Responses of retinal ganglion cells to locally applied glutamate were similar in normal and degenerated retinas and exhibited moderate to robust evoked responses that varied between cells, from moderate firing rates to high-frequency repetitive bursting. This article explores important parameters for a prototype neurotransmitter-based retinal prosthesis, including effective glutamate concentrations, monotonic dependence of responses on duration of ejection, and differential effects on different RGC types.
Abstract
Purpose.
To investigate the suitability of glutamate as a potential agent for a neurotransmitter-based retinal prosthesis.
Methods.
Retinal ganglion cells (RGCs) from P35-70 albino Sprague-Dawley (normal) and P60-254 S334ter-4 (photoreceptor degeneration) rats were recorded extracellularly in flattened eye cup preparations, to assess their responses to glutamate, applied locally via micropipettes.
Results.
Brief local application of glutamate effectively excited RGCs in both normal and degenerated retinas. Epiretinal surface application of glutamate was less likely to excite RGCs than was subsurface application (20 μm below the epiretinal surface). Glutamate evoked RGC firing rates, and the response patterns were similar for epiretinal surface and subsurface applications. Subsurface application of 2 mM glutamate effectively excited cells within 130 μm of the ejection sites. Response latencies averaged 281 ms and were significantly longer for OFF RGCs than for ON RGCs in normal retinas (P = 0.025). Suppression of activity was observed at shorter latencies (∼100 ms) after glutamate application in most of the spontaneously active RGCs. Responses to each glutamate application were similar, and the duration of activity was directly dependent on the duration of application. RGC responses varied from recurrent high-frequency bursts to sustained firing at rates above 40 spikes/s, in normal and degenerated retinas. Paired, sequential applications of glutamate evoked two distinguishable responses, with interstimulus intervals as low as 200 ms. Overall, RGC response sensitivity to glutamate was similar in normal and degenerated retinas.
Conclusions.
Glutamate is an excellent candidate for a neurotransmitter-based retinal prosthesis, as its local application effectively stimulates RGCs with high spatial and temporal resolution.
An effective retinal prosthesis would improve the lives of hundreds of thousands of patients with retinitis pigmentosa (RP) or millions of blind patients with advanced age-related macular degeneration (ARMD). Research is being conducted worldwide to employ electrical stimulation as a means of modulating neural activity within the inner retina to restore vision in such patients.1–12 Significant progress has been made toward the development of long-term, implantable retinal prostheses, because of the application of advances in microelectronics, packaging, and electrode fabrication methods. Acute and chronic human testing has revealed three important findings. First, prescreened patients with retinal degenerations, including RP and ARMD, perceive white flashes of light in response to epiretinal or subretinal electrical stimulation. Second, retinal prostheses are well tolerated in patients with long-term implantation of these devices. Third, although the temporal features of electrical stimuli correlate well with electrophosphene percepts, patients demonstrate greater difficulty in perceiving shapes when more complex spatial patterns of electrical stimulation are required.2,13,14 In patients with advanced retinal degeneration, electrophosphenes often necessitate high electrical currents and large electrodes. The requirement for relatively large electrodes limits the effective resolution of electrically based retinal prostheses. Small electrodes for high-resolution devices are prone to high charge densities and compliance voltages that may electrochemically dissociate water into hydrogen peroxide.15–17 Thus, small-diameter electrodes are more likely to induce retinal tissue damage from free radicals that are toxic to the lipid membranes of neurons and glia. In addition to these electrochemical limitations, electrical stimulation is nonspecific, in that it stimulates ON and OFF retinal ganglion cell somata and axons, bipolar cells, and, very likely, amacrine cells in nonphysiological sequences that may reduce contrast and spatial localization within the retina. Thus, electrical stimulation has limitations in encoding important sensory features used in normal central visual processing.
To the extent that the spatial resolution of these devices remains poor, relatively few patients will benefit from them. Without a high-resolution retinal prosthesis, patients with advanced age-related macular degeneration would probably not benefit from such devices. We hypothesize that many of these limitations can be circumvented by using more naturalistic means of stimulating retinal ganglion cells (RGCs) for retinal prosthesis. Natural vision is encoded as neurotransmitter signals with glutamate as the primary transmitter.
Glutamate, which is generally accepted as the transmitter released by photoreceptor, bipolar, and retinal ganglion cells, may provide a more natural means of stimulating RGCs. Ionotropic and metabotropic glutamate receptor subtypes are differentially expressed by various retinal neuronal cell types. Glutamate receptors have been shown to participate in normal visual activation of RGCs,18,19 and include kainate, AMPA, and NMDA receptor subtypes.20–23 Bath application and iontophoretic application of glutamate have produced mixed effects on RGCs.18,24–26 When delivered via bath application, glutamate has been shown to evoke an initial hyperpolarization, followed by a depolarization in seven of nine RGCs, while evoking only a hyperpolarization in the other two.18 Iontophoretic application of glutamate and kainate consistently excites RGCs in the cat.24 However, other glutamate agonists, AMPA, quisqualate, and NMDA often also inhibit RGCs, probably through activation of inhibitory neurons.24,26 Excitation of RGCs by exogenous glutamate may also occur through glutamatergic excitation of other cells in the retinal circuit, including bipolar and amacrine cells.
Damping the response to exogenous glutamate application are the excitatory amino acid transmitter (EAAT) pumps that rapidly remove extracellular glutamate and maintain a high signal-to-noise ratio.27 These systems are likely to have reserve capacity to remove exogenously applied extracellular glutamate, since in retinal degeneration, photoreceptors that constitutively release glutamate have been lost.
In this study, we investigated the feasibility of activating RGCs through a neurotransmitter-based retinal prosthesis via local, small-volume epiretinal application of glutamate-containing solutions in flattened eye cup preparations in both normal retinas and retinas from an animal model (S334ter) of RP (degenerated retinas). In S334ter line 4 (S334ter-4) rats, rhodopsin with a truncated C-terminal region is expressed in photoreceptors, which results in photoreceptor loss and a decrease of >85% in ONL thickness by postnatal day (P)180.28 RGCs were examined in retinas from S334ter-4 rats older than P180 to determine whether sequelae associated with advanced photoreceptor degeneration affects the sensitivity of RGCs to glutamate.
