD and CD, respectively. The rates from Nunavik were higher, specifically for CD.
Even if previous comparisons are based on age-standardized rates or crude rates, it is noteworthy that they are not exactly comparable because the data came from different sources, such as self-reported questionnaires versus medical files in our study. These comparisons are, nevertheless, interesting to obtain an overview of CVD morbidity and an estimate of the phenomenon’s magnitude.
Moreover, the elevated prevalence of CVD found in our investigation corroborates a recent report (44) from the Institut national de santé publique du Québec, which showed that the age-adjusted mortality rate from all CVDs was the highest (450/100,000 person-years) in Nunavik compared with other regions of the province. The mortality rate from all CVDs in the province of Quebec was reported to be 218/100,000 person-years (44).
Based on the mortality data, IHD and CD have been represented as having different burdens in the Inuit population – the former being lower than in the non-native population, and the latter being higher (20,45–47). With a similar prevalence (less than 3%), our data did not reveal any difference between both diseases. Furthermore, other comparisons of the crude rates of these diseases to non-native populations have challenged previous results. However, an ongoing study evaluating the accuracy of death certificates will adequately answer this question.
Another significant finding was that the Inuit population from Nunavik cumulated several major cardiovascular risk factors such as smoking, obesity and hypertension. Smoking is a common habit among the Inuit; more than 84% of individuals reported smoking, and this rate was alarmingly high among young people. Since the 1992 health survey, an increase of approximately 10% has been recorded, whereas a decline of tobacco consumption was recently noted in the rest of Canada (37).
The prevalence of obesity and overweight in the Inuit also warrants urgent action – 28% of Inuit participants were obese. The proportion was higher than that recorded among the general Canadian population (23%) (48). However, as clearly evoked recently by Young et al (49,50), the impact of obesity needs to be explored in detail because associated metabolic parameters common among obese Caucasians are less disrupted among the Inuit. The low prevalence rate of the MetS found in the present study again supports previous observations. Nevertheless, the complex anthropometry of Arctic populations requires further exploration.
Interestingly, whereas no sex difference was seen in obesity levels measured by BMI, a clear distinction appeared when abdominal obesity was considered. Women had higher rates of abdominal obesity. This last point draws attention to Inuit women who cumulate most major cardiovascular determinants such as smoking, elevated BP and sedentary lifestyles. Poor health status among women has already been observed among Alaskan Inuit women (51).
Hypertension, the leading disease reported in the medical files of participants (12%), was lower (27%) than in the adult Canadian population 35 to 64 years of age (52) but similar to that in the adult population (aged 20 years and older) of the province of Quebec (13.8%) (37). In parallel, among people without CVD, the prevalence of elevated BP also appeared to be increased. Altogether, HBP reached values of those recently recorded in this country (52). Moreover, the prevalence of HBP among older Inuit (55 years of age or older) was higher in Nunavik than in Alaska (63% versus 34%) (51). Notwithstanding previous rates in people without CVD, BP values always seemed to be lower than those reported among residents from the southern part of Quebec (25).
In 1992, the results showed that the blood lipid profile of the Inuit from Nunavik was healthier than that of Quebecers (33). However, with the expected increase in consumption of westernized foods, researchers have predicted a deterioration of their blood lipid profile. Twelve years later, mean HDL-C and TAG are still in the normal range. Furthermore, we observed a surprising increase of HDL-C concentration across age categories, particularly among women. This latest result is of great interest because 72% of Inuit women are abdominally obese, and Ghandehari et al (53) reported a negative association between HDL-C and WC among American adults.
This encouraging blood profile, corroborated by the generally low Framingham risk score, suggests that the Inuit population has a weak propensity for developing CAD. Nevertheless, our recent finding of high trans-fat dietary intake (54) and its deleterious interaction with blood lipid profile (55) could raise the risk of CAD among the Inuit Nunavik population.
The prevalence of diabetes was estimated to be 4.7%. This rate is comparable with values in the rest of Canada (5.5%) (39) and those recorded recently among Quebecers (3.7%) (37). In the present analysis, diabetes was considered to be a risk factor for CVD. Its prevalence fell to less than 1% after excluding individuals with declared CVD. Thus, diabetes does not represent an important concern for the Inuit population without CVD.
Previous interpretations should be considered in light of the fact that the circulatory system diseases presented here are extracted from the medical files, and the diagnoses were not validated by another physician. This might trigger less precision in the description of the disease. Consequently, because of the small Nunavik population size, these impressions might have introduced an information bias that may have had a significant impact on the prevalence recorded. This limitation will be corrected in the follow-up of our cohort by rigorous evaluation of cardiovascular events. Moreover, comparisons with Canadian Community Health Survey data on risk factors are limited due to the nature of data that are self-reported by the patient in the survey and reported from medical files or directly measured in our study. Nevertheless, these comparisons are essential to complete the general portrait of CVD burden among the Inuit population. Finally, sedentary lifestyle was self-reported. Such an assessment of the absence of physical activity reduces the potential bias introduced by social desirability.
