This study demonstrates ganglion cell layer thinning in the pericentral area and corresponding loss of retinal nerve fiber layer thickness in the peripheral macula in patients with type 1 diabetes with no or minimal diabetic retinopathy compared with control subjects. These results support the concept that diabetes has an early neurodegenerative effect on the retina, which occurs even though the vascular component of diabetic retinopathy is still minimal.
Abstract
Purpose.
To determine which retinal layers are most affected by diabetes and contribute to thinning of the inner retina and to investigate the relationship between retinal layer thickness (LT) and diabetes duration, diabetic retinopathy (DR) status, age, glycosylated hemoglobin (HbA1c), and the sex of the individual, in patients with type 1 diabetes who have no or minimal DR.
Methods.
Mean LT was calculated for the individual retinal layers after automated segmentation of spectral domain-optical coherence tomography scans of patients with diabetes and compared with that in control subjects. Multiple linear regression analysis was used to determine the relationship between LT and HbA1c, age, sex, diabetes duration, and DR status.
Results.
In patients with minimal DR, the mean ganglion cell layer (GCL) in the pericentral area was 5.1 μm thinner (95% confidence interval [CI], 1.1–9.1 μm), and in the peripheral macula, the mean retinal nerve fiber layer (RNFL) was 3.7 μm thinner (95% CI, 1.3–6.1 μm) than in the control subjects. There was a significant linear correlation (R = 0.53, P < 0.01) between GCL thickness and diabetes duration in the pooled group of patients. Multiple linear regression analysis (R = 0.62, P < 0.01) showed that DR status was the most important explanatory variable.
Conclusions.
This study demonstrates GCL thinning in the pericentral area and corresponding loss of RNFL thickness in the peripheral macula in patients with type 1 diabetes and no or minimal DR compared with control subjects. These results support the concept that diabetes has an early neurodegenerative effect on the retina, which occurs even though the vascular component of DR is minimal.
Diabetic retinopathy (DR) is one of the leading causes of blindness in developed countries. The clinically visible onset of DR with microaneurysms, capillary nonperfusion, hemorrhages, and/or lipoprotein exudates has led to the assumption that DR is primarily a microvascular disease. Several studies have shown neural apoptosis, loss of ganglion cell bodies, glial reactivity, and reduction in thickness of the inner retinal layers in the earliest stages of DR. Some have proposed that diabetes causes retinal neuropathy through a microvascular mechanism.1–10 The structural neuropathy is corroborated by previous functional studies showing neuroretinal deficits in patients with diabetes, even before the onset of visible vascular lesions, including electroretinogram abnormalities, loss of dark adaptation and contrast sensitivity, color vision disturbances, and abnormal microperimetry.11–20
The debate is still open as to whether diabetic retinal neuropathy is the effect of vascular diabetic retinopathy or is primarily caused by direct neurologic damage from chronic hyperglycemia. Relationships between specific inner retinal layer thicknesses and DR status, on the one hand, as a proxy for vascular damage, and specific inner retinal layer thicknesses and duration of diabetes, on the other hand, as a proxy for chronic hyperglycemia, may shed additional light on this debate.
The introduction of time domain-optical coherence tomography (TD-OCT) has made it possible to image the human retina longitudinally in vivo and to measure retinal thickness (RT) with high accuracy. Several groups have shown that total RT is decreased in diabetic patients with no or minimal DR, compared to that in normal control subjects.12,21–26 Automated segmentation of intraretinal layers in TD-OCT images has enabled calculation of the mean thickness of the individual layers of the retina. The results have shown that the decreased total RT in patients with type 1 diabetes with minimal DR is due to thinning of the inner retina in the pericentral and peripheral area of the central macula.26 However, researchers in these studies were unable to accurately differentiate between the individual layers that constitute the inner retinal layer (i.e., retinal nerve fiber layer, ganglion cell layer, inner plexiform layer, and inner nuclear layer), because of the limited resolution of TD-OCT. The identification of the retinal layers that are affected by diabetes may help elucidate the underlying mechanism of inner retinal damage and guide the development of neuroprotective therapeutic strategies.