Methods
The Wayne State University Animal Investigation Committee approved the care and use of all animals in the study. In addition, the animals were maintained and treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Flattened wholemount retinas were prepared from male Sprague-Dawley rats, 35 to 70 days old (Harlan Sprague-Dawley, Indianapolis, IN) and heterozygote S334ter-4 rats (60–254 days old). The animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Enzymatic vitrectomy of both eyes was performed by intravitreal injection of 2 μL of 4 IU/mL plasmin (dissolved in Ames medium; Sigma-Aldrich, St. Louis, MO). All subsequent procedures were performed in dim red light. The animals remained anesthetized until decapitation and enucleation, 20 minutes after plasmin injections. The eyes were transferred to cold (4°C) Ames medium, which was constantly aerated with 95%/5% O2/CO2. The eyes were circumferentially dissected to remove the cornea and lens. Four to five radial cuts in the resulting eye cup allowed the eye to be flattened. The eye cups were transferred to the perfusion chamber on a fixed stage for recordings. The retinas were held in place with bridal veil and constantly perfused (8 mL/min) with warm (34°C), aerated (95%/5% O2/CO2) Ames medium.
RGC activity was recorded extracellularly (Multiclamp 700B amplifier; Molecular Devices, Sunnyvale, CA), and the signals were digitized at 10 kHz (Digidata 1322 and pClamp 9; Molecular Devices). Recording electrodes were filled with 2 M NaCl and had resistances ranging from 2 to 20 MΩ. Glass micropipettes for local drug application had tip openings between 1 and 2 μm and were filled with solutions containing 400 μM to 10 mM glutamate dissolved in Ames medium. Recording and drug pipettes were manipulated into the tissue by using two robotic micropositioners (MP285; Sutter Instruments, Novato, CA). Microscopic visualization was achieved with a fixed-stage, water-immersion video microscope on a motorized base (BX51; Olympus, Center Valley, PA and MT-500; Sutter Instruments). A high-speed, infrared-sensitive, progressive-scan CCD camera was used to visualize the retinal preparation with an invisible 800-nm light (Sensicam QE camera; Cooke Corp., Romulus, MI, with Camware video capture software; PCO Imaging, Kelheim, Germany). Visual stimuli were delivered into the microscope's afocal optical path with a digital video projector with 800 × 600-pixel resolution (Samsung, Ridgefield Park, NJ), controlled via a digital video pattern generator (VSG 2/3; Cambridge Systems, Kent, UK). Images of electrode positions were captured to determine the distances between recording and drug application sites. The drugs were applied with a pressure ejection module (PPM-2; Neuro Phore BH-2; Harvard Apparatus, Holliston, MA). In the first set of experiments on the retinas from Sprague-Dawley rats, glutamate was applied at the surface of the retina just above the ganglion cell layer. In the second set of experiments on retinas from Sprague-Dawley and S334ter-4 rats, glutamate was applied with the tip of the electrode 15 to 20 μm below the retinal surface.
Retinal ganglion cell responses to brief full-field illumination were classified as ON, OFF, or unresponsive. The dependence of RGC responses on the duration and interejection interval of pressure-puffed glutamate solutions were characterized. Glutamate was applied after recording at least 2 seconds of background activity, and the same parameters were repeated sequentially 20 times. The interval between the start of each trial was 10 seconds. Response latencies were determined from the onset of the command pulse to the first spike of responses, when the cells exhibited low spontaneous rates. In cells with sustained spontaneous firing activity, response latency was defined as the time from the ejection command pulse to the first bin in peristimulus histograms that exceeded background activity.
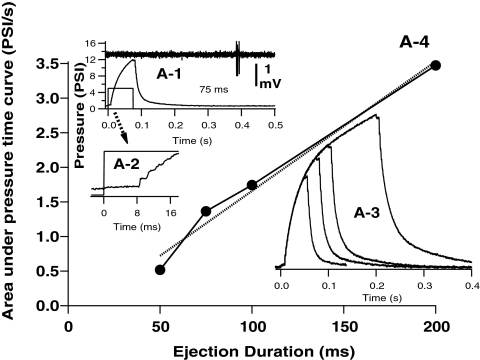
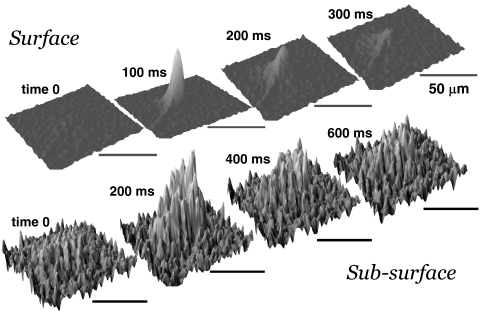
Pressure applied to the drug pipette was measured at the output of the PPM-2 outlet and digitized simultaneously with recordings. The pressure pulse for drug application initially increased exponentially from baseline after a fixed 9-ms electronic switching delay from the command pulse onset (Fig. 1). The area under the pressure versus time curve increased approximately linearly with ejection duration (Fig. 1; A4), within the range of the durations tested (50–400 ms). The spatial spread of ejection solutions within the retina was characterized after physiological recordings were completed using epifluorescent imaging (FITC excitation/emission filter) of 0.01% fluorescein included in the ejection solution. Epifluorescence images were sequentially collected at rates of 5 to 10 frame/s, and differential fluorescence (Δf/f) images were computed by subtracting a pre-ejection baseline control image, offline (Fig. 2).
Figure 1.
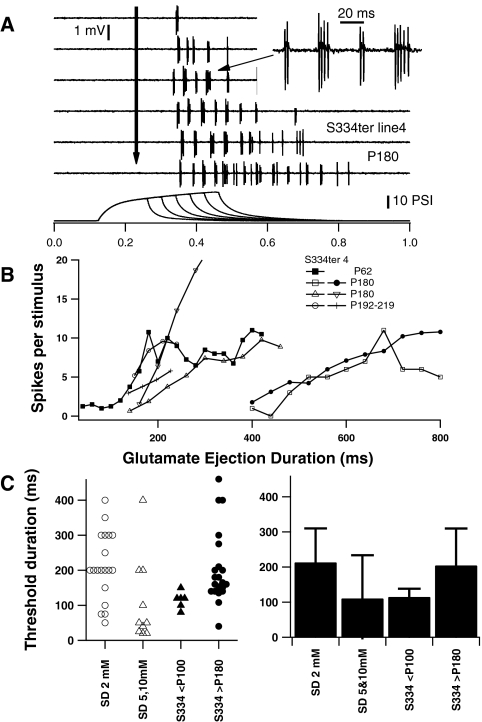
Pressure application of glutamate solutions from pipettes was controlled by TTL command pulses (A-1, A-2, square pulses). After an initial delay of approximately 9 ms from the command onset (A-2, command and pressure waves), pressure (measured at the BH-2 output) initially increases at an exponential rate (τ = 35–38 ms) over time, due to capacitance in the tubing. Pressure waves for different duration commands (e.g., 50-, 75-, 100-, and 200-ms command pulses, A-3) follow similar time courses. The area (A-4, data, ●; linear fit, dashed line) under the pressure ejection curves increased linearly with trigger pulse durations >50 ms. Excitatory responses of RGC cells (A-1, extracellular recording trace above command and pressure traces) typically occurred after shorter (<200ms) ejections were completed.
