The present study was designed to assess the neuroprotective action of the nonselective HDAC inhibitor trichostatin A (TSA) in an adult rodent model of retinal ischemia.
Abstract
Purpose.
The pathogenesis of retinal ischemia results from a series of events involving changes in gene expression and inflammatory cytokines. Protein acetylation is an essential mechanism in regulating transcriptional and inflammatory events. The purpose of this study was to investigate the neuroprotective action of the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) in a retinal ischemic model.
Methods.
To investigate whether HDAC inhibition can reduce ischemic injury, rats were treated with TSA (2.5 mg/kg intraperitoneally) twice daily on days 0, 1, 2, and 3. Seven days after ischemic injury, morphometric and electroretinographic (ERG) analyses were used to assess retinal structure and function. Western blot and immunohistochemical analyses were used to evaluate TSA-induced changes in histone-H3 acetylation and MMP secretion.
Results.
In vehicle-treated animals, ERG a- and b-waves from ischemic eyes were significantly reduced compared with contralateral responses. In addition, histologic examination of these eyes revealed significant degeneration of inner retinal layers. In rats treated with TSA, amplitudes of ERG a- and b-waves from ischemic eyes were significantly increased, and normal inner retina morphology was preserved. Ischemia also increased the levels of retinal TNF-α, which was blocked by TSA treatment. In astrocyte cultures, the addition of TNF-α (10 ng/mL) stimulated the secretion of MMP-1 and MMP-3, which were blocked by TSA (100 nM).
Conclusions.
These studies provide the first evidence that suppressing HDAC activity can protect the retina from ischemic injury. This neuroprotective response is associated with the suppression of retinal TNF-α expression and signaling. The use of HDAC inhibitors may provide a novel treatment for ischemic retinal injury.
Retinal ischemia plays a central role in a number of retinal degenerative diseases, such as diabetic retinopathy, glaucoma, and retinal artery occlusion. These blinding diseases likely involve both necrotic and apoptotic processes, and a variety of cellular events are thought to contribute to the degenerative response in affected persons, among them excessive accumulation of extracellular glutamate, ionic imbalance, generation of reactive oxidative and nitrosative species, release of proinflammatory cytokines, and secretion of active matrix metalloproteinases (MMPs).1 Studies have also provided evidence that these destructive events can be ameliorated by the activation of adenosine and opioid receptors, the upregulation of heat shock proteins and growth factors, and the modulation of proapoptotic and antiapoptotic proteins.2–7
In recent years, protein acetylation, like phosphorylation, has been recognized as playing a significant role in the regulation of cellular activity. In this system, protein acetylation is regulated by histone acetyltransferases (HATs) and protein deacetylation by histone deacetylases (HDACs). Initial studies identified that acetylation of nuclear histones mediates change in gene expression by modulating chromatin condensation. However, a growing list of nonhistone nuclear and cytoplasmic proteins have also been identified as substrates for both HATs and HDACs.8,9 As a result, increases in histone acetylation can enhance or repress gene expression and can alter downstream signaling events associated with receptor activation.
Acetylation imbalance within tissues has been shown to contribute to the pathogenesis of cancer, cardiovascular disease, and inflammatory disorders.10,11 In the brain, studies have provided evidence that hyperacetylation induced by HDAC inhibition is neuroprotective in models of Huntington disease,12–14 amyotrophic lateral sclerosis,15 spinal muscular atrophy,16 ischemic injury, and stroke.17 Cellular studies have shown that the administration of HDAC inhibitor can limit microglial activation, reduce TNF-α secretion, and upregulate neuroprotective proteins such as heat shock protein 70. Histone deacetylase inhibitors have also been shown to limit the cellular response to these inflammatory cytokines by suppressing the activation of NFκB and JAK/STAT and the secretion of MMPs.18–22
In the eye, studies have shown that histone deacetylation by HDACs is critical for the expression of several photoreceptors and the apoptotic genes in the developing retina.23,24 However, in the adult retina, hyperacetylation induced by HDAC inhibition did not lead to the upregulation of genes in the apoptotic pathway.24 Other retinal studies have shown that acetylation may also play a role in the differentiation of retinal ganglion cells.25 Taken together, these studies provide evidence that protein acetylation plays a central role in regulating retina development; however, events modulated by protein acetylation in the adult retina have not been investigated. The present study was designed to assess the neuroprotective action of the nonselective HDAC inhibitor trichostatin A (TSA) in an adult rodent model of retinal ischemia.
Materials and Methods
Adult male and female Brown Norway rats (age range, 3–5 months; weight range, 150–200 g; Charles River Laboratories, Inc.; Wilmington, MA) were used in this study. Rats were maintained in an environmental cycle of 12-hours light and 12-hours dark. Animal handling was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research; and the study protocol was approved by the Animal Care and Use Committee at the Medical University of South Carolina. For neuroprotection studies, TSA (2.5 mg/kg) or vehicle (0.1% dimethyl sulfoxide [DMSO]) was administered by intraperitoneal injection 1 hour before and 4 hours after ischemic injury on the day studies were initiated. On postischemic days 1, 2, and 3, TSA or vehicle was administered between 8:00 and 9:00 am and again between 3:00 and 4:00 pm.