Spectral domain (SD)-OCT allows imaging of the macula, with higher scan resolution and reduced motion artifacts compared with TD-OCT. TD-OCT collects 400 axial measurements per second with an axial resolution of approximately 10 μm. The scan rate of SD-OCT is at least 18,000 axial measurements per second with an axial resolution of 5 μm, so that SD-OCT allows detailed three-dimensional (3-D), close-to-isotropic volumetric scans. The primary advantage of the more isotropic imaging in measuring retinal thickness is that fewer assumptions have to be made about the tissue between measured samples, potentially leading to more accurate RT measurements. The high resolution of SD-OCT allows for enhanced definition of the retinal layers. We have recently developed a fully three-dimensional, automated algorithm to segment multiple surfaces in SD-OCT scans in the retina based on differences in refractive index, with an accuracy comparable to that of human experts.27
The purpose of this study was twofold: to determine in detail which retinal layers are most affected by diabetes and contribute to the thinning of the inner retina and to investigate the possible relationship between thinning of the inner retina and duration of diabetes mellitus (DM), DR status, age, glycosylated hemoglobin (HbA1c), and the sex of the individual, by using high-resolution SD-OCT and retinal layer segmentation in patients with type 1 diabetes and no or minimal DR compared with normal control subjects.
Materials and Methods
Patients
Patients recruited from the outpatient clinic of the department of Internal Medicine at the Academic Medical Center (University Hospital, Amsterdam, the Netherlands) between July 2004 and June 2005 and participating in an ongoing longitudinal observational study, were asked to participate in this observational cross-sectional study. Normal control subjects were age-matched with patients; did not have a diagnosis of any ocular disease, diabetes, or other systemic disease; and were randomly recruited from persons accompanying patients visiting the outpatient clinic of the Department of Ophthalmology. The study adhered to the tenets of the Declaration of Helsinki. Investigative Review Board approval was obtained at both the AMC and the University of Iowa, and all participants gave written informed consent.
Patients were included if they had a diagnosis of type 1 DM and either no DR or minimal DR, as determined by a retinal specialist through indirect fundoscopy, slit lamp stereo biomicroscopy, and stereoscopic fundus photography. Minimal DR was defined as the presence of at least one microaneurysm and/or hemorrhage in the central retina in the absence of peripheral lesions (stage 2 of the International Clinical Diabetic Retinopathy Disease Severity Scale [ICDRSS]).28 No DR was defined as no microaneurysms in the central retina and no lesions in the periphery (stage 1 of the ICDRSS). Exclusion criteria were refractive errors more than S+5 D or less than S−8 D, visual acuity below 20/25, significant media opacity, previous ocular surgery, and a previous diagnosis of glaucoma, uveitis, or retinal disease. Visual acuity was measured with an Early Treatment Diabetic Retinopathy Study chart at 4 m. Best corrected visual acuity was recorded as the Snellen equivalent. After pupil dilation with 0.5% phenylephrine hydrochloride and 0.1% tropicamide, both eyes were examined with stereoscopic slit lamp biomicroscopy with a handheld lens (SuperField; Volk Optical, Inc., Mentor, OH) for signs of DR, and OCT images were obtained (3-D OCT-1000; Topcon Corp., Tokyo, Japan). In addition, stereoscopic fundus photographs were taken (TRC-50IX; Topcon Corp.). Age, sex, duration of diabetes (in years), and mean HbA1c were obtained from the patients' charts. The mean HbA1c was calculated from all HbA1c measurements in the year preceding the ophthalmic examination.
OCT Imaging and Layer Segmentation
Subjects were imaged with SD-OCT (3-D OCT-1000; Topcon Corp.). A 3-D volume scan protocol (6 × 6 × 2.2 mm), consisting of 128 (y), to 512 (x), to 650 (z) voxels, was used. Nine intraretinal surfaces were segmented in all subjects by our algorithm, which is a fully 3-D graph search approach.27 This graph-theoretic approach detects the optimal set of feasible 3-D surfaces in the OCT volume with respect to a cost function. The cost function definition defines the better of two feasible surface sets. Intuitively, for each surface, a “cost'' is assigned to each voxel, to reflect the unlikelihood (the complement of the likelihood) that the voxel belongs to a particular surface, with the surface cost being defined as the cost summation of all surface voxels. The cost of a set of feasible surfaces is defined by summation of the individual surface costs. In this work, the cost functions were defined by 3-D edge detectors that favor a dark-to-light or a light-to-dark transition. As an example, consider the use of a 3-D edge detector favoring a dark-to-light transition for the internal limiting membrane. This approach corresponds to defining the surface at the points in which the intensity changes from a dark to a light intensity, subject to the feasibility constraint that the defined 3-D surface is smooth and appropriately interacts with the other detected surfaces (e.g., the surface is not allowed to cross a second surface or make large jumps in position).
