Patients with chronic progressive external ophthalmoplegia (CPEO) have significantly higher levels of cytochrome c oxidase (COX)-deficient fibers in extraocular muscles (EOMs) compared with skeletal muscle. Our data suggest that this could be due to a lower mutational threshold for COX deficiency in EOMs—a key factor that most likely contributes to their selective involvement in mitochondrial genetic disorders.
Abstract
Purpose.
Chronic progressive external ophthalmoplegia (CPEO) is a prominent, and often the only, presentation among patients with mitochondrial diseases. The mechanisms underlying the preferential involvement of extraocular muscles (EOMs) in CPEO were explored in a comprehensive histologic and molecular genetic study, to define the extent of mitochondrial dysfunction in EOMs compared with that in skeletal muscle from the same patient.
Methods.
A well-characterized cohort of 13 CPEO patients harboring a variety of primary and secondary mitochondrial (mt)DNA defects was studied. Mitochondrial enzyme function was determined in EOM and quadriceps muscle sections with cytochrome c oxidase (COX)/succinate dehydrogenase (SDH) histochemistry, and the mutation load in single muscle fibers was quantified by real-time PCR and PCR-RFLP assays.
Results.
CPEO patients with mtDNA deletions had more COX-deficient fibers in EOM (41.6%) than in skeletal muscle (13.7%, P > 0.0001), and single-fiber analysis revealed a lower mutational threshold for COX deficiency in EOM. Patients with mtDNA point mutations had a less severe ocular phenotype, and there was no significant difference in the absolute level of COX deficiency or mutational threshold between these two muscle groups.
Conclusions.
The more pronounced mitochondrial biochemical defect and lower mutational threshold in EOM compared with skeletal muscle fibers provide an explanation of the selective muscle involvement in CPEO. The data also suggest that tissue-specific mechanisms are involved in the clonal expansion and expression of secondary mtDNA deletions in CPEO patients with nuclear genetic defects.
Chronic progressive external ophthalmoplegia (CPEO) is a common manifestation of patients with mitochondrial diseases, and it is often the defining clinical feature, overshadowing other skeletal muscle or neurologic involvement. It is a progressive disorder in which all extraocular muscles (EOMs) are affected, characterized by bilateral, generalized restriction of eye movements (ophthalmoplegia) and drooping of the upper eyelids (ptosis).1 As a group, EOMs have the same embryologic origin, with the myocytes developing from mesodermal precursor cells adjacent to the developing optic cup and stalk.2 They consist of four paired recti and two oblique muscles that insert directly onto the globe to generate movements in various positions of gaze, whereas the levator palpebrae superioris muscle elevates and regulates the height of the upper eyelid.
CPEO is caused by a range of mitochondrial (mt)DNA defects, all of which ultimately compromise oxidative phosphorylation3,4: (1) primary mtDNA mutations that are commonly single, large-scale mtDNA rearrangements5 but also single-nucleotide substitutions in specific transfer (t)RNA genes6–8 and (2) pathogenic mutations in nuclear-encoded mtDNA maintenance genes leading to secondary, multiple mtDNA deletions.9 Skeletal limb muscle biopsy typically reveals cytochrome c oxidase (COX)-deficient fibers, some of which demonstrate characteristic subsarcolemmal accumulation of abnormal mitochondria, the classic ragged-red fiber.10 Studies of single muscle fibers have also confirmed high levels of the pathogenic mtDNA defects in these COX-deficient fibers, above a critical threshold level of >60% mutation load.11–13
The molecular mechanisms underpinning the selective involvement of EOMs in CPEO are poorly understood. To address this fundamental pathophysiological question, we performed a histologic and molecular genetic study comparing EOM samples taken from patients with CPEO during corrective strabismus and ptosis surgery, with skeletal muscle biopsies obtained during the patient's diagnostic work-up. Furthermore, using quantitative single fiber assays, we measured mtDNA mutational load and wild-type copy number in EOM and skeletal muscle fibers, to determine whether there was any difference in the threshold at which a biochemical defect becomes apparent at the cellular level.
Materials and Methods
Patients and Sample Preparation
We studied 13 patients with CPEO who had been referred to the UK National Commissioning Group, Rare Mitochondrial Diseases of Adults and Children, diagnostic laboratory in Newcastle upon Tyne (http://www.mitochondrialncg.nhs.uk/). Skeletal muscle biopsies of vastus lateralis (quadriceps) muscle had been obtained after informed consent for diagnostic purposes, and the molecular genetic work-up of these patients followed a published diagnostic algorithm.10 Eight patients had single, large-scale mtDNA deletions of various sizes, two had pathogenic mtDNA point mutations (m.3243A>G and m.4298G>A7) within tRNA genes, and three patients had multiple mtDNA deletions secondary to a mutation in a nuclear gene: one patient with recessive POLG1 mutations (p.A467T and p.R1096C), one patient with a dominant PEO1 mutation (p.R334Q), and one patient with an as yet unidentified nuclear genetic defect (Table 1).
