Abstract
BACKGROUND:
The minimal and optimal amount of physical activity associated with cardiovascular health benefits in young people is unknown.
OBJECTIVE:
To determine the dose-response relationship between moderate-to-vigorous physical activity (MVPA) with high-risk low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and triglyceride values in youth.
METHODS:
The study sample consisted of 1235 adolescents (12 to 19 years of age) from the 2003/2004 and 2005/2006 cycles of the United States National Health and Nutrition Examination Survey. Objective measures of MVPA were obtained over seven days with accelerometers. LDL cholesterol, HDL cholesterol and triglycerides were measured from a fasting blood sample. High-risk values for these lipids/lipoproteins were determined using age- and sex-specific thresholds. Logistic regression models were used to determine the dose-response relationships between MVPA and high-risk lipid levels.
RESULTS:
ORs for high-risk HDL cholesterol and triglyceride values decreased in a curvilinear manner with increasing minutes of MVPA. Compared with no MVPA (0 min), the ORs for high-risk HDL cholesterol values at 15 min, 30 min and 60 min per day of MVPA were 0.29 (95% CI 0.13 to 0.67), 0.24 (95% CI 0.10 to 0.64) and 0.21 (95% CI 0.07 to 0.61), respectively. The corresponding ORs for high-risk triglyceride values were 0.40 (95% CI 0.18 to 0.76), 0.22 (95% CI 0.06 to 0.66) and 0.10 (95% CI 0.01 to 0.51). There was no discernible dose-response relationship between MVPA and LDL cholesterol.
CONCLUSIONS:
Small amounts of MVPA were associated with a large reduction in the likelihood of having high-risk HDL cholesterol and triglyceride values in this representative sample of adolescents.
Keywords: Adolescent, HDL cholesterol, LDL cholesterol, Physical activity, Triglycerides
Abstract
HISTORIQUE :
On ne connaît pas la quantité minimale et optimale d’activité physique qui s’associe à des bienfaits sur la santé cardiovasculaire chez les jeunes.
OBJECTIF :
Déterminer la relation dose-effet entre une activité physique modérée à vigoureuse (APMV) et les valeurs de cholestérol à lipoprotéines de basse densité (LDL), de cholestérol à lipoprotéines de haute densité (HDL) et de triglycérides chez les jeunes.
MÉTHODOLOGIE :
L’échantillon de l’étude se composait de 1 235 adolescents (de 12 à 19 ans) ayant participé aux cycles 2003–2004 et 2005–2006 de la National Health and Nutrition Examination Survey des États-Unis. Les chercheurs ont obtenu des mesures objectives de l’APMV pendant sept jours à l’aide d’accéléromètres. Ils ont mesuré le cholestérol LDL, le cholestérol HDL et les triglycérides à partir d’un échantillon de glycémie à jeun. Ils ont déterminé les valeurs à haut risque de ces lipides et lipoprotéines au moyen de seuils propres à l’âge et au sexe. Ils ont utilisé les modèles de régression logistique pour déterminer la relation dose-effet entre l’APMV et les taux de lipides à haut risque.
RÉSULTATS :
Les RRR des valeurs de cholestérol HDL et de triglycérides à haut risque ont diminué de manière curvilinéaire avec l’augmentation des minutes d’APMV. Par rapport à l’absence d’APMV (0 min), les valeurs du RRR de cholestérol HDL à haut risque en cas d’APMV 15 min, 30 min et 60 min par jour s’élevaient à 0,29 (95 % IC 0,13 à 0,67), 0,24 (95 % IC 0,10 à 0,64) et 0,21 (95 % IC 0,07 à 0,61), respectivement. Les RRR correspondant aux valeurs des triglycérides à haut risque s’élevaient à 0,40 (95 % IC 0,18 à 0,76), 0,22 (95 % IC 0,06 à 0,66) et 0,10 (95 % IC 0,01 à 0,51). Il n’y avait pas de relation dose-effet discernable entre l’APMV et le cholestérol LDL.
CONCLUSIONS :
De petites quantités d’APMV s’associaient à une importante réduction de la probabilité de présenter des valeurs de cholestérol HDL et de triglycérides à haut risque dans cet échantillon représentatif d’adolescents.