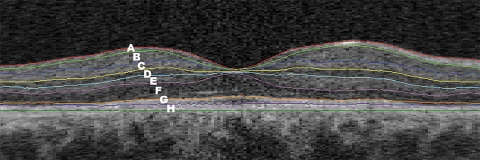
The layers that could be identified between intraretinal surfaces were interpreted as follows (from the inner to outer surface, as labeled in Fig. 1): A, retinal nerve fiber layer (RNFL); B, ganglion cell layer (GCL); C, inner plexiform layer (IPL); D, inner nuclear layer (INL); E, outer plexiform layer (OPL); F, outer nuclear layer (ONL)+inner segment photoreceptors (IS); G, outer segment photoreceptors (OS); and H, retinal pigment epithelium (RPE).
Figure 1.
Macular B-scan with intraretinal surfaces, as indicated by the colored lines and corresponding retinal layers. A, RNFL; B, GCL; C, IPL; D, INL; E, OPL; F, ONL+IS; G, OS; and H, RPE.
Thickness Measurement of the Separate Layers
After automated segmentation of the retinal layers, one of the authors (HvD) who was masked to the status of diabetes and demographic features, defined two retinal areas: the pericentral area, a donut-shaped ring centered on the fovea with an inner diameter of 1 mm and an outer diameter of 3 mm, and the peripheral area, with an inner diameter of 3 mm and outer diameter of 6 mm. The mean thickness of each layer in the pericentral and peripheral areas was calculated automatically with ImageJ 1.41, a public domain, Java-based image-processing program developed by Wayne Rasband at the National Institutes of Health.29
Statistical Analysis
Analysis of variance (ANOVA) was used to assess differences in mean age between diabetic patients with no and minimal DR and controls (SPSS 16.0.2 for Windows; SPSS, Chicago, IL). Mean HbA1c and duration of diabetes were compared between patients with no and minimal DR by using the unpaired t-test.
Mean RT and mean layer thickness (LT) of diabetic patients with minimal DR, diabetic patients with no DR, and control subjects were compared by ANOVA, followed by a Bonferroni post hoc analysis to correct for multiple comparisons.
Confidence intervals were computed at the P = 0.05 level. Correlation analysis between LT differences and duration of diabetes was performed by calculating the Pearson correlation coefficient. A multiple linear regression model was used to determine the relationship between retinal LT, duration of DM and DR status, while correcting for HbA1c, age, and sex.
Results
Demographics
In total, 40 patients with type 1 diabetes were included, of which 38 have already been described in a previous study in which TD-OCT was used instead of SD-OCT.26 Nineteen patients had no DR and 20 had minimal DR. There was a significant difference in mean duration of diabetes (P = 0.01) between the patients with minimal DR and those with no DR (Table 1). There was no significant difference in age and sex in the both patient and control groups. Patients were in average glycemic control (mean HbA1c = 8.4%; SD = 1.2%).
Table 1.
Demographics of Patients with Type 1 Diabetes and No or Minimal DR and Control Subjects
| Parameters | No DR (n = 19) | Minimal DR (n = 20) | Control Subjects (n = 40) |
|---|---|---|---|
| Age, y | 30 ± 11 | 37 ± 10 | 33 ± 9 |
| Sex, M:F | 7:12 | 10:10 | 24:16 |
| Duration of DM, y | 14 ± 7* | 22 ± 10* | NA |
| HbA1c, % | 8.4 ± 1.5 | 8.3 ± 0.8 | — |
Data are the mean micrometers ± SD for all subjects in each group. NA, not applicable; —, not performed.
Significant difference as compared between the patients with type 1 DM with minimal DR and no DR.
Retinal LT Analysis
Differences in Regional Retinal LT.
The differences (in micrometers) in regional retinal LT between patients with type 1 DM and no or minimal DR and normal control subjects in the pericentral and peripheral areas of the macula are given in Tables 2 and 3. Significant differences were found in the GCL in the pericentral area of the macula, which was 5.1 μm thinner in patients with minimal DR than in normal control subjects (95% confidence interval [CI], 1.1–9.1). The RNFL in the peripheral area of the macula was 3.7 μm thinner in patients with minimal DR than in the normal control subjects (95% CI, 1. 3–6.1). The other retinal layers did not show a significant difference in LT. Patients with diabetes but no DR showed no significant differences in any LT in any area compared with normal control subjects.