Table 1.
Clinical and Molecular Genetic Characteristics of the CPEO Cohort
| Patient | Genetic Defect | Molecular Defect | Age at Onset (y) | Family History | EOM Restriction | Ptosis Severity | Other Neurological Features | EOM Muscle Investigated | % COX-Deficient Fibers in Skeletal Muscle | % COX-Deficient Fibers in EOM |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Single mtDNA deletion | 3.9-kb deletion | 19 | No | −3 | Moderate | Mild myopathy | Levator | 10 | 50 |
| 2 | Single mtDNA deletion | 5.0-kb common deletion | 13 | No | −4 | Severe | None | Levator | 10 | 42 |
| 3 | Single mtDNA deletion | 5.0-kb common deletion | 27 | No | −3 | Severe | None | Levator | 1 | 28 |
| 4 | Single mtDNA deletion | 4.1-kb deletion | 19 | No | −3 | Moderate | Mild myopathy, myalgia, fatigue | Medial rectus | 15 | 44 |
| 5 | Single mtDNA deletion | 2.3-kb deletion | 54 | No | −3 | Severe | Mild myopathy, fatigue, migraine | Levator | 10 | 55 |
| 6 | Single mtDNA deletion | 2.9-kb deletion | 49 | No | −3 | Severe | Mild myopathy, fatigue, cerebellar dysfunction | Levator | 15 | 50 |
| 7 | Multiple mtDNA deletions | p.A467T and p.R1096C POLG mutations | 17 | No | −3 | Severe | Mild myopathy, myalgia, peripheral neuropathy, cerebellar dysfunction | Levator | 20 | 41 |
| 8 | Multiple mtDNA deletions | Unknown | 43 | No | −3 | Severe | Cerebellar dysfunction | Levator | 10 | 25 |
| 9 | Multiple mtDNA deletions | p.R334Q PEOI mutation | 48 | Yes | −3 | Moderate | Mild myopathy, fatigue | Levator | 4 | 51 |
| 10 | Single mtDNA deletion | 5.0-kb common deletion | 21 | No | −4 | Severe | Myopathy | Levator | 21 | 25 |
| 11 | Mitochondrial tRNA point mutation | m.4298G>A | 45 | No | −2 | Moderate | Mild myopathy, fatigue, cerebellar dysfunction | Medial Rectus | 25 | 28 |
| 12 | Single mtDNA deletion | 5.0 kb common deletion | 30 | No | −4 | Severe | Mild myopathy, myalgia, fatigue, cerebellar dysfunction | Levator | 35 | 46 |
| 13 | Mitochondrial tRNA point mutation | m.3243 A>G | 39 | Yes | −2 | Severe | Mild myopathy, fatigue, cerebellar dysfunction, deafness | Levator | 20 | 18 |
All patients were assessed by an experienced multidisciplinary team of neurologists and ophthalmologists to define the clinical phenotype and disease severity. The restriction of eye movements was quantified from −1 to −4, with −1 indicating only mild limitation and −4 indicating the worst impediment, with the eye being unable to move from the primary position of gaze.14 Ptosis severity was graded according to the height of the palpebral aperture (PA): severe (PA < 4 mm), moderate (PA 4–6 mm), and mild (PA > 6mm).15 Levator muscle samples were obtained at the time of ptosis surgery and medial rectus muscles were resected at the time of strabismus surgery for ocular misalignment. All muscle samples were mounted for sectioning and immediately frozen in isopentane cooled in liquid nitrogen. This study had the relevant institutional ethics approval and complied with the Declaration of Helsinki.