Retinal Ischemia
Before the induction of retinal ischemia, rats were anesthetized by intraperitoneal injection of ketamine (75 mg/kg) and xylazine (8 mg/kg) (Ben Venue Laboratories, Bedford, OH), and corneal analgesia was created by the application of proparacaine (0.5%; 5 μL; Akorn, Inc., Buffalo Grove, IL). Body temperature was maintained at 37°C by means of a heating pad (Harvard Apparatus, Holliston, MA). Retinal ischemia was created using methods previously described by Whitlock et al.26 Briefly, the anterior chamber was cannulated with a 30-gauge needle that was connected to a container of sterile normal saline by polyethylene tubing. To induce retinal ischemia, the reservoir was elevated to raise the intraocular pressure (IOP) above systolic blood pressure (155–160 mm Hg) for 45 minutes. Each pressure then returned to normal, and the eye was examined to ensure that retinal blood flow was reestablished. The IOP was monitored by an in-line pressure transducer connected to a computer. The contralateral eye was left untreated to serve as the control.
Electroretinography
To quantitate baseline and postischemic retinal function, electroretinography was performed. Baseline values were obtained 1 day before ischemic injury and 7 days after injury. For these studies, rats were dark-adapted overnight. The following day, rats were anesthetized with intraperitoneal ketamine and xylazine administration, as described, and pupils were dilated with a 10-μL drop of a solution containing phenylephrine HCl (2.5%) and tropicamide (1%; Akorn, Inc.). A needle ground-electrode was placed subcutaneously in the animal's back, and a reference electrode was placed on the animal's tongue. A contact lens gold-ring electrode was held in place on the cornea with a drop of methylcellulose. A stimulus-intensity series of electroretinograms was recorded in response to single-flash intensities, using 40-dB attenuation (low-intensity flash), through no attenuation (high-intensity flash). Responses were an average of two flashes with an interstimulus interval of 2 minutes. Electroretinograms were recorded by means of a universal testing and electrophysiologic system (UTAS-2000; LKC Technologies, Gaithersburg, MD). Amplitudes of ERG a- and b-waves from ischemic eyes of TSA-treated animals were compared with contralateral control responses and corresponding responses from vehicle-treated animals.
Histology
For morphometric analyses, rats were euthanatized by overdoses of pentobarbital. Eyes were then enucleated and fixed for 1 hour in 4% paraformaldehyde in 0.1 M phosphate-buffered saline at 4°C. The eyes were opened at the ora serrata, and fixation continued for 24 hours. After fixation, the anterior segment was removed, and the posterior eyecup was dehydrated and embedded in paraffin. Retinal cross-sections (5-μm thick) were then cut and stained with hematoxylin and eosin (Sigma Chemical Co., St. Louis, MO). Retinal sections were photographed and measured approximately 2 to 3 disc diameters from the optic nerve by means of a fluorescence microscope (Axioplan-2; Zeiss; Maple Grove, MN).
Retinal TNF-α Assay
To determine whether HDAC inhibition alters the expression of the proinflammatory cytokine TNF-α in the ischemic retina, rats were treated with TSA (2.5 mg/kg, administered intraperitoneally) or vehicle (0.1% DMSO) 1 hour before unilateral retinal ischemia. Animals were allowed to reperfuse for 4 hours. After euthanatization, the retinas were dissected free, placed in lysis buffer containing protease inhibitors, and stored at −80°C for subsequent determination of TNF-α. Retinal extracts were then centrifuged, and the supernatant was analyzed for TNF-α by means of enzyme-linked immunosorbent assay (eBiosciences, San Diego, CA; R&D Systems; Minneapolis, MN). Samples for TNF-α concentration were calculated from a standard curve and normalized to total retinal protein, as determined by Bradford protein assay (Bio-Rad, Hercules, CA).
Retinal HDAC Activity
To assess changes in overall retinal HDAC activity induced by TSA, rats were euthanatized with overdoses of pentobarbital 2 hours after the administration of TSA (2.5 mg/kg) or vehicle (0.1% DMSO). Retinas were then dissected free and placed in lysis buffer containing protease inhibitors and TSA (100 μM). Western blot analysis was performed on equivalent amounts of proteins. Protein acetylation was detected by overnight incubation of membranes at 4°C with primary polyclonal acetyl-lysine histone-H3 antibody (no. 7677; Cell Signaling Technology, Beverly, MA) followed by washing and 2-hour incubation with horseradish peroxidase (HRP)-conjugated secondary antibody. Membranes were treated with chemiluminescent reagent, and band intensity was measured with an imaging system (Versadoc; Bio-Rad). Membranes were then stripped and probed for total histone-H3 levels using monoclonal anti–histone-H3 (no. 05–499; Upstate, Lake Placid, NY) and HRP-conjugated secondary antibody. After this, band densities were again measured using chemiluminescent protocol.
