The authors explored the role of IL-12p35 and p40 in the progression of herpes stromal keratitis (HSK) and identified a previously unrecognized IL-12p40–independent, proinflammatory function of IL-12p35 in late HSK progression.
Abstract
Purpose.
Interleukin (IL)-12p40 can couple with IL-12p35 or p19 chains to form the molecules IL-12p70 and IL-23, respectively, which promote TH1 cytokine responses. IL-12p35 can bind to EBI3 to form the anti-inflammatory molecule IL-35, but a proinflammatory function of IL-12p35 independent of IL-12p40 has not been described. Here such a function in a mouse model of herpes stromal keratitis (HSK), a CD4+ TH1 cell–dependent corneal inflammation, is demonstrated.
Methods.
Corneas of wild-type (WT), IL-12p40−/−, IL-12p35−/−, and IL-12p35−/−p40−/− (double knockout) mice were infected with the RE strain of HSV-1, and HSK was monitored based on corneal opacity, neovascularization, leukocytic infiltrate, and cytokine/chemokine levels.
Results.
All mouse strains developed moderate HSK by 11 days after infection (dpi). However, from 11 to 21 dpi, HSK progressed in WT and IL-12p40−/− mice but regressed in IL-12p35−/− and IL-12p35−/−p40−/− mice. HSK regression was characterized by reductions in neutrophils and CD4+ T cells and attenuation of blood vessels, which was associated with reduced levels of the chemokines KC (CXCL3), Mip-2 (CXCL2), and MCP-1 (CCL2) and the angiogenic factor vascular endothelial growth factor.
Conclusions.
HSK development does not require IL-12p40 and is thus independent of IL-12p70 and IL-23. However, late HSK progression does require a previously unrecognized IL-12p40–independent, proinflammatory function of IL-12p35.
The IL-12 cytokine family, consisting of the heterodimers IL-12, -23, -27, and -35, has received increased attention because of its diverse and complex functions in immunity. IL-12 consists of a p40 and a p35 subunit1 and stimulates the differentiation and activation of naive CD4+ T cells toward a TH1 phenotype, promoting IFN-γ production.2 The role of IL-12 in disease has been confounded by the discovery of IL-23, which consists of the same p40 subunit coupled to a unique p19 subunit.3 IL-23 promotes both the proliferation of effector/memory TH1 cells and the maintenance of TH17 cells whose signature cytokine is IL-17.4 Interestingly, homodimerization of p40 yields a unique molecule capable of anti-inflammatory function through blockade of the IL-12Rβ15,6 but also of proinflammatory function as a chemoattractant for dendritic cells and macrophages.7,8 Muddying the waters further, p35 can also interact with a second binding partner, Epstein-Barr virus–induced gene-3 (EBI3), forming the inhibitory cytokine IL-35.9,10 IL-35 promotes the proliferation of and IL-10 production by CD4+CD25+ FoxP3+ natural Tregs, inhibits the proliferation of CD4+CD25− effector cells, and inhibits the differentiation of TH17 cells. Thus, IL-35 is considered an anti-inflammatory cytokine. The final current member of the family, IL-27, consists of EBI3 and p28 (IL-30) and enhances TH1 polarization of naive CD4 T cells.11
Herpes stromal keratitis (HSK) is a potentially blinding HSV-1–induced immunopathologic disease of the cornea. Previous work with athymic, SCID, and T cell–depleted mice demonstrated that CD4+ T cells are essential for HSK initiation and progression.12–15 CD4+ T cells infiltrating diseased corneas produce the TH1 cytokine IFN-γ, which regulates HSK.16,17 As is often the case, this predominantly TH1 response is associated with concurrent production of the anti-inflammatory molecule IL-10.18,19 The latter counteracts the proinflammatory effects of TH1 cytokines, and its overexpression in the cornea can ameliorate HSK.20 The TH2 cytokine IL-4 is either not detected in corneas with HSK or is detected during the late recovery stage.18,21 The TH17 cytokine IL-17 has been implicated in HSK in mice and humans and was shown to induce corneal fibroblast production of chemokines that are important regulators of HSK.22,23
The role of the primary TH1-driving cytokine IL-12 in HSK has been investigated previously with conflicting results. IL-12p40 mRNA and protein increases in response to HSV-1 corneal infection,24 and the protein is released by inflammatory cells rather than by infected epithelial cells.25 However, during the period of HSK development (7–22 days postinfection [dpi]), IL-12p40 mRNA levels decrease in the cornea, and, to our knowledge, IL-12p70 protein levels have not been measured. Transgenic expression of IL-12p35/p40 fusion protein under the glial fibrillary acidic protein promoter (expressed by nerve tissue) after ocular infection with the highly neurovirulent HSV-1 strain McKrae resulted in reduced viral titers in eyes and trigeminal ganglia and increased survival in mice.26 However, another study in which the corneas of IL-12p35−/− and IL-12p40−/− mice were infected with HSV-1 McKrae found no difference in corneal viral load reduced HSK severity among IL-12p35−/− mice, and no HSK among IL-12p40−/− mice that had survived lethal infection at 28 dpi.27 The use of the highly neurovirulent McKrae strain of HSV-1, coupled with the study of HSK at a single time point only in animals that had survived lethal infection, limits the translation of these results to human infection.
IL-23 has also recently been studied in the context of HSK. Mice deficient in p19 developed more severe lesions at a higher incidence than their wild-type (WT) counterparts.28 This study concluded that the lack of IL-23 resulted in a drastically increased IL-12-driven TH1 CD4+ T cell response, though no direct evidence implicating IL-12 in the enhanced HSK was provided.
