In this study, the authors examined conjunctival gene expression of matrix metalloproteinases and proinflammatory cytokines after trichiasis surgery. Recurrent trichiasis was associated with the ratio of MMP-1 to TIMP-1, which may favor the accumulation of fibrotic tissue after surgery.
Abstract
Purpose.
Trachoma, the leading infectious cause of blindness, is a chronic inflammatory scarring condition. Blindness follows the development of trichiasis, which is treated surgically. Unfortunately, it frequently recurs, compromising the treatment. In this study, gene expression analysis was used to examine factors that may be involved in the inflammation and tissue remodeling after surgery.
Methods.
Subjects were examined before and at 1 and 4 years after surgery. Conjunctival swab samples were collected for bacterial culture, Chlamydia trachomatis PCR, and RNA isolation at 1 year. Quantitative real-time PCR was performed to measure the expression of tumor necrosis factor-α (TNF), interleukin-1β (IL1B), matrix metalloproteinase-1 (MMP1), MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinase 1 (TIMP-1), TIMP-2, and hypoxanthine phosphoribosyl transferase-1 (HPRT1).
Results.
Two hundred forty individuals with trachomatous trichiasis were recruited. One year after surgery, recurrent trichiasis was associated with a reduced MMP-1/TIMP-1 ratio (P = 0.029). IL1B expression was elevated in the presence of either conjunctival bacterial infection (P = 0.011) or inflammation (P = 0.002). TNF expression was greater in the Mandinka ethnic group (P < 0.0001), and it was increased when clinical inflammation was associated with nonchlamydial bacterial infection (P = 0.012). MMP-9 expression increased when conjunctival inflammation was associated with bacterial infection (P = 0.007).
Conclusions.
Recurrent trichiasis was associated with a reduced MMP-1 to TIMP-1 ratio, which may favor the accumulation of fibrotic tissue. Nonchlamydial bacterial infection may induce factors that contribute to conjunctival tissue remodeling and recurrent trichiasis in trachoma. Prospective studies are needed to assess the potential importance of these and other factors in progressive disease.
Trachoma is the leading infectious cause of blindness worldwide.1 The disease process begins in early childhood, with recurrent episodes of chronic follicular conjunctivitis (active trachoma) triggered by the bacterium Chlamydia trachomatis. Chronic inflammation promotes conjunctival scarring, and after some years the eyelids may turn inward (entropion) so that the lashes scratch the surface of the eye (trichiasis). Thus, the cornea is compromised and blinding opacification may develop through various mechanisms, including direct trauma and secondary bacterial infection.2
Trachoma is a public health problem in more than 50 countries, predominantly in Sub-Saharan Africa, the Middle East, and Asia.3 The most recent global estimates from the World Health Organization (WHO) suggest that approximately 40 million people have active trachoma and 8.2 million have nonsurgically treated trichiasis.4 During 2002, 1.3 million people were estimated to be blind from trachoma and probably a further 1.8 million had low vision.1 The WHO is leading a global initiative to eliminate blinding trachoma by 2020 through the implementation of the SAFE strategy: surgery for trichiasis, antibiotics for infection, facial cleanliness, and environmental improvements to reduce transmission of the organism.5
Several alternative surgical procedures are used for treating trachomatous trichiasis (TT). These generally involve incising the entropic lid, followed by outward rotation and suturing of the distal fragment in the corrected position. When surgery succeeds in correcting the trichiasis, there is relief from pain and the risk of blinding corneal opacification is reduced. Unfortunately, trichiasis frequently returns: reported recurrence rates range from 10% at 1 year to 60% at 3 years.2,6–9 This tendency to recur is a major challenge for trachoma control, as it compromises the potential of surgery to prevent blindness. Recurrent TT broadly falls into two categories: early recurrence related to surgical failure and late recurrence, probably due to progressive fibrotic disease. Different factors may contribute to recurrence at different stages: preoperative disease severity, inherent limitations of the surgical procedure, the surgeon's ability, wound-healing responses, infection, and chronic inflammatory-fibrogenic responses.2,8 The relative importance of the biological disease factors and their contribution at different stages during recurrence of trichiasis is unknown. A better understanding of the process of recurrent trichiasis is therefore needed to refine and develop strategies for its prevention and treatment.
Chronic inflammation is a key event in the development of scarring in many different tissues and disease entities such as cirrhosis and pulmonary fibrosis.10 Similarly, chronic conjunctival inflammation is probably central in blinding trachoma. Long-term epidemiologic studies examining the development of conjunctival scarring in trachoma have identified a subgroup of people who have severe inflammatory trachoma (TI) on repeated examination. These individuals are at greatest risk of the development of scarring and trichiasis in later life.11–13 Adults with scarring sequelae, particularly trichiasis, frequently have clinically inflamed tarsal conjunctiva.14 Moreover, several studies have found an association between recurrent trichiasis after surgery and clinically visible tarsal conjunctival inflammation.2,6,8 The general consensus is that conjunctival inflammation and fibrosis in trachoma are mainly driven by C. trachomatis infection. However, other stimuli such as infection with other bacterial species, dryness of the ocular surface and the physical irritation from the anatomic changes due to trichiasis, may also contribute.14–16 There could also be an element of autoimmune hypersensitivity.17 In addition it is also possible that conjunctival inflammation in trachoma is maintained by an autoinflammatory process involving a positive feedback loop of cytokines and enzymes involved in extracellular matrix (ECM) turnover.18
We conducted a study to investigate conjunctival inflammatory cytokine and tissue remodeling responses 1 year after surgery. Specifically, we explored the hypotheses that (1) recurrent trichiasis is associated with evidence of altered ECM regulation and (2) clinically visible inflammation is associated with an increased proinflammatory cytokine response. We used quantitative real-time reverse transcriptase PCR to estimate the conjunctival gene expression levels. The expression levels of these genes were tested for association with the clinical status after posterior lamellar tarsal rotation surgery.
Methods
Ethical Permission
This study adhered to the tenets of the Declaration of Helsinki. The study was approved by the Gambian Government/Medical Research Council Joint Ethics Committee (SCC 858). Informed consent was obtained before enrollment of each subject.