Figure 2.
Pressure application of drug solutions were confined to small regions of the retina. Surface plots before (time 0) and from the start of 100-ms command pulse show that surface (top images, 10 frames/s) and subsurface (bottom images, 5 frames/s) ejections were restricted to a small area of the retina (radii of ejections were less than 100 μm) and did not spread substantially over time. The fluorescence profiles for subsurface ejections were irregular due to cellular exclusion of dye. Scale bars, 50 μm.
Data are expressed as means ± standard errors (SE). Comparisons between groups were statistically examined with Student's t-tests and multiple factor ANOVA. Significance levels were set at P = 0.05.
Results
RGC Responses to Locally Applied Glutamate in Normal (SD Rat) Retinas
The effects of locally applied glutamate at 0.4-, 0.8-, 2.0-, 5.0-, and 10-mM concentrations were tested in normal Sprague-Dawley rat retinas, to determine the basic parameters for effective stimulation of retinal ganglion cells. Local exogenous application of glutamate was examined in 15 Sprague-Dawley rat retinas in flattened eye cup preparations and effectively excited RGCs. Application of glutamate at concentrations between 2 and 10 mM, either at the retinal surface or 15 to 20 μm below the surface (subsurface) excited 28 (65%) of 43 RGCs (Table 1). Subsurface ejection of 2 or 5 mM glutamate was effective in 18 (67%) of 27 cells, whereas surface application of 2 to 10 mM glutamate was effective in 10 (62.5%) of 16 cells. Lower concentrations of glutamate (400 and 800 μM) applied at the surface were generally ineffective, exciting one of the five RGCs examined. Responses of individual RGCs to glutamate application were recorded for an average of 35 minutes, and the recordings lasted up to 98 minutes before the recording electrode was moved to a new cell or isolation was lost.
Table 1.
Effectiveness of Locally Applied Glutamate on Exciting RGCs in Normal and Degenerated Retinas
| Glutamate Concentration (mM) |
||||
|---|---|---|---|---|
| 0.4–0.8 | 2.0 | 5.0 | 10.0 | |
| Normal Retinas (SD Rat) | ||||
| All RGCs, 60% (29/48) | 20% (1/5) | 67.9% (19/28) | 50% (3/6) | 67% (6/9) |
| Surface | 20% (1/5) | 50% (3/6) | 100% (1/1) | 67% (6/9) |
| Subsurface | 73% (16/22) | 40% (2/5) | ||
| Subsurface: RGCs within 50 μm | 100% (8/8) | 100% (1/1) | ||
| S334ter Line 4 | ||||
| Early stages of photoreceptor loss | ||||
| Subsurface: P60–P100 | 75% (6/8) | |||
| Substantial photoreceptor loss | ||||
| Subsurface: P180–P254 | 60% (24/40) | |||
| Subsurface: P180–P220 | 52.9% (18/34) | |||
| Subsurface: P240–P254 | 100% (6/6) | |||
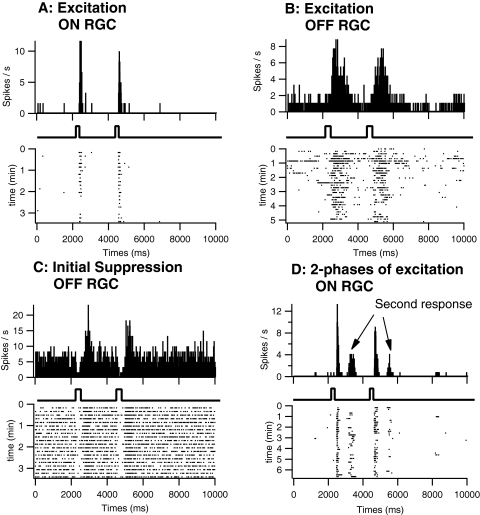
Three effects of glutamate on RGCs were observed (Fig. 3). First, glutamate application induced an increase in firing rate among all RGCs tested (Figs. 3A, 3B). Second, RGCs with high spontaneous firing rates (n = 6) exhibited an initial suppression of spontaneous firing, which was followed by excitation (Fig. 3C). Third, a secondary phase of excitation at longer latency was occasionally observed (Fig 3D).
Figure 3.
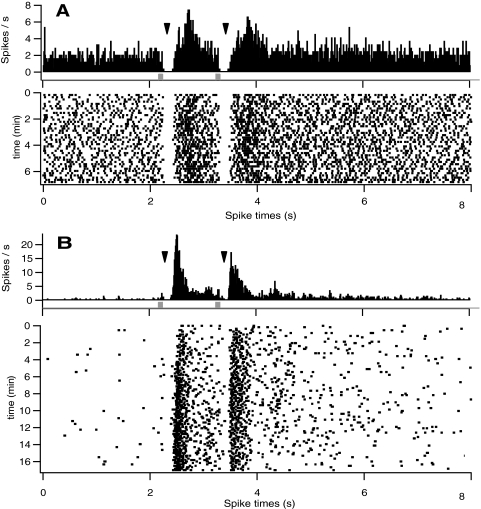
Local application of glutamate in flattened retinal eye cup preparations from normal retinas evoked excitatory, suppressive, and late excitatory responses in RGCs. Glutamate excited ON (A) and OFF (B) RGCs, activating firing of multiple spikes (raster plots at bottom of each panel) at relatively long latencies from the onset of command pulses (square pulses). Responses to a second ejection 2 seconds after initial application were similar but slightly decreased (see peristimulus histograms (PSTH), top of each panel; 30-ms bins). Although responses shown for OFF cells were longer in duration, overall the durations in ON and OFF RGCs were similar and were dependent on the duration of ejection. An initial suppression of firing was usually observed in RGCs with high spontaneous discharge rates (C). In a few cells, a longer latency secondary response to glutamate was evident (D). Data shown represent only a portion (duration for each cell shown on raster plot axis) of the overall recordings of each cell.
Glutamate excitation (2–10 mM) varied from 1 spike in response to short-duration ejections (<100 ms) to 25 spikes evoked by longer duration ejections (300–400 ms). The glutamate-evoked responses lasted up to 400 ms. The mean maximum response evoked by each glutamate application was approximately 12 to 13 spikes. Surface and subsurface glutamate application produced similar mean maximum response (surface: 13.4 ± 3.8 spikes; subsurface 12.1 ± 1.8 spikes; t-test; P = 0.75) and discharge rate (surface: 77.0 ± 24.8 spikes/s; subsurface 47.5 ± 12.1 spikes/s; t-test, P = 0.25). ON and OFF RGCs did not differ in discharge rate (ON: 46.8 ± 6.4 spikes/s; OFF: 54.7 ± 26.6 spikes/s; P = 0.73) and maximum number of spikes (ON: 14.9 ± 4.3; OFF: 12.0 ± 2.03; P = 0.58) evoked by glutamate application.
