Abstract
The glucose analogue fluorodeoxyglucose (FDG) has demonstrated enhanced uptake in the majority of tumours as a result of increased uptake and fixation by phosphorylation. It is the most widely used radiotracer in positron emission tomography (PET), being used in >90% of scans, and is useful for diagnosis, staging and detection of residual/recurrent cancer. However, there are limits to the utility of FDG, particularly in certain tumour types. The development of new radiotracers to study molecular processes such as proliferation, apoptosis, angiogenesis and hypoxia will complement FDG by providing additional information on the cell biology of tumours. The aim of this paper is to consider how the availability of new tracers, or new applications for existing PET/CT technologies, could deliver clinical benefit in cancer, using breast cancer as a paradigm.
Keywords: Assessing therapy response, breast cancer, diagnosis, identification of recurrence, pharmacological biomarker, positron emission tomography, predictive biomarker, staging, surrogate response biomarker, tumour subtyping
Introduction
The use of molecular/genetic markers to identify subtypes of specific cancers and the development of novel targeted drugs represent significant advances that will affect cancer, with the potential to realise a more stratified approach to cancer care. These advances, combined with simultaneous developments in imaging, will create new opportunities for positron emission tomography (PET) to help improve clinical outcomes in cancer. Breast cancer was chosen as an exemplar, as PET currently has a limited role in this indication, but this could expand significantly as a result of ongoing tumour characterisation at the molecular level, and the range of targeted therapies being developed for this disease. This article considers these new opportunities for PET.
Effect on patient care and opportunities for PET
Breast cancer remains the second most common cause of cancer-related death in British women, with more than 12,000 deaths per year[1] despite substantial improvements in management, including the introduction of mammography screening, contrast-enhanced magnetic resonance imaging (MRI), local treatment of early stage disease and new therapies. PET is not widely used in breast cancer management at present but this may change with two technical advances that increase instrument sensitivity: (1) PET scanners with time-of-flight capability can identify the location of annihilation events more accurately than conventional machines; this improves the spatial resolution of the reconstructed image by reducing background noise levels[2]; (2) dedicated breast PET/CT scanners with higher spatial resolution and photon sensitivity than whole-body scanners are currently being characterised[3].
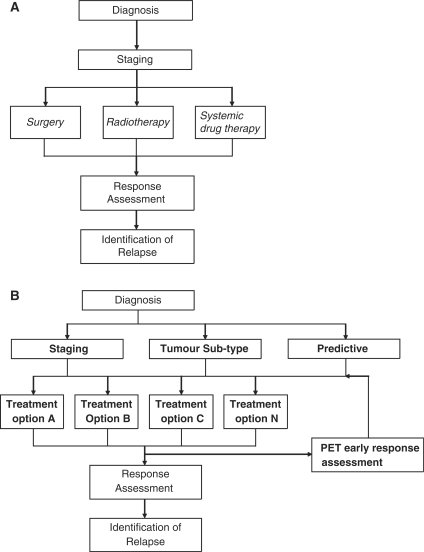
The developments of tumour subtyping and the availability of a broader range of treatments[4] are likely to lead to changes in how patients with breast cancer are managed clinically, as summarised in Fig. 1. Compared with traditional care pathways, the identification of cancer subtypes represents a new step. Next, based on tumour subtype, either alone or in combination with conventional staging information, specific treatments or combinations of treatments may be selected from amongst an increasing number of available targeted therapies. Furthermore, PET offers opportunities to measure functional aspects of the tumour microenvironment, predict response to treatment and assess response early rather than at the traditional time point on completion of therapy[5,6]. Earlier response assessment could potentially allow ineffective treatments to be changed, reducing the likelihood of treatment-associated morbidity. This approach creates new opportunities for PET at several points in the care pathway.
Figure 1.