Study Participants
The subjects had participated in a previously reported trial of perioperative azithromycin.2 Individuals requiring surgery for trichiasis were identified from the Gambian National Eye Care Programme (NECP) database, community ophthalmic nurse records, and village screening. Trichiasis surgery was performed by NECP ophthalmic nurses in district health centers. The posterior lamella tarsal rotation procedure (Trabut) was used in all cases.19
Clinical Assessment
The patients were assessed before and at 1 and 4 years after surgery. Clinical signs of trachoma were graded by using 2.5× binocular loupes and the detailed WHO trachoma grading system.20 The number of eyelashes touching the cornea and other parts of the globe in primary position were recorded. Evidence of epilation was recorded: broken/regrowing lashes or sections of eyelid without lashes. If evidence of epilation was found, the total extent of the epilation was scored as less than or more than half the length of the eyelid. Corneal opacification was considered visually significant if at least part of the pupil was obscured (CC2/CC3).20,21 Upper tarsal conjunctival inflammation was considered significant if there were prominent papillae and/or haziness of the tarsal blood vessel (P2/P3).6,14,20 The reason for this is that in heavily scarred conjunctiva there are often “islands” of intensely inflamed tissue between areas of dense scarring; therefore, the total area that can mount an intense response is less than the 50% threshold for P3.
One year after surgery, three conjunctival swab samples were collected. The conjunctiva was anesthetized with preservative-free proxymetacaine 0.5% eye drops (Minims; Chauvin Pharmaceuticals, Montpellier, France). The first swab (Dacron polyester-tipped swab; Hardwood Products Company, Guilford, ME) was collected for microbiologic culture from the inferior fornix and placed in sterile skimmed milk, Tryptone, glycerol, and glucose broth (STGG). The second swab sample was collected for RNA isolation from the upper tarsal conjunctival surface and placed directly into a tube containing 0.2 mL of RNA stabilizer (RNAlater; Ambion, Austin, TX). The third swab was collected for C. trachomatis PCR from the upper tarsal conjunctival surface and placed in a dry tube. Samples were kept on ice packs until frozen later the same day: −80°C for microbiologic culture and −20°C for the nucleic acid isolation samples.
Microbiologic Isolation
Conjunctival STGG samples were plated on blood agar (aerobic and anaerobic), McConkey agar (aerobic), gentamicin blood agar, and bacitracin chocolate agar. The gentamicin blood agar and the bacitracin chocolate agar were placed in a 5% carbon dioxide incubator. All plates were incubated at 37°C for 48 hours, and growing colonies were identified by using standard bacteriologic techniques. Staphylococcus epidermidis and Bacillus spp. were excluded from the analysis, as they were considered commensal rather than pathogenic at this site.
C. trachomatis PCR
Conjunctival swab samples collected for chlamydial detection were tested for C. trachomatis DNA by using a PCR-based assay (Amplicor CT/NG Test; Roche, Mannheim, Germany) with previously described modifications.22
RNA Extraction and Reverse Transcription
Total RNA was extracted from the conjunctival swab sample (RNeasy Mini Kit system; Qiagen, Hilden, Germany) according to the manufacturer's instructions, with the following modifications: The swabs were vortexed in RTL buffer and then discarded. Cellular material was homogenized by centrifugation (QIAshredder; Qiagen). Contaminating DNA was removed by DNase I digestion. RNA was eluted with 50 μL of RNase-free water and stored at −80°C. Total RNA was reverse transcribed to cDNA (Omniscript RT kit; Qiagen), according to the manufacturer's instructions.
Quantitative Real-Time PCR for Human Gene Expression
The expression of human genes was measured by two-step, real-time RT-PCR (QuantiTect SYBR Green PCR kit; Qiagen). Each 25-μL reaction contained 2.5 μL of cDNA solution (produced by the RT reaction described above), 12.5 μL of SYBR green PCR master mix (Quantitect; Qiagen), 8 μL of water, and 1 μL (final concentration 0.25 μM) each of the forward and reverse primers for the target of interest. The samples were tested in duplicate. With each run of the assay, a calibration curve was generated from a series of standards of the known concentration of the target sequence. Assays were performed on a sequence-detection system (model 5700; Applied Biosystems, Foster City, CA; or DNA Engine Opticon-2; Bio-Rad Laboratories, Hercules, CA), with the same instrument used to quantify a specific target in all specimens. The thermal cycle protocol used the following conditions: 95°C for 15 minutes (denaturation and activation the Taq DNA polymerase), followed by 45 cycles of (1) denaturation at 94°C for 15 seconds, (2) annealing for 30 seconds at the optimal temperature for each primer pair, and (3) extension at 72°C for 30 seconds. Fluorescence data were acquired at the end of each cycle. A melting curve analysis was performed after the final amplification cycle for the assessment of specific and nonspecific PCR products before accepting the quantitative estimate. Standards were produced by a series of 10-fold dilutions of a solution containing a known concentration of target DNA, as described previously.16 Standards were aliquoted and stored at −20°C. All standard curves contained a sequence of five serial dilutions ranging from 10 to 100,000 copies/μL, except for TNFa which ranged from 1 to 10,000 copies/μL.
Gene Expression Targets
Primers (sequences are available on request or from http://medgen.ugent.be/rtprimerdb/ a public domain database sponsored by Roche, Bio-Rad, and Eurogentec) were designed to amplify segments of transcripts for the following targets: hypoxanthine phosphoribosyl transferase-1 (HPRT-1), tumor necrosis factor-α (TNF), interleukin-1β (IL1B), matrix metalloproteinase-1 (MMP-1), MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), and TIMP-2. With the exception of HPRT-1, all primers were designed using Primer 3 software (http://www.genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi)23 and used default conditions. For each gene (except HPRT-1) one of the primers included a boundary between two adjacent exons in the cDNA generated from spliced mRNA. This feature reduced the risk of amplification of any contaminating genomic DNA. Finally the specificity of the primers was checked for homology with other known sequences by a nucleotide Blast Search (BLASTn; www.ncbi.nlm.nih.gov/blast/ provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD).
Statistical Analysis
To adjust for variations in the amount of RNA collected, we standardized the results by dividing by the amount of HPRT-1, a human reference gene, expressed in the same sample, which gives a variable left-censored cutoff for those samples below the lowest concentration included in the standard curve. For some targets, several samples had expression levels below 10 copies/μL, the lowest concentration included in the standard curves. These concentrations were estimated by linear extrapolation of the standard curves to a level of 1 copy/μL, and any samples outside this range were not included in the analysis. Summary statistics were calculated by including extrapolated values. The extrapolated values were not used in the regression models, but were replaced by an appropriate interval (1–10) to allow for the uncertainty caused by extrapolation from the standards curve. The data were analyzed in Stata version 9 (Stata Corp., College Station, TX). The regressions were performed with the intreg STATA command, which allows continuous and interval censored data to be mixed. Summary statistics are reported on untransformed responses, but logarithmic transformations of gene expression are used to improve the assumptions of regression. A family-wise error rate is maintained at the standard level of 5% by Bonferroni correction, which gives a significance level of 0.0016 for the 32 univariate analyses. Multivariate analyses were used to quantify the simultaneous effect of predictors, and terms P < 0.05 were retained without correction for multiplicity.