Mitochondrial Histochemistry and DNA Isolation from Single Muscle Fibers
All muscle samples were subjected to sequential COX/succinate dehydrogenase (SDH) histochemistry according to established diagnostic protocols.16,17 For laser microdissection, 20-μm cryostat sections were mounted on PEN (polyethylenenaphthalate) membrane slides (Leica Microsystems, Wetzlar, Germany), subjected to sequential COX/SDH histochemistry, and air-dried after ethanol dehydration. Single muscle fibers were cut into sterile 0.5-mL PCR tubes (Laser Microdissection [AS-LMD] System; Leica Microsystems) and lysed in standard lysis buffer.17
Real-Time PCR Quantification of mtDNA Deletions and mtDNA Copy Number
A real-time PCR assay13,18 with specific fluorogenic probes (TaqMan; Applied Biosystems, Inc. [ABI], Warrington, UK) was used to determine the relative amplification of mtDNA within the MTND1 and MTND4 genes. The MTND1 region is mostly spared in patients with mtDNA rearrangements, whereas MTND4 is deleted in >97% of all reported cases.19 Single-fiber lysates were analyzed in triplicate, and mtDNA deletion level was derived from the proportion of wild-type (MTND4) to total (MTND1) copy number by the established ΔΔCt method.18 Total and wild-type copy number were calculated with the standard curve method. For copy number analyses, single COX-positive and COX-deficient fibers were identified on COX/SDH-stained slides and then laser-microdissected from serial sections stained with SDH only. This method was used to ensure that all fibers were processed in the same way. Wild-type mtDNA density was calculated by dividing the copy number by the fiber area (in micrometers squared).
Quantification of m.3243A>G Mutation in Individual Muscle Fibers
The accurate m.3243A>G heteroplasmy level was determined with a last-fluorescent-cycle PCR restriction fragment length polymorphism (RFLP) assay.20 In brief, DNA lysate was amplified with primers H3155-3171 and L3353-3334 (nucleotides numbered according to the revised Cambridge Reference Sequence for human mtDNA21) in standard cycling conditions. Samples were subjected to a single cycle of PCR with a FAM- labeled forward primer as follows: initial denaturation at 95°C for 45 seconds, annealing at 61°C for 45 seconds, and extension at 72°C for 1 minute. After digestion at 37°C with HaeIII which recognizes a new restriction site introduced by the m.3243A>G mutation, labeled products were separated (model 3170 Genetic Analyzer System, ABI, analyzed with GeneMapper Software ver. 4.0; ABI).
Statistical Analysis
Group comparisons were made with unpaired t-tests (Prism ver.4 statistical software; GraphPad, San Diego, CA).
Results
Mitochondrial Histochemical Defect in EOM and Skeletal Muscle
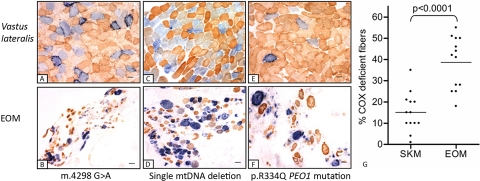
COX deficiency indicates mitochondrial oxidative dysfunction, and it is a valuable histochemical marker that is used to identify cells harboring deleterious levels of pathogenic mtDNA mutations.22 In 12 of the 13 CPEO patients studied, we detected a greater proportion of COX-deficient fibers in EOM than in skeletal muscle (Fig. 1). Analysis of the complete data set showed a higher mean percentage of COX-deficient fibers in EOM (38.7% ± 12.3%, mean ± SD) than in skeletal muscle (15.1% ± 9.1%, P < 0.0001, unpaired t-test). This difference was still significant in subgroup analysis, in which only CPEO patients with single mtDNA deletions were considered (EOM, 41.6% ± 10.8%; skeletal muscle, 13.7% ± 9.3%; P < 0.0001, unpaired t-test).
Figure 1.
(A–F) Sequential COX/SDH histochemistry performed on skeletal muscle (SKM) and EOM from patient 11 with an m.4298 G>A point mutation (A, B), patient 12 with a single 5.0-kb mtDNA deletion (C, D), and patient 9 with a nuclear encoded mutation in PEO1 (E, F). Blue: COX-deficient fibers brown: COX-positive fibers, with type I fibers being more darkly stained than type II. (G) Comparison of the percentage of COX-deficient fibers in skeletal muscle and EOM from the 13 CPEO patients in our study cohort. There was a significantly higher percentage of COX-deficient fibers in EOM than in skeletal muscle (P < 0.0001).