Emerging paradigm for cancer treatment. (A) The traditional cancer care pathway, in which imaging is used for diagnosis, staging and assessment of tumour response. The stage determines the treatment option: surgery for localised disease, radiotherapy for locoregional disease and chemotherapy for systemic disease. (B) The development of tumour subtyping and the availability of a wider range of targeted treatments is changing this paradigm such that treatment selection can be made on the basis of combined tumour subtype and staging. There is also an increased number of treatment options (A, B, C,…,N) which often comprise a combination of modalities and chemotherapeutic agents tailored to the patient’s subtype and stage. PET can be used as a functional baseline tool to predict response and can be used to assess early response after only one or two cycles of treatment.
Diagnosis
The poor sensitivity and high expense of PET means it is not a cost-effective primary screening method. Fluorodeoxyglucose (FDG) has low sensitivity for detection of small (<1 cm) and low-grade tumours, because breast tumours tend to have a low rate of glucose metabolism, resulting in many false-negative results. The uptake of FDG is higher in triple negative subtypes (i.e. basal-like) breast cancers than in ER+/PR+/HER2– tumours[7], but this finding is unlikely to affect the use of FDG-PET for staging because the subtype is not known at the time of diagnosis.
Staging
FDG-PET is currently not used in early stage breast cancer, but there is some evidence that it could be useful in more advanced cancers, specifically node-positive tumours greater than 2 cm in diameter (T2, N1–3), tumours greater than 5 cm (T3) and tumours with evidence of invasion of skin or chest wall (T4)[8]. MRI is advocated to assess the local extent of disease in the breast and although there a number of advocates for this to reduce reoperation rates and local recurrence, a recent large multicentre trial has suggested that MRI is not useful[9]. FDG-PET is useful for detecting locoregional disease[10], but does not eliminate the need for sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND)[11,12]; Metastasis is highly likely in axillary nodes with high FDG uptake, so patients require axillary dissection, whereas an axillary biopsy is still required with no uptake of FDG in the axilla. However, FDG-PET may be a useful test to select patients for SLNB or ALND[11]. Neither mammography nor ultrasound with guided biopsy have proved successful in robustly identifying patients with nodal disease. Contrast-enhanced MRI has been promising[13], and the use of ultrasmall iron oxide particles has been investigated with encouraging results, but neither technique is used in day to day practice.
In addition, the finding of greater FDG uptake in triple negative cancers[7] raises the possibility that FDG-PET may be useful in staging specific tumour subtypes. However, this targeted application of FDG-PET is yet to be subject to clinical trials.
New PET tracers may be able to improve on FDG by increasing the sensitivity, specificity or accuracy of staging late-stage tumours. FDG-PET has demonstrated some utility in detecting metastases. A range of novel PET tracers could generate data on different metastases to complement FDG-PET (e.g. [18F]fluoride is often superior at detecting bone metastases than FDG[14]). Other new probes could help to elucidate the biochemical nature of the metastasis (e.g. [18F]misonidazole which detects hypoxia) and whether this is homogeneous or heterogeneous. Moreover, some novel PET tracers (e.g. radiotracers that bind the ER or HER2) could be useful predictive biomarkers for breast cancer tumours, and aid identification of heterogeneous tumours that may not be fully responsive to single therapy treatment.
Tumour subtyping
There is ever increasing evidence that breast cancer is not one homogeneous disease, but composed of several related, but distinct, subtypes. Molecular profiling of breast cancer has identified at least five different subtypes of this disease, each of which displays a unique gene expression pattern[15], distinct prognosis[16], and different sensitivity to a range of anti-cancer drugs[16].
Luminal cancers are the most common subtype of breast cancer (60%); they characteristically express the oestrogen receptor (ER) and progesterone receptors (PR) and typically contain wild-type p53 protein. Luminal breast cancers are further subdivided into A and B subtypes; luminal A generally has a higher expression of ER-related proteins than B and lower expression of proteins that promote proliferation. Luminal breast cancers have a good overall prognosis, although luminal B has a somewhat poorer outcome than A. Luminal A breast cancers usually respond well to hormonal therapy, whereas conventional chemotherapy is often ineffective. In contrast, both endocrine therapy and chemotherapy are often beneficial in luminal B breast cancers.