Results
Study Participants
At baseline 451 individuals underwent trichiasis surgery (Fig. 1).2 In subjects with bilateral trichiasis, the eye with more severe preoperative trichiasis was included in the study. One year later, 426 (94%) of 451 were reassessed. At the 1-year time point, 240 consecutive individuals were recruited to this additional gene expression pathophysiology study. Their mean age was 58 years (range, 18–90) and the majority (71%) were women. Four years after the surgery, we reassessed 194 (81%) of the 240 subjects.
Figure 1.
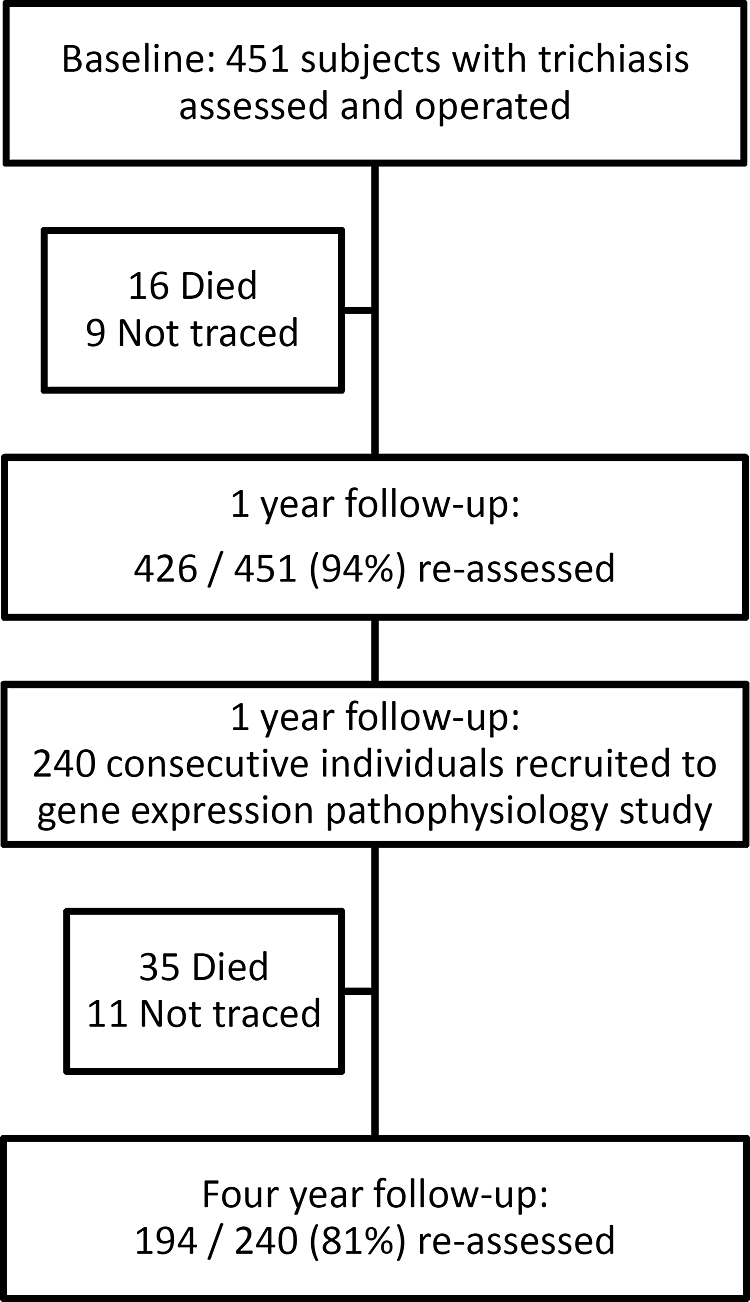
Patient flow chart.
Trichiasis Severity
The burden of the preoperative disease at baseline was relatively severe (Table 1): median of 7 lashes touching the eye (interquartile range: 5–17; total range: 0 [epilating]–74). Visually disabling central corneal opacification was present in 73 (30%) eyes. One year after surgery, 101 (42%) of the 240 individuals had recurrent trichiasis; however, the overall burden of trichiasis was much reduced (median: 0 lashes; interquartile range, 0–4). Among those with recurrent trichiasis, the 1-year trichiasis burden was much less (median: five lashes; interquartile range: 1–9; total range 0 [epilating]–47) compared with that before surgery in these individuals (median: 12 lashes; interquartile range: 5–25, total range 0 [epilating]–74). At 1 year, tarsal conjunctival inflammation (P2/P3) was observed in 91 (38%) of 240 study eyes. Of the 194 individuals examined at both 1 and 4 years after surgery, 117 did not have recurrent trichiasis at 1 year. By 4 years, 24 (20%) of these 117 people had incident recurrent trichiasis.
Table 1.
Trichiasis Severity before and 1 Year after Surgery
| Trichiasis Severity* | Before Surgery | 1 Year after Surgery |
|---|---|---|
| 0 (no trichiasis) | — — | 139 (57.9) |
| 0 (epilating) | 29 (12.1) | 18 (7.5) |
| 1–4 | 30 (12.5) | 35 (14.6) |
| 5–9 | 79 (32.9) | 24 (10.0) |
| 10–19 | 50 (20.8) | 9 (3.7) |
| 20+ | 52 (21.7) | 15 (6.3) |
Data are the number of subjects (% of the total group).
Number of lashes touching the globe.
Ocular Infection
At 1 year after surgery, 239 specimens were available for nonchlamydial bacterial culture. Nonchlamydial bacterial pathogens were isolated from 33 (13.8%) of 239 specimens. Ten specimens had two bacterial species isolated and two specimens had three species isolated (Table 2). Streptococcus pneumoniae was the commonest organism grown (Table 2). C. trachomatis was detected (Amplicor CT/NG; Ambion) by PCR in 3 (1%) of 232 subjects tested, none of which were co-infected with other bacterial pathogens.