Quantification of mtDNA Deletion Levels
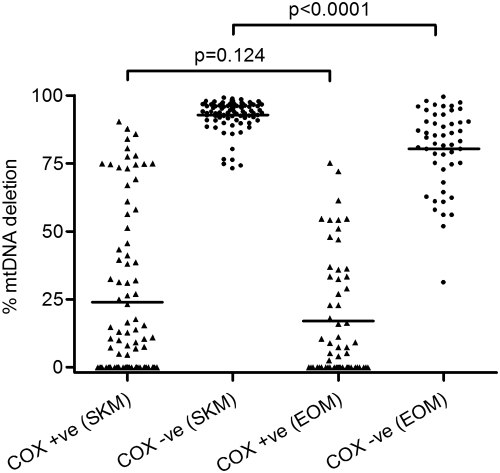
Since a higher proportion of EOM fibers were COX-deficient compared with skeletal muscle, this variation may be related to the absolute mutational threshold required for COX deficiency in these two tissues. We investigated this hypothesis in laser-microdissected COX-positive and COX-deficient fibers from both muscle groups and determined the mutational load within these single muscle fibers, focusing initially on those patients harboring single, large-scale mtDNA deletions. We investigated 89 COX-positive and 87 COX-deficient skeletal muscle fibers and 59 COX-positive and 50 COX-deficient EOM fibers from four patients (2, 3, 4, and 12; Table 1). The mean percentage mtDNA deletion level was significantly lower in the COX-deficient EOM fibers (80.3% ± 14.6%) than in the COX-deficient skeletal muscle fibers (92.8% ± 5.6%, P < 0.0001, unpaired t-test; Fig. 2). There was no significant difference in the mean percentages of deletion in COX-positive fibers from EOM (17.0% ± 21.9%) and skeletal muscle (24.0% ± 29.7%, P = 0.124, unpaired t-test; Fig. 2). This result implies that a much higher proportion of deleted mtDNA is required to cause COX deficiency in skeletal muscle than in EOM.
Figure 2.
mtDNA deletion load in single muscle fibers from EOM and skeletal muscle (SKM) samples of four patients: 2, 3, 4, and 12. The mean percentage deletions in COX-deficient EOM fibers was significantly lower than in COX-deficient skeletal muscle fibers (P < 0.0001). There was no difference in deletion load in the COX-positive muscle fibers from these two groups (P = 0.124).
Quantification of Wild-Type Copy Number
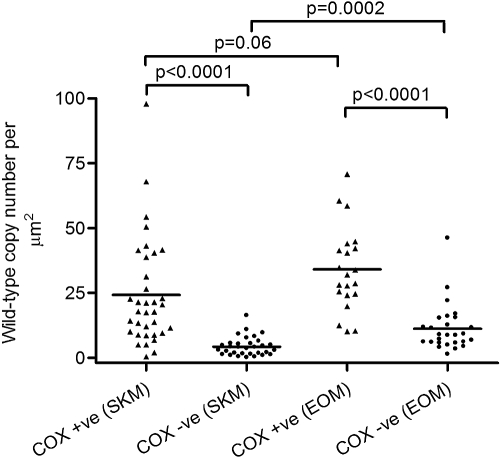
It has been proposed that it may not be the absolute level of deleted mtDNA molecules that determines whether a fiber is COX-negative, but the absolute level of wild-type mtDNA molecules remaining within that fiber.11 To explore this notion, we used real-time PCR analysis to quantify wild-type mtDNA copy number in individual COX-positive and COX-deficient EOM and skeletal muscle fibers. We laser-microdissected a total of 35 COX-positive and 32 COX-deficient skeletal muscle fibers, and 21 COX-positive and 29 COX-deficient EOM fibers from four patients (2, 3, 4, and 12; Table 1). The analysis showed a marked loss of wild-type mtDNA molecules in COX-deficient fibers from both skeletal muscle (24.2 ± 20.6 copies/μm2 in COX-positive fibers, 4.3 ± 3.6 copies/μm2 in COX-deficient fibers, P < 0.0001, unpaired t-test), and EOM (34.0 ± 16.1 copies/μm2 in COX-positive fibers, 11.2 ± 9.1 copies/μm2 in COX-deficient fibers, P < 0.0001, unpaired t-test). We also observed a significantly higher mean wild-type copy number in COX-deficient EOM fibers (11.2 ± 9.1 copies/μm2) than in COX-deficient skeletal muscle fibers (4.3 ± 3.6 copies/μm2, P = 0.0002, unpaired t-test), indicating that a higher absolute number of wild-type mtDNA molecules is necessary to maintain normal COX activity in EOM fibers than in skeletal muscle fibers (Fig. 3). There was no significant difference in the mean wild-type copy number in COX-positive EOM fibers (34.0 ± 16.1 copies/μm2) compared with that in COX-positive skeletal muscle fibers (24.2 ± 20.6 copies/μm2, P = 0.06, unpaired t-test).
Figure 3.