The HER2 subtype refers to breast cancer tumours that overexpress the HER2 receptor, irrespective of ER and PR expression. HER2 breast cancers comprise approximately 20% of breast tumours, generally contain a high proportion (40–80%) of mutated p53 protein and have a poor prognosis. HER2 breast cancer tumours are sensitive to anthracycline and taxane-based chemotherapy, but treatment of these tumours has been dramatically improved by the introduction of HER2-targeted therapies such as trastuzumab.
The basal-like subtype encompasses approximately 10% of breast cancers, and the majority of these patients are described as having triple negative receptor status, because they do not contain significant levels of ER, PR or HER2. Basal-like breast cancers have a high proportion of mutated p53 protein, low levels of BRCA1, are more likely to overexpress poly(ADP-ribose) polymerase (PARP) and generally have a poor prognosis. Many, but not all, cases of this breast cancer subtype respond to chemotherapy treatments such as anthracyclins or taxanes, suggesting that its poor prognosis is probably due to the intrinsic biology of the subtype.
Normal breast-like cancer has the lowest occurrence (approximately 5%) of all of the breast cancer subtypes and is characterised by low expression levels of ER and luminal epithelial genes, high expression of basal epithelial genes and usually has a reasonably good clinical prognosis. Not all authorities recognise it as a separate and well-defined subset.
The understanding that breast cancer is composed of several subtypes with different molecular and clinical properties is a significant advance, because this allows the possibility of selecting anti-cancer treatment(s) that are likely to have a higher probability of success in each subtype, as well as developing specific therapies to treat each breast cancer subtype. Some of the promising new therapies currently being developed are described below and shown in Fig. 2.
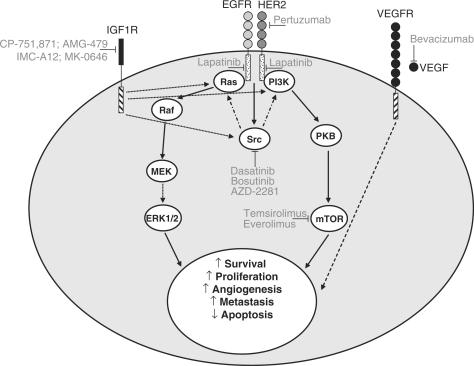
Figure 2.
Promising new anti-cancer agents in breast cancer. The figure summarises some of the most promising agents that are being developed for breast cancer, and how inhibition of each target may affect some of the key signalling pathways and biological processes in an individual tumour cell. ↓ represents activation, ⊥ represents inhibition. Abbreviations: EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; IGF1R, insulin-like growth factor 1 receptor; MEK, MAPK/extracellular signal-regulated kinase kinase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B; VEGF(R), vascular endothelial growth factor (receptor).
Angiogenesis inhibitors
Tumour growth and dissemination depends on the development of a neovasculature and vascular endothelial growth factor (VEGF) is a key stimulator of angiogenesis. The VEGF receptor is overexpressed in some breast cancers, and pathways related to angiogenesis are overrepresented in the basal subtype[17], suggesting a potential therapeutic role for angiogenesis inhibitors. Bevacizumab inhibits growth in several different solid tumours by binding to and sequestering VEGF, and having demonstrated activity in advanced breast cancer, is now being tested in the setting of early, curable breast cancer[18]. Other agents that modulate VEGF signalling (e.g. sorafenib which inhibits the VEGF receptor, B-Raf and c-kit) are also being considered for breast cancer.