Armed with recent advances in the study of the IL-12 cytokine family, we set out to elucidate the role of these cytokines in HSK using mice deficient in IL-12p35 or IL-12p40 or double knockouts deficient in both p35 and p40 subunits. Mice received corneal infections with the RE strain of HSV-1, which does not kill Balb/c mice. However, the infectious dose used in these studies induced epithelial corneal lesions and latent infections in the trigeminal ganglion of 100% of mice and HSK in at least 80% of mice. Our results show that neither IL-12 nor IL-23 is necessary for HSK development, but HSK progression and maintenance requires an IL-12p40–independent function of IL-12p35 that to our knowledge has not been previously recognized.
Materials and Methods
Animals
Female WT, IL-12p35−/−, and IL-12p40−/− BALB/c mice 6 to 8 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). The IL-12p35−/− and IL-12p40−/− mice were bred through four generations to produce IL-12p35−/−p40−/− double-knockout mice. IL-12p35 and IL-12p40 genes were individually genotyped to confirm knockout status using the following primers: IL-12p35 (forward, 5′-CTGAATGAACTGCAGGACGA-3′; reverse, 5′-ATACTTTCTCGGCAGGAGCA-3′; expected size, 172 base pairs) and IL-12p40 (forward, 5′-CTTGGGTGGAGAGGCTAT TC-3′; reverse, 5′-AGGTGAGATGACAGGAGATC-3′; expected size, 280 base pairs). All experimental animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Corneal HSV-1 Infection
Mouse corneas were scarified using a 30-gauge needle under deep anesthesia induced by intraperitoneal injection of 2.0 mg ketamine hydrochloride and 0.04 mg xylazine (Phoenix Scientific, St. Joseph, MO) in 0.2 mL HBSS (Mediatech, Inc., Herndon, VA). Intact virions from HSV-1 strain RE grown in Vero cells were isolated on gradients (Optiprep; Accurate Chemical & Scientific, Westbury, NY) according to the manufacturer's instructions and were titrated as plaque-forming units (pfu) on Vero cell monolayers using a standard viral plaque assay, as previously described.29 HSV-1 RE was applied to the scarified corneas in 3 μL RPMI (Lonza, Walkersville, MD) at a dose predetermined to induce 80% HSK incidence. With different viral preparations, this dose ranged from 1 × 103 to 1 × 104 pfu (determined by a standard viral plaque assay). We advocate using the lowest infectious dose that induces a consistently high level of corneal disease because HSK becomes less CD4+ T-cell dependent at higher doses.30
HSK Scoring System
Mice were monitored for HSK on alternate days between 7 and 21 dpi by slit lamp examination. A standard scale ranging from 1 to 4 based on corneal opacity was used: 1+, mild corneal haze; 2+, moderate opacity; 3+, complete opacity; 4+, corneal perforation. Disease incidence was defined as HSK score greater than or equal to 2 by 15 dpi. The extent of neovascularization was also recorded.
Periocular Skin Disease Scoring System
Mice were monitored for blepharitis on alternate days between 7 and 21 dpi by slit lamp examination. A standard scale ranging from 1 to 5 based on periocular skin disease score was used: 1+, confined blepharitis; 2+, moderate regional blepharitis with 1 to 2 mm skin involvement; 3+, blepharitis with 1 to 2 mm skin involvement, with vesicles; 4+, blepharitis with more than 2 mm periocular skin involvement, without vesicles; 5+, blepharitis with more than 2 mm periocular skin involvement, with vesicles.
Flow Cytometric Analysis
Harvested corneas were incubated in PBS-EDTA at 37°C for 10 minutes and then separated from overlying epithelium and digested in collagenase type 1 (84 U/cornea; Sigma-Aldrich Co., St. Louis, MO) for 2 hours at 37°C. Cells were dispersed by trituration, and suspensions were filtered through a 40-μm cell strainer cap (BD Labware, Bedford, MA). Suspensions were incubated with anti–mouse CD16/CD32 (Fcγ III/II receptor; clone 2.4G2; BD PharMingen, San Diego, CA), then stained with various leukocyte surface markers for 30 minutes on ice. The following markers were used: PerCP-conjugated anti–CD45 (30-F11), phycoerythrin-conjugated anti–CD4 (RM4–5), APC–Cy7-conjugated anti–CD8α (53–6.7), FITC-conjugated anti–CD69 (H1.2F3), and anti–CD25 (7D4) (all BD PharMingen), and APC-conjugated anti–Gr-1 (RB6–8C5) (Caltag; Carlsbad, CA). All isotype antibodies were obtained from BD PharMingen. Intracellular staining for Foxp3 (FJK16s) was performed after permeabilization with solution (Cytofix/Cytoperm; eBiosciences, San Diego, CA) for 2 hours. After staining, cells were fixed with 1% paraformaldehyde (Electron Microscopy Services, Chicago, IL) and analyzed on a flow cytometer (FACSAria with FACSDIVA data analysis software; BD Biosciences).
Regulatory T-Cell Depletion
Mice received a single intraperitoneal injection 100 μg anti–CD25 mAb (clone PC61) or control mAb (HLA-DR5) in 500 μL 1× PBS or received PBS alone 3 days before infection.