Our study aimed to provide an overview of prevalence rates of CVD and potentially associated risk factors among the Nunavik Inuit. Our main findings draw attention to risk factors among women who cumulate many CVD determinants and indicate the precarious health status of women from this Canadian Arctic population. The age/sex relationship with other factors such as obesity (general and visceral) and lipid profile is interesting, and a key to the evolution (increase or decrease) of CVD burden in this population. Despite the cross-sectional nature of the present analysis, sex-adapted public intervention is crucial to reduce their risk.
Acknowledgments
The authors thank the Inuit people of Nunavik for their extensive cooperation in this survey. The Nunavik Inuit Health Survey could not have been undertaken without the financial support of the Ministère de la santé et des services sociaux du Québec, the Nunavik Regional Board of Health and Social Services, the Department of Indian and Northern Affairs of Canada, the Canadian Foundation for Innovation, the Network of Centres of Excellence of Canada (ArcticNet), the Nasivvik Aboriginal Capacity and Developmental Research Environments (ACADRE) Inuit Centre and the Canadian Institutes of Health Research. The valuable assistance of Inuit representatives – both members of the Survey Advisory Committee and Inuit leaders from each community – is acknowledged. The authors are also indebted to all the professionals, technicians, students, interviewers and clerical staff who worked at each stage of the survey process. Our gratitude is extended to the staff of the Canadian Coast Guard Ship Amundsen. Dr M-L Chateau-Degat is grateful for the support provided by an Institute of Aboriginal Peoples’ Health Fellowship from the Canadian Institutes of Health Research.
REFERENCES
- 1.Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;1:1143–5. doi: 10.1016/s0140-6736(71)91658-8. [DOI] [PubMed] [Google Scholar]
- 2.Corcoran AC, Rabinowitch IM. A study of the blood lipids and blood protein in Canadian Eastern Arctic Eskimos. Biochem J. 1937;31:343–8. doi: 10.1042/bj0310343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerregaard P, Young KT. The Circumpolar Inuit: Health of a Population in Transition. Copenhagen: Munksgaard; 1998. [Google Scholar]
- 4.Mouratoff GJ, Scott E. Diabetes Mellitus in Eskimos. JAMA. 1967;199:107–12. doi: 10.1001/jama.199.13.107. [DOI] [PubMed] [Google Scholar]
- 5.Mouratoff GJ, Scott EM. Diabetes mellitus in Eskimos after a decade. JAMA. 1973;266:1345–6. [PubMed] [Google Scholar]
- 6.Sagild U, Littaur J, Jespersen SC. Epidemiological studies in Greenland 1962–64. 1. Diabetes mellitus in Eskimos. Acta Medica Scandinavica. 1966;179:29–39. doi: 10.1111/j.0954-6820.1966.tb05430.x. [DOI] [PubMed] [Google Scholar]
- 7.Scott EM, Griffith IV. Diabetes mellitus in Eskimos. Metabolism. 1957;6:320–5. [PubMed] [Google Scholar]
- 8.Duhaime G. La catastrophe et l’État. Histoire démographique et changements sociaux dans l’Arctique. Inuit Studies. 1989;13:75–114. [Google Scholar]
- 9.Duhaime G. Revenu personnel, destin collectif: La structure du revenu des Inuits de l’Arctique du Québec. Canadian Arctic Studies. 1991;23:21–39. [Google Scholar]
- 10.Pars T, Osler M, Bjerregaard P. Contemporary use of traditional food among Greenlandic Inuit. Arctic. 2001;54:22–31. [Google Scholar]
- 11.Proulx J-FVE. 5-16. Health and well-being in Nunavik: An historical overview. In: Jetté M, editor. Report of the Santé Québec Health Survey Among the Inuit of Nunavik. Montreal: Santé Québec, Gouvernement du Québec; 1994. pp. 5–16. [Google Scholar]
- 12.Blanchet C, Dewailly E, Chaumette P, et al. Sustainable Food Security in the Arctic: State of Knowledge. Edmonton: CCI Press; 2002. Chapter 2: Diet profile of circumpolar Inuit; pp. 47–60. [Google Scholar]
- 13.Hodgins S. Health and What Affects it in Nunavik: How is the Situation Changing? Kuujjuaq: Direction de la santé publique. Nunavik Regional Board of Health and Social Services; 1997. [Google Scholar]
- 14.Kuhnlein HV, Receveur O. Dietary change and traditional food systems of indigenous peoples. Annu Rev Nutr. 1996;16:417–42. doi: 10.1146/annurev.nu.16.070196.002221. [DOI] [PubMed] [Google Scholar]