Wild-type mtDNA copy number in single muscle fibers from EOM and skeletal muscle (SKM) samples of four patients: 2, 3, 4, and 12. The mean wild-type mtDNA copy number was significantly higher in COX-deficient EOM fibers than in COX-deficient skeletal muscle fibers (P = 0.0002)
Quantification of m.3243A>G Point Mutation Levels
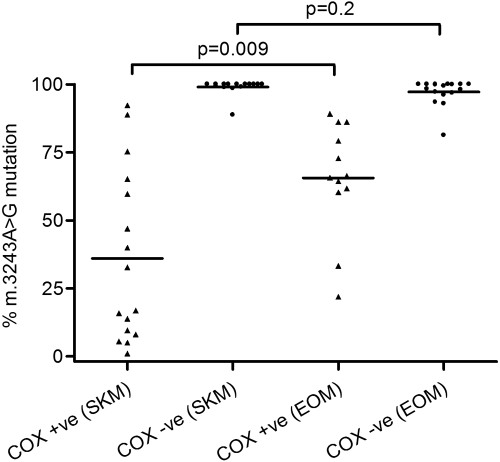
Of the 13 CPEO patients investigated in this study, only patient 13, who had the m.3243A>G single nucleotide substitution in the MTTL1 gene, did not show a greater proportion of COX-deficient fibers in the EOM than in skeletal muscle. We quantified m.3243A>G mutation levels in both COX-positive (n = 11 EOM; n = 16 skeletal muscle) and COX-deficient (n = 17 EOM; n = 16 skeletal muscle) fibers (Fig. 4). We observed no significant difference in the level of the m.3243A>G mutation in COX-deficient EOM fibers (97.3% ± 4.7%), compared with COX-deficient skeletal muscle fibers (99.1% ± 2.8%, P = 0.2, unpaired t-test), although there was a significantly higher mutational load in COX-positive EOM fibers (65.6% ± 31.5%) compared with COX-positive skeletal muscle fibers (36.1% ± 31.5%, P = 0.009, unpaired t-test; Fig. 4). This finding suggests no difference in the mutation threshold for COX deficiency in EOM compared with skeletal muscle for m.3243A>G.
Figure 4.
Level of the m.3243A>G point mutation in single muscle fibers dissected from the EOM and skeletal muscle (SKM) of patient 13. The m.3243G>A mutation was nearly homoplasmic in COX-deficient EOM and skeletal muscle fibers, with no significant difference in the threshold of COX deficiency in these two muscle groups (P = 0.2).
Disease Severity
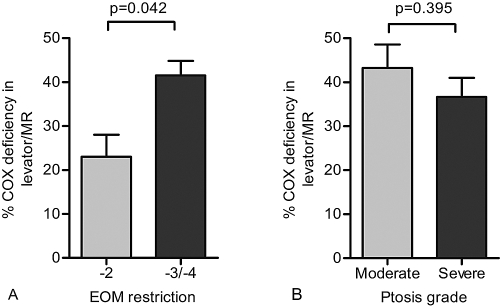
The two patients with the least severe EOM restriction (score of −2) both harbored the mtDNA point mutations m.3243G>A and m.4298G>A. The percentage of COX-deficient EOM fibers in the moderate (−2) group was significantly lower than that in the severe (−3/−4) group (23% ± 7. % vs. 41.6% ± 10.8%, P = 0.042, unpaired t-test; Fig. 5A), suggesting a correlation between the level of COX deficiency and ophthalmoplegia score. However, there was no significant association between COX deficiency and ptosis grade (moderate = 43.3% ± 10.6%, severe = 36.7% ± 12.9%, P = 0.395, unpaired t-test; Fig. 5B).
Figure 5.
Comparison of the level of COX deficiency in EOM samples with (A) degree of restriction of eye movements and (B) ptosis severity. Bars, mean ± SEM. MR, medial rectus.