Selected eyes were fixed in 4% paraformaldehyde and embedded in paraffin, and 5-μm retinal cross-sections were cut. Sections were blocked with 4% bovine serum albumin for 2 hours at room temperature, washed, and incubated with primary polyclonal acetyl-lysine histone-H3 antibody overnight at 4°C. Sections were then washed and incubated for 2 hours at room temperature with an FITC-labeled secondary antibody. For negative controls, staining with primary antibody was omitted, and sections were stained only with FITC-labeled secondary antibody. Retinal sections were observed and photographed by means of a fluorescence microscope (Axioplan-2; Zeiss, Maple Grove, MN).
MMP Analysis
To assess whether TSA can influence receptor-signaling events that are associated with ischemia, primary cultures of purified human astrocytes were evaluated. Postmortem donor eyes were obtained from Life-Point Ocular Tissue Division (Charleston, SC), and astrocytes were purified from human optic nerve head explants as described by Yang et al.27 The purity of the astrocyte culture was determined by positive immunostaining for the astrocyte markers glial fibrillary acidic protein and NCAM, a cell surface adhesion molecule, in each culture. Confluent astrocyte cultures were treated with TNF-α (10 ng/mL) for 24 hours in the presence or absence of TSA (100 nM). At the end of the incubation period, the media were collected (Amicon Ultra Concentrator; Millipore, Billerica, MA), and the secretion of MMP-1 and MMP-3 was determined by Western blot analysis. Levels of MMP-1 and -3 were detected after overnight incubation at 4°C with primary polyclonal antibody (no. M4177 or M5052; Sigma). Blots were washed, incubated with HRP-conjugated secondary antibody for 2 hours at room temperature, and treated with chemiluminescent reagent for detection. Band intensity was measured with an imaging system (Versadoc; Bio-Rad).
Statistical Analysis
Statistical comparisons were made with the Student's t-test for paired data or with ANOVA using the Dunnett posttest (GraphPad Software, Inc., San Diego, CA). P ≤ 0.05 was considered significant.
Results
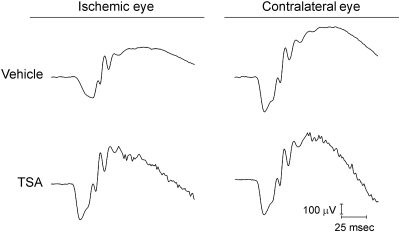
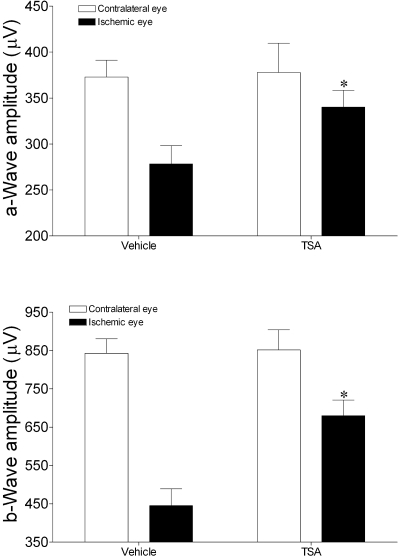
As shown in Figures 1 and 2, retinal ischemia in vehicle-treated rats produced significant reductions of 47% in the mean b-wave amplitudes when compared with the contralateral control eyes (ischemic eyes 444 ± 44 μV vs. contralateral control eyes 842 ± 38 μV; P < 0.05). A smaller, but significant, decline of 25% in a-wave amplitudes was also measured in these eyes (ischemic eyes 278 ± 20 μV vs. contralateral control eyes 372 ± 18 μV; P < 0.05). As shown in Figure 1, reduction in the amplitude of oscillatory potentials was also observed in the electroretinograms from ischemic eyes when compared to the contralateral control eye. In animals that received TSA, mean a- and b-wave amplitudes from ischemic eyes were significantly greater (P < 0.05) than in the corresponding ischemic eyes of vehicle-treated animals. In the ischemic eye from animals treated with TSA, oscillatory potentials were also larger in amplitude than in the corresponding vehicle-treated eye. No significant difference between mean a- and b-wave amplitudes from contralateral (nonischemic) eyes was measured between the TSA and the vehicle-treated animals. Although a trend toward reduced implicit time in the control eye of TSA-treated animals was measured, these changes were not significant (data not shown).
Figure 1.
Representative ipsilateral and contralateral electroretinograms 7 days after unilateral ischemic retinal injury. Animals were treated with vehicle or TSA (2.5 mg/kg) twice daily on days 0, 1, 2, and 3. Electroretinograms were obtained by averaging two responses to full-intensity flashes with an interstimulus interval of 2 minutes.
Figure 2.