- 15.Labbé J. Les Inuits du Nord québécois et leur santé: Projet Nord. Québec: CHUL and Ministère de la santé et des services sociaux, Gouvernement du Québec; 1987. [Google Scholar]
- 16.Nobmann E, Byers T, Lanier EO, Hankin JH, Jackson MY. The diet of Alaska Native adults: 1987–1988. Am J Clin Nutr. 1992;55:1024–32. doi: 10.1093/ajcn/55.5.1024. [DOI] [PubMed] [Google Scholar]
- 17.Adler AI, Boyko EJ, Schraer CD, Murphy NJ. Lower prevalence of impaired glucose tolerance and diabetes associated with daily seal oil or salmon consumption among Alaska Natives. Diabetes Care. 1994;17:1498–501. doi: 10.2337/diacare.17.12.1498. [DOI] [PubMed] [Google Scholar]
- 18.Murphy NJ, Schraer C, Thiele MC. Dietary change and obesity associated with glucose intolerance in Alaska Natives. J Am Diet Assoc. 1995;95:676–82. doi: 10.1016/S0002-8223(95)00184-0. [DOI] [PubMed] [Google Scholar]
- 19.Schraer C, Adler A, Mayer A, Halderson K, Trimble B. Diabetes complications and mortality among Alaska Natives: 8 years of observation. Diabetes Care. 1997;20:314–21. doi: 10.2337/diacare.20.3.314. [DOI] [PubMed] [Google Scholar]
- 20.Bjerregaard P, Mulvad G, Pedersen HS. Cardiovascular risk factors in Inuit of Greenland. Int J Epidemiol. 1997;26:1182–90. doi: 10.1093/ije/26.6.1182. [DOI] [PubMed] [Google Scholar]
- 21.Moffat M, Young KT. Nutritional patterns of Inuit in the Keewatin region of Canada. Arctic Med Res. 1994;53:298–300. [Google Scholar]
- 22.Québec Santé. Report of the Santé Québec Health Survey among the Inuit of Nunavik: 1992. Montréal: Ministère de la Santé et des services sociaux, Gouvernement du Québec; 1994. [Google Scholar]
- 23.Blanchet C, Dewailly E, Ayotte P, Bruneau S, Receveur O, Holub BJ. Contribution of selected traditional and market foods to the diet of Nunavik Inuit women. Can J Diet Pract Res. 2000;61:50–9. [PubMed] [Google Scholar]
- 24.Cote S, Dodin S, Blanchet C, et al. Very high concentrations of n-3 fatty acids in peri- and postmenopausal Inuit women from Greenland. Int J Circumpolar Health. 2004;63(Suppl 2):298–301. doi: 10.3402/ijch.v63i0.17923. [DOI] [PubMed] [Google Scholar]
- 25.Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Fish consumption and blood lipids in three ethnic groups of Quebec (Canada) Lipids. 2003;38:359–65. doi: 10.1007/s11745-003-1070-4. [DOI] [PubMed] [Google Scholar]
- 26.Dewailly E, Blanchet C, Lemieux S, et al. n-3 fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001;74:464–73. doi: 10.1093/ajcn/74.4.464. [DOI] [PubMed] [Google Scholar]
- 27.Hegele RA, Tully C, Young TK, Connelly PW. V677 mutation of methylenetetrahydrofolate reductases and cardiovascular disease in Canadian Inuit. Lancet. 1997;349:1221–2. doi: 10.1016/s0140-6736(05)62414-2. [DOI] [PubMed] [Google Scholar]
- 28.Hegele RA, Young TK, Connelly PW. Are Canadian Inuit at increased genetic risk for coronary heart disease? J Mol Med. 1997;75:364–70. doi: 10.1007/s001090050122. [DOI] [PubMed] [Google Scholar]
- 29.Middaugh JP. Cardiovascular deaths among Alaskan Natives, 1980–86. Am J Public Health. 1990;80:282–5. doi: 10.2105/ajph.80.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjerregaard P, Dewailly E, Young TK, et al. Blood pressure among the Inuit (Eskimo) populations in the Arctic. Scand J Public Health. 2003;31:92–9. doi: 10.1080/14034940210133924. [DOI] [PubMed] [Google Scholar]
- 31.Blanchet C, Genest J, Moisan J, Sauve L, Schiffrin E. Report of the Santé Québec Health Survey among the Inuit of Nunavik. Québec: Santé Québec, gouvernement du Québec; 1992. Chapter 10: Health factor leading to cardiovascular disease; pp. 67–124. [Google Scholar]
- 32.Dewailly E, Chateau-Degat ML, Ékoé JM, Ladouceur R, Rochette L. Nunavik Inuit Health Survey 2004, Qanuippitaa? How are we? Québec: Institut national de santé publique du Québec (INSPQ) & Nunavik Regional Board of Health and Social Services (NRBHSS); 2007. Status of Cardiovascular Disease and Diabetes in Nunavik. [Google Scholar]
