Based on the characterization of the ERK1/2- and Akt-signaling pathways involved in vitreous-induced lens fiber cell differentiation, it is clear that FGF is the key growth factor involved in this process, and that other ocular growth factors, such as IGF, PDGF, and EGF, may be involved in fine tuning the signaling required for the initiation and/or maintenance of lens fiber differentiation in situ.
Abstract
Purpose.
Although some of the factors and signaling pathways that are involved in induction of fiber differentiation have been defined, such as FGF-mediated MAPK/ERK and PI3-K/Akt signaling, the factors in the vitreous that regulate this differentiation process in vivo have yet to be identified. The purpose of this study was to better understand the role of growth factors in vitreous that regulate this process by further characterizing the signaling pathways involved in lens fiber differentiation.
Methods.
Rat lens epithelial explants were used to compare the ability of vitreous, IGF-1, PDGF-A, EGF, and FGF-2 to stimulate the phosphorylation of ERK1/2 and Akt leading to fiber differentiation, in the presence or absence of selective receptor tyrosine kinase (RTK) inhibitors.
Results.
Similar to vitreous, FGF induced a sustained ERK1/2 signaling profile, unlike IGF, PDGF, and EGF, which induced a more transient (shorter) activation of ERK1/2. For Akt activation, IGF was the only factor that induced a profile similar to vitreous. IGF, PDGF, and EGF potentiated the effects of a low dose of FGF on lens fiber differentiation by extending the duration of ERK1/2 phosphorylation. In the presence of selective RTK inhibitors, although the sustained vitreous-induced ERK1/2 signaling profile and subsequent fiber differentiation was perturbed, the results also showed that, although prolonged ERK1/2 phosphorylation was necessary, it was not sufficient for fiber differentiation to proceed.
Conclusions.
These results are consistent with FGF's being the key growth factor involved in vitreous-induced signaling leading to lens fiber differentiation; however, they also indicate that other vitreal growth factors such as IGF may be involved in fine-tuning ERK1/2- and Akt-phosphorylation to the level that is necessary for initiation and/or maintenance of lens fiber differentiation in vivo.
The lens has a distinctive architecture, with an anterior monolayer of lens epithelial cells overlying a mass of elongated fiber cells. The lens grows as lens epithelial cells proliferate at the lens equator and differentiate into secondary fiber cells. The surrounding ocular media, the aqueous humor that bathes the lens epithelial cells and the vitreous humor that bathes the lens fiber cells, have been shown to be important in the maintenance of this distinctive polarity and architecture of the lens.1 The ocular media contain members of several growth factor families (see Ref. 2) including, insulin-like growth factor (IGF),3 fibroblast growth factor (FGF),4 platelet-derived growth factor (PDGF),5 epidermal growth factor (EGF),6 hepatocyte growth factor (HGF),7 and vascular endothelial growth factor (VEGF).8,9 Studies over the past two decades have linked these growth factors in various ways to the process of lens fiber differentiation.
Extensive experiments with lens epithelial explants have shown that FGF induces lens fiber cell differentiation.10 In vitro, FGF has been shown to induce many of the morphologic and molecular changes associated with fiber differentiation, including cell elongation, the loss of cytoplasmic organelles, denucleation, and the accumulation of fiber-specific β- and γ-crystallins.11–13 Of interest, these experiments showed that FGF could also induce lens epithelial cell proliferation, with a low dose of FGF inducing cell proliferation and a much higher concentration required to induce fiber differentiation.14 Based on this, and together with studies examining the distribution of FGF in the eye, it was proposed that an anteroposterior gradient of FGF in the eye may determine lens polarity,10,15 with a low concentration of FGF in aqueous stimulating proliferation and a higher concentration of FGF in the vitreous inducing lens fiber differentiation. In situ experiments using transgenic mice to misexpress FGFs and/or FGF receptors, were also shown to impair the normal development and growth of the lens,16–22 further underpinning the important and essential role for FGF in lens differentiation.
To date, FGF has been demonstrated to be the only growth factor able to induce lens fiber differentiation; however, other growth factors such as IGF and/or PDGF and Wnts have been shown to potentiate FGF-induced lens fiber differentiation in vitro.23–26 In transgenic mice that overexpressed IGF in the lens, the germinative and transitional zones were shown to expand, but no inappropriate differentiation of lens epithelial cells was observed.27 Overexpression of PDGF in the lens resulted in enlarged lenses that developed cataracts.28 The lens epithelium of these mice became multilayered with some evidence of fiber differentiation changes28; however, subsequent in vitro experiments confirmed that PDGF cannot induce this fiber differentiation process without FGF.25 Taken together, these in vitro and in vivo experiments suggest that IGF or PDGF do not directly induce lens fiber differentiation, but may play some role in this process.
High-affinity receptors for FGF, IGF, PDGF, and EGF belong to different subclasses of RTKs29; however, they all can activate similar intercellular signaling pathways, including the ERK1/2 and PI3-K pathways through their association with common adaptor proteins.30–32
It has been established that ERK1/2 signaling is essential for both lens cell proliferation and differentiation.33–35 More recent studies have shown that the duration of ERK1/2 phosphorylation is associated with a specific cell fate in the lens.36 Although both FGF and vitreous can induce lens fiber differentiation, their ability to stimulate phosphorylation of ERK1/2 and Akt differs to some degree.36,37 As other vitreous-derived growth factors can also stimulate the phosphorylation of ERK1/2 and Akt signaling pathways, we propose that a combination of factors participate in vitreous-induced lens fiber differentiation. To better define the role of these growth factors in the vitreous, we compared their ability to phosphorylate ERK1/2 and Akt in lens epithelial cells, either independently or in combination with FGF. Our findings demonstrate a correlation between the different ERK/Akt phosphorylation profiles and the degree of growth factor-induced fiber differentiation. The results clearly show that IGF, PDGF, and EGF can all prolong and potentiate FGF-induced ERK1/2 activation, leading to lens fiber differentiation. Our blocking studies using a range of selective RTK inhibitors to block vitreous-induced ERK/Akt signaling and fiber differentiation show that FGF is an essential growth factor involved in vitreous-induced lens fiber differentiation and that IGF, PDGF, and EGF may also be involved in this process by regulating ERK1/2 and Akt phosphorylation. Finally, these studies indicate that prolonged phosphorylation of ERK1/2 is necessary but not sufficient for fiber differentiation to proceed.
Methods
All experimental procedures conformed to the National Health and Medical Research Council (NHMRC, Australia) guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All protocols were approved by the Animal Ethical Review Committee of the University of Sydney, Australia.