Cytokine/Chemokine Analysis by Multiplex Bead Array
Each cornea was excised at 17 dpi and quartered in sterile PBS, and pieces were transferred to tubes containing 300 μL PBS + complete protease inhibitor (Complex Mini Protease Inhibitor; Roche Applied Science, Indianapolis, IN) and sonicated (Fisher Model 100 Sonic Dismembrator; Fisher Scientific, Pittsburgh, PA) four times for 15 seconds each. The sonicator tip was rinsed with 75 μL PBS + protease inhibitor, yielding a final volume of 600 μL/sample. To remove cellular debris, samples were microcentrifuged twice. Assay (Bio-Plex; Bio-Rad, Hercules, CA) was performed according to the manufacturer's instructions, or samples were sent for analysis (Luminex; Millipore, St. Louis, MO). The cytokines and chemokines assayed were IL-6, KC, MCP-1, MIP-2, and VEGF.
Statistical Analysis
Statistical analysis software (Prism; GraphPad, San Diego, CA) was used for all statistical analyses. Where indicated, P values were calculated using the Student's t-test when comparing two groups. P < 0.05 was considered significant. Results are presented as mean ± SEM.
Results
Herpes Stromal Keratitis
The corneas of IL-12p35−/−, IL-12p40−/−, IL-12p35−/−p40−/− (double-knockout), and WT mice were infected with HSV-1. Compared with WT mice, the viral burden in the corneas of IL-12p35−/−p40−/− mice was higher at 2 and 4 dpi but was ultimately cleared with similar kinetics (Table 1). Mice lacking either IL-12p35 or IL-12p40 alone had viral burdens that were similar to those of WT mice and cleared HSV-1 from their corneas with similar (IL-12p40−/−) or slightly delayed (IL12p35−/−) kinetics. All mouse strains cleared virus from their tear film by 10 dpi.
Table 1.
WT, IL-12p35−/−, IL-12p40−/−, and IL-12p35−/− p40−/− Mice Clear HSV-1 from the Cornea with Similar Kinetics
| Mouse Type | Corneal Viral Burden ± SEM, pfu/mL* (virus-positive mice, %)** |
||||
|---|---|---|---|---|---|
| 2 dpi | 4 dpi | 6 dpi | 8 dpi | 10 dpi | |
| WT | 5938.17 ± 1741 (100) | 3115.61 ± 984 (100) | 678.27 ± 455.4 (100) | 0 (0) | 0 (0) |
| IL-12p35−/− | 4765.33 ± 1554 (100) | 1112.38 ± 275.9 (100) | 272.31 ± 57.06 (100) | 18 ± 11.08 (30.7) | 0 (0) |
| IL-12p40−/− | 20364.2 ± 8083 (100) | 4358.33 ± 1306 (100) | 1614.26 ± 555.9 (100) | 0 (0) | 0 (0) |
| IL-12p35−/−p40−/− | 32796.2 ± 8688 (100) | 16435.2 ± 6576 (100) | 1616.60 ± 585.6 (100) | 0 (0) | 0 (0) |
WT, IL-12p35−/−, IL-12p40−/−, and IL-12p35−/−p40−/− mice were infected with the lowest possible dose of HSV-1 RE that induced 90% disease incidence. At 2 dpi through 10 dpi, standard plaque assay was performed on swabs taken from the cornea every other day.
The n value per mouse group ranged between 8 and 20 mice, indicative of at least two independent experiments.
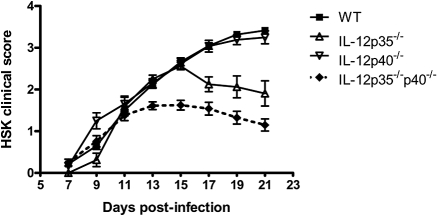
All four strains of mice developed moderate to severe HSK marked by increasing corneal opacity and peripheral neovascularization by 15 dpi (Fig. 1). HSK severity progressed steadily through 21 dpi in both WT and IL-12p40−/− mice with complete opacity, expanded neovascularization encroaching from the periphery into the central cornea, and corneal edema. In contrast, HSK severity began to regress by 15 dpi and 11 dpi in IL-12p35−/− mice and IL-12p35−/−p40−/− mice, respectively. Disease regression in both groups of IL-12p35–deficient mice was marked by rapidly decreased peripheral opacity with thinning of peripheral vasculature and a more gradual decrease in central opacity. All mice strains developed periocular skin disease similar in kinetics and severity to WT, with a peak of 1.5 ± 0.5 disease severity score (data not shown).
Figure 1.
Mice lacking IL-12 develop HSK. IL-12p35−/−, IL-12p40−/−, IL-12p35−/−p40−/− (double knockout), and WT mice infected with HSV-1 RE were scored for HSK by slit-lamp examination from 7 to 21 dpi. Data shown reflect n values of at least five mice per group and are representative of two or more independent experiments.