Dependence of RGC Responses on Distance from Ejection in Normal (SD Rat) Retinas
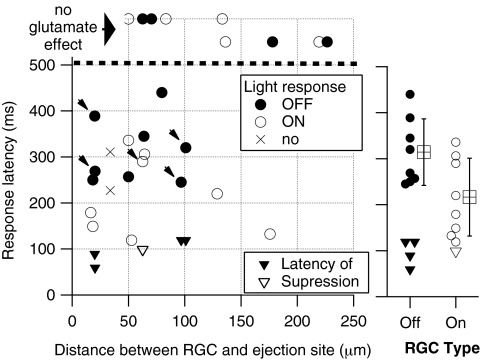
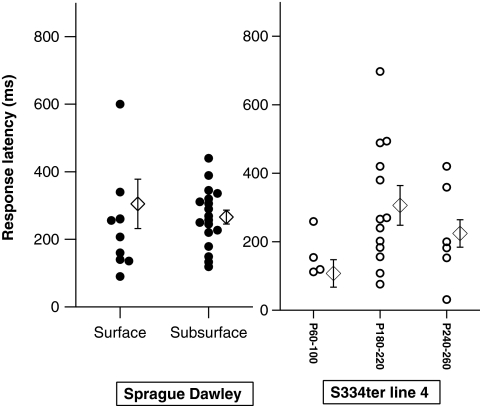
The probability of eliciting RGC responses to the exogenous glutamate application was inversely dependent on the distance between the recorded cell and the stimulation site and was directly related to the concentration of applied glutamate (Table 1, Fig. 4). Within 50 μm of the application site, subsurface ejection excited all RGCs tested, whereas surface application excited approximately 50% of tested cells. Lower glutamate concentrations were necessary to excite RGCs with the subsurface application (Table 1). Subsurface glutamate application excited 81% (17/21) of RGCs when applied within 130 μm of recorded cell somata, whereas only 17% (1/6) of RGCs responded when glutamate application was between 133 and 226 μm from the cell soma (Fig. 4). The response latency between the glutamate ejection command pulse to the first RGC response spike was dependent on cell type, but not on the location (distance and depth) or concentration of glutamate application. The mean latency of the first RGC response spike was 281 ± 30 ms, among all RGCs recorded. The average latency of responses did not differ significantly between surface (305 ± 73 ms) and subsurface (266 ± 21 ms) application (t-test, P = 0.61). The latency of the first spike also was not dependent on the distance between the cell soma and the site of drug application, but was dependent on the type of RGC (based on their light responses, Fig. 4). ON RGCs exhibited a mean latency of 230.8 ± 81 ms (range, 119–340 ms, n = 11), whereas OFF RGCs exhibited significantly longer first-spike latencies of 346.1 ± 117 ms (range, 245–600 ms, n = 9; t-test, P = 0.025).
Figure 4.
Glutamate excitation by subsurface application of glutamate in normal retinas depended on the proximity of RGCs to ejection sites, whereas latency of excitatory responses depended on RGC type. The majority (17/21) of cells recorded less than 130 μm from subsurface ejection sites were excited by glutamate. Most (five of six) cells recorded >130 μm from ejection sites were not affected by glutamate ejections (symbols, top). The latency of responses to glutamate varied considerably, with little dependence on the distance between ejection and recording sites. The average latency of responses (right) was significantly longer for OFF than ON cells, although the distribution of latencies did overlap. The average latency of suppression was shorter than excitation of OFF and ON cells. Arrows, latency of excitation in units that exhibited an initial suppression of firing.
Suppressive Effects of Glutamate Application in Normal (SD Rat) Retinas
Local exogenous glutamate application induced an initial suppression of firing activity in five of six RGCs, with moderate to high baseline spontaneous firing rates (Figs. 3, 5). This suppression was observed in all OFF (four of four) cells and one of two ON RGCs with high spontaneous activity. The mean latency between the glutamate ejection command pulse and the onset of suppressed RGC firing activity was 108 ± 13 ms (Fig. 5). The mean duration of spike suppression was 202 ± 115 ms (n = 5). The mean latency to excitation (297 ms) in cells with high spontaneous firing activity (where a transient suppression of spike activity was observed) was not significantly different from RGCs with lower spontaneous firing rates (277 ms; t-test, P = 0.67). With extracellular recording methods, it was not possible to determine whether there was transient suppression of excitability at the onset of glutamate applications in most RGCs with low spontaneous firing activity; however, we did observe transient suppression in one cell with low spontaneous firing rates by compiling responses recorded over 17 minutes (Fig. 5).
Figure 5.
Suppression of spontaneous firing activity of ON RGCs in normal retinas to subsurface glutamate ejection. Suppression (arrowheads) of firing followed by excitation was readily observed in response to a 50-ms ejection of 5 mM glutamate in a spontaneously active RGC (A). In another ON RGC with a low of spontaneous firing, an initial suppression of firing after glutamate application was also detected after summation of responses to 100 pairs of applications (B). Note that responses to glutamate were consistent over long recording periods, which lasted up to 98 minutes.
Temporal Effectiveness of Glutamate Stimulation in Normal (SD Rat) Retinas
The response delay and magnitude of each response to two sequential applications of glutamate were quite consistent. Response latencies to the first and second glutamate (surface, 2–10 mM; subsurface, 2–5 mM) application (between 500 and 4000 ms apart) were always similar. Responses to a second application of glutamate, 4 seconds after the first application, resulted in similar or slightly reduced total response in the majority of cells, with the second response level an average of 80% of first responses. One cell exhibited a marked decrease (less than 10% of the first) response, although three other cells exhibited a large (>70%) increase in responses to the second application of glutamate. The variation in relative response magnitude was not related to the magnitude or the latency of the first response to glutamate, or the type of RGC (ON/OFF).
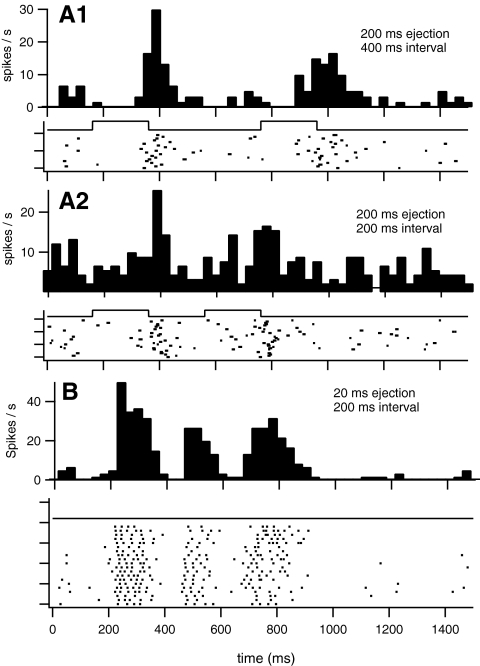
Sequential application of glutamate at intervals shorter than 500 ms between applications were also examined in 13 RGCs. Discrete responses to each application of glutamate were observed in five cells when ejections were separated by 200 ms (Fig. 6; surface application in four cells). In eight cells (three surface, five subsurface applications) discrete responses were evoked when ejections were separated by 400 or 500 ms. Cells with ON responses (n = 5: two surface; three subsurface) all required at least 400 ms between pulses to evoke two discrete response epochs. The minimum separation between ejections to evoke two discrete responses had no apparent dependence on response latency, maximum discharge rate, or spikes per stimulus.