Although the clinical manifestations of atherosclerotic cardiovascular disease do not typically occur until old age, the process of atherosclerosis begins in childhood (1). Children and youth with elevated risk factors for atherosclerosis, such as abnormal low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels, are at increased risk of having these risk factors as adults (2–4). In addition, plasma lipid levels during childhood and adolescence predict the degree of atherosclerosis in early adulthood (5). It is, therefore, important to maintain a healthy plasma lipid profile even at young ages.
Physical activity is an important component of the therapeutic lifestyle recommendations for preventing and treating abnormal plasma lipid levels (hereafter referred to as dyslipidemia) in children and youth (6–8). Recent guidelines from the American Heart Association (9) and the Department of Health and Human Services (10) state that children and youth should engage in 60 min of moderate-to-vigorous physical activity (MVPA) on a daily basis to maintain good cardiovascular health. The evidence-based advisory report from which the Department of Health and Human Services guidelines were developed note that children and youth who engage in large amounts of MVPA have a more favourable cardiovascular risk factor profile than sedentary youth (10). However, the nature of the dose-response relationship between MVPA and plasma lipid levels in youth remains to be determined, and the minimal and optimal amount of physical activity required for good cardiovascular health in youth is unclear (10). Therefore, the purpose of the present study was to determine the dose-response relationship between MVPA and dyslipidemia in youth.
METHODS
Data source
The study sample included adolescents (12 to 19 years of age) from the 2003/2004 and 2005/2006 cycles of the National Health and Nutrition Examination Survey (NHANES) who completed both the home interview and mobile examination centre portions of the survey. Of the 2202 eligible participants, 738 did not have acceptable physical activity data (explained below) and an additional 229 did not have fasting blood measures. The current study was, therefore, limited to 1235 participants (659 boys and 576 girls). There were no statistical differences in sex and race between participants who were included in the analysis and those who were excluded due to missing data (P>0.1).
NHANES is a representative cross-sectional survey of the United States population. Participants were identified using a complex stratified, multistage probability sampling technique (11). Informed consent was obtained from all participants, or from their parents or guardians if they were under the age of consent. The study protocol was approved by the National Center for Health Statistics (USA). The secondary analysis presented in the current report was approved by the Queen’s University Health Sciences Research Ethics Board (Kingston, Ontario).
Physical activity
Physical activity was measured objectively using ActiGraph AM-7164 accelerometers (ActiGraph, USA). ActiGraph AM-7164 accelerometers are uniaxial monitors used to measure vertical accelerations between 0.05 G and 2.00 G in magnitude at a frequency between 0.25 Hz and 2.50 Hz. These parameters reduce spurious data caused by vibrations from outside sources and restrict monitoring to normal human movement (12). The filtered movement is stored as an activity count for a user-specified ‘epoch’ interval. Epochs were set to 1 min to determine the participant’s minute-by-minute physical activity intensity.
Accelerometers were attached with an elastic belt to the right hip and worn for seven consecutive days (12). Thus, for each participant, there were 10,080 epoch values (one for each minute of the week), with each of these values corresponding to their intensity of movement during that minute. Participants were asked to wear their accelerometer during all waking hours, except when it would get wet such as while bathing or during water sports. Epoch values of 0 would have been recorded while the accelerometers were not worn.
The 10,080 epoch values were downloaded from the accelerometers by the US Centers for Disease Control and Prevention and checked for biologically implausible data. Additional data reduction was completed by the study authors before analysis. Specifically, participants were only included in the analysis if they had complete monitoring data for at least four days, including at least one weekend day. Days were considered to be complete if there was at least 10 h of monitoring time (12). Periods of 20 min or longer with zero counts were assumed to indicate nonwear time and did not count toward the total wear time. Among adolescents, four days of physical activity monitoring using accelerometers has a test-retest reliability correlation coefficient of 0.70 (12).