Corneal Inflammatory Infiltrate
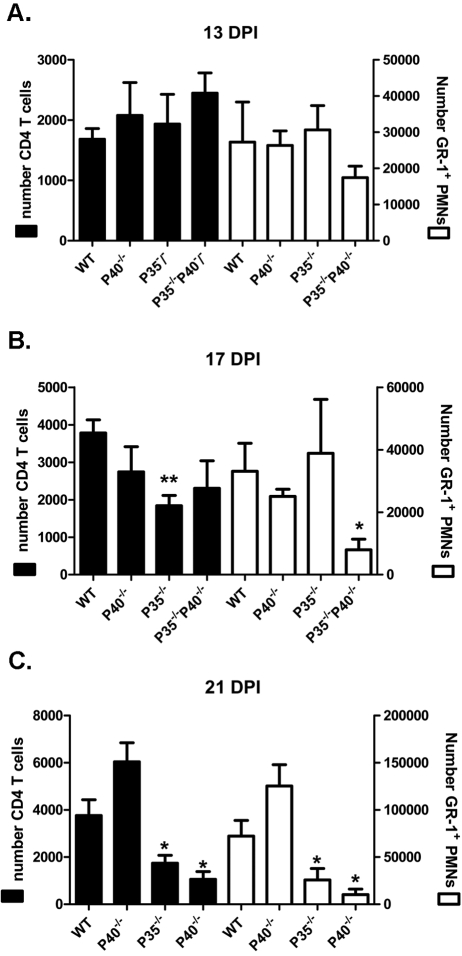
Early in HSK development (13 dpi), the infected corneas of WT, IL-12p35−/−, IL-12p40−/−, and IL-12p35−/−p40−/− mice showed comparable infiltrates of CD4+ T cells and Gr1bright neutrophils (Fig. 2A) that constituted most of the bone marrow-derived CD45+ cells in the cornea (data not shown). At 17 dpi, IL-12p35−/−p40−/− mice exhibited a significant reduction in neutrophilic infiltrate and a reduction in the mean number of CD4+ T cells in the cornea that did not achieve statistical significance (Fig. 2B). The p35−/− mice showed a reduced CD4+ T cell infiltrate at 17 dpi, but their neutrophilic infiltrate was similar to that of WT controls. The corneal infiltrate of p40−/− mice at 17 dpi was also not significantly different from that of WT mice. At the peak of HSK severity (21 dpi), both IL-12p35−/− and IL-12p35−/−p40−/− mice showed significantly reduced numbers of CD4+ T cells and neutrophils within their corneal infiltrates relative to WT mice (Fig. 2C). In both IL-12p35−/− and IL-12p35−/−p40−/− mice, reduction in clinical HSK severity preceded changes in the composition of the inflammatory infiltrate in the cornea by approximately 2 days.
Figure 2.
Mice lacking IL-12p35 have a reduced corneal leukocytic infiltrate. At 13, 17, and 21 dpi, corneas were dispersed into single-cell suspensions and stained with anti–CD45, CD4, and Gr-1 mAb. Cell suspensions were analyzed by flow cytometry. Data are represented as mean ± SEM number of CD4+ T cells (left axis) and GR-1bright neutrophils (right axis). Data reflect the average of two independent experiments with n values of at least four corneas per group. *P < 0.05; **P < 0.01.
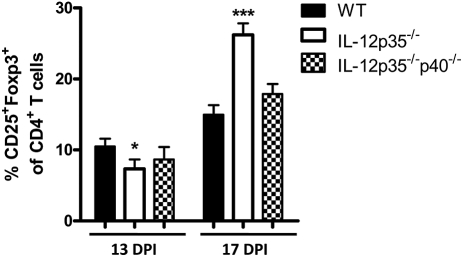
We hypothesized that HSK regression would be associated with an increased frequency of CD4+ CD25+ FoxP3+ Tregs in the corneas of p35-deficient mice. In fact, the CD4+ T-cell population in the corneas of IL-12p35−/− mice did show an increased frequency of CD25+ FoxP3+ cells during HSK regression at17 dpi (Fig. 3). However, an increased frequency of Tregs was not observed in the IL-12p35−/−p40−/− mice despite more rapid HSK regression (Fig. 3).
Figure 3.
IL-12p35−/− corneas contain an increased Treg population during disease regression. Corneas were dispersed into single-cell suspensions at 13 and 17 dpi and were stained with anti–CD4, CD25, and Foxp3 mAb. Corneal suspensions were analyzed by flow cytometry. Data are represented as mean ± SEM percentages. CD25+FoxP3+ cells in the CD4+ T-cell population. Groups consisted of five or more corneas, and results reflect the average of two independent experiments. *P < 0.05; ***P < 0.001.
The Role of Natural Tregs in HSK Regression in IL-12p35−/− Mice
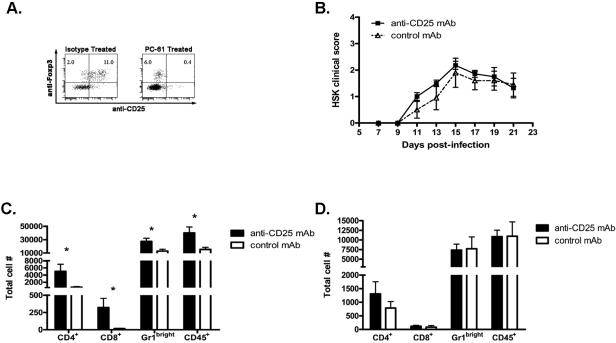
To determine whether the increased frequency of CD4+ CD25+ FoxP3+ cells in infected corneas of 12p35−/− mice was responsible for HSK regression, we sought to determine whether in vivo depletion of CD25+ cells before HSV-1 corneal infection would alter the course of HSK in these mice. A single treatment with 100 μg anti–CD25 mAb or control mAb 3 days before HSV-1 corneal infection effectively depleted CD25+ Foxp3+ cells from corneas through 21 dpi (Fig. 4A). However, Treg depletion did not significantly impact the course of HSK in IL-12p35−/− mice, with both depleted and nondepleted mice exhibiting HSK regression (Fig. 4B). As established in previous studies,31,32 Treg depletion did significantly increase the leukocytic infiltrate in infected corneas of WT mice (Fig. 4C) but did not significantly influence the size of the infiltrate in corneas of IL-12p35−/− mice (Fig. 4D). Thus, though CD25+ Tregs do modulate HSK severity in WT mice, they do not account for HSK regression in mice lacking IL-12p35. However, there remained in the corneas of the anti–CD25 mAb–treated IL-12p35−/− mice a substantial population of CD4+ FoxP3+ cells that did not express CD25 (Fig. 4A). These cells did not stain with anti-rat immunoglobulin, suggesting that CD25 was not simply masked by the rat anti–mouse CD25 mAb used for depletion. A contribution of these FoxP3+ cells to HSK regression cannot be ruled out.