Effect of TSA or vehicle treatment on ERG a- and b-wave amplitudes 7 days after unilateral ischemic retinal injury. Animals were treated with vehicle or TSA (2.5 mg/kg) twice daily on days 0, 1, 2, and 3. Data are expressed as mean ± SE. *P < 0.05, significant difference between ipsilateral responses in the vehicle- and TSA-treated animals. No significant differences in contralateral responses were measured. n ≥ 7.
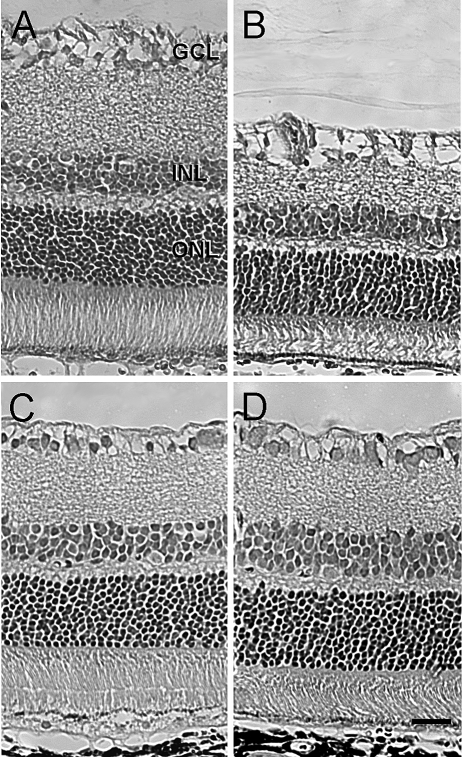
Morphologic changes induced by 45 minutes of ischemia in each group were also evaluated 7 days after ischemic injury (Fig. 3; Table 1). In control (vehicle-treated) rats, eyes that received ischemic injury showed a significant decrease in overall retinal thickness of 30%. This reduction in thickness was attributed primarily to significant thinning of the inner plexiform and inner nuclear layers and to degeneration of the cell bodies in the ganglion cell layer. Mild disorganization of the outer nuclear layer and photoreceptors was also observed. In contrast, retinas from animals that received ischemic injury and were treated with TSA exhibited no significant thinning of the inner plexiform and inner nuclear layers or significant loss of cell bodies within the ganglion cell layer. The outer nuclear and plexiform layers showed normal patterns of organization; however, photoreceptors often exhibited signs of misalignment. Contralateral (nonischemic) eyes from both vehicle- and TSA-treated rats were normal in appearance.
Figure 3.
Effect of TSA on retinal morphologic changes 7 days after unilateral ischemia. Animals were treated with vehicle or TSA (2.5 mg/kg) twice daily on days 0, 1, 2, and 3. (A, B) Photomicrographs of retinal cross-sections of contralateral and ischemic eyes from vehicle-treated animals. (C, D) Photomicrographs of retinal cross-sections of contralateral and ischemic eyes from TSA-treated animals. All micrographs were taken 2 to 3 disc diameters from the optic nerve. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 20 mm.
Table 1.
Thickness of Retina and Individual Layers Measured 7 Days after Ischemia
| Retina (μm) | IPL (μm) | INL (μm) | OPL (μm) | ONL (μm) | RGCs (Cells/200 μm) | |
|---|---|---|---|---|---|---|
| Control | 185 ± 8.0 | 45.8 ± 2.1 | 24.5 ± 2.4 | 12.1 ± 1.7 | 48.4 ± 3.2 | 22 ± 1.3 |
| Ischemia | 131 ± 7.1* | 21.5 ± 2.7* | 19.1 ± 1.2* | 10.3 ± 0.6 | 44.4 ± 4.5 | 13 ± 1.2* |
| TSA | 175 ± 17.4 | 40.5 ± 1.3 | 28.9 ± 3.5 | 11.1 ± 0.7 | 48.9 ± 6.6 | 19 ± 1.2 |
| TSA + ischemia | 165 ± 11.1 | 35.9 ± 4.8 | 26.8 ± 3.1 | 11.4 ± 0.5 | 43.6 ± 4.3 | 18 ± 1.2 |
Values are mean ± SE (n = 4–7). Values were compared between groups using one-way ANOVA with Dunnett's posttest. IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RGC, retinal ganglion cell.
P < 0.05; significant difference from control eye.