An epoch threshold of 3000 counts/min or more was used to denote minutes of MVPA (13). This threshold is based on previous validation studies conducted in a similar age group and corresponds to a metabolic equivalent of 3.0, which approximates the energy expended while walking at a brisk pace (13). For each complete day of monitoring, data were filtered to determine the number of minutes spent engaged in bouts of MVPA (5 min or more) and sporadic MVPA (less than 5 min). To calculate a bout of MVPA, the number of consecutive minutes in which participants spent at least 80% of the time above the threshold for MVPA (eg, for a 10 min bout, 8 min of that time would have to be above 3000 counts/min and this would count as 10 min) was determined. Furthermore, bouts were required to be at least 5 min in duration. The 80% threshold allowed us to account for the occasional rest period observed during bouts of physical activity (eg, the short break between a goal in soccer and the restart of the game). Sporadic physical activity was calculated and summed over the course of the day as those minutes outside of bouts in which participants achieved the 3000 counts/min threshold. The total MVPA for each day was then calculated as the sum of bouts and sporadic activity. Total minutes of MVPA were then averaged over the four- to seven-day monitoring period to create a final variable for each participant that reflected their average daily MVPA in minutes.
Plasma lipids and lipoproteins
The study outcomes consisted of the plasma lipids that can be readily measured in the clinical setting and that are the primary and secondary targets for lipid management: LDL cholesterol, HDL cholesterol and triglycerides. Blood samples were obtained after an overnight fast during a mobile examination centre visit. A brief description of blood analysis procedures is described below. Detailed methodology can be obtained elsewhere (14).
Total serum cholesterol was measured enzymatically through a series of coupled reactions using cholesteryl ester hydrolase, cholesterol oxidase and peroxidase. Triglycerides were measured enzymatically through a series of coupled reactions in which triglycerides were hydrolyzed to produce glycerol, which is then oxidized once more to produce hydrogen peroxide. Absorbance (set at 500 nm) was measured to determine levels of serum triglycerides. HDL cholesterol was measured using the direct HDL method of Roche/Boehringer-Mannheim Diagnostics (United States) and analyzed using the direct HDL cholesterol immunoassay method (11). The bias for the HDL cholesterol values in the 2003/2004 NHANES cycle were acceptable (less than 4%) and did not need to be corrected; however, values from the 2005/2006 cycle were biased (greater than 4%) compared with control samples (Soloman Park Research Laboratories, Kirkland, Washington, USA) (11). These values were corrected using the following formula:
LDL cholesterol was estimated from the Friedewald equation (15) as the following:
Previous intervention research has suggested that physical activity has a minimal effect on plasma lipids in individuals whose values are within the normal healthy range (16,17). Therefore, the analyses in the present paper focused on predicting individuals with ‘high-risk’ lipid values. High-risk LDL cholesterol, HDL cholesterol and triglyceride values were determined using the age- and sex-specific values that were developed by Jolliffe and Janssen (18). These thresholds were developed using growth curve modelling and correspond at entry into adulthood (20.0 years) to the adult cut-points established by the United States National Cholesterol Education Program Expert Panel (LDL cholesterol 4.14 mmol/L or greater, HDL cholesterol 1.04 mmol/L or lower, and triglycerides 2.26 mmol/L or greater) (19).
Covariates
Demographic information regarding ethnicity (non-Hispanic white, non-Hispanic black, Hispanic and other), age (to the nearest month), sex and socioeconomic status were used as covariates. The poverty-to-income ratio was used as a measure of socioeconomic status. It was based on family income and size, and is a ratio of the family’s appropriate poverty threshold.
Better overall dietary habits are associated with increased levels of physical activity (20) and favourable plasma lipid levels (21). Total caloric content, and the intake of sugar, dietary fibre and saturated fats were used as covariates. Dietary values were obtained by averaging the results from two 24 h food recall questionnaires. The dietary variables were included in the analyses as continuous measures.
Statistical analysis
Statistical analysis was performed using SAS 9.1 (SAS Institute Inc, USA), and took into account the sample weights and complex survey design of the NHANES survey. Because the MVPA and triglyceride values were positively skewed, the descriptive data have been presented as medians and interquartile ranges. Unpaired t tests were used to determine age and sex differences in MVPA. The MVPA values were log transformed for the t test analyses.