Figure 4.
Regulatory T cells do not cause disease attenuation in IL-12p35−/− mice. IL-12p35−/− and WT mice were approximately 80% depleted of Treg cells by treatment with anti–CD25 mAb (PC61) 3 days before infection with HSV-1 RE (A, comparing depletion in WT mice). WT and IL-12p35−/− mice were followed up for HSK (data not shown and B, respectively). At 21 dpi, dispersed corneas were stained with anti–CD4, CD8, CD45, and GR-1 and were analyzed by flow cytometry (WT, C; IL-12p35−/−, D). Data are represented as mean ± SEM number of cells per cornea. Results represent the average of two independent experiments with an n value of at least six mice per group. *P < 0.05.
Influence of IL-12p35 on the Cytokine and Chemokine Profile of Infected Corneas
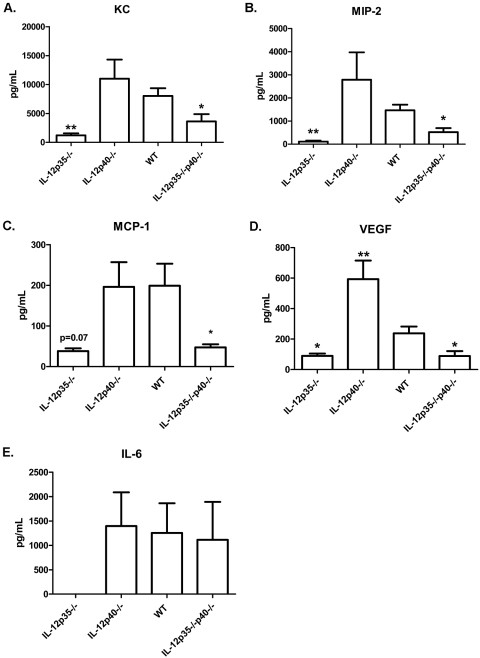
To determine whether HSK progression and regression were associated with different chemokine and cytokine profiles, corneas of WT, IL-12p40−/−, IL-12p35−/−, and IL-12p35−/−p40−/− mice were excised at 17 dpi, and cytokine and chemokine proteins were quantified using a multiplex bead array assay (Fig. 5). During HSK regression (17 dpi), the corneas of both IL-12p35−/− and IL-12p35−/−p40−/− mice exhibited significantly reduced expression of the neutrophil chemoattractants KC/CXCL3 (Fig. 5A) and MIP-2/CXCL2 (Fig. 5B) compared with those of WT mice. This correlated with a reduction in the neutrophilic infiltrate in IL-12p35−/−p40−/− mice and slightly preceded their reduction in IL-12p35−/− mice (Fig. 2B). In conjunction with a reduced CD4+ T-cell infiltrate, the corneas of IL-12p35−/− and IL-12p35−/−p40−/− mice also exhibited significantly reduced levels of the chemotactic factor MCP-1/CCL2 (Fig. 5C), a chemokine that one study suggested regulates CD4+ T-cell infiltration into infected corneas.33 The attenuation of blood vessels in infected corneas of IL-12p35−/− and IL-12p35−/−p40−/− mice was also associated with significantly reduced levels of the angiogenic factor VEGF (Fig. 5D). Compared with infected corneas of WT mice, infected corneas of IL-12p40−/− mice exhibited elevated levels of MIP-2/CXCL2 and VEGF but similar levels of KC/CXCL3 and MCP-1/CCL2. The increased levels of MIP-2/CXCL2 and VEGF preceded the increased leukocytic infiltrate at 21 dpi (Fig. 2C) but were not associated with increased clinical HSK scores (Fig. 1). Of interest was the lack of detectable levels of IL-6 within the corneas of the IL-12p35−/− mice though levels of this cytokine were similar in WT, IL-12p40−/−, and IL-12p35−/−p40−/− mice (Fig. 5E).
Figure 5.
Absence of IL-12 alters expression of cytokines and chemoattractants in corneas. WT, IL-12p35−/−, IL-12p40−/−, and IL-12p35−/−p40−/− corneas were harvested at 17 dpi. Corneas were homogenized by sonic dismembranation in PBS + protease inhibitor and analyzed by multiplex bead array for cytokine and chemokine expression. Data are represented as mean ± SEM picogram per milliliter of analyte. Groups consisted of five or more corneas, and results were averaged between two independent experiments. *P < 0.05; **P < 0.01.