Table 2.
Mean Layer Thickness Measurements of the Individual Intraretinal Layers in the Pericentral Area of the Macula in Patients with Type 1 DM and No or Minimal DR Compared with Control Subjects
| Parameters | No DR (n = 19) | Mean Difference | 95% CI | Minimal DR (n = 20) | Mean Difference | 95% CI | Control Subjects (n = 40) |
|---|---|---|---|---|---|---|---|
| RNFL | 23.6 | 0 | −1.4–1.4 | 22.3 | 1.3 | −0.1–2.7 | 23.6 |
| GCL | 50.8 | −1.4 | −5.5–2.7 | 44.3 | 5.1 | 1.1–9.1 | 49.4 |
| IPL | 40.2 | 0 | −2.6–2.6 | 38.1 | 2.1 | −0.4–4.6 | 40.2 |
| INL | 38.6 | −0.9 | −3.2–1.4 | 36.3 | 1.4 | −0.9–3.7 | 37.7 |
| OPL | 30.0 | 0.3 | −2.6–3.0 | 31.4 | −1.1 | −4.0–1.6 | 30.3 |
| ONL+IS | 88.3 | 2.6 | −4.7–9.8 | 88.1 | 2.8 | −4.3–9.9 | 90.9 |
| OS | 24.2 | 0.3 | −1.9–2.4 | 24.9 | −0.4 | −2.5–1.7 | 24.5 |
| RPE | 36.7 | −1.6 | −3.4–0.2 | 35.7 | −0.6 | −2.4–1.1 | 35.1 |
Data are the mean micrometers ± SD for all subjects in each group. The bold data indicate a statistically significant difference between patients and control subjects (P < 0.01).
Table 3.
Mean Layer Thickness Measurements of the Individual Intraretinal Layers in the Peripheral Area of the Macula in Patients with Type 1 DM and Minimal or No DR Compared with Control Subjects
| Parameters | No DR (n = 19) | Mean Difference | 95% CI | Minimal DR (n = 20) | Mean Difference | 95% CI | Control Subjects (n = 40) |
|---|---|---|---|---|---|---|---|
| RNFL | 35.8 | 0.8 | −1.7–3.1 | 32.9 | 3.7 | 1.3–6.1 | 36.6 |
| GCL | 30.5 | −0.6 | −2.8–1.5 | 28.7 | 1.2 | −0.9–3.3 | 29.9 |
| IPL | 34.9 | 0.2 | −2.5–2.8 | 32.9 | 2.2 | −0.4–4.7 | 35.1 |
| INL | 30.5 | −0.6 | −2.5–1.2 | 28.9 | 1.0 | −0.8–2.8 | 29.9 |
| OPL | 25.3 | 0.2 | −1.1–1.4 | 26.0 | −0.5 | −1.7–0.7 | 25.5 |
| ONL+IS | 73.4 | 2.3 | −3.2–7.7 | 74.6 | 1.1 | −4.3–6.4 | 75.7 |
| OS* | 24.6 | −1.0 | −3.5–1.6 | 23.0 | 0.6 | −1.9–3.1 | 23.6 |
| RPE | 33.5 | −0.8 | −2.8–1.3 | 34.2 | −1.5 | −3.4–0.5 | 32.7 |
Data are the mean micrometers ± SD for all subjects in each group. The bold data indicate a statistically significant difference between patients and control subjects (P < 0.01).
Minimal DR, n = 16; no DR, n = 14; healthy control subjects, n = 31, measurements with artifacts excluded.
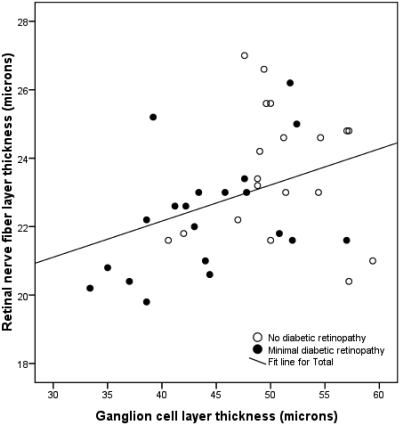
Relationships between GCL and RNFL.
In the pooled population of patients with no or minimal DR, the relationship between individual LTs was assessed. There was a significant and marked linear correlation (R = 0.54, P < 0.01) between GCL thickness in the pericentral area of the macula and RNFL thickness in the peripheral area of the macula (Fig. 2), in all patients with diabetes.