Logistic regression was used to predict the ORs of having ‘high-risk’ LDL cholesterol, HDL cholesterol and triglyceride values according to the level of MVPA. Thirty-six different logistic regression models were run for each of the plasma lipid outcomes. Each of these models contained a different combination of fractional polynomial models for the continuous MVPA measure. The model with the highest Akaike Information Criterion value was considered to be the model with the best fit, and the parameters from this model were used to plot the dose-response curve between MVPA and ‘high-risk’ plasma lipid/lipoprotein values. This analysis strategy has been described by Bagnardi et al (22) and is concerned with finding the best-fitting model rather than simply determining the significance of the relationship between the exposure (MVPA) and the outcome (plasma lipids). Bootstrapping was used to estimate the 95% CIs for the logistic regression analyses. All of the logistic regression analyses included ethnicity, age, sex, socioeconomic status and the dietary variables as covariates.
RESULTS
Basic descriptive characteristics of the study sample are shown in Table 1. The median physical activity value was 15.7 min/day with an interquartile range of 7.0 min/day to 30.7 min/day. Boys engaged in significantly more MVPA than girls (median of 29.4 min/day versus 12.7 min/day, P<0.0001). Only 4.1% of youth met physical activity guidelines of 60 min/day (10), and 34.8% of boys and 71.1% of girls accumulated less than 15 min/day of MVPA.
TABLE 1.
Descriptive characteristics of participants
| Characteristic | Total (n=1235) | Boys (n=659) | Girls (n=576) |
|---|---|---|---|
| Ethnicity | |||
| Non-Hispanic white | 24.4 | 24.8 | 23.9 |
| Hispanic | 36.4 | 34.3 | 38.8 |
| Non-Hispanic black | 35.3 | 37.5 | 32.8 |
| Other | 4.0 | 3.5 | 4.6 |
| Age, years | |||
| 12–13 | 28.3 | 29.3 | 27.1 |
| 14–16 | 36.9 | 36.3 | 37.7 |
| 17–19 | 35.0 | 34.5 | 35.2 |
| MVPA, min/day | |||
| 0–14 | 51.8 | 34.8 | 71.1 |
| 15–29 | 25.9 | 30.5 | 20.7 |
| 30–59 | 17.9 | 27.0 | 7.6 |
| ≥60 | 4.4 | 7.8 | 0.6 |
Data presented as %. MVPA Moderate-to-vigorous physical activity
Descriptive details for the plasma lipids are presented in Table 2. A small proportion of the participants (4.3% of boys and 5.4% of girls) had high-risk triglyceride values and even fewer (2.0% of boys and 3.8% of girls) had high-risk LDL cholesterol values. A higher proportion of participants had high-risk HDL cholesterol values (18.1% of boys and 12.2% of girls).
TABLE 2.
Descriptive information for low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and triglyceride measures
| Lipid variable |
Total (n=1235) |
Boys (n=659) |
Girls (n=576) |
|||
|---|---|---|---|---|---|---|
| Median (interquartile range) | Measures with a high-risk value, % | Median (interquartile range) | Measures with a high-risk value, % | Median (interquartile range) | Measures with a high-risk value, % | |
| LDL cholesterol* | 2.25 (1.84–2.71) | 2.8 | 2.17 (1.81–2.66) | 2.0 | 2.38 (1.86–2.74) | 3.8 |
| HDL cholesterol* | 1.32 (1.16–1.60) | 15.4 | 1.27 (1.14–1.55) | 18.1 | 1.40 (1.19–1.66) | 12.2 |