Table 2.
Conjunctival Bacterial Culture Isolates 1 Year after Surgery
| Organism | All Organisms* |
Number of Organisms Cultured† |
|||
|---|---|---|---|---|---|
| n | (%) | 1 | 2 | 3 | |
| Streptococcus pneumoniae | 14 | (31) | 10 | 4 | – |
| Viridans group streptococci | 1 | (2) | 1 | – | – |
| Streptococcus Spp | 4 | (9) | 1 | 1 | 2 |
| Streptococcus Group A | 1 | (2) | 1 | – | – |
| Streptococcus Group C | 4 | (9) | 1 | 3 | – |
| Staphylococcus aureus | 6 | (13) | 3 | 2 | 1 |
| Moraxella | 4 | (9) | 3 | 1 | – |
| Haemophilus influenzae | 6 | (13) | 2 | 3 | 1 |
| Coliform spp | 2 | (4) | – | 1 | 1 |
| Pseudomonas aeruginosa | 1 | (2) | – | – | 1 |
| Klebsiella | 1 | (2) | – | 1 | – |
| Serratia | 1 | (2) | 1 | – | – |
Conjunctival bacterial culture results were available for 239 eyes. Forty-five separate bacterial isolates were grown from 33 culture positive swab samples.
Of the 33 eyes with organisms cultured, 23 had a single organism, 8 had two organisms and 2 had three organisms.
Conjunctival Gene Expression
The expression of mRNA for proinflammatory cytokines and tissue remodeling factors was quantitated in all 240 samples. Each target was detected in all specimens, with the exception of a single sample in which MMP1 expression was undetectable. All values are presented as a ratio to the expression of a reference gene, HPRT-1. The distributions of most targets were positively skewed; therefore, the ratios were transformed (log10) for statistical testing. Table 3 shows the number of samples in which values for the mRNA estimates were below the range of the standard curves. Four targets (lL1B, MMP-1, MMP-2, and MMP-9) had samples estimated to have <10 copies/μL. Those samples with results below 1 copy/μL were excluded from the analysis.
Table 3.
The Number of Subjects with Extrapolated Values by Gene of Interest
| Gene | None Detected* | Extrapolated copy numbers |
Total Included | |
|---|---|---|---|---|
| <1 Copy/μL* | 1–10 Copies/μL† | |||
| HPRT-1 | — | — | — | 240 |
| TNF | — | — | — | 240 |
| IL1B | — | (1) | 31 | 239 |
| MMP-1 | (1) | — | 7 | 239 |
| MMP-2 | — | (20) | 114 | 220 |
| MMP-9 | — | (3) | 30 | 237 |
| TIMP-1 | — | — | — | 240 |
| TIMP-2 | — | — | — | 240 |
Samples that appear in parentheses (<1 copy/μL) were excluded from the analysis.
Samples between 1 and 10 copies/μL were handled by interval analysis.
Univariate analyses compared the expression level of each gene for the presence of recurrent trichiasis at 1 year, nonchlamydial bacterial infection, and conjunctival inflammation. Each comparison was adjusted for the likely confounding factors of age, sex, and ethnicity. These factors are generally held to be potential confounders in trachoma, as they are major determinants in the exposure and response to C. trachomatis infection. Recurrent trichiasis 1 year after surgery was associated with a reduced MMP-1/TIMP-1 ratio (P = 0.0087), but the decrease was not significant after adjustment for multiplicity (Table 4). The development of recurrent trichiasis between the 1- and 4-year assessments was not associated with any statistically significant changes in the levels of mRNA transcripts measured at 1 year after surgery (data not presented). Significant univariate associations (P ≤ 0.0016) were found between the detection of nonchlamydial bacterial infection and increased expression of IL1B and MMP-9 (Table 5). Conjunctival inflammation (P2/P3) was associated with increased expression of IL1B and MMP-9 (Table 6). For the purpose of analysis, ethnicity was subdivided into four groups: Mandinka, Wolof, Fula, and Other. Table 7 is a comparison of gene expression levels between the four ethnic groups. The P-value is an omnibus test for differences between the four ethnic groups adjusted for age and sex. There were significant differences in expression levels between ethnic group for TNF, TIMP-1, and TIMP-2. The value of TNF expression was appreciably higher in the Mandinka ethnic group than in the Wolof group.
Table 4.
Conjunctival Gene Expression Levels 1 Year after Surgery by Recurrent Trichiasis Status*
| Gene | No Recurrent Trichiasis (n = 139) |
P‡ | Recurrent Trichiasis (n = 101) |
||||
|---|---|---|---|---|---|---|---|
| Mean† | 95% CI | Median | Mean† | 95% CI | Median | ||
| TNF | 1.29 | 0.99–1.58 | 0.71 | 0.67 | 2.28 | 0.73–3.84 | 0.62 |
| IL1B | 0.60 | 0.29–0.90 | 0.15 | 0.60 | 1.06 | 0.21–1.90 | 0.12 |
| MMP-1 | 3.15 | 1.56–4.73 | 0.42 | 0.069 | 1.41 | 0.58–2.25 | 0.34 |
| MMP-2 | 0.08 | 0.00–0.17 | 0.02 | 0.24 | 0.05 | 0.03–0.07 | 0.02 |
| MMP-9 | 0.32 | 0.19–0.46 | 0.15 | 0.061 | 0.35 | 0.27–0.44 | 0.19 |
| TIMP-1 | 14.99 | 11.2–18.8 | 6.08 | 0.98 | 14.79 | 9.70–19.9 | 6.78 |
| TIMP-2 | 5.10 | 4.44–5.76 | 4.14 | 0.79 | 5.01 | 4.27–5.74 | 3.68 |
| MMP-1/TIMP-1 | 0.14 | 0.11–0.18 | 0.06 | 0.0087 | 0.08 | 0.07–0.11 | 0.05 |
Conjunctival gene expression level of the gene of interest is expressed as a ratio to the HPRT-1 expression level in the same sample.
Arithmetic mean of untransformed response.
Interval regression, with P-values adjusted for age, sex, and ethnicity.
Table 5.