Figure 2.
Correlation between pericentral GCL and peripheral RNFL thicknesses in patients with type 1 diabetes and minimal or no DR (R = 0.54, P < 0.01).
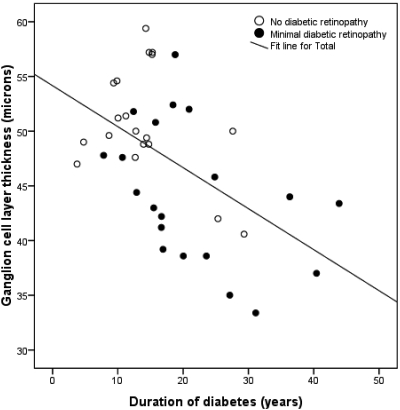
Relationship between GCL Thickness and Duration of DM.
In the pooled population of patients with no or minimal DR, the relationship was assessed between GCL thickness and duration of diabetes. GCL thickness versus duration of diabetes, not corrected for HbA1c, age, sex, and retinopathy status is plotted in Figure 3, showing a significant inverse linear correlation between GCL thickness and duration of diabetes.
Figure 3.
Correlation between GCL thickness and duration of diabetes in patients with type 1 diabetes and minimal or no DR (R = 0.53, P < 0.01).
Relationship between GCL Thickness and Duration of DM, Retinopathy Status, Age, HbA1C, and Sex.
A multiple linear regression model was used to assess the relationship between GCL thickness, duration of diabetes, and DR status, while correcting for HbA1c, age, and sex. In Table 4 the standardized coefficients of the explanatory variables—HbA1c, age, sex, duration of DM, and retinopathy status—using GCL thickness as the dependent variable are presented, which shows the DR status to be the best explanatory variable.
Table 4.
Standardized Regression Coefficients Derived from Multiple Linear Regression Analysis with Ganglion Cell Layer Thickness as the Dependent Variable
| Independent Variable | Standardized Coefficients | P |
|---|---|---|
| HbA1c | 0.05 | 0.76 |
| Age | 0.01 | 0.96 |
| Sex | −0.01 | 0.93 |
| Duration DM | −0.38 | 0.06 |
| DR status | −0.35 | 0.03 |
R = 0.62, P < 0.01. Regression coefficients are presented in standardized (z-score) form.
Discussion
The findings in this study demonstrate that, in this population, the GCL in the pericentral area and the RNFL in the peripheral area of the macula were thinner in patients with minimal DR than in normal control subjects. There was a significant linear correlation between the GCL thickness in the pericentral area and the RNFL thickness in the peripheral area of the macula. The duration of DM was correlated significantly and inversely with GCL thickness. In the multiple linear regression analysis including age, sex, HbA1c, diabetes duration, and DR status, DR status was the most important explanatory variable.
The results suggest that the thinning of the inner retina in patients with minimal retinopathy, which we described earlier,26 is caused primarily by a thinning of the GCL in the pericentral area of the macula, and secondary thinning of the RNFL more peripherally in the macula, because of axonal loss from the central ganglion cells. The ganglion cell axons in the RNFL travel in bundles toward the optic nerve head without any tendency to cross to adjacent bundles or disperse.30 Therefore, the most pronounced difference in RNFL thickness due to loss of ganglion cells in the pericentral area is expected to be found in the nasal region of the peripheral area of the macula. This result was confirmed in an additional analysis showing a significant difference in RNFL thickness (5.5 μm; 95% CI, 1–9.9) in the nasal region of the peripheral area of the macula in patients with minimal DR compared with that in normal control subjects, whereas the difference in the temporal region was minimal (0.4 μm; 95% CI, −1.7–2.4).
The results indicate an early neurodegenerative effect on the retina in diabetes, which occurs, even though the vascular component of diabetic retinopathy remains minimal. The mean duration of DM in patients with minimal DR in this study was 8 years longer than that in the patients without DR (Table 1). This result shows that both processes, DR and neurodegeneration, develop slowly over time and that both are late complications of DM, which suggests that both processes are closely linked. However, the exact nature of their interdependence is not known. Each process, once established, probably contributes to the progression of the other. Therefore, neuronal apoptosis may be an important target for new therapeutic interventions.