Discussion
mtDNA defects are responsible for a heterogeneous group of multisystemic disorders, and the prominent and often restricted EOM involvement in CPEO remains an intriguing pathologic feature. In our study cohort, two patients had a pure CPEO phenotype. Although the remainder had other neurologic features such as myopathy, exercise intolerance, and cerebellar dysfunction, these effects were mostly mild and the greatest impact on these patients' quality of life was due to their marked ophthalmoplegia and ptosis.23 Although the underlying mechanisms have not been properly investigated, the discrepancy in clinical severity between EOM and limb muscle has been ascribed to the fundamental differences between these two muscle groups in fiber composition, innervation patterns, and basic contractile physical properties. EOMs have a higher mitochondrial content,24 higher fatigue resistance,25 smaller motor units, higher blood flow,26,27 and higher motor neuron discharge rates,28 all of which suggest that they have a more sustained metabolic demand than do skeletal muscles. Our study is the first to directly compare the mitochondrial biochemical defect (COX-deficiency) in EOM and skeletal muscle specimens from patients (n = 13) with a clinical and molecular diagnosis of CPEO. Although COX-deficient muscle fibers accumulate in EOMs during normal aging,29,30 the levels of COX deficiency in both skeletal muscle and EOM of patients with CPEO were significantly higher,31 thus highlighting the pathogenic load of these genetic defects in our patient cohort. In 11 of the 13 patients, all of whom had mtDNA deletions, we identified a significantly higher proportion of COX-deficient fibers in EOM than in skeletal muscle. The lower threshold of COX deficiency in the EOMs that was demonstrated in single-fiber analysis, by both mutational load and absolute wild-type copy number analyses, offers a plausible explanation for this finding.
Two CPEO patients in this study had pathogenic mutations in mtDNA maintenance genes (POLG1 and PEO1) known to cause secondary, multiple mtDNA deletions.3,9 These deletions are somatic and accumulate over time. We and others have shown that at the single-cell level, this accumulation is associated with clonal expansion of one, sometimes two, deleted mtDNA species.32–34 The threefold greater percentage of COX-deficient fibers in EOM therefore implies that mtDNA deletions accumulate to deleterious levels at a faster rate in EOM fibers than in skeletal muscle fibers. Although this study has shown a lower threshold for COX-deficiency in EOM, other mechanisms could lead to the higher proportion of COX-deficient fibers observed in EOM, such as a higher rate of deletion formation and a faster rate of clonal expansion. These factors are not mutually exclusive, and additional investigations are warranted to clarify their respective contributions.
Levator palpebrae superioris (n = 11) and medial rectus (n = 2) specimens were analyzed as a single group, as they develop from the same mesodermal precursor cells and share similar histologic features.35,36 In the same patient, it is therefore not unreasonable to assume that both of these muscles would harbor comparable proportions of COX-deficient fibers. Compared with patients with mtDNA deletions, the two patients with mtDNA point mutations had less severe limitation of eye movements, and the reduced severity was associated with a lower absolute level of COX deficiency in the EOM samples. However, there was no significant difference in either the percentage of COX-deficient fibers between skeletal muscle and EOM or the threshold for COX deficiency in these two tissues. This result is in marked contrast to the clinical findings, with both patients having severe restriction in their ocular motility compared with only mild proximal limb muscle weakness. These molecular findings indicate that at the cellular level, EOMs are more vulnerable than skeletal muscle when faced with a similar level of metabolic impairment, very likely because of their unique developmental and biological characteristics.
The results of our study suggest that decreasing the mutational load in EOMs to below the biochemical threshold could lead to a functional benefit by improving the range of eye movements. Potential therapeutic strategies could involve increasing the level of wild-type mtDNA by recruiting EOM satellite cells, which are thought to harbor lower levels of mutant mtDNA, to differentiate into mature myofibers.37,38 EOMs are easily accessible, and the proliferation of satellite cells may be induced by injection of specific myotoxic stimuli such as bupivacaine.39 It is therefore of great interest that in a recent study of patients with diplopia due to lateral rectus palsy, injection of the paretic muscle with bupivacaine led to muscle hypertrophy, increased force generation, and an improvement in ocular alignment.40
A limitation of our study is the relatively small number of patients investigated and the heterogeneous nature of the underlying genetic defects, although the majority (8/13) had single mtDNA deletions. However, CPEO is a relatively rare condition, and only a select group of patients will undergo corrective strabismus or ptosis surgery during the course of the disease. These factors contribute to the difficulty in obtaining EOM tissues, further compounded by the need for skeletal muscle to allow direct comparative analysis between the two muscle groups. Nevertheless, our study has provided important molecular insights into the selective vulnerability of EOMs in CPEO; future collaborative studies may clarify these basic factors further. A better understanding of the underlying disease process is crucial in developing effective therapeutic strategies for patients with CPEO, a disorder associated with significant morbidity and for which treatment is currently mostly supportive.
Footnotes
Supported by the Newcastle University Centre for Brain Ageing and Vitality, which is supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC), and MRC (Medical Research Council) as part of the cross-council Lifelong Health and Wellbeing Initiative (LCG); a Wellcome Trust Programme grant, and funding from the Newcastle upon Tyne Hospitals NHS Trust (DMT, RWT). PYWM is an MRC Research Fellow in neuro-ophthalmology. Diagnostic testing was funded by the U.K. National Commissioning Group to provide the “Rare Mitochondrial Disorders of Adults and Children” Diagnostic Service.