Epidermal growth factor receptor inhibitors
A number of new inhibitors that target members of the epidermal growth factor receptor (EGFR) family are in clinical development. The most promising of these are lapatinib, a dual EGFR1 and 2 (HER2) inhibitor and pertuzumab, a monoclonal antibody that prevents the dimerisation of HER2[19]. EGFR1 expression is observed only in basal-like and HER2 positive breast cancer[20], making these subtypes prospective targets for lapatinib therapy. Lapatinib has recently been approved for treatment of HER2+ breast cancers, but has not been particularly effective in basal-like breast cancers, suggesting that this subtype is not dependent on EGF signalling. Pertuzumab will only be effective in the HER2 subtype, and is likely to be used as an alternative to herceptin, or more probably in combination with that drug.
Insulin-like growth factor inhibitors
Insulin-like growth factor 1 receptor (IGF1R) is associated with the growth, invasion and metastasis of breast cancer[21]. At present, the relative importance of the IGF1R signalling pathway in each breast cancer subtype is not clear. The IGF1R pathway has been implicated in resistance to cytotoxic agents and targeted therapies such as those that inhibit the ER, HER2 and the EGFR[22]. Several IGF1R inhibitors are in clinical development (e.g. monoclonal antibodies CP-751,871, IMC-A12, AMG-479 and MK-0646) and it has been suggested that these agents may be particularly effective at reversing or treating resistance to chemotherapeutic or targeted breast cancer treatment[22].
Src inhibitors
Src plays an important role in the cross-talk between proliferative signalling pathways downstream of receptors such as the ER, IGF1R and the EGFR family, and src kinase activity is enhanced in breast cancer patients, making it a potential target for targeted therapeutics. Several promising anti-cancer agents are being developed for this target, including dasatinib, bosutinib and AZD0530[23]. Preclinical data suggest that Src inhibitors may be effective as a single agent in basal-like tumours[24], or a part of a combination therapy with other targeted agents[23] in multiple breast cancer subtypes.
Inhibitors of the PI3K/PKB/mTOR pathway
Phosphatidylinositol 3-kinase (PI3K), protein kinase B (PKB) and mammalian target of rapamycin (mTOR) are inter-connected signalling proteins that are involved in cell survival. PI3K/PKB activates various anti-apoptotic pathways, whereas mTOR (a downstream effector of PI3K/PKB) regulates cell cycle transition. These proteins play a key signalling role downstream of many cell surface receptors such as HER2 and the EGFR, and other signalling systems that drive proliferation including ER. They have also been implicated in resistance to tamoxifen and oestrogen deprivation[25]. Development of PI3K/PKB inhibitors is still at an early stage, whereas several mTOR inhibitors (e.g. the rapamycin derivatives temsirolimus and everolimus) are further advanced[18]. It has been suggested that combining mTOR inhibitors with agents that modulate oestrogen signalling or HER2/EGFR signalling pathways may be an effective strategy[18].
PARP inhibitors
Activation of PARP-1 is part of a key cellular response to single-strand DNA breaks, and stimulates DNA repair through base excision repair. PARP-1 also binds to double-strand DNA breaks preventing accidental recombination of homologous DNA. PARP inhibitors (e.g. AZD2281[18]) could improve several breast cancer chemotherapy regimes by enhancing the activity of DNA damaging agents and helping to overcome DNA repair-mediated resistance. PARP inhibitors could be particularly effective in basal-like tumours, which tend to have low levels of BRCA1[20]; BRCA1 dysfunction can sensitise cells to inhibition of PARP activity[26].
Identification of tumour subtypes is currently performed using histological techniques. Several molecular tests that assess the gene expression pattern are now available[27]. These are cheaper than PET and have the potential to produce a much bigger dataset resulting in better prognostic profiling. Although identification of molecular imaging correlates for specific tumour subtypes is conceivable, it is unlikely that PET will be used routinely to identify breast cancer subtypes. However, PET may provide complementary biological information about a specific tumour that could further advance the subtyping process by providing additional prognostic information. Efficient management of breast cancer requires good prognostic tests to allow the appropriate treatment (e.g. endocrine therapy or chemotherapy) to be administered to the patients who are most likely to benefit from each therapy. This use of PET has some distinctive properties that could overcome problems associated with biopsies such as: absence of biopsies, impracticality of taking biopsies, sampling errors and when there are differences of target expression between the primary tumour and metastasis. Moreover, in cases where a metastasis is hard to biopsy, PET may help to confirm that the metastasis has the same subtype characteristics.