| Triglycerides* | 0.86 (0.59–1.12) | 4.8 | 0.82 (0.58–1.12) | 4.3 | 0.88 (0.60–1.11) | 5.4 |
Median LDL cholesterol, HDL cholesterol and triglyceride values are presented in mmol/L. To convert LDL cholesterol and HDL cholesterol values from mmol/L to mg/dL, multiply by a factor of 38.67. To convert triglyceride values from mmol/L to mg/dL, multiply by a factor of 88.5
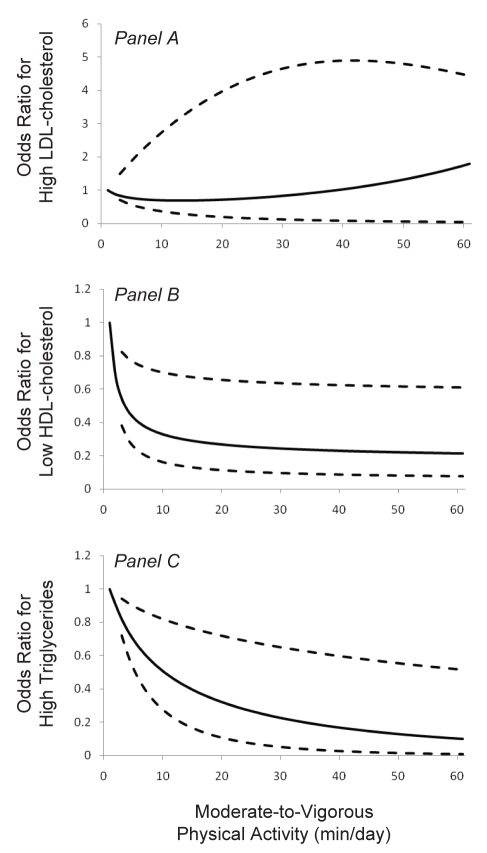
Relationships between MVPA and high-risk plasma lipid values were modelled for MVPA values, ranging from 1 min/day to the 98th percentile (Figure 1). There was no discernable dose-response relationship between MVPA and high-risk LDL cholesterol because the 95% CIs crossed one throughout the MVPA range (Figure 1A). The likelihood of having high-risk HDL cholesterol values decreased in a curvilinear manner with increasing minutes of MVPA (Figure 1B). At 15 min/day, 30 min/day and 60 min/day of MVPA, the ORs for high-risk HDL cholesterol were 0.29 (95% CI 0.13 to 0.67), 0.24 (95% CI 0.10 to 0.64) and 0.21 (95% CI 0.07 to 0.61), respectively, compared with 1 min/day of MVPA. The likelihood of having high-risk triglyceride values also decreased in a curvilinear manner with increasing minutes of MVPA (Figure 1C). At 15 min/day, 30 min/day and 60 min/day of MVPA, the ORs for high-risk triglycerides were 0.40 (95% CI 0.18 to 0.76), 0.22 (95% CI 0.06 to 0.66) and 0.10 (95% CI 0.01 to 0.51), respectively, compared with 1 min/day of MVPA.
Figure 1).
Odds ratio (OR) for high-risk low-density lipoprotein (LDL) cholesterol values (Panel A), high-risk high-density lipoprotein (HDL) cholesterol values (Panel B) and high-risk triglyceride values (Panel C) according to average min/day of moderate-to-vigorous physical activity (MVPA). A value of 1 min/day represents the referent value (OR 1.0) for MVPA. Solid lines represent the predicted ORs and dotted lines represent the 95% CIs
DISCUSSION
The objective of the present study was to examine the dose-response relationship between MVPA and dyslipidemia in youth. A curvilinear dose-response relationship was observed with high-risk HDL cholesterol and triglyceride values such that most of the effect was observed within the first 15 min to 30 min per day of MVPA. There was no clear relationship between MVPA and high-risk LDL cholesterol values.
The observation of decreased odds for high-risk HDL cholesterol and triglycerides with increasing MVPA is consistent with previous observational studies in youth (4). Intervention studies have also demonstrated improvements in HDL cholesterol (8) and trigycerides (23) with increases in exercise in youth. Research examining the relationship between physical activity and LDL cholesterol in both adults and youth is contradictory (24,25). Within the adult population, endurance athletes have LDL cholesterol levels that are only 8% to 10% lower than their sedentary counterparts (25). Conversely, HDL cholesterol levels are 40% to 50% higher in highly active adults compared with inactive adults (25). Thus, the observation in the present study that MVPA was related to high-risk HDL cholesterol and triglyceride values, but not high-risk LDL cholesterol values, is consistent with existing knowledge.