Disclosure: L.C. Greaves, None; P. Yu-Wai-Man, None; E.L. Blakely, None; K.J. Krishnan, None; N.E. Beadle, None; J. Kerin, None; M.J. Barron, None; P.G. Griffiths, None; A.J. Dickinson, None; D.M. Turnbull, None; R.W. Taylor, None
References
- 1.Richardson C, Smith T, Schaefer A, Turnbull D, Griffiths P. Ocular motility findings in chronic progressive external ophthalmoplegia. Eye 2005;19:258–263 [DOI] [PubMed] [Google Scholar]
- 2.Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci 2005;46:4200–4208 [DOI] [PubMed] [Google Scholar]
- 3.Bau V, Zierz S. Update on chronic progressive external ophthalmoplegia. Strabismus 2005;13:133–142 [DOI] [PubMed] [Google Scholar]
- 4.Schapira AH. Mitochondrial disease. Lancet 2006;368:70–82 [DOI] [PubMed] [Google Scholar]
- 5.Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988;331:717–719 [DOI] [PubMed] [Google Scholar]
- 6.Moraes CT, Ciacci F, Silvestri G, et al. Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscul Disord 1993;3:43–50 [DOI] [PubMed] [Google Scholar]
- 7.Taylor RW, Chinnery PF, Bates MJ, et al. A novel mitochondrial DNA point mutation in the tRNA(Ile) gene: studies in a patient presenting with chronic progressive external ophthalmoplegia and multiple sclerosis. Biochem Biophys Res Commun 1998;243:47–51 [DOI] [PubMed] [Google Scholar]
- 8.Taylor RW, Schaefer AM, McFarland R, Maddison P, Turnbull DM. A novel mitochondrial DNA tRNA(Ile) (A4267G) mutation in a sporadic patient with mitochondrial myopathy. Neuromuscul Disord 2002;12:659–664 [DOI] [PubMed] [Google Scholar]
- 9.Hirano M, DiMauro S. ANT1, twinkle, POLG, and TP: new genes open our eyes to ophthalmoplegia. Neurology 2001;57:2163–2165 [DOI] [PubMed] [Google Scholar]
- 10.Taylor RW, Schaefer AM, Barron MJ, McFarland R, Turnbull DM. The diagnosis of mitochondrial muscle disease. Neuromuscul Disord 2004;14:237–245 [DOI] [PubMed] [Google Scholar]
- 11.Durham SE, Samuels DC, Cree LM, Chinnery PF. Normal levels of wild-type mitochondrial DNA maintain cytochrome c oxidase activity for two pathogenic mitochondrial DNA mutations but not for m. 3243A→G. Am J Hum Genet 2007;81:189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoubridge EA, Karpati G, Hastings KE. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell 1990;62:43–49 [DOI] [PubMed] [Google Scholar]
- 13.He L, Chinnery PF, Durham SE, et al. Detection and quantification of mitochondrial DNA deletions in individual cells by real-time PCR. Nucleic Acids Res 2002;30:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivian AJ, Morris RJ. Diagrammatic representation of strabismus. Eye 1993;7:565–571 [DOI] [PubMed] [Google Scholar]
- 15.Taherian K, Atkinson PL, Shekarchian M, Scally AJ. Comparative study of the subjective and objective grading of ptosis surgery outcomes. Eye 2007;21:639–642 [DOI] [PubMed] [Google Scholar]
- 16.Old SL, Johnson MA. Methods of microphotometric assay of succinate dehydrogenase and cytochrome c oxidase activities for use on human skeletal muscle. Histochem J 1989;21:545–555 [DOI] [PubMed] [Google Scholar]
- 17.Taylor RW, Barron MJ, Borthwick GM, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest 2003;112:1351–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan KJ, Bender A, Taylor RW, Turnbull DM. A multiplex real-time PCR method to detect and quantify mitochondrial DNA deletions in individual cells. Anal Biochem 2007;370:127–129 [DOI] [PubMed] [Google Scholar]
- 19.Brandon MC, Lott MT, Nguyen KC, et al. MITOMAP: a human mitochondrial genome database: 2004 update. Nucleic Acids Res 2005;33:D611–D613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyle A, Taylor RW, Durham SE, et al. Depletion of mitochondrial DNA in leucocytes harbouring the 3243A→G mtDNA mutation. J Med Genet 2007;44:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson S, Bankier AT, Barrell BG, et al. Sequence and organization of the human mitochondrial genome. Nature 1981;290:457–465 [DOI] [PubMed] [Google Scholar]