Reagents were obtained from the following sources: recombinant human FGF-2 and EGF (R&D Systems, Minneapolis, MN); recombinant human IGF-1 (Gropep, Thebarton, SA, Australia); recombinant human PDGF-A (Peprotech, Rocky Hill, NJ); sheep anti-rabbit IgG-HRP-conjugated antibody, mouse anti-phospho-ERK1/2 (pERK1/2; Thr202/Tyr204) antibody, rabbit anti-phospho-Akt (pAkt, Ser473) antibody, and rabbit anti-ERK1/2 antibody (p44/p42 MAP kinase; Cell Signaling Technology, Beverly, MA); bisbenzimide (Hoechst dye, Calbiochem, San Diego, CA); goat anti-mouse IgG-HRP-conjugated (Zymed, South San Francisco CA); goat anti-rabbit IgG conjugated with fluorescein-isothiocyanate (FITC; Sigma-Aldrich, Castle Hill, NSW, Australia); a protein assay kit (Micro BCA; Pierce Biotechnology/Thermo Scientific, Sydney, NSW, Australia); and RTK inhibitors SU5402, AG1296, AG1024, and PD153035 (Calbiochem, La Jolla, CA).
Preparation of Lens Epithelial Explants
All tissue culture was performed in medium 199 with Earle's salts (M199; Trace Scientific, Sydney, NSW, Australia), supplemented with 0.1% bovine serum albumin (BSA; Sigma-Aldrich), 0.1 μg/mL l-glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, and 2.5 μg/mL amphotericin B (Amphostat B; all from Trace Scientific, NSW, Australia). Lens explant methodology has been described previously.37 In brief, 10-day old Wistar rats were killed. The eyes were removed and placed in prewarmed culture medium under sterile conditions. Under a dissection microscope, the lenses were isolated and their posterior lens capsule was torn to remove the fiber cell mass. The remaining lens capsule with the adherent epithelial cell monolayer was pinned flat to the base of the culture dish with fine forceps. Culture medium was replaced with 1 mL of fresh, equilibrated (37°C, 5% CO2) M199 before further treatment.
Bovine eyes (Wilberforce Meats, Wilberforce, NSW, Australia) were collected as described previously.37 In brief, the eyes were kept on ice after harvesting and for transportation. After collecting aqueous humor, an incision along the sclera near the ciliary body was made to remove the anterior portion of the eye with the lens attached. Vitreous humor from multiple eyes was pooled in a sterile Petri dish and homogenized with three passages through a 19-gauge needle using a syringe. The samples were then stored at −20°C until needed.
Lens epithelial explants were cultured in M199 containing 50 ng/mL of IGF, 15 ng/mL of PDGF, 5 ng/mL of EGF, 5 ng/mL of FGF (low dose), 100 ng/mL of FGF (high dose), or 50% vitreous humor. For all β-crystallin immunofluorescence labeling studies, explants were cultured for 5 days. For Western blot analysis, the explants were collected at various time points over a 24-hour culture period.
Inhibitor Treatment
For blocking studies, inhibitors were added 2 hours before growth factor or vitreous was added to the cells. Once added, the inhibitor (20 μM SU5402, 5 μM AG1024, 1 μM AG1296, or 50 nM PD153035) remained present with the growth factors for the duration of the culture period. For control explants, an equivalent volume of DMSO (manufacturer's recommended solvent for the inhibitors) was substituted for the inhibitor.
SDS-PAGE and Western Blot Analysis
SDS and Western blot methodologies have been described previously.37 After the culture period (between 5 minutes and 24 hours), the explants were rinsed three times in cold PBS, and cell proteins were extracted in lysis buffer (1 mM EDTA, 10 mM Tris-HCl [pH 7.5], 0.15 M NaCl, 1% Igepal [Sigma-Aldrich], 1 mM Na3VO4 [Sigma-Aldrich], and protease inhibitor cocktail [Roche, Basel, Switzerland]) for 60 minutes at 4°C. Protein concentration was quantified using the protein assay (MicroBCA), according to the manufacturer's instructions (Pierce Biotechnology/ThermoFisher). Each experiment was performed at least three times and representative blots are presented.
For relabeling of the blots, the primary blots were stripped with stripping buffer containing 2% SDS, 60 mM Tris (pH 6.7), and 100 mM β-mercaptoethanol, for 20 minutes at 60°C. After three rinses with TBST (0.1% Tween 20 in Tris-buffered saline), the blots were incubated in blocking buffer and further immunolabeled as necessary.
For data analysis, representative Western blots from three separate experiments were examined. The intensity of the protein label was quantified (AlphaEaseFc Software; Alpha Innotech, San Leandro, CA) by a published method.37 In brief, each band was measured in triplicate. The relative density was obtained using the formula: (DpAkt/DERK1/2)sample/(DpAkt/DERK1/2)control, where D represents the density of each band. DpAkt/DERK1/2 adjusts the density of each band against reference bands (e.g., for total ERK1/2, which we used as a standard).
Immunofluorescence
Explants to be used for immunofluorescent labeling were cultured for 5 days. At the end of the culture period, the explants were fixed in 10% NBF for 20 minutes and rinsed in PBS supplemented with BSA (PBS/BSA), followed by immunofluorescent labeling for β-crystallin.37 Explants were counterstained with 3 μg/mL Hoechst dye (Calbiochem) in PBS and viewed with an epifluorescence microscope (DMLB; Leica Microsystems, Gladesville, NSW, Australia). Images were captured with a digital camera and accompanying software (DC100; Leica Microsystems).
Results
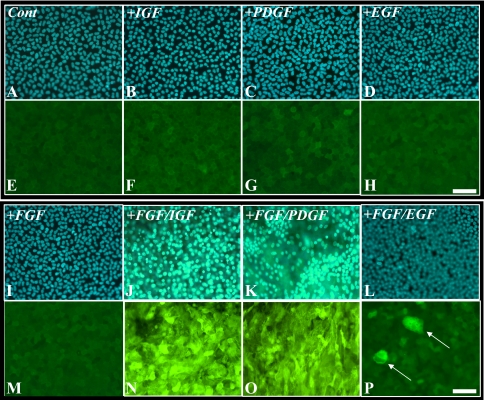
In the presence of IGF or PDGF or of EGF, fiber differentiation was not induced in rat lens epithelial explants, with epithelial cells remaining as a monolayer and not expressing β-crystallin (Figs. 1F–H), similar to cells in control explants (Fig. 1E). A low-proliferating dose of FGF (5 ng/mL) was also not sufficient to induce fiber cell differentiation in the lens epithelial explants (Figs. 1I, 1M); however, in combination with IGF (FGF/IGF), PDGF (FGF/PDGF), or EGF (FGF/EGF), features of a fiber cell differentiation response, such as cell enlargement, multilayering, and the accumulation of β-crystallin, were evident (Figs. 1N–P). Compared with FGF/IGF (Fig. 1N), explants treated with FGF/EGF presented a relatively weaker fiber differentiation response (Fig. 1P), with only a few cells throughout the explant showing multilayering and accumulating β-crystallin (Fig. 1P, arrows). This result was also found when EGF was used at higher concentrations (up to 100 ng/mL, data not shown). To better characterize this phenomenon of FGF potentiation leading to fiber cell differentiation, we used immunolabeling to compare phosphorylated ERK1/2 and Akt in response to these different growth factor conditions.
Figure 1.