Figure 6.
Sequential application of glutamate in normal retinas produced separable RGCs responses with intervals between ejections as short as 200 ms. Discrete responses observed in a RGC to 200-ms ejections of 10 mM ejected at the epiretinal surface separated by 200 (A2) or 400 (A1) ms. A second cell (B) exhibiting two discrete responses to 20-ms glutamate ejections separated by 200 ms also exhibited a secondary response that was also observed when the ejections were separated by 1 second (not shown).
Secondary Excitation in Normal (SD Rat) Retinas
A secondary late phase of excitation was observed in three cells (Fig. 3D). This late response was consistent in latency and duration for individual cells, but exhibited varied latency and duration between cells. For example in two cells, the latencies to the secondary response were 389 and 270 ms, whereas the durations of this activity were 1329 and 195 ms, respectively.
RGC Responses in Degenerated (S334ter-4 Rat) Retinas
The effect of subsurface locally applied 2 mM glutamate was then examined in degenerated retinas from S334ter-4 rats in flattened eye cup preparations. Glutamate was applied at a concentration of 2 mM, as this was the lowest concentration that was effective in exciting over 50% of RGCs in normal retinas. All ejections were subsurface, as this location was more effective than surface application in normal retinas. Responses to glutamate were examined in 11 retinas from P180 to P254 S334ter-4 rats and 2 retinas from P60 and P100 S334ter-4 rats. Application of 2 mM glutamate, 15 to 20 μm below the surface (subsurface) excited 24 of 40 RGCs (60%, Table 1) in retinas from P180 to P254 animals, and 6/8 (75%) in retinas from P60 to P100 S334ter-4 rats. Glutamate evoked only suppression of activity in 1 RGC within a retina from a P60 S334ter-4 rat. Similar to normal retinas, an initial suppression of spontaneous activity followed by excitation was observed in 11 spontaneously active RGCs (P60–P100, 3 RGCs; P180–P254, 8 RGCs). It should be noted that the mean spontaneous RGC firing rates in degenerated S334ter-4 retinas did not increase in relation to age or degenerated state (mean rates ± SE: P60–P100, 9.8 ± 3.6 spikes/s; P180–P220, 4.5 ± 10.35 spikes/s; and P240–P280, 3.0 ± 7.3 spikes/s). A secondary response to glutamate was observed in only 1 RGC, and this cell was in a retina from a P60 S334ter-4 rat.
RGC Response Dependence on Ejection Duration in S334ter-4 Rat Retinas
RGC responses were directly controlled by varying the duration of glutamate ejection. The number of spikes and duration of RGC responses to glutamate increased with duration of ejection (Fig. 7). The latency of responses, as may be expected, did not vary with the duration of ejection. In addition, the patterns of firing appeared to be relatively consistent during shorter and longer duration applications, and throughout longer responses. However, in responses to long duration (> 300 ms) glutamate applications, activity levels began to decrease at the end of the responses (Fig. 7). The shortest duration of glutamate ejection (threshold) which evoked a response varied between the cells. The threshold duration was relatively long (>400 ms) for some RGCs in both normal and S334ter-4 retinas. The mean threshold duration for 2 mM glutamate ejection to evoke a response in RGCs (Fig. 7C) was not significantly different between Sprague-Dawley (213.2 ± 22.2 ms) and degenerated (P180–P254, S334ter-4, 204.6 ± 23 ms) retinas (t-test, P = 0.79).
Figure 7.
Excitation of RGCs by glutamate application increased with the duration of ejection, with a minimum duration (threshold) required. The duration of the response and number of spikes evoked increased with the duration of ejection (A, B), but the firing pattern remained similar throughout the responses (A, RGC in a P180 S334ter-4 retina). The threshold duration for 2 mM glutamate ejection to evoke RGCs responses (C) was similar for RGCs in retinas from Sprague-Dawley (P32–P64), and S334ter-4 (>P180) rats. The mean threshold duration for 5 and 10 mM glutamate in Sprague-Dawley retinas and 2 mM glutamate in S334ter-4 <P100 were not significantly lower than those in Sprague-Dawley retinas with 2 mM glutamate (C, right; mean ± SD).
The duration of RGC responses and the spikes evoked by each glutamate application increased with the duration of ejection to a maximum, which was usually 100 to 200 ms longer than threshold durations (Fig. 7B). Since the duration of responses was directly dependent on the duration of glutamate ejection and the activity of many RGCs exhibited considerable variation (often bursts of activity with varied interburst intervals), the mean glutamate-evoked firing rate only partially describes RGC responses to glutamate. Therefore, we examined the distribution of interspike intervals in RGC glutamate responses.
Latency of RGC Glutamate Responses in Normal and Degenerated Retinas
The latency of the first spike in RGC responses from the glutamate ejection command pulse was also compared in normal and degenerated retinas (Fig 8). The mean latency of responses in normal retinas (281 ± 30 ms) was not significantly different from those in degenerated retinas (S344ter-4 >P180; 280 ± 39 ms; t-test, P = 0.64). However, the variation in the latency of responses was significantly greater in s344-ter retinas (Bartlett, P = 0.019), which was mainly due to three cells in degenerated retinas with glutamate response latencies of >400 ms.
Figure 8.
The latency of responses to local application of glutamate ejection measured from command pulse onset was not significantly different between surface and subsurface application in normal retinas, nor between RGCs in retinas from Sprague-Dawley and S334ter-4 rats (mean ± SE, diamonds).