- 33.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: Background and recommendations for the United States. Am J Clin Nutr. 2000;72:1074–81. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 35.Abbott D, Campbell N, Carruthers-Czyzewski P, et al. Guidelines for measurement of blood pressure, follow-up, and lifestyle counselling. Canadian Coalition for High Blood Pressure Prevention and Control. Can J Public Health. 1994;85(Suppl 2):S29–43. [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 37.Lee DS, Chiu M, Manuel DG, et al. Trends in risk factors for cardiovascular disease in Canada: Temporal, socio-demographic and geographic factors. CMAJ. 2009;181:E55–66. doi: 10.1503/cmaj.081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu K, Chen Z, Lipscombe LL. Prevalence and incidence of hypertension from 1995 to 2005: A population-based study. CMAJ. 2008;178:1429–35. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Clinical practice Guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32:S1–152. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 40.National Institutes of Health Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary. Bethesda: National Institutes of Health, National Heart, Lung and Blood Institute; 2001. (NIH publ. no. 01-3670) [Google Scholar]
- 41.Canada S.2001 Census of population Statistic Canada, 2001<http://www.12.statcan.ca/english/census01/product/highlight/SAC/> (Accessed on March 18, 2009).
- 42.Manuel DG, Leung M, Nguyen K, Tanuseputro P, Johansen H. Burden of cardiovascular disease in Canada. Can J Cardiol. 2003;19:997–1004. [PubMed] [Google Scholar]
- 43.Public Health Agency of Canada Cardiovascular diseases surveillance – online Public Health Agency of Canada, 2004<http://204.187.39.30/surveillance/Profiles.aspx> (Accessed on October 12, 2008).
- 44.Institut National de Santé Publique. Portrait de santé du Québec et de ses régions 2006: Les statistiques – 2ième rapport national sur l’état de santé de la population. Québec: Gouvernement du Québec; 2006. [Google Scholar]
- 45.Bjerregaard P, Young TK, Hegele RA. Low incidence of cardiovascular disease among the Inuit – what is the evidence? Atherosclerosis. 2003;166:351–7. doi: 10.1016/s0021-9150(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 46.Young TK, Moffatt ME, O’Neil JD. Cardiovascular diseases in a Canadian Arctic population. Am J Public Health. 1993;83:881–7. doi: 10.2105/ajph.83.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjerregaard P, Dyerberg J. Mortality from ischaemic heart disease and cerebrovascular disease in Greenland. Int J Epidemiol. 1988;17:514–9. doi: 10.1093/ije/17.3.514. [DOI] [PubMed] [Google Scholar]
- 48.Mongeau L, Audet N, Aubin J, Baraldi R. L’excès de poids dans la population québecoise de 1987 à 2003. Québec: INSPQ; 2005. [Google Scholar]
- 49.Young TK. Are the circumpolar Inuit becoming obese? Am J Hum Biol. 2007;19:181–9. doi: 10.1002/ajhb.20617. [DOI] [PubMed] [Google Scholar]
- 50.Young TK, Bjerregaard P, Dewailly E, Risica PM, Jorgensen ME, Ebbesson SE. Prevalence of obesity and its metabolic correlates among the circumpolar Inuit in 3 countries. Am J Public Health. 2007;97:691–5. doi: 10.2105/AJPH.2005.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebbesson SO, Adler AI, Risica PM, et al. Cardiovascular disease and risk factors in three Alaskan Eskimo populations: The Alaska-Siberia project. Int J Circumpolar Health. 2005;64:365–86. doi: 10.3402/ijch.v64i4.18014. [DOI] [PubMed] [Google Scholar]
- 52.Wolf-Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–7. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 53.Ghandehari H, Le V, Kamal-Bahl S, Bassin SL, Wong ND. Abdominal obesity and the spectrum of global cardiometabolic risks in US adults. Int J Obes (Lond) 2009;33:239–48. doi: 10.1038/ijo.2008.252. [DOI] [PubMed] [Google Scholar]
- 54.Counil E, Dewailly E, Bjerregaard P, Julien P.Trans-polar-fat: All Inuit are not equal Br J Nutr 2008April11–4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Counil E, Julien P, Lamarche B, Chateau-Degat ML, Ferland A, Dewailly E. Association between trans-fatty acids in erythrocytes and pro-atherogenic lipid profiles among Canadian Inuit of Nunavik: Possible influences of sex and age. Br J Nutr. 2009;102:766–76. doi: 10.1017/S0007114509297182. [DOI] [PubMed] [Google Scholar]