Explants treated for 5 days with no growth factors (A, E) or a combination of different growth factors: 50 ng/mL IGF (B, F); 15 ng/mL PDGF (C, G); 5 ng/mL EGF (D, H); 5 ng/mL FGF (I, M), or a combination of a low dose of FGF (5 ng/mL) with IGF (FGF/IGF, J, N), PDGF (FGF/PDGF, K, O), or EGF (FGF/EGF, L, P), immunofluorescently labeled for β-crystallin (E–H, M–P) or counterstained with Hoechst dye (A–D, I–L). Only combinations of FGF with IGF (J, N), PDGF (K, O), or EGF (L, P, arrows) induced cell multilayering and accumulation of β-crystallin. Scale bar, 50 μm.
Stimulation of RTK Signaling Leading to Lens Fiber Cell Differentiation
Fibroblast Growth Factor.
As previously reported, in lens epithelial cells treated only with a low dose of FGF, Akt phosphorylation was slightly increased above basal levels, gradually peaking at 2 hours.36 In contrast, ERK1/2 was phosphorylated within 5 minutes and maintained for 4 hours, demonstrating a shorter profile than that induced by a high fiber-differentiating dose of FGF (data not shown, see Ref. 36).
Insulin-like Growth Factor.
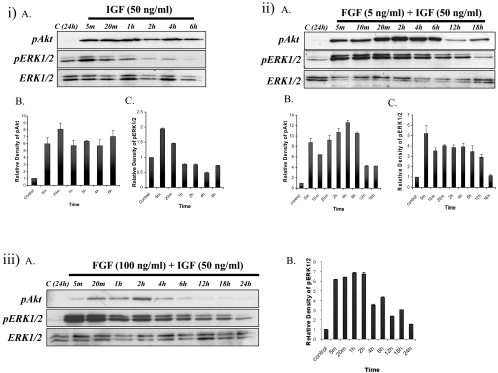
In IGF-treated explants, Akt phosphorylation was strongly induced within 5 minutes and was maintained for up to 6 hours (Figs. 2iA, top; 2iB). ERK1/2 phosphorylation was also stimulated and peaked within 5 minutes, persisting for up to1 hour, after which it dropped to basal levels (Figs. 2iA, middle; 2iC).
Figure 2.
(i) Representative Western blots (iA) of explants cultured with no growth factor (control) or IGF from 5 minutes up to 6 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), or total ERK1/2 (bottom). Quantification of relative density of phosphorylated Akt (iB) and ERK1/2 (iC) showed the same trend as Western blot analysis. (ii) Representative Western blots (iiA) of explants cultured with no growth factor (control) or IGF+FGF from 5 minutes up to 18 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and total ERK1/2 (bottom). Quantification of the relative density of phosphorylated Akt (iiB) and ERK1/2 (iiC) showed the same trend as the Western analysis. (iii) Explants treated with a high dose of FGF (100 ng/mL)+IGF (50 ng/mL). (iiiA) Explants cultured without growth factor (control) or with a combination of a high dose of FGF+IGF from 5 minutes up to 24 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and total ERK1/2 (bottom). (iiiB) Quantification of the relative density of ERK1/2.
Fibroblast Growth Factor/Insulin-like Growth Factor.
Similar to IGF treatment alone, FGF/IGF treatment activated Akt phosphorylation within 5 minutes and maintained it for up to 6 hours, followed by a return to basal levels (Figs. 2iiA, top; 2iiB). In contrast to IGF treatment alone, ERK1/2 phosphorylation was potentiated with FGF/IGF, being stimulated within 5 minutes and maintained for up to 12 hours (Figs. 2iiA, middle; 2iiC). We also found that by combining IGF with a higher differentiating dose of FGF (100 ng/mL), ERK1/2 phosphorylation (but not Akt phosphorylation) was further potentiated and was sustained for approximately 18 hours (Figs. 2iiiA, 2iiiB).
Plate-Derived Growth Factor.
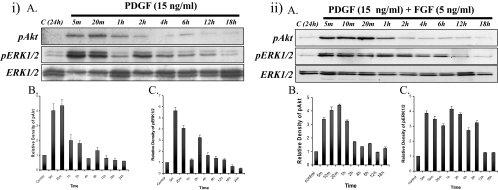
With the addition of only PDGF, Akt phosphorylation in lens epithelial cells was activated within 5 minutes and maintained for up to 1 hour, before dropping to basal levels (Figs. 3iA, top; 3iB). ERK1/2 was phosphorylated within 5 minutes and maintained for up to 20 minutes before returning to basal levels over 1 hour, with another pulse of activation at 2 hours (Figs. 3iA, middle; 3iC).
Figure 3.
Explants treated with PDGF (15 ng/mL) or a low dose of FGF (5 ng/mL)+PDGF (15 ng/mL). (i) Representative Western blots of explants cultured without growth factor (control) or with PDGF (iA) from 5 minutes up to 24 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and total ERK1/2 (bottom). Strong Akt phosphorylation was detected in 5 minutes and continued for up to 2 hours, followed by a return to basal levels. ERK1/2 phosphorylation was transiently activated within 5 minutes and peaked by 20 minutes. This level returned to basal level by 1 hour. Total ERK1/2 showed that the same amount of protein was loaded in each lane. Quantification of the relative density of phosphorylated Akt (iB) and ERK1/2 (iC) showed the same trend as the Western analysis. (ii) Representative Western blots of explants cultured without growth factor (control) or with PDGF+FGF (iiA) from 5 minutes up to 18 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), or total ERK1/2 (bottom). Quantification of the relative density of phosphorylated Akt (iiB) and ERK1/2 (iiC) showed the same trend as the Western analysis.
Fibroblast Growth Factor/Plate-Derived Growth Factor.
Western blot analysis showed that Akt was activated within 5 minutes and maintained for up to 1 hour (Figs. 3iiA top; 3iiB). ERK1/2 phosphorylation was activated within 5 minutes and maintained for up to 6 hours, longer than the profile observed for either PDGF or a low dose of FGF alone (Figs. 3iiA, middle; 3iiC).
Epithelial Growth Factor.
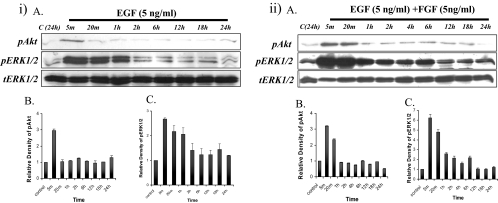
EGF was shown by Western blot to transiently activate Akt phosphorylation within 5 minutes, followed by a decrease to basal levels by 20 minutes (Figs. 4iA, top; 4iB). EGF-induced ERK1/2 phosphorylation was detected within 5 minutes and was maintained for up to 1 hour before returning to basal levels (Figs. 4iA, middle; 4iC).
Figure 4.
Explants treated with EGF (5 ng/mL) or a low dose of FGF (5 ng/mL)+EGF (5 ng/mL). (i) Representative Western blots of explants cultured without growth factor (control) or with EGF (iA) from 5 minutes up to 24 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and total ERK1/2 (bottom). Quantification of the relative density of phosphorylated Akt (iB) and ERK1/2 (iC) showed the same trend as the Western analysis. (ii) Representative Western blots of explants cultured without growth factor (control) or with EGF+FGF (iiA) from 5 minutes up to 24 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and total ERK1/2 (bottom). Quantification of the relative density of phosphorylated Akt (iiB) and ERK1/2 (iiC) showed the same trend as the Western analysis.