Discussion
The established regulatory role of TH1 cytokines in HSK immunopathology strongly implicates the involvement of the IL-12 cytokine family. Indeed, a previous study using the same BALB/c WT, IL-12p40−/−, and IL-12p35−/− mice used in our study supported a role for IL-12 in HSK by showing reduced HSK in IL-12p35−/− mice and no HSK in IL-12p40−/− mice.27 The authors concluded that IL-12 was required for HSK. Those findings stand in stark contrast to the findings in this report. In our hands, IL-12p40−/− mice developed HSK with similar kinetics and severity to those seen in WT control mice. Indeed, infected corneas of IL-12p40−/− mice exhibited a more robust inflammatory infiltrate at the peak of HSK (21 dpi) compared to their WT counterparts. These findings demonstrate that in our HSK model neither IL-12 nor IL-23 had a requisite role in HSK development because both molecules incorporate an IL-12p40 chain. We further established that the IL-12p35 chain has a requisite role in the progression of HSK beyond 11 dpi, which is independent of the IL-12p40 chain as indicated by the transient nature of HSK in corneas of IL-12p35−/− mice and IL-12p35/p40 double knockout mice. The genotypes of all the mice used in these experiments were confirmed by PCR, and the pattern of HSK was observed in multiple experiments.
We surmise that a likely explanation for the differences in findings of the two studies lies in the virus used to infect the mice. Osorio et al. used the McKrae strain of HSV-1 at an infectious dose of 2 × 105 pfu to infect corneas. The McKrae strain is highly neurovirulent, and, at the dose used, only 20% of WT and IL-12p40−/− mice and 50% of IL-12p35−/− mice survived to the time of HSK evaluation. Thus, in that study, HSK was evaluated only in the few mice that survived infection; the general health of the surviving mice was not described. Our study used a less neurovirulent RE strain of HSV-1 at a much lower infectious dose that induced HSK in 80% to 100% of WT mice while permitting 100% survival with no clinically apparent disease other than HSK. We previously established that HSK is highly dependent on the function of CD4+ T cells at the RE HSV-1 infectious dose used in these studies,30 and our model better reflects human disease in which infections are rarely fatal and HSK usually occurs in otherwise healthy persons. One interesting parallel between the two studies is that the IL-12p35 chain appears to function independently of IL-12p40, prolonging HSK in our study and enhancing the lethality of HSV-1 infection in the previous study. These findings are consistent with an important role for IL-12p35 in regulating immunopathology in the cornea and in the CNS independently of IL-12p40.
We did not observe patterns of viral clearance from the cornea that would seem to explain the HSK regression in mice lacking the IL-12p35 chain. Neither the higher early viral titers with normal kinetics of clearance seen in the IL-12p35, p40−/− mice nor the normal early viral titers and slightly delayed clearance seen in IL-12p35−/− mice would seem to predispose them to transient HSK.
The IL-12p40 chain can form an IL-12p40 homodimer that has been shown to inhibit T-cell responses by binding to the IL-12Rβ1 chain and inhibiting the binding of IL-12 and IL-23.5,6 We considered the possibility that IL-12p35−/− mice have a propensity to produce more IL-12p40 homodimer, which might account for the transient nature of HSK in these mice. This possibility was addressed by monitoring HSK in mice that lack both the p35 and the p40 subunits. We observed a transient pattern of HSK in the double-knockout mice that was similar to that seen in IL-12p35−/− mice. These findings demonstrated that HSK regression was caused by lack of the IL-12p35 subunit rather than by altered function of the IL-12p40 subunit. The increased level of infiltrate within the IL-12P40−/− corneas is in agreement with the recent study in IL-23–deficient mice, in which more severe HSK lesions develop than develop in WT mice.28 However, the observation that mice deficient in both IL-12p35 and p40 regress in HSK earlier than do IL-12p35−/− mice suggests more complex relationships exist for p40 in the development of HSK.
We were intrigued by the dramatic increase in the frequency of Foxp3+ Tregs in the infected corneas of IL-12p35−/− mice during HSK regression. The reduced overall CD4+ T-cell population in the infected corneas of IL-12p35−/− mice during HSK regression, combined with the elevated frequency of Tregs among the CD4+ T cells, suggest a very high Treg/effector T-cell ratio in the infected corneas of these mice. The cytokine TGF-β regulates the differentiation of CD4+ T cells to Tregs and TH17 cells, with costimulation by IL-6 favoring the latter.34,35 We noted dramatically reduced levels of IL-6 in the corneas of IL-12p35−/− mice during HSK regression compared with those of WT mice with progressive HSK. The known presence of TGF-β in the cornea36,37 and the low levels of IL-6 in the corneas of IL-12p35−/− mice might provide a cytokine milieu that favors the differentiation or expansion of Tregs.
However, depletion of CD25+ cells failed to influence HSK regression in IL-12p35−/− mice. The possible explanation that our anti–CD25 treatment effected HSK regression by inadvertently depleting CD4+ effector T cells along with Tregs appears highly unlikely. Most CD4+ CD25+ cells in infected corneas coexpressed Foxp3 (not shown), suggesting that predominantly Tregs would be depleted by anti–CD25 treatment. Moreover, similar anti–CD25 mAb treatment increased CD4+ T-cell numbers and augmented the overall leukocytic infiltrate in HSV-1–infected corneas of WT mice, suggesting that the CD4+ T cells that mediate HSK do not express CD25. To further corroborate this evidence, IL-12p35−/−p40−/− did not exhibit any increase in Treg frequency in their corneas, despite HSK regression. Thus, although the frequency of CD4+ CD25+ Foxp3+ Tregs is dramatically increased in infected corneas of IL-12p35−/− mice during HSK regression, these cells are either inactive or their effector molecules inhibit an IL-12p35–dependent activation pathway. However as noted, depletion of CD25+ cells leaves a substantial population of CD4+ FoxP3+ CD25− cells in the cornea that might contribute to HSK regression.