- 22.Sciacco M, Bonilla E, Schon EA, DiMauro S, Moraes CT. Distribution of wild-type and common deletion forms of mtDNA in normal and respiration-deficient muscle fibers from patients with mitochondrial myopathy. Hum Mol Genet 1994;3:13–19 [DOI] [PubMed] [Google Scholar]
- 23.Yu Wai Man CY, Smith T, Chinnery PF, Turnbull DM, Griffiths PG. Assessment of visual function in chronic progressive external ophthalmoplegia. Eye 2006;20:564–568 [DOI] [PubMed] [Google Scholar]
- 24.Carry MR, Ringel SP, Starcevich JM. Mitochondrial morphometrics of histochemically identified human extraocular muscle fibers. Anat Rec 1986;214:8–16 [DOI] [PubMed] [Google Scholar]
- 25.Fuchs AF, Binder MD. Fatigue resistance of human extraocular muscles. J Neurophysiol 1983;49:28–34 [DOI] [PubMed] [Google Scholar]
- 26.Spencer RF, Porter JD. Structural organization of the extraocular muscles. Rev Oculomotor Res 1988;2:33–79 [PubMed] [Google Scholar]
- 27.Wooten GF, Reis DJ. Blood flow in extraocular muscle of cat. Arch Neurol 1972;26:350–352 [DOI] [PubMed] [Google Scholar]
- 28.Robinson DA. Oculomotor unit behavior in the monkey. J Neurophysiol 1970;33:393–403 [DOI] [PubMed] [Google Scholar]
- 29.Muller-Hocker J. Cytochrome c oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. J Neurol Sci 1990;100:14–21 [DOI] [PubMed] [Google Scholar]
- 30.Muller-Hocker J, Schneiderbanger K, Stefani FH, Kadenbach B. Progressive loss of cytochrome c oxidase in the human extraocular muscles in ageing: a cytochemical-immunohistochemical study. Mutat Res 1992;275:115–124 [DOI] [PubMed] [Google Scholar]
- 31.Yu-Wai-Man P, Lai-Cheong J, Borthwick GM, et al. Somatic mitochondrial DNA deletions accumulate to high levels in aging human extraocular muscles. Invest Ophthalmol Vis Sci 2010;51:3347–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moslemi AR, Melberg A, Holme E, Oldfors A. Clonal expansion of mitochondrial DNA with multiple deletions in autosomal dominant progressive external ophthalmoplegia. Ann Neurol 1996;40:707–713 [DOI] [PubMed] [Google Scholar]
- 33.Bender A, Krishnan KJ, Morris CM, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 2006;38:515–517 [DOI] [PubMed] [Google Scholar]
- 34.Reeve AK, Krishnan KJ, Elson JL, et al. Nature of mitochondrial DNA deletions in substantia nigra neurons. Am J Hum Genet 2008;82:228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sevel D. The origins and insertions of the extraocular muscles: development, histologic features, and clinical significance. Trans Am Ophthalmol Soc 1986;84:488–526 [PMC free article] [PubMed] [Google Scholar]
- 36.Porter JD, Baker RS. Muscles of a different ‘color’: the unusual properties of the extraocular muscles may predispose or protect them in neurogenic and myogenic disease. Neurology 1996;46:30–37 [DOI] [PubMed] [Google Scholar]
- 37.Clark KM, Bindoff LA, Lightowlers RN, et al. Reversal of a mitochondrial DNA defect in human skeletal muscle. Nat Genet 1997;16:222–224 [DOI] [PubMed] [Google Scholar]
- 38.Fu K, Hartlen R, Johns T, Genge A, Karpati G, Shoubridge EA. A novel heteroplasmic tRNAleu(CUN) mtDNA point mutation in a sporadic patient with mitochondrial encephalomyopathy segregates rapidly in skeletal muscle and suggests an approach to therapy. Hum Mol Genet 1996;5:1835–1840 [DOI] [PubMed] [Google Scholar]
- 39.Andrews RM, Griffiths PG, Chinnery PF, Turnbull DM. Evaluation of bupivacaine-induced muscle regeneration in the treatment of ptosis in patients with chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome. Eye 1999;13:769–772 [DOI] [PubMed] [Google Scholar]
- 40.Scott AB, Alexander DE, Miller JM. Bupivacaine injection of eye muscles to treat strabismus. Br J Ophthalmol 2007;91:146–148 [DOI] [PMC free article] [PubMed] [Google Scholar]