The significant linear correlation between GCL thickness and duration of diabetes suggests that neurodegeneration—loss of GCL—is primarily caused by a prolonged disturbance of the glucose metabolism, perhaps even irrespective of the presence of vasculopathy. Figure 3 shows that longer duration of DM without DR can result in a relatively thin GCL. This assumption is in line with results in earlier reports. A previous study showed that, in diabetic patients with no or mild DR, the macular and foveal thickness is significantly thinner with longer duration of DM.21 Moreover, neuroretinal functional deficits, such as an abnormal electroretinogram, loss of dark adaptation and contrast sensitivity, color vision disturbances, and abnormal microperimetry were described in several studies in patients with diabetes before the onset of visible vascular lesions.11–20 As the inner retina is part of the central nervous system, the results are also in line with the findings of a previous study describing decreased functional connectivity and cognition in diabetic patients with and without microvascular complications. In this study, the authors hypothesized that chronic hyperglycemia, even in the absence of clinically detectable microvascular complications, can negatively affect cognitive functioning and cerebral communication.31 Although the pathophysiological substrate is yet undefined, there is evidence that chronic hyperglycemia and not the number of hypoglycemic events is the cause of cognitive deterioration and structural brain changes in patients with diabetes.31–35
With the introduction of SD-OCT, regional differences in LT can be determined more reliably. TD-OCT (Stratus-OCT; Carl Zeiss Meditec, Dublin, CA) is limited because only six B-scans are obtained, and the data must be interpolated over relatively large areas. The faster scanning time and increased depth resolution of the SD-OCT allow for a substantial improvement of retinal thickness mapping resolution, with less movement artifacts. The results of this study substantially confirm those in a previous study with the Stratus-OCT, in which thinning of the inner retina was described in the same group of patients with type 1 diabetes and minimal DR.26 Neurodegeneration seems to be a generalized process that occurs throughout the macular area and is not confined to local abnormalities, as is the case with funduscopically visible signs of retinopathy. However, the use of SD-OCT makes it possible to measure individual layers at higher resolution and indicates that the thinning of the inner retina in the macula is primarily due to loss of ganglion cells.
There are limitations to the present study. First, the grading of no or minimal background retinopathy was made by a single reader from a single set of stereoscopic central retinal photographs combined with clinical evaluation, instead of the gold standard—that is, seven-field stereo fundus photography read by independent, trained graders.36 However, the patients definitely did not have advanced retinopathy, indicating that the results do apply to the earliest stage of DR.
Second, the HbA1c of the normal subjects was not known. Type 2 diabetes is often subclinical; therefore it cannot be excluded that some normal control subjects actually had type 2 diabetes and elevated blood sugar levels, although the prevalence of diabetes at this age is low. Also, the presence of undiagnosed diabetes would most likely lead to underestimation, not overestimation, of the difference in inner retinal thickness between patients and control subjects.
In summary, this study demonstrates selective loss of thickness of the GCL in the pericentral area and corresponding loss of RNFL thickness in the peripheral area of the macula in patients with type 1 diabetes and minimal DR compared with control subjects. These results support the concept that early DR includes a neurodegenerative component. The hypothesis that diabetes causes retinal neuropathy independent of retinopathy is intriguing (although larger studies are needed) and potentially links retinal neuropathy to other diabetic neuropathies.
Footnotes
Supported by the Netherlands Organization for Health Research and Development, National Institutes of Health Grant R01-EY017066, Research to Prevent Blindness, and The Edward en Marianne Blaauwfonds.