Conjunctival Gene Expression Levels 1 Year after Surgery by Bacterial Infection Status*
| Gene | Uninfected (n = 206) |
P‡ | Bacterial Infection (n = 33) |
||||
|---|---|---|---|---|---|---|---|
| Mean† | 95% CI | Median | Mean† | 95% CI | Median | ||
| TNF | 1.58 | 0.82–2.34 | 0.60 | 0.0028 | 2.54 | 1.29–3.80 | 0.77 |
| IL1B | 0.77 | 0.32–1.22 | 0.12 | 0.0002 | 0.96 | 0.43–1.49 | 0.34 |
| MMP-1 | 2.72 | 1.58–3.86 | 0.41 | 0.0087 | 0.62 | 0.15–1.08 | 0.19 |
| MMP-2 | 0.07 | 0.02–0.12 | 0.02 | 0.37 | 0.05 | 0.03–0.06 | 0.04 |
| MMP-9 | 0.23 | 0.19–0.27 | 0.15 | <0.0001 | 0.99 | 0.45–1.53 | 0.65 |
| TIMP-1 | 15.89 | 12.4–19.4 | 6.81 | 0.084 | 9.11 | 5.53–12.7 | 5.23 |
| TIMP-2 | 4.96 | 4.46–5.46 | 3.91 | 0.83 | 5.70 | 3.93–7.47 | 4.88 |
| MMP-1/TIMP-1 | 0.10 | 0.10–0.15 | 0.07 | 0.029 | 0.07 | 0.04–0.11 | 0.04 |
Conjunctival gene expression level of the gene of interest is expressed as a ratio to the HPRT-1 expression level in the same sample.
Arithmetic mean of untransformed response.
Interval regression, with P-values adjusted for age, sex, and ethnicity.
Table 6.
Conjunctival Gene Expression Levels* 1 Year after Surgery by Tarsal Conjunctival Inflammation Status
| Gene | Noninflamed (n = 149) |
P‡ | Inflamed (n = 91) |
||||
|---|---|---|---|---|---|---|---|
| Mean† | 95% CI | Median | Mean† | 95% CI | Median | ||
| TNF | 1.66 | 0.65–2.66 | 0.58 | 0.42 | 1.79 | 1.11–2.47 | 0.83 |
| IL1B | 0.39 | 0.15–0.64 | 0.11 | 0.0006 | 1.44 | 0.49–2.40 | 0.20 |
| MMP-1 | 3.08 | 1.56–4.60 | 0.38 | 0.27 | 1.33 | 0.61–2.05 | 0.37 |
| MMP-2 | 0.03 | 0.02–0.04 | 0.02 | 0.035 | 0.12 | 0.00–0.24 | 0.02 |
| MMP-9 | 0.21 | 0.17–0.25 | 0.13 | <0.0001 | 0.54 | 0.33–0.75 | 0.22 |
| TIMP-1 | 16.54 | 12.7–20.4 | 7.05 | 0.16 | 12.21 | 7.13–17.3 | 5.58 |
| TIMP-2 | 5.27 | 4.65–5.90 | 4.31 | 0.32 | 4.71 | 3.91–5.50 | 3.32 |
| MMP-1/TIMP-1 | 0.13 | 0.10–0.16 | 0.06 | 0.78 | 0.10 | 0.08–0.13 | 0.06 |
Conjunctival gene expression level of the gene of interest is expressed as a ratio to the HPRT-1 expression level in the same sample.
Arithmetic mean of untransformed response.
Interval regression, with P-values adjusted for age, sex, and ethnicity.
Table 7.
Conjunctival Gene Expression Levels by Ethnic Group, 1 Year after Surgery*
| Gene | Mandinka (n = 139) |
Wolof (n = 57) |
Fula (n = 18) |
Other (n = 26) |
P‡ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean† | 95% CI | Mean† | 95% CI | Mean† | 95% CI | Mean† | 95% CI | ||
| TNF | 2.24 | 1.09–3.39 | 0.65 | 0.39–0.90 | 1.47 | 0.79–2.15 | 1.32 | 0.75–1.90 | <0.0001 |
| IL1B | 0.57 | 0.29–1.02 | 0.76 | 0.06–1.45 | 2.41 | 1.88–6.71 | 0.93 | 0.24–2.10 | 0.38 |
| MMP-1 | 3.16 | 1.55–4.78 | 0.96 | 0.52–1.45 | 1.43 | 0.17–2.69 | 2.30 | 0.35–4.96 | 0.23 |
| MMP-2 | 0.04 | 0.03–0.05 | 0.13 | 0.06–0.32 | 0.05 | 0.03–0.14 | 0.07 | 0.00–0.13 | 0.049 |
| MMP-9 | 0.38 | 0.24–0.52 | 0.24 | 0.16–0.32 | 0.33 | 0.12–0.53 | 0.32 | 0.15–0.49 | 0.37 |
| TIMP-1 | 17.78 | 14.2–21.4 | 7.51 | 4.80–10.2 | 17.54 | 7.45–42.5 | 13.92 | 2.16–25.7 | <0.0001 |
| TIMP-2 | 5.73 | 5.04–6.42 | 3.68 | 2.92–4.43 | 4.40 | 2.50–6.29 | 4.95 | 3.51–6.39 | 0.0001 |
| MMP-1/TIMP-1 | 0.11 | 0.09–0.14 | 0.14 | 0.10–0.18 | 0.16 | 0.03–0.28 | 0.10 | 0.05–0.14 | 0.42 |
Conjunctival gene expression level of the gene of interest is expressed as a ratio to the HPRT-1 expression level in the same sample.
Arithmetic mean of untransformed response.
Interval regression, with P-values adjusted for age, sex, and ethnicity.
Multivariate models for the expression level of the different mRNA transcripts were derived (Table 8). These simultaneously included confounding factors (age, sex, and ethnicity) and three other factors: recurrent trichiasis at 1 year, bacterial infection, and tarsal conjunctival inflammation. As interactions between these factors were considered to be biologically plausible, they were also examined. Terms were retained at the 5% level. In addition, if there was a significant interaction, the individual component terms were also retained. The most parsimonious models are presented.
Table 8.