Fibroblast Growth Factor/Epithelial Growth Factor.
Western blot analysis showed that Akt phosphorylation induced by FGF/EGF was activated within 5 minutes and maintained for up to 20 minutes, followed by a drop to basal levels (Figs. 4iiA, top; 4iiB). ERK1/2 phosphorylation was activated within 5 minutes, peaked at 20 minutes, before dropping to a reduced level that was maintained for up to 6 hours, before returning to basal levels (Figs. 4ii, middle; 4iiC).
Overall, our results showed that IGF, and to a lesser extent PDGF and EGF, can potentiate a low-proliferating dose of FGF to induce some fiber differentiation changes in lens epithelial explants. To better understand the contribution of these growth factors in vitreous, we used selective growth factor receptor inhibitors to block signaling for each respective growth factor.
Inhibition of RTK Signaling
Inhibition of FGFR Signaling (SU5402).
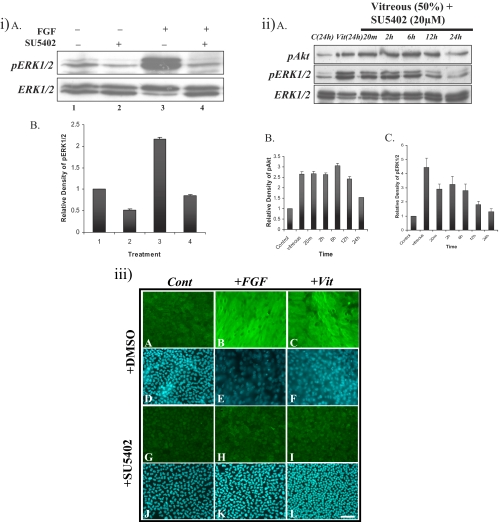
To test the selectivity of SU5402 to FGFR, 20 μM SU5402 was added 2 hours before the cells were treated with FGF. Western blot analysis showed that FGF-induced ERK1/2 phosphorylation was retained at basal levels after 20 minutes (Figs. 5iA, top; 5iB), indicating that SU5402 can block FGF-induced ERK1/2 phosphorylation. Immunolabeling showed that in the presence of SU5402, cells remained as a monolayer and did not express β-crystallin (Figs. 5iiiH, 5iiiK), similar to cells in control explants (Figs. 5iiiA, 5iiiD, 5iiiG, 5iiiJ); indicating that SU5402 can effectively block FGF-induced lens fiber differentiation. Cells in explants treated with FGF in the absence of SU5402, differentiated and accumulated β-crystallin (Figs. 5iiiB, 5iiiE).
Figure 5.
Effect of the FGFR inhibitor SU5402 on vitreous-induced lens fiber differentiation. (iA) Representative Western blots of explants cultured without growth factor (lanes 1 and 2) or with FGF (lanes 3 and 4) in the presence of DMSO (lanes 1 and 3) or 20 μM SU5402 (lanes 2 and 4) for 20 minutes. SU5402 completely blocked FGF-induced ERK1/2 phosphorylation. Quantification of the relative density of phosphorylated ERK1/2 (iB) showed the same trend as the Western analysis. (iiA) Representative Western blots of explants cultured without growth factor (control) or with 50% vitreous with 20 μM SU5402 from 20 minutes to 24 hours, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and ERK1/2 (bottom). Akt and ERK1/2 were phosphorylated within 20 minutes and maintained for up to 12 and 6 hours, respectively. Quantification of the relative density of phosphorylated Akt (iiB) and ERK1/2 (iiC) showed the same trend as the Western analysis. (iii) Representative micrographs of lens explants cultured with 20 μM SU5402. Explants were cultured without growth factors (A, D, G, J), with FGF (100 ng/mL; B, E, H, K), or with vitreous (50%; C, F, I, L), in the presence (G–L) or absence (A–F) of 20 μM SU5402 added 2 hours before FGF or vitreous treatment. The explants were assayed for β-crystallin accumulation after 5 days. Those cultured without SU5402 were treated with an equivalent volume of DMSO (A, D). When SU5402 was added 2 hours before FGF or vitreous treatment, lens fiber differentiation was blocked (H, I). Scale bar, 50 μm.
When we examined vitreous-induced Akt and ERK1/2 phosphorylation profiles in the presence of SU5402, Akt phosphorylation was activated within 5 minutes and maintained for up to 12 hours (Figs. 5iiA, top; 5iiB). ERK1/2 phosphorylation was activated within 5 minutes and also maintained for 12 hours. The intensity of ERK1/2 phosphorylation began to drop at 12 hours, reaching basal levels by 24 hours (Figs. 5iiA, middle; 5iiC). This finding shows that in the presence of SU5402, vitreous-induced ERK1/2 activation was decreased, and the duration of the phosphorylation was markedly shortened. Although blocking FGF signaling with SU5402 inhibits but cannot completely block vitreous-induced ERK1/2 phosphorylation, immunofluorescence results showed that SU5402 can effectively block vitreous-induced fiber differentiation. Cells remained as a monolayer and did not express β-crystallin (Figs. 5iiiI, 5iiiL), similar to cells in control explants (Figs. 5iiiG, 5iiiJ). Cells in explants treated with vitreous in the absence of SU5402, differentiated, and accumulated β-crystallin (Figs. 5iiiC, 5iiiF).
Inhibition of IGFR Signaling (AG1024).
In the presence of AG1024, ERK1/2 phosphorylation was reduced in control and IGF-treated explants (Figs. 6iA, top, lanes 2, 4; 6iB), indicating that IGF signaling may contribute to maintenance of basal levels of ERK1/2 phosphorylation in lens epithelial cells. Blocking IGF-induced ERK1/2 phosphorylation; however, had no obvious impact on the lens epithelial cell phenotype (Figs. 6iii, 6iiiB, 6iiiD).
Figure 6.
Effect of the IGFR inhibitor AG1204 on vitreous-induced lens fiber differentiation. (iA) Representative Western blots of explants cultured without (lanes 1 and 2) or with (10 ng/mL; lanes 3 and 4) IGF in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of 5 μM AG1024 added 2 hours before growth factor treatment. AG1024 blocked IGF-induced ERK1/2 phosphorylation. (iB) Quantification of the relative density of phosphorylated ERK1/2 showed the same trend as the Western analysis. (iiA) Representative Western blots of explants cultured without (lanes 1 and 2) or with (lanes 3 and 4) FGF in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of 5 μM AG1024 added 2 hours before growth factor treatment. AG1204 did not block FGF-induced ERK1/2 phosphorylation. (iiB) Quantification of the relative density of phosphorylated ERK1/2 that showed the same trend as the Western analysis. (iii) Representative micrographs of lens explants cultured without (A–D) or with (E–H) FGF, in the presence (B, D, F, H) or absence (A, C, E G) of 5 μM AG1024 added 2 hours before FGF treatment. AG1024 did not block FGF-induced lens fiber differentiation (F). (iv) Representative micrographs of lens explants cultured with vitreous and 5 μM AG1024. Explants were cultured without (A–D) or with (E–H) vitreous, in the presence (B, D, F, H, I, J) or absence (A, C, E, G) of 5 μM AG1024, added 2 hours before vitreous treatment. AG1024 reduced the accumulation of β-crystallin and cell elongation, but did not completely block the elongation. Some bare patches were observed over the explants (F, arrows), indicative of cell death/loss. Scale bar, (A–H) 50 μm; (I, J) 200 μm. (vA) Representative Western blots of explants cultured without or with vitreous in the presence or absence of 5 μM AG1024 added 2 hours before vitreous treatment, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and total ERK1/2 (bottom). Akt was phosphorylated within 20 minutes and maintained for up to 18 hours. The duration of ERK1/2 phosphorylation was reduced to 6 hours. Quantification of the relative density of phosphorylated Akt (vB) and ERK1/2 (vC) showed the same trend as the Western analysis.