Kelley and Kelley (26) completed a meta-analysis that included 12 randomized controlled trials investigating the effect of exercise training on plasma lipid levels in youth eight to 16 years of age. They concluded that aerobic exercise training has no effect on non-HDL cholesterol (calculated as total cholesterol minus HDL cholesterol) or HDL cholesterol. The lack of a significant effect of exercise training on plasma lipids in this meta-analysis may be partially attributable to the inclusion criteria. Specifically, this meta-analysis only included studies that recruited youth with normal preintervention lipid levels. Previous findings in adults have suggested that physical activity interventions are most effective at improving plasma lipids in those with abnormal lipid values before intervention (27–29). Our study focused on predicting high-risk plasma lipid values and, with this analytical approach, we were able to demonstrate relationships of MVPA with HDL cholesterol and triglycerides in youth.
We are aware of only two previous studies (7,16) that have examined the dose-response relationship between physical activity and plasma lipids in children and youth. Tolfrey et al (16) published the results of a 12-week intervention study that was conducted using a small sample (n=36) of 10- to 12-year-old adolescents. Participants were prescribed either low-volume (approximately 60 min/week) or moderate-volume (approximately 78 min/week) exercise programs. Not surprisingly, given the small total volume of exercise prescribed and the small difference (approximately 18 min per week) in exercise volume between the groups, the researchers failed to find a dose-response or threshold effect of physical activity on total cholesterol, LDL cholesterol, HDL cholesterol or triglycerides. The second study (7) was a cross-sectional study of 2358 young Finnish people nine to 24 years of age. Participants were divided into tertiles based on their level of self-reported MVPA. Plasma triglyceride and HDL cholesterol levels improved across physical activity tertiles in this study. Although the results suggested that a dose-response relationship between MVPA and plasma lipids exists, the authors were not able to explore the pattern of the dose-response relationship (eg, linear or curvilinear). Only three physical activity categories were compared, and each of these categories included a wide range of physical activity scores. The present study examined MVPA on a continuum, which enabled us to better characterize the dose-response relationships.
The shape of the dose-response curves between MVPA and high-risk HDL cholesterol and triglyceride values offers encouraging news for clinicians and other health care practitioners who are concerned about promoting physical activity in their young patients. The majority of the benefits of MVPA on lipids were observed within the first 15 min to 30 min per day. This is far less than the 60 min of daily MVPA that have been recommended by many countries and organizations (9,30–32), and the 90 min of MVPA that is recommended in Canada’s physical activity guidelines for children and youth (33). While it would be ideal to reach MVPA levels that conform to these guidelines, clinicians and health care practitioners need to recognize that getting a very sedentary young person to engage in even minimal levels of MVPA may offer tremendous benefits for their cardiovascular health. In the present study, which used objective measures of MVPA, almost one-half of the youth studied accumulated 15 min or less of MVPA per average day; one-quarter accumulated 7 min per day or less. This implies that a large percentage of youth are extremely sedentary and need to engage in more MVPA to promote good health.
To our knowledge, the present study is the first to use a representative study sample and/or an objective measure of physical activity to examine the relationship between MVPA and plasma lipids in youth. Accelerometers provide a reliable and objective measure of physical activity in a ‘real-life’ setting. A limitation of previous observational studies is that information regarding the level and intensity of physical activity was often obtained through subjective, self-report questionnaires (7). It is well documented that most children and youth over-report their time spent engaging in physical activity (34).Over-reporting of physical activity may have masked the true relationship between physical activity and lipid/lipoprotein level in the past. The statistical modelling approach used in the current study allowed us to use the model that best represented the relationship between MVPA and high-risk plasma lipid values, thereby producing dose-response curves that best represent the data.
Limitations
As with any study, the present study had a number of limitations. The primary study limitation was that it was observational and cross-sectional in nature. This limits our ability to make any causal inferences about the relationship between MVPA and plasma lipids. Furthermore, although accelerometers are objective in nature and have been validated in previous population research (35), they are not a perfect measure of physical activity. Accelerometers are insensitive measure devices when body movement in the hip region is independent of physical activity intensity (such as weight lifting or bicycling) (36). The accelerometry measurement period ranged from four to seven days for each participant, and although this measurement period has a high test-retest reliability (12), it may not accurately reflect habitual activity levels. Thus, limiting our physical activity measurement period to four to seven days may have contributed to measurement error for physical activity. This measurement error would have resulted in biased estimates of the associations between activity and lipid values. Finally, it is important to note that the present study only examined the relationship between MVPA and the traditional plasma lipids. We did not consider emerging lipid and lipoprotein risk factors, such as apolipoprotein B and small, dense LDL particles. Nonetheless, the available information suggests that the relationship between physical activity and apolipoprotein B in adolescents is similar to that between physical activity and LDL cholesterol (37).