RGC Response Patterns to Glutamate in Normal and Degenerated Retinas
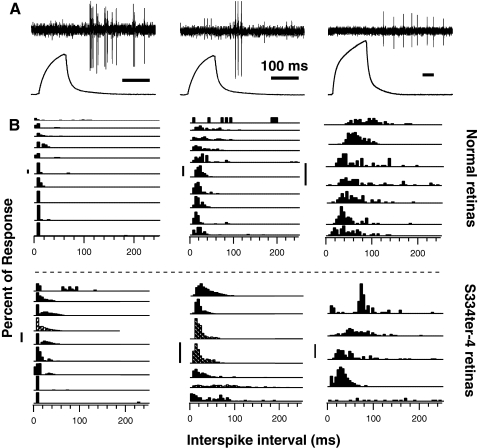
Interspike interval (ISI) histograms of glutamate-evoked RGCs responses were broadly separated into three patterns (Fig. 9; ISI analysis excluded units with high spontaneous activity). RGC responses to glutamate exhibited ISI histograms with (1) transient responses with peaks in ISIs of short (<10 ms) interspike intervals, (2) responses with most activity having firing rates between 25 spikes/s (40-ms ISI) and 50 spikes/s (20-ms ISI), or (3) responses with more broad ISIs with median ISI greater than 50 ms (Fig. 9). RGCs that had transient ISI response patterns exhibited bursts of activity during which firing rates were 140 to 300 spikes/s in both normal and degenerated retinas (Fig. 9). The distribution of firing patterns was similar in normal and degenerated retinas, with the exception that more RGCs in normal retinas (12.5%, 4/27) had median interspike intervals of less than 10 ms (rates >100 spikes/s), compared with degenerated retinas (4.5%, 1/23). However, the mean of the mean interspike intervals in responses to glutamate were not different in normal (64.1 ± 9.25 ms; n = 32) and degenerated (65.5 ± 9.70 ms; n = 21; t-test, P = 0.92) retinas.
Figure 9.
Responses of RGCs to glutamate varied from transient responses, with bursts having interspike intervals less than 10 ms (A, left), to transient firing having bursts with interspike intervals of 10 to 20 ms (A, middle), to lower sustained firing activity without burst firing (A, right, time bars = 100 ms). ISI histograms reflected these patterns varying from the ISI histograms with peaks at less than 10 ms (transient bursting patterns; B, left) to ISI patterns with peaks at 10 to 20 ms and wider interspike intervals (B, right). Similar ISI patterns of RGC responses were observed in Sprague-Dawley (B, above dashed line) and S334ter-4 retinas (B, below dashed line; striped bars, S334ter-4 <P100; vertical scale bars, 20%). Each histogram is from a different RGC.
Therefore, the percentage of RGCs responding to 2 mM glutamate, the threshold duration of glutamate application to activate RGCs, the latency of responses to glutamate application, and the duration and pattern of RGC activation by glutamate all showed that RGCs in degenerated retinas were effectively activated by local application of glutamate. Glutamate activated 60% of RGCs in degenerated retinas (P180–P254) versus 73% in normal retinas.
Discussion
In this study, we examined the responses of RGCs in normal and photoreceptor degenerated retinas to locally applied glutamate as an initial step, to determine the feasibility of a neurotransmitter-based retinal prosthesis. The study included an analysis of the spatiotemporal parameters necessary for effective stimulation of RGCs by locally applied glutamate in normal rat retinas as well as in rats with progressive stages of retinal degeneration associated with the primary rhodopsin S334ter-4 mutation. Specifically, we identified the proximity requirement of application sites to the cell soma, as well as the dependency of RGC responses on glutamate concentration and RGC type. We also identified the latency, duration, interspike intervals, and consistency of responses. We observed that comparable RGC response properties were identified between normal and S334ter-4 rats in response to local, temporally controlled glutamate applications, even at advanced stages of retinal degeneration.
Our study shows that the concentrations of locally applied glutamate necessary to effectively stimulate RGCs are slightly lower than those previously reported. Glutamate effectively excited RGCs when applied within 50 μm of cells, consistent with the lateral spread of ejected fluids measured via (Δf/f) fluorescence imaging. Therefore, we hypothesize that activation of glutamate receptors on the somata and proximal dendritic field of RGCs partially explains the limited spatial stimulus–response spread. In addition, EAAT pumps that naturally maintain extracellular glutamate concentrations at low levels may temporally damp and spatially constrain RGC responses to local extracellular glutamate puffs. In other studies, bath application required 10 to 20 mM glutamate to depolarize intracellularly recorded rabbit RGCs.18 In the feline, in vivo model, iontophoretic application of glutamate in one study was reported to inconsistently stimulate RGCs,25 whereas it consistently excited RGCs in another in vivo cat study.24 In this study, we noted that surface ejections were less effective overall than subsurface application and were most effective with application of 5 to 10 mM glutamate. Application of glutamate 15 to 20 μm below the inner retinal surface was more reliable in exciting RGCs and was effective with 2 mM glutamate in both normal and degenerated retinas. The requirement for millimolar glutamate concentrations is likely, at least in part due to glutamate uptake by EAATs present in the retinal glia,29 as in isolated rat RGCs, 50 μM glutamate was effective in eliciting glutamate receptor currents.30 We examined glutamate thresholds for spiketrain generation and the dose–response effects on temporal dynamics of spike latency, duration, and interspike intervals. Consequently, we believe it is logical that the thresholds we observed were higher. In addition, the high tissue perfusion rate (8 mL/min) to maintain the retinal preparation, in vitro may contribute to the rapid removal of ejected glutamate. Therefore, lower concentrations of locally applied glutamate from a neurotransmitter-based retinal prosthesis may be effective in vivo. Our prior work in mathematically modeling glutamate concentrations necessary for spiketrain responses identified that synaptic concentrations of glutamate between 0.5 and 11 mM would be necessary.31 This finding is consistent with those in the present study.
The properties of RGC responses in normal and degenerated retinas to locally applied glutamate, including latency, duration, and evoked firing pattern, provide important information related to the potential effectiveness of neurotransmitter-based retinal prosthesis. The latency of RGC responses to local exogenous glutamate application depends on both technical and physiological factors. In the present study, latencies were measured from the onset of the ejection command pulse. However, after the command pulse, there is an initial delay of 9 ms before pressure is actuated. Subsequently, in the present study, the pressure increased exponentially (τ = 35–38 ms). Therefore, the onset of glutamate ejection may be delayed by approximately 45 ms, because of switching and the delay introduced by capacitance in the ejection system. However, we observed that the latency of responses in normal retinas ranged from 100 to 400 ms (mean, 281 ms). Accounting for delays in ejection, we expect that the true RGC response latencies are likely to be between 55 and 355 ms. RGC response latencies in degenerated retinas were similar to those in normal retinas, but demonstrated greater variability. Latency of responses to bath and iontophoretic application of glutamate cannot be readily compared to those in the present studies, as the onset of glutamate application to bath application18 is slow, and the latency of RGC responses (activity counted in 1-second bins) to iontophoresed glutamate were not determined.24
Studies in normal rabbit retinas have shown that the latency of RGC responses to subretinal electrical stimulation was dependent on RGC cell type, as well as anodal versus cathodal stimulation patterns. Response latencies were clustered at short (5–10 ms) and long (25–60 ms) time points.32 The mean latencies of type I and III RGCs in normal and RD1 mouse retinas to subretinal electrical stimulation were approximately 10 ms, whereas type II RGCs had mean response latencies of 104 ms in normal and 54 ms in RD1 mouse retinas.33 In comparison, the latencies we measured of RGC responses to locally applied glutamate are often longer than electrically evoked responses, with differences ranging between 50 and 300 ms, depending on RGC type. Possible reasons for the relatively long RGC response latencies to locally applied glutamate may be inferred from the study of other glutamate agonists.