Multivariate Models for the Expression of Each Gene of Interest 1 Year after Surgery*
| Gene | Factor | P |
|---|---|---|
| TNF | Ethnicity | <0.0001 |
| Inflammation | 0.25 | |
| Bacterial infection | 0.52 | |
| Inflammation×bacterial infection | 0.012 | |
| IL1B | Inflammation | 0.002 |
| Bacterial infection | 0.011 | |
| MMP-1 | Bacterial infection | 0.018 |
| MMP-2 | No significant model | — |
| MMP-9 | Inflammation | 0.062 |
| Bacterial infection | 0.96 | |
| Inflammation×bacterial infection | 0.007 | |
| TIMP-1 | Ethnicity | <0.0001 |
| Age | 0.031 | |
| TIMP-2 | Ethnicity | 0.0003 |
| Inflammation | 0.011 | |
| Bacterial infection | 0.009 | |
| Inflammation×bacterial infection | 0.001 | |
| MMP-1/TIMP-1 | Recurrent trichiasis | 0.029 |
| Age | 0.035 | |
| Bacterial infection | 0.048 |
The most parsimonious models are presented and were derived by testing for interactions between recurrent trichiasis, infection, and inflammation, retaining terms at the 5% level. Interaction terms in italics. P-values were not adjusted for multiplicity and main effects were retained if the interaction was significant.
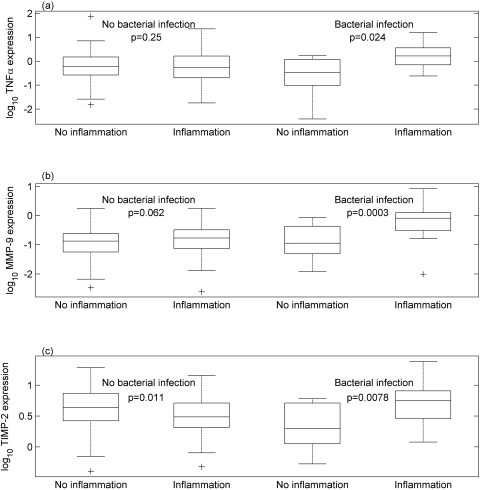
Adjusting for the effects of age and bacterial infection, recurrent trichiasis was associated with a reduced ratio of MMP-1 to TIMP-1 (P = 0.029; Table 8). TNF expression was increased in the Mandinkas and there was an interaction between inflammation and bacterial infection; TNF expression was increased in clinically inflamed conjunctiva when it was associated with bacterial infection (Table 8, Fig. 2a). The expression of IL1B was independently associated with inflammation and bacterial infection (Table 8). Unlike TNF, conjunctival inflammation in the absence of bacterial infection was also associated with increased IL1B. The expression of MMP-1 was significantly reduced in the presence of nonchlamydial bacterial infection. MMP-9 expression was increased when bacterial infection and inflammation were present simultaneously (Table 8, Fig. 2b). Both TIMP-1 and -2 were expressed at higher levels in the Mandinkas. TIMP-2 expression was increased with both inflammation and bacterial infection, and there was an interaction between these two factors (Fig. 2c).
Figure 2.
Augmentation of the gene expression response* to bacterial infection in the presence of conjunctival inflammation† for (a) TNF, (b) MMP-9, and (c) TIMP-2. *Conjunctival expression level of the gene of interest is expressed as a ratio to the HPRT-1 expression level in the same sample. †No inflammation versus inflammation for each bacterial infection group, adjusted for ethnicity for TNF and TIMP-2. Interaction P-values for inflammation and bacterial infection are given in Table 8.
Discussion
This is the first study to explore immunopathogenic factors that may be involved in the recurrence of TT after tarsal rotation surgery. We measured the gene expression levels of proinflammatory cytokines (TNF-α and IL-1β) and downstream factors involved in the regulation of the extracellular matrix (MMP-1, -2, and -9 and TIMP-1 and -2). One year after surgery, we found that recurrent trichiasis was associated with a reduction in the ratio of MMP-1 to TIMP-1 expression (P = 0.0087), although because of the large number of tests the significance was above the Bonferroni corrected P ≤ 0.0016 required to maintain a family-wise error rate of 5%. No factor measured at 1 year predicted the development of new recurrence between 1 and 4 years after surgery.
The MMPs are a family of proteolytic enzymes involved in the regulation of the ECM during development, normal tissue turnover, wound-healing, and disease. Moreover, the dysregulation of MMPs is known to be important in a wide variety of disease processes, including several affecting the surface of the eye.24 Their function is tightly regulated at several levels: transcription, posttranscriptional activation, and inhibition by specific TIMPs. Therefore, it is also important to consider the relative expression levels of an MMP to its corresponding TIMP, as it may reflect the overall functional activity of the enzyme. MMP-1 is a proteolytic enzyme responsible for the degradation of type 1 collagen. A reduction in the ratio of MMP-1/TIMP-1 expression would shift the balance away from the breakdown of type 1 collagen, favoring its accumulation in developing scar tissue. It has been shown in vitro that transforming growth factor (TGF)-β, a key cytokine in many scarring processes, induces the expression of TIMP-1 and suppresses the expression of MMP-1, favoring ECM anabolism.25,26 The regulation of TGF-β is complex, occurring mainly at the posttranscriptional level; therefore, gene expression analysis of TGF-β itself would not necessarily reflect its activity. However, the shift in the balance between MMP-1 and TIMP-1 may be a downstream effect of increased TGF-β activity in people with recurrent trichiasis.
Chronic inflammation is important in the pathophysiology of many scarring diseases. The epidemiology of trachoma suggests that recurrent severe conjunctival inflammation in childhood is a major risk factor for subsequent scarring and trichiasis.11,12 Tarsal conjunctival inflammation has also been associated with trichiasis and its recurrence after surgery in several studies.2,6,8 This raises the question of what contribution conjunctival inflammation makes to the development of recurrent trichiasis after surgery and what processes are activated at the cellular and molecular levels. One year after surgery, we found increased expression of IL1B associated with both clinical inflammation and conjunctival bacterial infection. There are 10 members of the IL-1 family; the dominant forms are IL-1α (the intracellular and surface form) and IL-1β (the secreted form). Previously, we have found IL1B expressed at higher levels in individuals (mostly children) with clinically active trachoma.16,27 Immunohistochemical staining of conjunctival specimens from children with active trachoma has demonstrated that both IL-1α and -1β are found in conjunctival epithelium and macrophages within the substantia propria.28 IL-1β has also been found at higher concentrations in tear fluid from persons with trichiasis and inflammation, compared with control subjects.29 In vitro models have demonstrated increased production of IL-1α and -1β in response to C. trachomatis infection, leading to the activation of an IL-1-dependent inflammatory cascade.30,31 Infection with other bacteria also stimulates the production of IL-1, consistent with our finding. This evidence points to IL-1β as a key inflammatory mediator in trachoma, involved in the promotion of chronic inflammation that leads to progressive scarring and also in the activation of factors, such as MMPs, that are involved in tissue remodeling.