CONCLUSIONS
The present study helped clarify the dose-response relationship between MVPA and lipid/lipoprotein levels in youth. Although MVPA was not related to LDL cholesterol levels, there were substantive reductions in the likelihood of having high-risk HDL cholesterol and triglyceride values with even modest amounts of MVPA (eg, 15 min to 30 min per day). Because youth can achieve significant cardiovascular health benefits with even modest amounts of MVPA, physical activity guidelines for youth may need to recognize that getting young people to engage in less than the currently recommended 60 min/day or more of MVPA is beneficial. Lower MVPA targets may act as an incentive for currently sedentary youth.
Footnotes
FUNDING: This research was funded by an operating grant provided by the Canadian Institutes of Health Research (MOP 84478). Ian Janssen is supported by a New Investigator Award from the Canadian Institutes of Health Research and an Early Researcher Award from the Ontario Ministry of Research and Innovation.
REFERENCES
- 1.Natural history of aortic and coronary atherosclerotic lesions in youth. Findings from the PDAY Study. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb. 1993;13:1291–8. doi: 10.1161/01.atv.13.9.1291. [DOI] [PubMed] [Google Scholar]
- 2.Eisemann J, Welk G, Wickel E, Blair S. Stability of variables associated with the metabolic syndrome from adolescence to adulthood: The aerobics center longitudinal study. Am J Hum Biol. 2004;16:690–6. doi: 10.1002/ajhb.20079. [DOI] [PubMed] [Google Scholar]
- 3.Bao W, Srinivasan SR, Valdez R, Greenlund KJ, Wattigney WA, Berenson GS. Longitudinal changes in cardiovascular risk from childhood to young adulthood in offspring of parents with coronary artery disease: The Bogalusa Heart Study. JAMA. 1997;278:1749–54. [PubMed] [Google Scholar]
- 4.Katzmarzyk PT, Malina RM, Bouchard C. Physical activity, physical fitness, and coronary heart disease risk factors in youth: The Quebec Family Study. Prev Med. 1999;29:555–62. doi: 10.1006/pmed.1999.0592. [DOI] [PubMed] [Google Scholar]
- 5.Guo S, Beckett L, Chumlea WC, Roche AF, Siervogel RM. Serial analysis of plasma lipids and lipoproteins from individuals 9–21 y of age. Am J Clin Nutr. 1993;58:61–7. doi: 10.1093/ajcn/58.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Craig SB, Bandini LG, Lichtenstein AH, Schaefer EJ, Dietz WH. The impact of physical activity on lipids, lipoproteins, and blood pressure in preadolescent girls. Pediatrics. 1996;98:389–95. [PubMed] [Google Scholar]
- 7.Raitakari OT, Taimela S, Porkka KV, et al. Associations between physical activity and risk factors for coronary heart disease: The Cardiovascular Risk in Young Finns Study. Med Sci Sports Exerc. 1997;29:1055–61. doi: 10.1097/00005768-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Tolfrey K, Campbell IG, Batterham AM. Exercise training induced alterations in prepubertal children’s lipid-lipoprotein profile. Med Sci Sports Exerc. 1998;30:1684–92. doi: 10.1097/00005768-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr. 2003;142:368–72. doi: 10.1067/mpd.2003.205. [DOI] [PubMed] [Google Scholar]
- 10.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington: U.S. Department of Health and Human Services; 2008. 2008. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services and National Center for Health Statistics. National Health and Nutrition Examination Survey Questionnaire, Examination Protocol, Laboratory Protocol. Hyattsville: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 12.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: How many days of monitoring are needed? Med Sci Sports Exerc. 2000;32:426–31. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Treuth MS, Sherwood NE, Baranowski T, et al. Physical activity self-report and accelerometry measures from the Girls health Enrichment Multi-site Studies. Prev Med. 2004;38(Suppl):S43–9. doi: 10.1016/j.ypmed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services and National Center for Health Statistics National Health and Nutrition Examination Survey Laboratory Procedure Manual Hyattsville: Department of Health and Human Services. Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Tolfrey K, Jones AM, Campbell IG. Lipid-lipoproteins in children: An exercise dose-response study. Med Sci Sports Exerc. 2004;36:418–27. doi: 10.1249/01.mss.0000117132.70711.2b. [DOI] [PubMed] [Google Scholar]