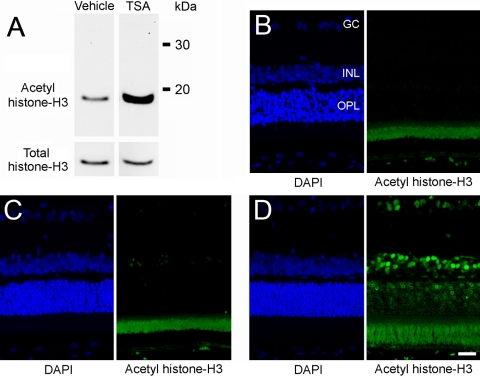
In the present study, TSA was administered by intraperitoneal injection. Hence, the amelioration of ischemic retinal injury as measured in this study may reflect retinal or systemic responses to TSA. To begin to assess whether systemic administration of TSA could result in the hyperacetylation of retinal proteins, rats were treated with TSA (2.5 mg/kg) or vehicle, and the levels of acetylated histone-H3 in the retina was examined by Western blot analysis and immunohistochemistry 2 hours after administration. Western blot analysis revealed relatively low levels of acetylated histone-H3 in the retinas of vehicle-treated animals; however, 2 hours after intraperitoneal administration of TSA, the level of retina histone-H3 acetylation was increased more than 10-fold (Fig. 4A). Immunohistochemical staining for histone-H3 acetylation in nonischemic retinas revealed staining of nuclei in the retinal pigment epithelium and select cell bodies in the inner nuclear layer. No staining was observed in cell bodies of the outer nuclear or ganglion cell layers (Fig. 4C). In animals treated with TSA, labeled nuclei were observed in all retinal nuclear layers and in the cell bodies of the RPE (Fig. 4D).
Figure 4.
Effect of TSA on retinal acetylation of histone-H3. Animals were treated with vehicle or TSA (2.5 mg/kg, administered intraperitoneally) 2 hours before tissue analysis. (A) Western blot analysis of retinal lysate for total and acetylated histone-H3. (B) DAPI staining of cell bodies and negative control (no primary antibody) for immunohistochemical staining of retinal acetylated histone-H3. Note the autofluorescence of the photoreceptor layer. (C) DAPI staining of cell bodies and immunohistochemical staining for retinal acetylated histone-H3 from vehicle-treated animals. (D) DAPI staining of cell bodies and immunohistochemical staining for retinal acetylated histone-H3 from TSA-treated animals. GC, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 20 mm.
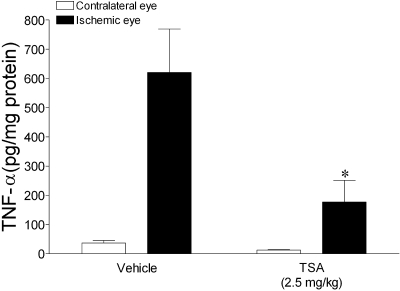
Recent studies have provided evidence that TNF-α plays a central role in the pathogenesis of a number of degenerative retinal diseases, including retinal ischemia.28–30 To assess whether TSA administration alters ischemia-induced TNF-α retinal expression, animals were pretreated with vehicle or TSA (2.5 mg/kg) 1 hour before ischemic injury. Rats were then allowed to recover for 4 hours, and retinal TNF-α was determined. In vehicle-treated rats, the mean level of TNF-α in control (nonischemic) retinas was 36 ± 8.8 ng/mg protein; however, 4 hours after ischemic/reperfusion, mean TNF-α levels in the retina were increased 16-fold (Fig. 5). This ischemia-induced increase in TNF-α was blocked by TSA pretreatment. A trend toward lower TNF-α levels was also measured in the control nonischemic eyes of TSA-treated animals. Immunohistochemical evaluation of ischemic eyes from vehicle- or TSA-treated animals 4 hours after ischemia/reperfusion did not identify any ED-1–positive inflammatory cells within the posterior segment of these eyes (data not shown).
Figure 5.
Effect of TSA or vehicle treatment on total retinal TNF-α levels after unilateral ischemic retinal injury. Animals were treated with vehicle or TSA (2.5 mg/kg, administered intraperitoneally) and TNF-α measured 4 hours after ischemia. TNF-α levels were normalized to total retinal protein. Data are expressed as mean ± SE. *P < 0.05, significant difference between ipsilateral responses in control and TSA-treated animals. n ≥ 3.
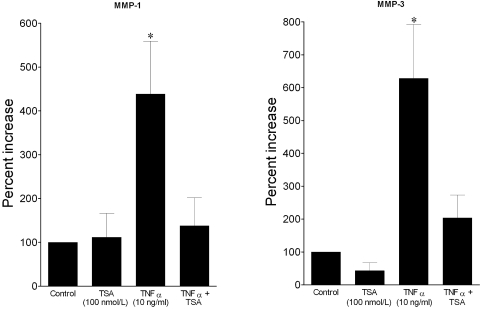
To examine whether TSA can also influence the cellular responses of inflammatory cytokines, human astrocytes were treated with TNF-α (10 ng/mL) for 24 hours in the presence or absence of TSA (100 nM), and the secretion of MMP-1 and MMP-3 was evaluated. As shown in Figure 6, the addition of TNF-α produced a significant increase in both MMP-1 and MMP-3 over control levels. Cotreatment with TSA blocked the TNF-α–induced increase in MMP-1 and MMP-3 levels. Treatment with TSA alone did not significantly alter the basal secretion of MMP-1 or MMP-3. Examination of cells after treatment with TSA, alone or in combination with TNF-α, did not produce any observable change in cellular morphology or number (data not shown).
Figure 6.