The expression of TNF was characterized by an interaction between bacterial infection and clinically apparent conjunctival inflammation. Individuals with both bacterial infection and conjunctival inflammation had increased TNF expression. However, in the absence of bacterial infection, TNF expression was not increased, even if the conjunctiva appeared inflamed clinically. Several earlier studies have investigated the role of TNFα in trachoma. Younger individuals with clinically active trachoma (TF/TI) have increased expression of TNF in their conjunctiva.16,27 Immunohistochemical staining of conjunctival biopsies from children with active trachoma found the main source of TNFα to be macrophages within the substantia propria.28 TNFα was also more frequently detected and at higher concentration in tear fluid from people with conjunctival scarring than in the controls.29,32 We found that samples from the Mandinka subjects contained higher levels of TNF mRNA as part of their proinflammatory response. In two case–control studies from The Gambia, researchers found that a single nucleotide polymorphism (SNP) in the TNF promoter (TNF-308A) was linked to an increased risk of scarring complications (TNF2 allele) and was associated with elevated levels of TNFα in tears or increased production in vitro after stimulation.32,33 The same polymorphism has been associated with adverse outcomes in several infectious diseases, including malaria.34 In a human cell culture system, the TNF2 promoter was associated with a marked increase in transcription.35 Of interest, this risk allele (TNF2) is found more frequently in the Mandinka population in West Africa.32 Epidemiologic studies from The Gambia found that progression from mild conjunctival scarring to more advanced entropion and trichiasis occurred more rapidly in the Mandinka population than in other ethnic groups.36 Overall, these findings suggest a potential role for TNFα in pathways leading to trachomatous scarring, as a key cytokine in the inflammatory response leading to tissue damage and scarring.
We found the expression of MMP-9 was increased when tarsal conjunctival inflammation was associated with bacterial infection. Other studies have investigated MMP-9 in trachoma. In children with clinically active trachoma we have found increased MMP-9 expression with increasing severity of conjunctival inflammation.16 Immunohistochemistry indicates that the main source of MMP-9 in the conjunctiva in active trachoma are macrophages.37 There is evidence that genetically inherited variations in MMP-9 may contribute to differences in disease outcome.38 An SNP in MMP-9, which leads to a structural change adjacent to the fibronectin binding domain (FN2), was associated with a reduced risk of scarring sequelae, particularly trichiasis, in heterozygotes. MMP-9 works on a diverse group of substrates: it degrades ECM components (type IV and V collagens, gelatin, fibronectin, and elastin) and activates several cytokines involved with inflammation and fibrosis (TNF, IL-1β, and TGF-β).39 Of interest, MMP-9 production is induced by several cytokines, including TNF, IL-1β, and TGF-β. This raises the possibility that an inflammatory profibrotic state could be perpetuated through the proteolytic activation of these cytokines and MMP-9, in a positive autofeedback loop. Lipopolysaccharide (LPS) and other bacterial antigens are important inducers of MMP-9 expression by macrophages and other inflammatory cells.39 This effect may explain the marked increase in MMP-9 expression when both bacterial infection and inflammation are present. Overall, the current evidence suggests that MMP-9 is important in the pathogenesis of trachoma.
Conjunctival bacterial infection is frequently associated with trichiasis, becoming more common as the severity of trichiasis increases.2,15 Tarsal conjunctival inflammation is often found in association with such infections. In contrast, C. trachomatis has been infrequently detected in such cases. These associated findings led to the suggestion that infection by other bacterial species, particularly during the cicatricial stages of trachoma, may also cause chronic inflammation, contributing to progressive scarring.14 In this study, we found some limited evidence supporting this hypothesis. Bacterial infection was associated with increased expression of TNF, IL1B, MMP-1, and MMP-9. In the case of TNF and MMP-9, there was an interaction between infection and inflammation; only when both occurred together was the expression increased.
In summary, we found that recurrent trichiasis 1 year after surgery was associated with a reduced ratio of the proteolytic enzyme MMP-1 to its inhibitor TIMP-1. We suggest that this altered balance may be driven by TGF-β and favor the accumulation of collagen in scar tissue which could contribute to recurrent trichiasis through progressive cicatrization. In addition, we found evidence that nonchlamydial bacterial infection is associated with increased expression of factors that may be important in progressive conjunctival scarring. Prospective studies with gene expression analysis at several time points are needed to more fully understand the key factors that drive progressive conjunctival scarring in trachoma, both before and after surgery. It is conceivable that such studies may identify factors in the pathogenic pathway of progressive scarring that are amenable to pharmacologic inhibition. Such therapy would have parallels with the antifibrotic treatment used in other forms of ophthalmic surgery, such as glaucoma filtration surgery.24 In cases of trichiasis at high risk of recurrence (repeat surgery, severe conjunctival inflammation, and severe entropion/trichiasis), the inhibition of scarring after trichiasis surgery may reduce rates of recurrence and, ultimately, avoidable blindness.
Acknowledgments
The authors thank the ophthalmic nurses of the Gambian National Eye Care Programme and the field staff from the Medical Research Council Laboratories for their hard work, often under challenging conditions.
Footnotes
Supported by Grant 01-030 from the International Trachoma Initiative with additional support from the Wellcome Trust/Burroughs Wellcome Fund (059134).