Although AG1024 was shown to reduce IGF-induced ERK1/2 phosphorylation, it had no effect on FGF-induced ERK1/2 phosphorylation (Fig. 6iiA, top, lanes 3, 4); however, it appeared to reduce the number of differentiating, β-crystallin-reactive cells in FGF-treated explants (Fig. 6iiiF). This finding implies a role for endogenous IGF signaling in FGF-induced lens fiber differentiation.
In explants treated with vitreous in the presence of AG1024, although several cells continued to elongate and accumulate β-crystallin (Figs. 6ivF, 6ivH), the differentiation response was clearly reduced in comparison with explants treated with vitreous alone (Figs. 6ivE, 6ivG). There also appeared to be some cell loss in these vitreous-treated explants (Fig. 6ivF, arrows); this was more apparent at lower magnification (Figs. 6ivI, 6ivJ, arrows). In this context it should be noted that AG1024 alone seemed to have no deleterious effects on control explants (Figs. 6ivB, 6ivD).
Vitreous-induced Akt phosphorylation in the presence of AG1024 was activated within 20 minutes and maintained for up to 18 hours (Figs. 6vA, top; 6vB). In contrast, the duration of ERK1/2 phosphorylation was reduced, being activated within 20 minutes and only maintained for up to 6 hours, before returning to basal levels (Figs. 6vA, middle; 6vC).
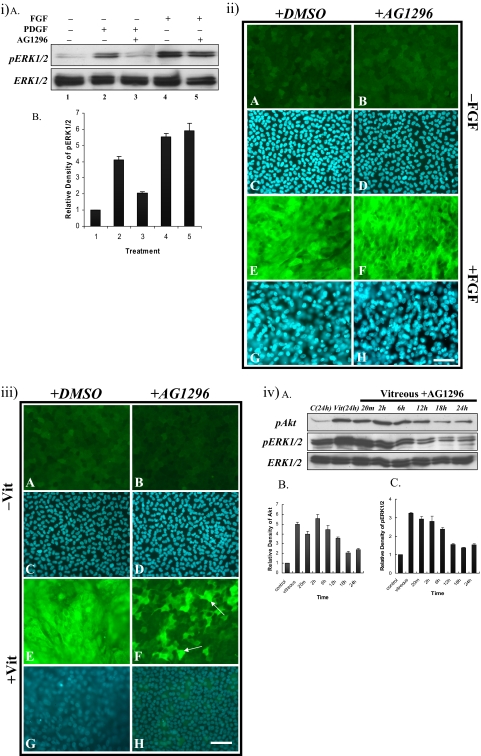
Inhibition of PDGFR Signaling (AG1296).
We examined the selectivity of AG1296 by using Western blot and immunofluorescent staining. AG1296 (1 μM) effectively decreased PDGF-induced ERK1/2 phosphorylation within 20 minutes (Figs. 7iA, top, lanes 2, 3; 7iB), but not FGF-induced ERK1/2 phosphorylation (Figs. 7iA, top, lanes 4, 5; 7iB). In the presence of AG1296, FGF-treated cells still elongated and expressed β-crystallin (Figs. 7iiF, 7iiH), similar to explants treated with only a high fiber-differentiating dose of FGF (Figs. 7iiE, 7iiG). Cells in explants treated without FGF, with or without AG1296, remained as an epithelial monolayer (Figs. 7iiA–D).
Figure 7.
Effect of PDGFR inhibitor, AG1296 on vitreous-induced lens fiber differentiation. (iA) Representative Western blots of explants cultured without growth factor (lane 1) with PDGF (15 ng/mL; lanes 2 and 3) or FGF (100 ng/mL; lanes 4 and 5) in the presence (lanes 3 and 5) or absence (lanes 2 and 4) of 1 mM AG1296, added 2 hours before growth factor treatment. AG1296 blocked PDGF-induced ERK1/2 phosphorylation, but had no effect on FGF-induced ERK1/2 phosphorylation. Quantification of the relative density of phosphorylated ERK1/2 (iB) showed the same trend as the Western analysis. (ii) Representative micrographs of lens explants cultured with 1 mM AG1296 and FGF (100 ng/mL). The explants were cultured without (A–D) or with (E–H) FGF, in the presence (B, D, F, H) or absence (A, C, E, G) of 1 mM AG1296 added 2 hours before FGF treatment. AG1296 did not block FGF-induced fiber differentiation (F). (iii) Representative micrographs of lens explants cultured with AG1296 and vitreous. Explants were cultured without (A–D) or with (E–H) vitreous, in the presence (B, D, F, H) or absence (A, C, E, G) of 1 mM AG1296 added 2 hours before vitreous treatment. AG1296 reduced vitreous-induced lens fiber differentiation. Some cells still expressed β-crystallin (F, arrows). Scale bar, 50 μm. (ivA) Representative Western blots of explants cultured without (control) or with vitreous in the presence or absence of 1 mM AG1296, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), and total ERK1/2 (bottom). Duration of ERK1/2 phosphorylation was reduced to 6 hours. Quantification of the relative density of phosphorylated Akt (ivB) and ERK1/2 (ivC) showed the same trend as the Western analysis.
To examine the function of PDGF in vitreous-induced lens fiber differentiation, we added AG1296 to the explants before adding vitreous. Unlike cells in explants treated with vitreous alone that differentiated and showed strong expression of β-crystallin (Figs. 7iiiE, 7iiiG), the cells in explants cultured with AG1296 and vitreous did not elongate; however, some did still express β-crystallin (Figs. 7iiiF, 7iiiH, arrows). Cells in explants treated without vitreous, with or without AG1296, remained as an epithelial monolayer (Figs. 7iiiA–D).
When we used Western blot to examine the phosphorylation of Akt and ERK1/2 in vitreous-treated explants in the presence of AG1296, we found that Akt phosphorylation was activated within 20 minutes and maintained for up to 12 hours (Figs. 7ivA, top; 7ivB). ERK1/2 phosphorylation was still activated within 20 minutes; however, it was only maintained for up to 6 hours (Figs. 7ivA, middle; 7ivC), suggesting that PDGF in the vitreous may contribute to lens fiber differentiation by promoting an extended ERK1/2 phosphorylation profile.