Glutamate agonists have mixed effects on RGCs, including inhibition that is mediated through interneurons (most likely, amacrine cells). Although the kainate glutamate receptor agonist kainate consistently excited RGCs,18,24,32 AMPA and quisqualate, an AMPA and metabotropic glutamate group I (mGlur1/5) receptor agonist, inhibit many RGCs (rabbit,26 primate,32 and cat24). Inhibition of RGCs by quisqualate is reversed by blocking either glycine or GABA receptors.24,26 Glutamate also depolarizes amacrine cells,18 which are the major glycinergic and GABAergic interneurons in the inner plexiform layer. Therefore, glutamate application is likely to have direct and indirect effects on RGCs. Bath application of glutamate produced an initial hyperpolarization in seven of nine RGCs but hyperpolarizing responses in only two.18 The initial suppression of RGC firing that we observed in spontaneously active cells may also be due to activation of inhibitory neurons. The latency of excitation in these cells was similar to the latency of other RGCs. Therefore, we hypothesize that glutamate application quickly activates a prominent indirect inhibitory input to most or all RGCs, perhaps through amacrine cells, that counteracts direct glutamatergic excitation of RGCs immediately after local application. We observed that this suppression was immediately followed by a burst of RGC excitation around the ejection site. It is possible that glutamate also generates RGC spiketrain suppression in distal regions away from local glutamate application where glutamate concentrations are too low to elicit RGC spiketrain activity. The role, temporal dynamics, and spread of inhibition in and around the ejection site warrant further investigation. This issue is particularly important for determining the optimal separation of ejection sites in a prototype retinal prosthetic device. In addition, these inhibitory effects may be important in producing spatial contrast. Furthermore, reducing inhibition via GABA blockade may allow lower concentrations of glutamate to effectively stimulate RGCs and may change the receptive field response parameters for locally applied exogenous glutamate stimulation. We are planning future studies to further examine the role of amacrine cell stimulation on direct RGC responses to glutamate.
Our observations that ON and OFF retinal ganglion cells respond with different response latencies may also allow designers of neurotransmitter-based retinal prostheses to create temporal separation of ON and OFF responses, which may provide central neurons with important visually relevant cues. The latency of OFF RGCs was significantly longer than the latency of ON RGCs. Response latencies of OFF RGCs were also longer than ON RGC latencies for anodal electrical subretinal stimulation, but the reverse was found for cathodal stimulation.3 The differences in ON and OFF RGC responses may be due to differential localization of glutamate receptors, differences in cell morphology such as dendritic field size between cell types, and the activation of inhibitory interneurons, which have differential effects in ON and OFF RGCs. In degenerated retinas, response latencies to glutamate stimulation were similar to those observed in normal retinas, but due to the paucity of visual responses, differences in latencies between ON and OFF RGCs could not be assessed in degenerated retinas. We also do not know whether there is a loss of specific RGC cell types in degenerated retinas. Therefore, the feasibility of inducing differential excitation among specific RGC types in degenerated retinas must be studied further.
We observed that the duration, but not the temporal firing pattern of RGC responses varied with the duration of glutamate ejections. Consequently, we believe that designers of neurotransmitter-based retinal prostheses will be able to effectively modulate RGC spiketrain durations by varying the duration of each glutamate application. Although we observed a high variability in the threshold duration necessary to evoke responses between RGCs, response durations within individual cells varied monotonically with ejection duration. RGC spiketrain response duration in normal and degenerated retinas increased with duration of local application of 2 mM glutamate between 50 to 350 ms. Therefore, varying the duration of application could be an effective means of regulating the output of the retina in a neurotransmitter-based retinal prosthesis. Glutamate-evoked RGC firing patterns were different among cells but where consistent for a given cell. Recent studies suggest that many RGCs exhibit a specific firing pattern that is evident as a consistent shape of ISI histograms for a given cell type, independent of visual stimulus.34 In the present study, we observed that RGC responses to stimulation with light and glutamate exhibited very similar ISI histograms, suggesting that glutamate-evoked excitation is effective in producing naturalistic RGC response patterns that may be recognized in higher order processing centers. Factors that limit the repetition rate of glutamate application may include the ejection and response durations, the influences of glutamate-evoked RGC response suppression (inhibitory) effects, and the temporal ejection characteristics inherent in the neurotransmitter-based retinal prosthesis design. RGC responses to a second application of glutamate, 2 and 4 seconds after the initial response, were usually slightly reduced. Future studies should be extended past paired ejections, to examine the effects of multiple sequential ejections and the interactions between ejections from multiple sites. EAAT uptake mechanisms are critically important in damping RGC responses to locally applied exogenous glutamate in healthy and degenerated retinas. These systems maintain low extracellular glutamate levels, improving the signal-to-noise ratio of glutamate neurotransmission and preventing glutamate excitotoxicity. During our experiments, we observed no prolonged responses to glutamate stimulation, suggesting that EAAT pumps were able to maintain low extracellular glutamate during microfluidic stimulation of RGCs in normal and degenerated retinas. Safe limits of prolonged glutamate stimulation must be determined in long-term in vivo models.
We have applied the data from this and ongoing studies on glutamate responses in degenerated retinas to design a prototype neurotransmitter-based retinal prosthesis. This study is the first of its kind to correlate RGC responses in normal and degenerated retinas with stimulation performance parameters required of a neurotransmitter-based retinal prosthesis. Our observations demonstrate that neurotransmitter-based retinal prostheses can afford fine control over the spatial and temporal responses of RGCs in normal and degenerated retinas. We believe that by virtue of their ability to modulate RGC responses neurotransmitter-based retinal prostheses may more closely mimic natural visual stimulation.
Acknowledgments
The authors thank Matthew M. LaVail, PhD (University of California San Francisco, School of Medicine, Beckman Vision Center), for the S334ter-4 homozygote breeders.
Footnotes
Supported by the Ligon Research Center of Vision, the National Eye Institute (R21 EY018709-01), and an unrestricted grant from Research to Prevent Blindness.