Effects of HDAC inhibitor TSA on TNF-α–induced MMP-1 and MMP-3 secretion from human optic nerve astrocytes. Serum-deprived astrocytes were treated with vehicle or TSA for 60 minutes, followed by incubation with TNF-α for 24 hours. Media were then collected, concentrated, and analyzed by Western blot analysis. Data shown are the mean ± SE of densitometry measurements. *P < 0.05, significant difference from control. n ≥ 4.
Discussion
The complexity of ischemic retinal degeneration is underscored by the fact that the resultant retinal pathology depends not only on the duration of ischemia but also on which vascular bed is affected (i.e., choroidal, retinal, or optic nerve head) and by differences in retinal cell responses to the ischemic and postischemic environments. However, retinal preconditioning studies have shown that brief periods of noninjurious retinal ischemia or hypoxia can provide robust retinal protection to subsequent ischemic injury. Associated with this neuroprotective response is the upregulation and downregulation of several genes.2,31–35 Hence, the modulation of retinal gene expression can increase the retina's resistance to ischemic injury.
The reversible acetylation of histones plays a critical role in gene expression and in many other cellular events. Protein acetylation of conserved lysine residues in the histone tail by HATs enhances gene expression by neutralizing the positively charged histones and relaxing the histone-histone and histone-DNA interactions that limit transcription factor access to the DNA. However, deacetylation often accompanies the suppression of gene expression by promoting chromatin condensation and limiting transcription factor access. Since the discovery that p53 is a substrate for HATs and HDACs, there has been a rapidly growing list of proteins other than histones that undergo reversible acetylation.8,9 These findings have established that protein acetylation and deacetylation, like phosphorylation, have multiple roles in regulating cellular processes.
To date, 18 HDACs in four general classes have been identified in humans. These enzymes have been shown to modulate transcription,36,37 cell cycle progression,38,39 differentiation,40 and apoptosis.41 Class I HDACs (HDAC1, 2, 3, and 8) are found in almost all tissues, whereas class II HDACs (HDAC4, 5, 6, 7, 9, and 10) have a restricted tissue distribution. Class III HDACs are homologous to yeast silent information regulator 2 (Sir2), are NAD+ dependent, and include SIRT1 to SIRT7. HDAC11 alone represents class IV HDACs. Both class I and class II HDACs are found in the nucleus and the cytosol and are inhibited by TSA.
In the retina, organ culture studies using developing retina explants (p2–p15) have demonstrated that inhibition of HDAC activity by TSA results in hyperacetylation of retinal proteins, downregulation of transcription factors necessary for rod development, upregulation of apoptotic factors, and increased cell death after 20 hours of exposure.23,24 However in adult explants (p60), the TSA-induced upregulation of apoptotic genes and cellular apoptosis was not observed.24 In the present in vivo study, we found that 2 hours after systemic administration of TSA (2.5 mg/kg), adult rats increased their retinal histone acetylation, and this increase in acetylation was observed in cell bodies throughout the retina. In normal (nonischemic) eyes, administration of TSA for 3 days did not produce any significant change in retinal function, as measured by electroretinography or morphology. These in vivo results are consistent with previous organ culture studies that support the idea that acute inhibition of retinal HDACs can be tolerated in adult animals. In addition, these data provide the initial evidence that the retinal efficacy of systemically administered TSA is not limited by the pharmacokinetic restrictions imposed by the inner and outer blood-retina barriers.
Although studies in the brain have provided evidence that HDAC inhibitors are neuroprotective, their potential usefulness in ameliorating retinal degenerative changes has received little attention. As shown in Figures 2 and 3, treatment with TSA starting 1 hour before ischemic injury provided significant neuroprotection. This neuroprotective response was measured by an improvement in both a- and b-wave amplitudes of the electroretinogram and morphologic preservation of the retina. These results support the idea that inhibition of HDAC activity can ameliorate ischemic injury to both the outer and the inner retinal regions.
Our understanding of the pathophysiologic events that lead to ischemic retinal degeneration remain incomplete; however, recent studies have shown that expression and secretion of the inflammatory cytokine TNF-α play a central role in this process.42 This degenerative response to elevated TNF-α levels likely involves the stimulation of TNFR1 and the downstream activation of the intrinsic apoptotic pathway as well as caspase-independent processes through the secretion of metalloproteinases and reactive-oxygen species.43–45 Inhibition of HDAC activity has been shown to suppress TNF-α expression induced by LPS.23 In addition, HDAC inhibitors have been shown to modulate downstream signaling associated with TNFR1 activation, such as caspases, NFκB, and JAK/STAT.18,19 To investigate the neuroprotective actions associated with HDAC inhibition, we evaluated how pretreatment with TSA alters ischemia-induced expression of TNF-α in the retina. Previous studies42 have shown that in the normal rat retina, TNF-α levels are very low but rise rapidly (3–12 hours) after ischemic injury. As shown in Figure 5, we measured a significant rise in total retinal TNF-α 4 hours after ischemic injury. This early increase in TNF-α level was blocked by pretreatment with TSA. These studies provided initial evidence that TSA administration produces a significant anti-inflammatory effect in the retina, similar to that observed in other tissues. A variety of cell types, including activated macrophages, astrocytes, microglia, and neuronal cells, under stress or ischemic conditions have been proposed for the enhanced production of TNF-α. To evaluate whether invading macrophages are responsible for the elevated TNF-α levels in the ischemic retina, we stained retinal sections of normal and ischemic eyes with ED-1 antibody (a marker for activated-macrophages). No positive staining with ED-1 antibody 4 hours after ischemia was observed (data not shown). We hypothesized that retinal and optic nerve head astrocytes and microglial cells are the major sources of acute TNF-α production and the site of TSA actions in suppressing TNF-α expression.