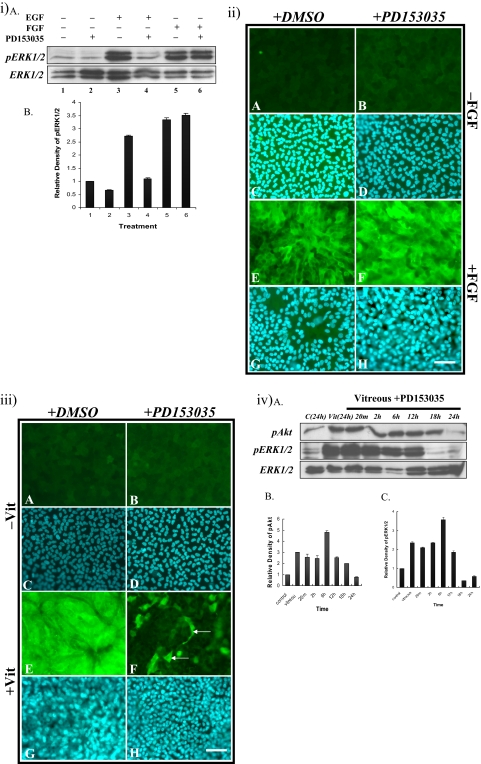
Inhibition of EGFR Signaling (PD153035).
Western blot analysis showed that ERK1/2 phosphorylation in control explants was not influenced by treatment with PD153035 (Figs. 8iA, top, lanes 1, 2; 8iB). EGF was able to induce ERK1/2 phosphorylation within 20 minutes (Figs. 8iA, top, lane 3; 8iB) and the reaction was readily blocked to basal levels in the presence of PD153035 (Figs. 8iA, top, lane 4; 8iB). FGF-induced ERK1/2 phosphorylation was not effected in the presence of PD153035 (Figs. 8iA, top, lanes 5, 6; 8iB). Consistent with this, FGF still induced fiber cell differentiation and the accumulation of β-crystallin in the presence of PD153035 (Figs. 8iiF, 8iiH).
Figure 8.
(iA) Representative Western blots of explants cultured without growth factor (lanes 1 and 2), with EGF (lanes 3 and 4), or with FGF (lanes 5 and 6) in the presence (lanes 2, 4, and 6) or absence (lanes 1, 3, and 5) of 50 nM PD153035, added 2 hours before growth factor treatment. Quantification of the relative density of (iB) phosphorylated ERK1/2 showed the same trend as the Western analysis. (ii) Representative micrographs of lens explants cultured without (A–D) or with (E–H) 100 ng/mL FGF, in the presence (B, D, F, H) or absence (A, C, E, G) of 50 nM PD153035 added 2 hours before FGF treatment. PD153035 did not block FGF-induced fiber differentiation (F). (iii) Representative micrographs of lens explants cultured without (A-D) or with (E–H) vitreous, in the presence (B, D, F, H) or absence (A, C, E, G) of 50 nM PD153035, added 2 hours before vitreous treatment. PD153035 reduced vitreous-induced lens fiber differentiation. Some cells still expressed β-crystallin (F, arrows). (iv) Western blot analysis of explants treated with vitreous and PD153035. (ivA) Representative Western blots of explants cultured without or with vitreous in the presence or absence of 50 nM of PD153035, added 2 hours before vitreous treatment, assayed for phosphorylated Akt (top), phosphorylated ERK1/2 (middle), or total ERK1/2 (bottom). Akt and ERK 1/2 were phosphorylated within 20 minutes and continued for up to 18 and 12 hours, respectively. Quantification of the relative density of phosphorylated Akt (ivB) and ERK1/2 (ivC) showed the same trend as the Western analysis. Scale bar, 50 μm.
In vitreous-treated explants containing PD153035, some cells differentiated and expressed β-crystallin (Fig. 8iiiF, arrows), whereas others in the same explants did not, remaining as a monolayer (Fig. 8iiiF).
Vitreous-induced phosphorylation of Akt in explants treated with PD153035 was stimulated within 20 minutes and maintained for up to 18 hours (Figs. 8ivA, top; 8ivB). Moreover, ERK1/2 phosphorylation was activated within 20 minutes but was reduced in duration from 18 to 12 hours (Figs. 8ivA, middle; 8ivC).
Overall, the common feature of all inhibitor studies was that they reduced the duration of vitreous-induced ERK1/2 phosphorylation and that reduction blocked or at least diminished the fiber differentiation response.
Discussion
It is well established that vitreous humor or a high dose of FGF can induce lens fiber cell differentiation and that this process has been linked with the ability of both vitreous and FGF to promote a prolonged phosphorylation of ERK1/2.36 Although the lens epithelial cell response to vitreous or FGF appears to be similar, at both the morphologic and molecular levels, it is clear from the present study that the intracellular signaling pathways induced by the two differ. We have reported that an appropriate high dose of FGF can readily mimic the ERK1/2 phosphorylation profile induced by vitreous in lens cells36; however, unlike a fiber-differentiating dose of FGF, vitreous can also strongly stimulate the PI3-K/Akt signaling pathway, with phosphorylation of Akt persisting for up to 6 hours. This vitreous-induced Akt activation cannot be attributed to the presence of FGF, but may be attributable to IGF, as in the present study, it was the only growth factor able to stimulate a strong and sustained (up to 6 hours) Akt phosphorylation in lens explants, similar to vitreous. Surprisingly, we did not observe any decrease in the duration and level of Akt phosphorylation when we blocked the RTK signaling pathways, but an increase, longer than the Akt phosphorylation profile normally induced by vitreous. As Ras activates Raf and PI3-K/Akt in cells,38,39 given that ERK1/2 have been shown to repress SOS (the guanine nucleotide exchange factor for Ras), mediated by RSK2, to downregulate the activation of Ras,40 any repression of ERK1/2 phosphorylation (by blocking RTK signaling) may lead to the elevation of activated Ras, therefore increasing Akt phosphorylation in the cells. Overall, consistent with earlier studies supporting an essential role for PI3-K/Akt signaling in lens fiber differentiation,37 we propose that IGF or a combination of yet to be identified factors in the vitreous promote PI3-K signaling and Akt phosphorylation in the lens.