Relevant biological processes for which PET probes are available include hypoxia, multidrug resistance, angiogenesis and receptor expression. Radiotracers that detect hypoxia (e.g. [18F]MISO and [64Cu]diacetyl-bis(N4-methylthiosemicarbazone)) may produce a specialised role for PET, because hypoxic tumours tend to be more aggressive and less likely to respond to a range of treatments[28]. Indeed, recent data have suggested that the basal-like subtype contains higher levels of proteins associated with hypoxia than other subtypes[29], suggesting that this could be associated with the poor prognosis in this subtype. Although [18F]MISO has been tested in breast cancer patients[30], a hypoxia PET study has not yet been used to stratify breast cancer patients; early identification of hypoxic tumours could improve clinical outcome by promoting the appropriate use of hypoxia-targeting therapies. Similarly, the availability of PET radiotracers that can detect multidrug resistance could permit early identification of tumours unlikely to respond to multiple chemotherapeutic agents. In a pilot study, 4-[18F]fluoro-paclitaxel was tested in breast cancer[31], but no subsequent studies have been carried out. Pathways related to angiogenesis are overrepresented in the basal subtype[17], suggesting that it may be possible to identify this subtype using an angiogenesis tracer. [18F]Galacto-RGD, which binds to a key integrin involved in angiogenesis, has been studied in breast cancer[32], but has not yet been used to identify tumour subtypes. The availability of novel radiotracers that bind to receptors (e.g. oestradiol derivatives or ligands that bind HER2) will give PET the potential to further stratify the patient population. A radiolabelled oestradiol derivative has been used successfully in breast cancer[33.34], but this tracer has not yet been used to link the relative level of the radiotracer to tumour subtype.
Tumour stem cells (TSC) are often associated with a poor prognosis, because they seem to be particularly resistant to chemotherapy and radiotherapy, and adept at stimulating angiogenesis. By detecting TSCs and identifying TSC subtypes, PET could be used to categorize patients who are more likely to be resistant to chemotherapy and radiotherapy. Suitable PET ligands could be developed to fulfil these roles as our understanding of TSC progresses.
Combined assessment of multiple imaging probes is likely to lead to more precise assessment of tumour biology and more accurate prognosis. For example, combined imaging of angiogenesis and metabolism with [15O]water and FDG has been shown to predict likely response of advanced breast cancer to neoadjuvant chemotherapy[35]. Similarly, a preliminary PET-CT study that used the CT component to assess angiogenesis by perfusion imaging, combined with FDG-PET for metabolism, observed that the relationship between tumour metabolism and blood flow may be related to tumour grade[36].
PET and the development of new therapies
Efficient development, testing and approval of most new anti-cancer agents will require an innovative approach and a new set of skills and tools. Traditionally, cytotoxic drugs have been optimised by identifying the maximum tolerated dose of the agent, and screening for efficacy based on tumour shrinkage. Subsequent clinical trials seek to identify an effective dose that is associated with an acceptable level of toxicity. Many of the targeted agents that are now being developed will probably be efficacious only in a specific subset of patients, and it is likely that neither toxicity nor significant tumour shrinkage will be suitable surrogate end points for detecting activity or selecting the appropriate dose. New tracers that are currently being developed for PET will assist the development of targeted anti-cancer agents by acting as predictive, pharmacological and surrogate response biomarkers, and extend the utility of this imaging technique making it well placed to facilitate development of new drugs.