In arthritis models, HDAC inhibitors have been shown to suppress not only the expression of TNF-α but also the secretion of MMPs resulting from TNF-α receptor activation in these cells.20–22 Because the expression and secretion of MMPs have also been linked to retinal degeneration,43,44 we evaluated whether TSA administration could modulate MMP secretion induced by TNF-α. As shown in Figure 6, the addition of TSA blocked TNF-α–induced expression and secretion of both MMP-1 and MMP-3 from cultured astrocytes. These studies support the idea that inhibiting HDAC activity may provide cytoprotection by stabilizing the extracellular matrix while maintaining the blood retinal barrier. The silencing of MMPs and TNF-α observed in this study may reflect the upregulation of transcriptional repressors or the requirement for the inclusion of specific HDACs in the transcriptome for these proteins.
In summary, our study demonstrates that pretreatment with the HDAC inhibitor TSA can significantly reduce retinal injury initiated by ischemia/reperfusion. This retinal protective action was associated with the suppression of retinal TNF-α expression. In vitro studies provided evidence that TSA can also inhibit the downstream action of TNF-α, suppressing the increases in MMPs associated with TNF-α receptor stimulation. These findings support the idea that the regulation of acetylation in the retina provides a viable neuroprotective strategy for the treatment of retinal diseases in which ischemia may play a role in the etiology of the disease.
Footnotes
Supported in part by National Institutes of Health Grants EY009741 (CEC) and HL095696 (DRM) and by an unrestricted grant from Research to Prevent Blindness to Storm Eye Institute, Medical University of South Carolina.
Disclosure: C.E. Crosson, None; S.K. Mani, None; S. Husain, None; O. Alsarraf, None; D.R. Menick, None
References
- 1.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 2004;23:91–147 [DOI] [PubMed] [Google Scholar]
- 2.Li B, Roth S. Retinal ischemic preconditioning in the rat: requirement for adenosine and repetitive induction. Invest Ophthalmol Vis Sci 1999;40:1200–1216 [PubMed] [Google Scholar]
- 3.Husain S, Potter DE, Crosson CE. Opioid receptor-activation: retina protected from ischemic injury. Invest Ophthalmol Vis Sci 2009;50:3853–3859 [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Roth S, Laser M, Ma JX, Crosson CE. Retinal preconditioning and the induction of heat-shock protein 27. Invest Ophthalmol Vis Sci 2003;44:1299–1304 [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Schlamp CL, Poulsen GL, Jackson MW, Griep AE, Nickells RW. p53 Regulates apoptotic retinal ganglion cell death induced by N-methyl-D-aspartate. Mol Vis 2002;8:341–350 [PubMed] [Google Scholar]
- 6.Libby RT, Li Y, Savinova OV, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet 2005;1:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casson RJ, Wood JP, Melena J, Chidlow G, Osborne NN. The effect of ischemic preconditioning on light-induced photoreceptor injury. Invest Ophthalmol Vis Sci 2003;44:1348–1354 [DOI] [PubMed] [Google Scholar]
- 8.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006;6:38–51 [DOI] [PubMed] [Google Scholar]
- 9.Yang XJ, Gregoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol Cell Biol 2005;25:2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L. Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol 2006;209:611–616 [DOI] [PubMed] [Google Scholar]
- 11.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 2002;110:479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira JM, Chen S, Almeida S, et al. Mitochondrial-dependent Ca2+ handling in Huntington's disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci 2006;26:11174–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrante RJ, Kubilus JK, Lee J, et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci 2003;23:9418–9427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardian G, Browne SE, Choi DK, et al. Neuroprotective effects of phenylbutyrate in the N171–82Q transgenic mouse model of Huntington's disease. J Biol Chem 2005;280:556–563 [DOI] [PubMed] [Google Scholar]
- 15.Petri S, Kiaei M, Kipiani K, et al. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 2006;22:40–49 [DOI] [PubMed] [Google Scholar]
- 16.Chang JG, Hsieh-Li HM, Jong YJ, Wang NM, Tsai CH, Li H. Treatment of spinal muscular atrophy by sodium butyrate. Proc Natl Acad Sci USA 2001;98:9808–9813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraco G, Pancani T, Formentini L, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol 2006;70:1876–1884 [DOI] [PubMed] [Google Scholar]
- 18.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 2005;307:269–273 [DOI] [PubMed] [Google Scholar]
- 19.Quivy V, Van Lint C. Regulation at multiple levels of NF-κB-mediated transactivation by protein acetylation. Biochem Pharmacol 2004;68:1221–1229 [DOI] [PubMed] [Google Scholar]