Disclosure: H.W. van Dijk, None; F.D. Verbraak, None; P.H.B. Kok, None; M.K. Garvin, P; M. Sonka, P; K. Lee, P; J.H. DeVries, None; R.P.J. Michels, None; M.E.J. van Velthoven, None; R.O. Schlingemann, None; M.D. Abràmoff, P
References
- 1.Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci 2004;45:2760–2766 [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 2006;55:2401–2411 [DOI] [PubMed] [Google Scholar]
- 3.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Invest 1998;102:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:283–290 [DOI] [PubMed] [Google Scholar]
- 5.Barber AJ, Antonetti DA, Kern TS, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci 2005;46:2210–2218 [DOI] [PubMed] [Google Scholar]
- 6.Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci 2006;47:3143–3150 [DOI] [PubMed] [Google Scholar]
- 7.Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci 2008;49:2635–2642 [DOI] [PubMed] [Google Scholar]
- 8.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci 2004;45:3330–3336 [DOI] [PubMed] [Google Scholar]
- 9.Park SH, Park JW, Park SJ, et al. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 2003;46:1260–1268 [DOI] [PubMed] [Google Scholar]
- 10.Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 2000;41:1971–1980 [PubMed] [Google Scholar]
- 11.Bearse MA, Jr, Adams AJ, Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res 2006;25:425–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronson-Castain KW, Bearse MA, Jr, Neuville J, et al. Adolescents with type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina 2009;29:618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Leo MA, Caputo S, Falsini B, et al. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care 1992;15:620–625 [DOI] [PubMed] [Google Scholar]
- 14.Dosso AA, Bonvin ER, Morel Y, Golay A, Assal JP, Leuenberger PM. Risk factors associated with contrast sensitivity loss in diabetic patients. Graefes Arch Clin Exp Ophthalmol 1996;234:300–305 [DOI] [PubMed] [Google Scholar]
- 15.Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci 1999;40:2638–2651 [PubMed] [Google Scholar]
- 16.Hardy KJ, Lipton J, Scase MO, Foster DH, Scarpello JH. Detection of colour vision abnormalities in uncomplicated patients with type 1 diabetes with angiographically normal retinas. Br J Ophthalmol 1992;76:461–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtenbach A, Flogel W, Erb C. Anomaloscope matches in patients with diabetes mellitus. Graefes Arch Clin Exp Ophthalmol 2002;240:79–84 [DOI] [PubMed] [Google Scholar]
- 18.Lopes de Faria JM, Katsumi O, Cagliero E, Nathan D, Hirose T. Neurovisual abnormalities preceding the retinopathy in patients with long-term type 1 diabetes mellitus. Graefes Arch Clin Exp Ophthalmol 2001;239:643–648 [DOI] [PubMed] [Google Scholar]
- 19.Ng JS, Bearse MA, Jr, Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci 2008;49:1622–1628 [DOI] [PubMed] [Google Scholar]
- 20.Realini T, Lai MQ, Barber L. Impact of diabetes on glaucoma screening using frequency-doubling perimetry. Ophthalmology 2004;111:2133–2136 [DOI] [PubMed] [Google Scholar]
- 21.Asefzadeh B, Fisch BM, Parenteau CE, Cavallerano AA. Macular thickness and systemic markers for diabetes in individuals with no or mild diabetic retinopathy. Clin Exp Ophthalmol 2008;36:455–463 [DOI] [PubMed] [Google Scholar]
- 22.Biallosterski C, van Velthoven ME, Michels RP, Schlingemann RO, DeVries JH, Verbraak FD. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol 2007;91:1135–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology 2008;115:533–539 [DOI] [PubMed] [Google Scholar]
- 24.Nilsson M, von WG, Wanger P, Martin L. Early detection of macular changes in patients with diabetes using Rarebit Fovea Test and optical coherence tomography. Br J Ophthalmol 2007;91:1596–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oshitari T, Hanawa K, Adachi-Usami E. Changes of macular and RNFL thicknesses measured by Stratus OCT in patients with early stage diabetes. Eye 2009;23(4):884–889 [DOI] [PubMed] [Google Scholar]
- 26.Van Dijk HW, Kok PH, Garvin M, et al. Selective loss of inner retinal layer thickness in patients with type 1 diabetes with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci 2009;50(7):3404–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garvin M, Abramoff M, Wu X, Russell S, Burns T, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence Tomography Images. IEEE Trans Med Imaging 2009;28(9):1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson CP, Ferris FL, III, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 29.Abràmoff M, Magalhâes P, Ram S. Image processing with ImageJ. Biophotonics 2004;11:36–42 [Google Scholar]
- 30.Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 2000;107:1809–1815 [DOI] [PubMed] [Google Scholar]
- 31.Van Duinkerken E, Klein M, Schoonenboom NS, et al. Functional brain connectivity and neurocognitive functioning in patients with long-standing type 1 diabetes with and without microvascular complications: a magnetoencephalography study. Diabetes 2009;58:2335–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156 [DOI] [PubMed] [Google Scholar]
- 33.Jacobson AM, Musen G, Ryan CM, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musen G, Lyoo IK, Sparks CR, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 2006;55:326–333 [DOI] [PubMed] [Google Scholar]
- 35.Wessels AM, Rombouts SA, Remijnse PL, et al. Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia 2007;50:1763–1769 [DOI] [PubMed] [Google Scholar]
- 36.Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98:786–806 [PubMed] [Google Scholar]