Our findings demonstrate that IL-12p35 and p40 regulate the production of several chemokines and cytokines in corneas with HSK. Levels of the macrophage chemoattractant MCP-1/CCL2 were significantly and equivalently reduced in infected corneas of IL-12p35−/− and IL-12p35−/−p40−/− mice at 17 dpi. This observation is consistent with a role for IL-12 in regulating production of this chemokine. A recent study does suggest a role of MCP-1 in regulating the infiltration of CD4 T cells into the HSK-inflamed cornea,33 though such studies are complicated by the fact that MCP1−/− mice exhibit enhanced IL-12 production and increased HSK.38,39 Together these findings suggest a regulatory circuit in which IL-12 induces MCP-1/CCL2 production, whereas MCP-1/CCL2 provides feedback inhibition of IL-12 production.
We also observed that IL-12p35 regulates neutrophil infiltration and production of the neutrophil chemoattractants KC/CXCL3 and MIP-2/CXCL2 because these chemokines were significantly reduced in infected corneas of IL-12p35−/− and IL-12p35/40−/− mice compared with WT mice. This function of IL-12p35 is independent of IL-12p40 because neutrophilic infiltration and levels of these chemokines were elevated in infected corneas of IL-12p40−/− mice. These findings are consistent with previous studies identifying KC/CXCL3 and, to a greater extent, MIP-2/CXCL2 as important factors for neutrophil recruitment and HSK development.40–42
Several studies have established a critical role for VEGF and IL-6–mediated neovascularization in HSK progression.43–47 Here we demonstrate that IL-12p35 independently of IL-12p40 regulates VEGF production in corneas with HSK. In fact, IL-12p40 appears to inhibit the induction of VEGF production by IL-12p35 as corneas of IL-12p40−/− mice exhibit dramatically increased VEGF production, whereas the IL-12p35/p40 double knockouts show reduced VEGF levels comparable to those seen in IL-12p35 single knockouts. The reduced levels of VEGF are consistent with our observations of attenuated peripheral vasculature as HSK regressed in these mice. This suggests that the lack of VEGF may reduce vascularization, which in turn could lead to regression in HSK disease severity. Our data also suggest that IL-6 production in corneas with HSK is inhibited by IL-12p40 in the absence of IL-12p35. Mice lacking IL-12p35 exhibited impaired IL-6 production, whereas those lacking IL-12p40 or both IL-12p40 and p35 exhibited IL-6 production at levels similar to those seen in WT corneas with HSK. Given that we observed HSK regression in both IL-12p35−/− with undetectable IL-6 and in IL-12p35/p40−/− mice with WT levels of IL-6, we conclude that IL-6 may be necessary but clearly is insufficient for HSK progression.
The current understanding of IL-12p35 synthesis indicates that this subunit is not released by unbound cells.48 The recent description of IL-35, a IL-12p35 EBI3 heterodimeric, sets a precedence for more p35 binding partners.10,48 The exact contribution of the IL-12p35 subunit to the maintenance of HSK remains unclear but reflects an exciting development in the study of the IL-12 cytokine family in the pathogenesis of HSK immunopathology.
Acknowledgments
The authors thank Jessica Spehar for technical assistance and Nancy Zurowski for flow cytometry acquisition.
Footnotes
Supported by National Eye Institute Grants R01 EY010359 (RLH) and P30-EY08099 (RLH); an unrestricted research grant from Research to Prevent Blindness, Inc. (RLH); a grant from the Eye and Ear Foundation of Pittsburgh (RLH); and a grant from Research to Prevent Blindness, Inc. (SJD).
Disclosure: G.M. Frank, None; S.J. Divito, None; D.M. Maker, None; M. Xu, None; R.L. Hendricks, None
References
- 1.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol 1991;146:3074–3081 [PubMed] [Google Scholar]
- 2.Heinzel FP, Rerko RM, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol 1995;155:730–739 [PubMed] [Google Scholar]
- 3.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000;13:715–725 [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003;278:1910–1914 [DOI] [PubMed] [Google Scholar]
- 5.Ling P, Gately MK, Gubler U, et al. Human Il-12 P40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol 1995;154:116–127 [PubMed] [Google Scholar]
- 6.Piccotti JR, Chan SY, Li KW, Eichwald EJ, Bishop DK. Differential effects of IL-12 receptor blockade with IL-12 p40 homodimer on the induction of CD4(+) and CD8(+) IFN-gamma-producing cells. J Immunol 1997;158:643–648 [PubMed] [Google Scholar]
- 7.Khader SA, Partida-Sanchez S, Bell G, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med 2006;203:1805–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell TD, Yan Q, Fan G, et al. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J Immunol 2003;171:6866–6874 [DOI] [PubMed] [Google Scholar]
- 9.Nieuwenhuis EE, Neurath MF, Corazza N, et al. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci U S A 2002;99:16951–16956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007;450:566–569 [DOI] [PubMed] [Google Scholar]
- 11.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 2002;16:779–790 [DOI] [PubMed] [Google Scholar]
- 12.Hendricks RL, Tumpey TM. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr Eye Res 1991;10:47–53 [DOI] [PubMed] [Google Scholar]