Predictive biomarkers to identify patient subpopulations
For targeted anti-cancer agents it is crucial that the tumour cell population being treated expresses the target of interest and that the tumour shows dependency on the target (e.g. trastuzumab in HER2 subtype). Several inexpensive non-imaging techniques can readily identify the presence of the target in tumours, assuming that an appropriate biopsy is available. Novel predictive biomarkers (e.g. radiolabelled oestradiol derivatives[33] or PARP-1 binding ligand[37] will be required for PET to fulfil these potential roles in studying the level and distribution of important targets.
Pharmacological biomarkers
PET has a number of attractive attributes that are likely to make it a very useful technique for studying new agents in vivo to support their development[38]. PET can examine sensitive molecular processes without perturbing them, so as novel radiotracers become available to study proliferation, apoptosis, angiogenesis and hypoxia, it will be possible to perform proof of concept pharmacodynamic studies to ascertain whether an agent is modulating tumour biology. For example, therapeutics that primarily inhibit cell growth (e.g. EGFR inhibitors) rather than promoting cell death could be studied with the tracer [18F]fluorothymidine (FLT), a surrogate marker of cell proliferation. Similarly, [18F]galacto-RGD, a surrogate marker of angiogenesis, may be useful for studying therapeutics that inhibit angiogenesis (e.g. bevacizumab). Moreover, the broad applicability of PET will permit detailed proof of mechanism studies, using radiolabelled versions of new breast cancer agents. This will be particularly useful for studying binding of agents to receptors, allowing values to be derived for receptor number, binding affinity and binding potential. This will provide confirmation that an agent interacts with its target (e.g. oestradiol derivatives binding to ER[33]), and by studying the level of receptor occupation it will be possible to optimise the dose and schedule of the therapeutic.
The generation of intricate pharmacokinetic data makes a considerable contribution to the drug development process, so pharmacokinetic PET studies are likely to become increasingly important to pharmaceutical companies. By applying mathematical modelling to PET data, pharmacokinetic information can be calculated on the kinetics, dosimetry and distribution of radiolabelled drugs in diseased and normal tissue, as well as plasma clearance rates[39]. This information can contribute to go/no go decisions on new therapeutics, by informing researchers if the agent goes to the target tissue or normal tissue, and whether it is retained in the body for long enough to have an effect on its biological target.
Another important parameter that can be generated from PET studies is the standardised uptake value (SUV). The SUV gives a measure of tracer uptake in the region of interest relative to a uniform distribution over the body. Use of SUVs eliminates the subjective nature of visual analysis to obtain a more objective assessment of treatment response[40]. SUVs can produce the added advantage of allowing responses to be identified earlier in the treatment cycle[41]. However, to obtain meaningful SUVs it is important that a standardised protocol is used throughout the study, and that the same scanner is used for protocols that involve longitudinal scans[40].
Surrogate response biomarkers to demonstrate drug efficacy
Since most targeted agents are not expected to produce significant tumour shrinkage, surrogate end points will be required to demonstrate clinical efficacy. Quantitative PET data are likely to play a key role in assessing response to therapy. In principle, most radiotracers that generate SUV values could be used as surrogate response biomarkers, including tracers that measure glucose metabolism, cell proliferation and apoptosis, as well as agents that bind to receptors such as HER2 and the ER. To date, decreases in the SUV of glucose metabolism[42–44], cell proliferation[45,46] and the oestrogen receptor[33,34] have been used to identify effective therapies in breast cancer patients. PET surrogate response biomarkers have been used to demonstrate the efficacy of diverse chemotherapy regimes[42,43,45,46], as well as different strategies to modulate oestrogen signalling[33,34] in breast cancer.