- 20.Nasu Y, Nishida K, Miyazawa S, et al. Trichostatin A, a histone deacetylase inhibitor, suppresses synovial inflammation and subsequent cartilage destruction in a collagen antibody-induced arthritis mouse model. Osteoarthritis Cartilage 2008;16:723–732 [DOI] [PubMed] [Google Scholar]
- 21.Young DA, Lakey RL, Pennington CJ, et al. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther 2005;7:R503–R512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Song Y, Jacobi JL, Tuan RS. Inhibition of histone deacetylases antagonized FGF2 and IL-1beta effects on MMP expression in human articular chondrocytes. Growth Factors 2009;27:40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen B, Cepko CL. Requirement of histone deacetylase activity for the expression of critical photoreceptor genes. BMC Dev Biol 2007;7:78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace DM, Donovan M, Cotter TG. Histone deacetylase activity regulates Apaf-1 and caspase 3 expression in the developing mouse retina. Invest Ophthalmol Vis Sci 2006;47:2765–2772 [DOI] [PubMed] [Google Scholar]
- 25.Schwechter BR, Millet LE, Levin LA. Histone deacetylase inhibition-mediated differentiation of RGC-5 cells and interaction with survival. Invest Ophthalmol Vis Sci 2007;48:2845–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci 2005;46:1092–1098 [DOI] [PubMed] [Google Scholar]
- 27.Yang P. Purification of astrocytes from adult human optic nerve heads by immunopanning. Brain Res Brain Res Protoc 2003;12:67–76 [DOI] [PubMed] [Google Scholar]
- 28.Perez-Guijo V, Santos-Lacomba M, Sanchez-Hernandez M, Castro-Villegas MC, Gallardo-Galera JM, Collantes-Estevez E. Tumour necrosis factor-alpha levels in aqueous humour and serum from patients with uveitis: the involvement of HLA-B27. Curr Med Res Opin 2004;20:155–157 [DOI] [PubMed] [Google Scholar]
- 29.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis (TNF) levels in proliferative diabetic retinopathy. Eye 2006;20:1366–1369 [DOI] [PubMed] [Google Scholar]
- 30.Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 2001;42:1787–1794 [PubMed] [Google Scholar]
- 31.Dreixler JC, Hagevik S, Hemmert JW, Shaikh AR, Rosenbaum DM, Roth S. Involvement of erythropoietin in retinal ischemic preconditioning. Anesthesiology 2009;110:774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng PH, Huang HS, Lee YJ, Chen YS, Ma MC. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J Neurochem 2009;108:741–754 [DOI] [PubMed] [Google Scholar]
- 33.Thiersch M, Raffelsberger W, Frigg R, et al. Analysis of the retinal gene expression profile after hypoxic preconditioning identifies candidate genes for neuroprotection. BMC Genomics 2008;9:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamphuis W, Dijk F, Bergen AA. Ischemic preconditioning alters the pattern of gene expression changes in response to full retinal ischemia. Mol Vis 2007;13:1892–1901 [PubMed] [Google Scholar]
- 35.Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Jr, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1a and heme oxygenase-1. Invest Ophthalmol Vis Sci 2007;48:1735–1743 [DOI] [PubMed] [Google Scholar]
- 36.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine seacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol 2008;9:206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walkinshaw DR, Tahmasebi S, Bertos NR, Yang XJ. Histone deacetylases as transducers and targets of nuclear signaling. J Cell Biochem 2008;104:1541–1552 [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Fu M, Mani S, Wadler S, Senderowicz AM, Pestell RG. Histone acetylation and the cell-cycle in cancer. Front Biosci 2001;6:D610–D629 [DOI] [PubMed] [Google Scholar]
- 39.Zhang ZK, Davies KP, Allen J, et al. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol Cell Biol 2002;22:5975–5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Sif S, Jones B, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 1999;10:345–355 [DOI] [PubMed] [Google Scholar]
- 41.Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature 2000;408:377–381 [DOI] [PubMed] [Google Scholar]
- 42.Berger S, Savitz SI, Nijhawan S, et al. Deleterious role of TNF-alpha in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci 2008;49:3605–3610 [DOI] [PubMed] [Google Scholar]
- 43.Mali RS, Cheng M, Chintala SK. Intravenous injection of a membrane depolarization agent causes retinal degeneration via matrix metalloproteinase-9. Invest Ophthalmol Vis Sci 2005;46:2125–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci 2005;46:4747–4753 [DOI] [PubMed] [Google Scholar]
- 45.Fatma N, Kubo E, Sen M, et al. Peroxiredoxin 6 delivery attenuates TNF-alpha-and-glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res 2008;1233:63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]