- 17.Tolfrey K, Jones AM, Campbell IG. The effect of aerobic exercise training on the lipid-lipoprotein profile of children and adolescents. Sports Med. 2000;29:99–112. doi: 10.2165/00007256-200029020-00003. [DOI] [PubMed] [Google Scholar]
- 18.Jolliffe CJ, Janssen I. Distribution of lipoproteins by age and gender in adolescents. Circulation. 2006;114:1056–62. doi: 10.1161/CIRCULATIONAHA.106.620864. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sports Exerc. 2000;32:963–75. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Roberts CK, Chen AK, Barnard RJ. Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis. 2007;191:98–106. doi: 10.1016/j.atherosclerosis.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol. 2002;159:1077–86. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 23.Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism. 2006;55:871–8. doi: 10.1016/j.metabol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong N, Simons-Morton B. Physical activity and blood lipids in adolescents. Pediatr Exerc Sci. 1994;6:381–405. [Google Scholar]
- 25.Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L.The acute versus the chronic response to exercise Med Sci Sports Exerc 200133S438–45.; discussion S452–3. [DOI] [PubMed] [Google Scholar]
- 26.Kelley GA, Kelley KS. Effects of aerobic exercise on non-high-density lipoprotein cholesterol in children and adolescents: A meta-analysis of randomized controlled trials. Prog Cardiovasc Nurs. 2008;23:128–32. doi: 10.1111/j.1751-7117.2008.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couillard C, Despres JP, Lamarche B, et al. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: Evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol. 2001;21:1226–32. doi: 10.1161/hq0701.092137. [DOI] [PubMed] [Google Scholar]
- 28.Lokey EA, Tran ZV. Effects of exercise training on serum lipid and lipoprotein concentrations in women: A meta-analysis. Int J Sports Med. 1989;10:424–9. doi: 10.1055/s-2007-1024937. [DOI] [PubMed] [Google Scholar]
- 29.Tran ZV, Weltman A, Glass GV, Mood DP. The effects of exercise on blood lipids and lipoproteins: A meta-analysis of studies. Med Sci Sports Exerc. 1983;15:393–402. [PubMed] [Google Scholar]
- 30.Physical Activity Guidelines Advisory Committee Report. Washington: US Department of Health and Human Services; 2008. [Google Scholar]
- 31.Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–7. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 32.Sallis J, Patrick K. Physical activity guidelines for adolescents: Consensus statement. Pediatr Exerc Sci. 1994;6:302–14. [Google Scholar]
- 33.Janssen I. Physical activity guidelines for children and youth. Can J Public Health. 2007;98(Suppl 2):S109–21. [PubMed] [Google Scholar]
- 34.Adamo K, Prince S, Tricco A, Connor-Gorber S, Tremblay M. A comparison of indirect versus direct measured for assessing physical activity in the pediatric population: A systematic review. Int J Pediatr Obes. 2009;4:2–27. doi: 10.1080/17477160802315010. [DOI] [PubMed] [Google Scholar]
- 35.Bjornson KF. Physical activity monitoring in children and youths. Pediatr Phys Ther. 2005;17:37–45. doi: 10.1097/01.pep.0000154107.30252.fe. [DOI] [PubMed] [Google Scholar]
- 36.Treuth MS, Schmitz K, Catellier DJ, et al. Defining accelerometer thresholds for activity intensities in adolescent girls. Med Sci Sports Exerc. 2004;36:1259–66. [PMC free article] [PubMed] [Google Scholar]
- 37.Suter E, Hawes MR. Relationship of physical activity, body fat, diet, and blood lipid profile in youths 10–15 yr. Med Sci Sports Exerc. 1993;25:748–54. [PubMed] [Google Scholar]