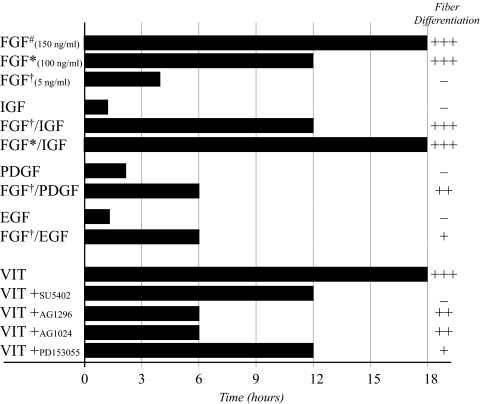
It is clear from this study that besides FGF, other growth factors that are known to be present in the ocular media, including IGF, PDGF, and EGF, may play a role in vitreous-induced lens fiber differentiation. These growth factors have all been reported to induce cell proliferation in lens explants,41,42 but cannot independently induce lens fiber differentiation (Fig. 1, Table 1). Consistent with earlier studies,25,43 our results show that FGF, FGF/IGF, FGF/PDGF, and now for the first time FGF/EGF, all have the ability to induce features of lens fiber differentiation, with some growth factors, such as IGF, being more potent than others, such as PDGF or EGF, in potentiating FGF-induced lens fiber differentiation. Consistent with this, the combination of any one of the growth factors examined, with a low dose of FGF, extended the duration of ERK1/2 phosphorylation, potentiating the effects of the proliferating dose of FGF (Table 1). Overall, results from this study clearly show that a prolonged phosphorylation of ERK1/2 is associated with fiber cell differentiation, with the factors inducing the longest duration of ERK1/2 phosphorylation providing the more potent fiber differentiation response in lens epithelial explants (Table 1). This is the first study to report that specific growth factors can potentiate the effects of FGF by extending its ability to induce the phosphorylation of ERK1/2 in lens cells. Other studies in different tissues have identified a similar synergistic effect of several growth factors in the activation of different signaling pathways, including CNTF and FGF in neural cells,44 TGFβ and FGF in bone organ culture,45 and FGF and IGF in neuroblastoma cells.46
Table 1.
pERK1/2 Duration in Lens Epithelial Explants
Summary of the duration of ERK1/2 phosphorylation over a 24-hour period, in cultured explants of lens epithelial cells exposed to different treatments. Each treatment is scored for its ability to induce lens fiber differentiation in vitro. −, no response; +, weak response, with some multilayering and β-crystallin accumulation; +++, strong response, with extensive multilayering, cell elongation, and β-crystallin accumulation. #Iyengar et al.36; *100 ng/mL; †5 ng/mL.
To better understand the role of specific vitreous-derived growth factors during the process of lens fiber differentiation, we used a range of RTK inhibitors to selectively block their effects. Consistent with many earlier studies, FGF signaling was essential for the induction of lens fiber differentiation as the FGFR inhibitor, SU5402, completely blocked vitreous-induced lens fiber differentiation. PDGF or EGF receptor inhibitors partially blocked vitreous-induced fiber differentiation, with many cells still expressing β-crystallin and differentiating, indicating that these growth factors are not essential for lens fiber differentiation. When we blocked IGFR-induced signaling, vitreous could still induce lens epithelial cell differentiation and expression of β-crystallin; however, unlike the effects of PDGF and EGF, some cells died during the culture period, suggesting that IGF signaling may be necessary for cell survival.47 This notion is supported by other studies that compared the long-term differentiating abilities of FGF and vitreous on lens explants; vitreous maintained the fiber differentiation process longer than FGF in vitro.48 In contrast to vitreous-treated explants, some fiber cells in explants induced by FGF degenerated over time,48 indicating a role for other vitreous-derived factors, like IGF, for maintenance of intact lens fiber cell viability.
We extended our blocking studies to examine the duration of vitreous-induced ERK1/2-phosphorylation in lens cells, in the presence of the different RTK inhibitors. Blocking any of the RTK signaling pathways using the different selective inhibitors for FGFR, PDGFR, IGFR, or EGFR significantly reduced the levels of vitreous-induced ERK1/2 phosphorylation (Table 1), suggesting that each growth factor may be important in sustaining (and/or possibly potentiating) the duration of ERK1/2 phosphorylation, leading to fiber differentiation. Of interest, although blocking FGFR signaling reduced the level of vitreous-induced ERK1/2 phosphorylation to levels that would normally be sufficient to promote fiber differentiation, the loss of this FGFR activity resulted in no fiber differentiation. Together with findings in previous studies,36 the results of this study suggest that prolonged phosphorylation of ERK1/2 is necessary but not sufficient for fiber differentiation to proceed. In relation to this finding, it should be noted that FGF receptors have been shown to be localized to fiber cell nuclei.49 Taken together with the fact that FGF signaling can also be mediated by translocation of its receptor to the cell nucleus,50 in the presence of SU5402, this process may be compromised and the fiber differentiation process may be prevented from proceeding, irrespective of the FGFR-mediated signaling (and sustained ERK1/2 phosphorylation) initiated at the cell surface.
Considering that all the vitreous-derived growth factors examined in this study are potentially involved in promoting the distinct ERK1/2 phosphorylation profile leading to fiber differentiation in situ, it is reasonable to suggest that they may each contribute, to some extent, to the activation of the ERK1/2 pathway for vitreous-induced differentiation. However, in light of the large volume of evidence from previous studies, it is clear that FGF is a necessary component of this vitreous effect, as only FGF alone or combinations of factors that include FGF, can induce fiber differentiation. Results from the FGFR inhibition (using SU5402) experiments in the present study are also consistent with this. Given the observation that, compared with the other factors, IGF showed a greater ability to potentiate FGF's phosphorylation of ERK, as well as better mimic the effect of vitreous on Akt phosphorylation, IGF may also play a prominent role in contributing to the initiation, promotion, and maintenance of lens fiber differentiation in situ.
In conclusion, although FGF is essential for the induction of lens fiber cell differentiation, the results of this study emphasize the significance of other ocular growth factors that act in concert with FGF and are necessary for the distinct vitreous-induced signaling leading to lens fiber differentiation.
Footnotes
Supported by the Sydney Foundation for Medical Research; the National Health and Medical Research Council (NHMRC, Australia); Grant R01 EY03177 from the National Institutes of Health, Bethesda, MD; the Ophthalmic Research Institute of Australia; and the Australian Federal Government through the Cooperative Research Centres Programme, undertaken as part of the Vision Cooperative Research Centre, New South Wales, Sydney, Australia.