- 13.Mercadal CM, Bousley DM, Destephano D, Rouse B. Herpetic stromal keratitis in the reconstituted SCID mouse model. J Virol 1993;67:3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalf JF, Hamilton DS, Reichert RW. Herpetic keratitis in athymic (nude) mice. Infect Immun 1979;26:1164–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newell CK, Martin S, Sendele D, Mercadal CM, Rouse BT. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol 1989;63:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Q, Hendricks RL. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J Exp Med 1996;184:1435–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stumpf TH, Shimeld C, Easty DL, Hill TJ. Cytokine production in a murine model of recurrent herpetic stromal keratitis. Invest Ophthalmol Vis Sci 2001;42:372–378 [PubMed] [Google Scholar]
- 18.Babu JS, Kanangat S, Rouse BT. T cell cytokine mRNA expression during the course of the immunopathologic ocular disease herpetic stromal keratitis. J Immunol 1995;154:4822–4829 [PubMed] [Google Scholar]
- 19.Keadle TL, Usui N, Laycock KA, Kumano Y, Pepose JS, Stuart PM. Cytokine expression in murine corneas during recurrent herpetic stromal keratitis. Ocul Immunol Inflamm 2001;9:193–205 [DOI] [PubMed] [Google Scholar]
- 20.Keadle TL, Stuart PM. Interleukin-10 (IL-10) ameliorates corneal disease in a mouse model of recurrent herpetic keratitis. Microb Pathog 2005;38:13–21 [DOI] [PubMed] [Google Scholar]
- 21.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol 1992;149:3035–3039 [PubMed] [Google Scholar]
- 22.Duan R, Remeijer L, van Dun JM, Osterhaus AD, Verjans GM. Granulocyte macrophage colony-stimulating factor expression in human herpetic stromal keratitis: implications for the role of neutrophils in HSK. Invest Ophthalmol Vis Sci 2007;48:277–284 [DOI] [PubMed] [Google Scholar]
- 23.Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol 2002;169:5897–5903 [DOI] [PubMed] [Google Scholar]
- 24.Kanangat S, Thomas J, Gangappa S, Babu JS, Rouse BT. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression: implications in immunopathogenesis and protection. J Immunol 1996;156:1110–1116 [PubMed] [Google Scholar]
- 25.Kumaraguru U, Rouse BT. The IL-12 response to herpes simplex virus is mainly a paracrine response of reactive inflammatory cells. J Leukoc Biol 2002;72:564–570 [PubMed] [Google Scholar]
- 26.Al Khatib K, Campbell IL, Carr DJ. Resistance to ocular herpes simplex virus type 1 infection in IL-12 transgenic mice. J Neuroimmunol 2002;132:41–48 [DOI] [PubMed] [Google Scholar]
- 27.Osorio Y, Wechsler SL, Nesburn AB, Ghiasi H. Reduced severity of HSV-1-induced corneal scarring in IL-12-deficient mice. Virus Res 2002;90:317–326 [DOI] [PubMed] [Google Scholar]
- 28.Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes Infect 2008;10:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med 2000;191:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepisto AJ, Frank GM, Xu M, Stuart PM, Hendricks RL. CD8 T cells mediate transient herpes stromal keratitis in CD4-deficient mice. Invest Ophthalmol Vis Sci 2006;47:3400–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol 2004;172:4123–4132 [DOI] [PubMed] [Google Scholar]
- 32.Divito SJ, Hendricks RL. Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis. Invest Ophthalmol Vis Sci 2008;49:1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SK, Choi BK, Kang WJ, et al. MCP-1 derived from stromal keratocyte induces corneal infiltration of CD4+ T cells in herpetic stromal keratitis. Mol Cells 2008;26:67–73 [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–238 [DOI] [PubMed] [Google Scholar]
- 35.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006;24:179–189 [DOI] [PubMed] [Google Scholar]
- 36.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res 1990;9:963–969 [DOI] [PubMed] [Google Scholar]
- 37.Wilson SE, Schultz GS, Chegini N, Weng J, He YG. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp Eye Res 1994;59:63–71 [DOI] [PubMed] [Google Scholar]
- 38.Thomas J, Kanangat S, Rouse BT. Herpes simplex virus replication-induced expression of chemokines and proinflammatory cytokines in the eye: implications in herpetic stromal keratitis. J Interferon Cytokine Res 1998;18:681–690 [DOI] [PubMed] [Google Scholar]
- 39.Kim B, Sarangi PP, Lee Y, Deshpande KS, Lee S, Rouse BT. Depletion of MCP-1 increases development of herpetic stromal keratitis by innate immune modulation. J Leukoc Biol 2006;80:1405–1415 [DOI] [PubMed] [Google Scholar]
- 40.Tumpey TM, Cheng H, Cook DN, Smithies O, Oakes JE, Lausch RN. Absence of macrophage inflammatory protein-1alpha prevents the development of blinding herpes stromal keratitis. J Virol 1998;72:3705–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumpey TM, Cheng H, Yan XT, Oakes JE, Lausch RN. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J Leukoc Biol 1998;63:486–492 [DOI] [PubMed] [Google Scholar]
- 42.Yan X-T, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci 1998;39:1854–1862 [PubMed] [Google Scholar]
- 43.Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol 2001;75:9828–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp Eye Res 2006;82:46–54 [DOI] [PubMed] [Google Scholar]
- 45.Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. CXCR2−/− mice show enhanced susceptibility to herpetic stromal keratitis: a role for IL-6-induced neovascularization. J Immunol 2004;172:1237–1245 [DOI] [PubMed] [Google Scholar]
- 46.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 1996;271:736–741 [DOI] [PubMed] [Google Scholar]
- 47.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Invest Ophthalmol Vis Sci 2002;43:737–743 [PubMed] [Google Scholar]
- 48.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci U S A 1991;88:4143–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]