Disclosure: M.J. Burton, None; R.L. Bailey, None; D. Jeffries, None; S.N. Rajak, None; R.A. Adegbola, None; A. Sillah, None; D.C.W. Mabey, None; M.J. Holland, None
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:844–851 [PMC free article] [PubMed] [Google Scholar]
- 2.Burton MJ, Kinteh F, Jallow O, et al. A randomised controlled trial of azithromycin following surgery for trachomatous trichiasis in the Gambia. Br J Ophthalmol 2005;89:1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Report of the 2nd global scientific meeting on trachoma. Geneva: World Health Organization; WHO/PBD/GET 03.1 [Google Scholar]
- 4.Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol 2009;93(5):563–568 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Future Approaches to Trachoma Control. Report of a Global Scientific Meeting Geneva, Switzerland; June 17–20, 1996; WHO//PBL/96.56 [Google Scholar]
- 6.Burton MJ, Bowman RJ, Faal H, et al. Long term outcome of trichiasis surgery in the Gambia. Br J Ophthalmol 2005;89:575–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandekar R, Mohammed AJ, Courtright P. Recurrence of trichiasis: a long-term follow-up study in the Sultanate of Oman. Ophthalmic Epidemiol 2001;8:155–161 [DOI] [PubMed] [Google Scholar]
- 8.West ES, Mkocha H, Munoz B, et al. Risk factors for postsurgical trichiasis recurrence in a trachoma-endemic area. Invest Ophthalmol Vis Sci 2005;46:447–453 [DOI] [PubMed] [Google Scholar]
- 9.West SK, West ES, Alemayehu W, et al. Single-dose azithromycin prevents trichiasis recurrence following surgery: randomized trial in Ethiopia. Arch Ophthalmol 2006;124:309–314 [DOI] [PubMed] [Google Scholar]
- 10.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004;4:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson CR, Marx R, Daghfous T, et al. In: Bowie WR. ed. Chlamydial Infections Cambridge: Cambridge University Press; 1990:271–278 [Google Scholar]
- 12.West SK, Munoz B, Mkocha H, et al. Progression of active trachoma to scarring in a cohort of Tanzanian children. Ophthalmic Epidemiol 2001;8:137–144 [DOI] [PubMed] [Google Scholar]
- 13.Wolle MA, Munoz BE, Mkocha H, et al. Constant ocular infection with Chlamydia trachomatis predicts risk of scarring in children in Tanzania. Ophthalmology 2009;116:243–247 [DOI] [PubMed] [Google Scholar]
- 14.Burton MJ, Bowman RJ, Faal H, et al. The long-term natural history of trachomatous trichiasis in the Gambia. Invest Ophthalmol Vis Sci 2006;47:847–852 [DOI] [PubMed] [Google Scholar]
- 15.Burton MJ, Adegbola RA, Kinteh F, et al. Bacterial infection and trachoma in the Gambia: a case control study. Invest Ophthalmol Vis Sci 2007;48:4440–4444 [DOI] [PubMed] [Google Scholar]
- 16.Burton MJ, Bailey RL, Jeffries D, et al. Cytokine and fibrogenic gene expression in the conjunctivas of subjects from a Gambian community where trachoma is endemic. Infect Immun 2004;72:7352–7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanborg RH, Boros DL, Whittum-Hudson JA, et al. Molecular mimicry and horror autotoxicus: do chlamydial infections elicit autoimmunity? Expert Rev Mol Med 2006;8:1–23 [DOI] [PubMed] [Google Scholar]
- 18.McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoSMed 2006;3:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reacher MH, Taylor HR. The management of trachomatous trichiasis. Rev Int Trach Pathol Ocul Trop Subtrop Sante Publique 1990;67:233–262 [PubMed] [Google Scholar]
- 20.Dawson CR, Jones BR, Tarizzo ML. Guide to Trachoma Control Geneva: World Health Organization; 1981 [Google Scholar]
- 21.Thylefors B, Dawson CR, Jones BR, et al. A simple system for the assessment of trachoma and its complications. Bull World Health Organ 1987;65:477–483 [PMC free article] [PubMed] [Google Scholar]
- 22.Burton MJ, Holland MJ, Faal N, et al. Which members of a community need antibiotics to control trachoma?—conjunctival Chlamydia trachomatis infection load in Gambian villages. Invest Ophthalmol Vis Sci 2003;44:4215–4222 [DOI] [PubMed] [Google Scholar]
- 23.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S. eds. Bioinformatics Methods and Protocols: Methods in Molecular Biology Totowa, NJ: Humana Press; 2000:365–386 [DOI] [PubMed] [Google Scholar]
- 24.Wong TT, Sethi C, Daniels JT, et al. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol 2002;47:239–256 [DOI] [PubMed] [Google Scholar]
- 25.Edwards DR, Murphy G, Reynolds JJ, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J 1987;6:1899–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall MC, Young DA, Waters JG, et al. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. J Biol Chem 2003;278:10304–10313 [DOI] [PubMed] [Google Scholar]
- 27.Faal N, Bailey RL, Sarr I, et al. Temporal cytokine gene expression patterns in subjects with trachoma identify distinct conjunctival responses associated with infection. Clin Exp Immunol 2005;142:347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.bu El-Asrar AM, Geboes K, Tabbara KF, et al. Immunopathogenesis of conjunctival scarring in trachoma. Eye 1998;12:453–460 [DOI] [PubMed] [Google Scholar]
- 29.Skwor TA, Atik B, Kandel RP, et al. Role of secreted conjunctival mucosal cytokine and chemokine proteins in different stages of trachomatous disease. PLoS Negl Trop Dis 2008;2:e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothermel CD, Schachter J, Lavrich P, et al. Chlamydia trachomatis-induced production of interleukin-1 by human monocytes. Infect Immun 1989;57:2705–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen SJ, Eckmann L, Quayle AJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest 1997;99:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conway DJ, Holland MJ, Bailey RL, et al. Scarring trachoma is associated with polymorphism in the tumor necrosis factor alpha (TNF-alpha) gene promoter and with elevated TNF-alpha levels in tear fluid. Infect Immun 1997;65:1003–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natividad A, Hanchard N, Holland MJ, et al. Genetic variation at the TNF locus and the risk of severe sequelae of ocular Chlamydia trachomatis infection in Gambians. Genes Immun 2007;8:288–295 [DOI] [PubMed] [Google Scholar]
- 34.McGuire W, Hill AV, Allsopp CE, et al. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature 1994;371:508–510 [DOI] [PubMed] [Google Scholar]
- 35.Wilson AG, Symons JA, McDowell TL, et al. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA 1997;94:3195–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowman RJ, Jatta B, Cham B, et al. Natural history of trachomatous scarring in The Gambia: results of a 12-year longitudinal follow-up. Ophthalmology 2001;108:2219–2224 [DOI] [PubMed] [Google Scholar]
- 37.El-Asrar AM, Geboes K, Al-Kharashi SA, et al. Expression of gelatinase B in trachomatous conjunctivitis. Br J Ophthalmol 2000;84:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natividad A, Cooke G, Holland MJ, et al. A coding polymorphism in matrix metalloproteinase 9 reduces risk of scarring sequelae of ocular Chlamydia trachomatis infection. BMC Med Genet 2006;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van den Steen PE, Dubois B, Nelissen I, et al. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) (review). Crit Rev Biochem Mol Biol 2002;37:375–536 [DOI] [PubMed] [Google Scholar]