Disclosure: Q. Wang, None; J.W. McAvoy, None; F.J. Lovicu, None
References
- 1.Coulombre JL, Coulombre AJ. Lens development: fiber elongation and lens orientation. Science 1963;142:1489–1490 [DOI] [PubMed] [Google Scholar]
- 2.McAvoy JW, Chamberlain CG. Growth factors in the eye. Prog Growth Factor Res 1990;2:29–43 [DOI] [PubMed] [Google Scholar]
- 3.Beebe DC, Silver MH, Belcher KS, Van Wyk JJ, Svoboda ME, Zelenka PS. Lentropin, a protein that controls lens fiber formation, is related functionally and immunologically to the insulin-like growth factors. Proc Natl Acad Sci USA 1987;84:2327–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development 1993;118:117–126 [DOI] [PubMed] [Google Scholar]
- 5.Cassidy L, Barry P, Shaw C, Duffy J, Kennedy S. Platelet derived growth factor and fibroblast growth factor basic levels in the vitreous of patients with vitreoretinal disorders. Br J Ophthalmol 1998;82:181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majima K. Presence of growth factor in human vitreous. Ophthalmologica 1997;211:226–228 [DOI] [PubMed] [Google Scholar]
- 7.Katsura Y, Okano T, Noritake M, et al. Hepatocyte growth factor in vitreous fluid of patients with proliferative diabetic retinopathy and other retinal disorders. Diabetes Care 1998;21:1759–1763 [DOI] [PubMed] [Google Scholar]
- 8.Shui YB, Wang X, Hu JS, et al. Vascular endothelial growth factor expression and signaling in the lens. Invest Ophthalmol Vis Sci 2003;44:3911–3919 [DOI] [PubMed] [Google Scholar]
- 9.Brooks HL, Jr, Caballero S, Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol 2004;122:1801–1807 [DOI] [PubMed] [Google Scholar]
- 10.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol 2005;280:1–14 [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain CG, McAvoy JW. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res 1987;6:1165–1169 [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain CG, McAvoy JW. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF). Growth Factors 1989;1:125–134 [DOI] [PubMed] [Google Scholar]
- 13.Lovicu FJ, McAvoy JW. Structural analysis of lens epithelial explants induced to differentiate into fibres by fibroblast growth factor (FGF). Exp Eye Res 1989;49:479–494 [DOI] [PubMed] [Google Scholar]
- 14.McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development 1989;107:221–228 [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain CG, McAvoy JW. Fiber differentiation and polarity in the mammalian lens: a key role for FGF. Prog Retin Eye Res 1997;16:443–478 [Google Scholar]
- 16.Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development 1995;121:3959–3967 [DOI] [PubMed] [Google Scholar]
- 17.Chow RL, Roux GD, Roghani M, et al. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development 1995;121:4383–4393 [DOI] [PubMed] [Google Scholar]
- 18.Robinson ML, Overbeek PA, Verran DJ, et al. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development 1995b;121:505–514 [DOI] [PubMed] [Google Scholar]
- 19.Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development 1998;125:3365–3377 [DOI] [PubMed] [Google Scholar]
- 20.Robinson ML, Ohtaka-Maruyama C, Chan CC, et al. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol 1998;198:13–31 [DOI] [PubMed] [Google Scholar]
- 21.Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development 2001;128:1617–1627 [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Yang T, Madakashira BP, et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol 2008;318:276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson NA, Chamberlain CG, McAvoy JW. IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of different ages. Invest Ophthalmol Vis Sci 1993;34:3303–3312 [PubMed] [Google Scholar]
- 24.Liu J, Chamberlain CG, McAvoy JW. IGF enhancement of FGF-induced fibre differentiation and DNA synthesis in lens explants. Exp Eye Res 1996;63:621–629 [DOI] [PubMed] [Google Scholar]
- 25.Kok A, Lovicu FJ, Chamberlain CG, McAvoy JW. Influence of platelet-derived growth factor on lens epithelial cell proliferation and differentiation. Growth Factors 2002;20:27–34 [DOI] [PubMed] [Google Scholar]
- 26.Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development 2004;131:1813–1824 [DOI] [PubMed] [Google Scholar]
- 27.Shirke S, Faber SC, Hallem E, et al. Misexpression of IGF-I in the mouse lens expands the transitional zone and perturbs lens polarization. Mech Dev 2001;101:167–174 [DOI] [PubMed] [Google Scholar]
- 28.Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A alters lens growth and development. Dev Biol 1996;180:554–565 [DOI] [PubMed] [Google Scholar]
- 29.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000;103:211–225 [DOI] [PubMed] [Google Scholar]
- 30.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003;284:31–53 [DOI] [PubMed] [Google Scholar]
- 31.Lamothe B, Yamada M, Schaeper U, Birchmeier W, Lax I, Schlessinger J. The docking protein Gab1 is an essential component of an indirect mechanism for fibroblast growth factor stimulation of the phosphatidylinositol 3-kinase/Akt antiapoptotic pathway. Mol Cell Biol 2004;24:5657–5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol 2004;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development 2001;128:5075–5084 [DOI] [PubMed] [Google Scholar]
- 34.Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol 2001;233:394–411 [DOI] [PubMed] [Google Scholar]
- 35.Zatechka SD, Jr, Lou MF. Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 1. The mitogenic and stress responses. Exp Eye Res 2002;74:703–717 [DOI] [PubMed] [Google Scholar]
- 36.Iyengar L, Wang Q, Rasko JE, McAvoy JW, Lovicu FJ. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation 2007;75:662–668 [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Stump R, McAvoy JW, Lovicu FJ. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp Eye Res 2009;88:293–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Viciana P, Warne PH, Dhand R, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 1994;370:527–532 [DOI] [PubMed] [Google Scholar]
- 39.Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol 1994;4:798–806 [DOI] [PubMed] [Google Scholar]
- 40.Douville E, Downward J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene 1997;15:373–383 [DOI] [PubMed] [Google Scholar]
- 41.Iyengar L, Patkunanathan B, Lynch OT, McAvoy JW, Rasko JE, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res 2006;83:667–678 [DOI] [PubMed] [Google Scholar]
- 42.Iyengar L, Patkunanathan B, McAvoy JW, Lovicu FJ. Growth factors involved in aqueous humour-induced lens cell proliferation. Growth Factors 2009;27:50–62 [DOI] [PubMed] [Google Scholar]
- 43.Klok E, Lubsen NH, Chamberlain CG, McAvoy JW. Induction and maintenance of differentiation of rat lens epithelium by FGF-2, insulin and IGF-1. Exp Eye Res 1998;67:425–431 [DOI] [PubMed] [Google Scholar]
- 44.Song MR, Ghosh A. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci 2004;7:229–235 [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee A, Dong SS, Clemens T, Alvarez J, Serra R. Co-ordination of TGF-beta and FGF signaling pathways in bone organ cultures. Mech Dev 2005;122:557–571 [DOI] [PubMed] [Google Scholar]
- 46.Russo VC, Andaloro E, Fornaro SA, et al. Fibroblast growth factor-2 over-rides insulin-like growth factor-I induced proliferation and cell survival in human neuroblastoma cells. J Cell Physiol 2004;199:371–380 [DOI] [PubMed] [Google Scholar]
- 47.Chandrasekher G, Sailaja D. Phosphatidylinositol 3-kinase (PI-3K)/Akt but not PI-3K/p70 S6 kinase signaling mediates IGF-1-promoted lens epithelial cell survival. Invest Ophthalmol Vis Sci 2004;45:3577–3588 [DOI] [PubMed] [Google Scholar]
- 48.O'Connor MD, Wederell ED, de Iongh R, Lovicu FJ, McAvoy JW. Generation of transparency and cellular organization in lens explants. Exp Eye Res 2008;86:734–745 [DOI] [PubMed] [Google Scholar]
- 49.de Iongh RU, Lovicu FJ, Hanneken A, Baird A, McAvoy JW. FGF receptor-1 (flg) expression is correlated with fibre differentiation during rat lens morphogenesis and growth. Dev Dyn 1996;206:412–426 [DOI] [PubMed] [Google Scholar]
- 50.Bryant DM, Stow JL. Nuclear translocation of cell-surface receptors: lessons from fibroblast growth factor. Traffic 2005;6:947–954 [DOI] [PubMed] [Google Scholar]