Disclosure: P.G. Finlayson, None; R. Iezzi, None
References
- 1.Fried SI, Hsueh HA, Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J Neurophysiol 2006;95:970–978 [DOI] [PubMed] [Google Scholar]
- 2.Humayun MS, de Juan E, Jr, Weiland JD, et al. Pattern electrical stimulation of the human retina. Vision Res 1999;39:2569–2576 [DOI] [PubMed] [Google Scholar]
- 3.Jensen RJ, Rizzo JF., 3rd Thresholds for activation of rabbit retinal ganglion cells with a subretinal electrode. Exp Eye Res 2006;83:367–373 [DOI] [PubMed] [Google Scholar]
- 4.Jensen RJ, Ziv OR, Rizzo JF., 3rd Thresholds for activation of rabbit retinal ganglion cells with relatively large, extracellular microelectrodes. Invest Ophthalmol Vis Sci 2005;46:1486–1496 [DOI] [PubMed] [Google Scholar]
- 5.Jensen RJ, Ziv OR, Rizzo JF. Responses of rabbit retinal ganglion cells to electrical stimulation with an epiretinal electrode. J Neural Eng 2005;2:S16–S21 [DOI] [PubMed] [Google Scholar]
- 6.Margalit E, Maia M, Weiland JD, et al. Retinal prosthesis for the blind. Surv Ophthalmol 2002;47:335–356 [DOI] [PubMed] [Google Scholar]
- 7.Pardue MT, Phillips MJ, Yin H, et al. Neuroprotective effect of subretinal implants in the RCS rat. Invest Ophthalmol Vis Sci 2005;46:674–682 [DOI] [PubMed] [Google Scholar]
- 8.Rizzo JF, 3rd, Wyatt J, Humayun M, et al. Retinal prosthesis: an encouraging first decade with major challenges ahead. Ophthalmology 2001;108:13–14 [DOI] [PubMed] [Google Scholar]
- 9.Ziv OR, Rizzo JF, Jensen RJ. In vitro activation of retinal cells: estimating location of stimulated cell by using a mathematical model. J Neural Eng 2005;2:S5–S15 [DOI] [PubMed] [Google Scholar]
- 10.Chow AY, Chow VY. Subretinal electrical stimulation of the rabbit retina. Neurosci Lett 1997;225:13–16 [DOI] [PubMed] [Google Scholar]
- 11.Chow AY, Pardue MT, Chow VY, et al. Implantation of silicon chip microphotodiode arrays into the cat subretinal space. IEEE Trans Neural Syst Rehabil Eng 2001;9:86–95 [DOI] [PubMed] [Google Scholar]
- 12.Eckhorn R, Wilms M, Schanze T, et al. Visual resolution with retinal implants estimated from recordings in cat visual cortex. Vision Res 2006;46:2675–2690 [DOI] [PubMed] [Google Scholar]
- 13.Rizzo JF, 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Perceptual efficacy of electrical stimulation of human retina with a microelectrode array during short-term surgical trials. Invest Ophthalmol Vis Sci 2003;44:5362–5369 [DOI] [PubMed] [Google Scholar]
- 14.Rizzo JF, 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci 2003;44:5355–5361 [DOI] [PubMed] [Google Scholar]
- 15.Cogan SF, Troyk PR, Ehrlich J, Plante TD. In vitro comparison of the charge-injection limits of activated iridium oxide (AIROF) and platinum-iridium microelectrodes. IEEE Trans Biomed Eng 2005;52:1612–1614 [DOI] [PubMed] [Google Scholar]
- 16.Cogan SF, Troyk PR, Ehrlich J, Plante TD, Detlefsen DE. Potential-biased, asymmetric waveforms for charge-injection with activated iridium oxide (AIROF) neural stimulation electrodes. IEEE Trans Biomed Eng 2006;53:327–332 [DOI] [PubMed] [Google Scholar]
- 17.Troyk P, Detlefsen D, Cogan S, et al. “Safe” charge-injection waveforms for iridium oxide (AIROF) microelectrodes. Conf Proc IEEE Eng Med Biol Soc 2004;6:4141–4144 [DOI] [PubMed] [Google Scholar]
- 18.Bloomfield SA, Dowling JE. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. II. Inner plexiform layer. J Neurophysiol 1985;53:714–725 [DOI] [PubMed] [Google Scholar]
- 19.Slaughter MM, Miller RF. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci 1983;3:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci 2002;22:2165–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marc RE. Kainate activation of horizontal, bipolar, amacrine, and ganglion cells in the rabbit retina. J Comp Neurol 1999;407:65–76 [PubMed] [Google Scholar]
- 22.Marc RE. Mapping glutamatergic drive in the vertebrate retina with a channel-permeant organic cation. J Comp Neurol 1999;407:47–64 [DOI] [PubMed] [Google Scholar]
- 23.Yang XL. Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol 2004;73:127–150 [DOI] [PubMed] [Google Scholar]
- 24.Boos R, Muller F, Wassle H. Actions of excitatory amino acids on brisk ganglion cells in the cat retina. J Neurophysiol 1990;64:1368–1379 [DOI] [PubMed] [Google Scholar]
- 25.Ikeda H, Sheardown MJ. Aspartate may be an excitatory transmitter mediating visual excitation of “sustained” but not “transient” cells in the cat retina: iontophoretic studies in vivo. Neuroscience 1982;7:25–36 [DOI] [PubMed] [Google Scholar]
- 26.Massey SC, Miller RF. Glutamate receptors of ganglion cells in the rabbit retina: evidence for glutamate as a bipolar cell transmitter. J Physiol 1988;405:635–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauen T. Diversity of glutamate transporter expression and function in the mammalian retina. Amino Acids 2000;19:53–62 [DOI] [PubMed] [Google Scholar]
- 28.Lee D, Geller S, Walsh N, et al. Photoreceptor degeneration in Pro23His and S334ter transgenic rats. Adv Exp Med Biol 2003;533:297–302 [DOI] [PubMed] [Google Scholar]
- 29.Higgs MH, Lukasiewicz PD. Glutamate uptake limits synaptic excitation of retinal ganglion cells. J Neurosci 1999;19:3691–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol 1988;396:75–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iezzi R, Finlayson PG. Neurotransmitter stimulation for retinal prosthesis: the artificial synapse chip. In: Dagnelie G. ed. Visual Prosthetics: Physiology, Bioengineering and Rehabilitation New York: Springer; In press [Google Scholar]
- 32.Cohen ED, Miller RF. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci 1994;11:317–332 [DOI] [PubMed] [Google Scholar]
- 33.Jensen RJ, Rizzo JF., 3rd Activation of retinal ganglion cells in wild-type and rd1 mice through electrical stimulation of the retinal neural network. Vision Res 2008;48:1562–1568 [DOI] [PubMed] [Google Scholar]
- 34.Zeck GM, Masland RH. Spike train signatures of retinal ganglion cell types. Eur J Neurosci 2007;26:367–380 [DOI] [PubMed] [Google Scholar]