Many anti-cancer agents can directly or indirectly affect the pathways, glucose transporters or metabolic enzymes controlling glycolysis[47], resulting in decreased FDG uptake into tumours. Therefore changes in the FDG uptake rate could provide very early evidence of drug activity for many of the agents being developed, such as inhibitors of angiogenesis, the IGF1R and PI3K/PKB/mTOR, where surrogate response biomarkers are not available to study the particular pathway under investigation. FDG-PET has several limitations, although some of these can be overcome by data from an accompanying CT scan. The PET image has limited spatial resolution and cannot accurately identify anatomical features. It can take more than 20 min for a full body scan, which can result in breathing movement artefacts. Some tumours and metastases are not detected as a result of poor tracer uptake. Some non-cancerous cells exhibit increased FDG uptake (e.g. tissues with high proportion of inflammatory cells), which can make it hard to distinguish between cancerous and non-cancerous tissue. It will be important to establish the limitations associated with each non-FDG radiotracer.
Qualification of each surrogate response biomarker (e.g. the proliferation marker FLT) will be necessary for quantitative studies to demonstrate the clinical efficacy of new agents. The availability of suitable radiotracers to study apoptosis, and anti-cancer targets such as the VEGF receptor, will permit increased apoptosis, or changes in the expression level of molecular targets, to be used as surrogate response biomarkers.
Assessing therapy response
Several different grading systems can be used to assess the histological response of a tumour to treatment. The Miller-Payne system is widely used; this compares tumour cellularity before and after treatment[48]. Tumours are graded on a 1 to 5 scale: 1, no response to treatment; 2, <30% reduction in cellularity; 3, 30–90% reduction in cellularity; 4, >90% and <100% reduction in cellularity; and 5, complete response.
One of the greatest clinical needs in breast cancer is to find an early and accurate way to determine which patients are responding to therapy. This could significantly improve clinical outcome by allowing non-responding patients to be prescribed a different anti-cancer treatment at an early stage, when it still has the potential to be effective. Several FDG studies have demonstrated that decreases in the SUV of glucose metabolism[42–44,49] correspond with response to chemotherapy treatments. In these studies it was possible to distinguish between responders and non-responders when response was evaluated after either one (early evaluation) or two or more cycles (midtherapy evaluation) of chemotherapy.
New radiotracers such as the proliferation marker FLT[45,46,50] and ligands that bind to the oestrogen receptor[33,34] have also been used as surrogate response biomarkers to evaluate therapy response. These tracers complement FDG by permitting the evaluation of response to targeted therapies[33,34,50] as well as chemotherapeutic ones. Radiotracers that can measure apoptosis/cell death, and other targeted agents, will also be beneficial, by introducing new PET assays to identify patients who are responding to therapy.
Dynamic contrast-enhanced MRI has also been used extensively to assess early treatment response with varying success (reviewed in Ref.[51]) but is still not used in routine practice. One of the main problems is the lack of agreement on the method to acquire and analyse the data so it is difficult to compare the results from one site with another.
Identification of recurrence
There is some evidence that FDG-PET may be useful for detecting recurrent breast cancer[52]; it is more sensitive than MRI, but less specific. PET is particularly good at detecting nodal and osseous recurrence[12], but is poor at detecting very small tumours[53]. PET can help to identify patients with single site metastatic disease who are more likely to benefit from surgery[12]. Sodium fluoride is being promoted as a better tool to detect bone metastases than technetium labelled radioisotopes. New PET tracers with increased specificity or sensitivity could be very useful for detecting recurrent disease. Whole-body MRI and diffusion-weighted image (DWI) have been explored to a limited extent for assessing recurrent disease[54], but are not used in routine practice.
Conclusion
FDG is by far the most extensively used PET radiotracer and has demonstrated clinical utility in various tumour types for diagnosis, staging or detection of residual/recurrent cancer. There are, however, some roles that FDG cannot perform because of low uptake into the tumour, high background signal or merely because of the inherent characteristics of the FDG radiotracer. The availability of new radiotracers, or new applications of established PET radiotracers, will increase the utility of PET by providing data from additional tumour types and previously inaccessible information on the cell biology of tumours. In breast cancer this should facilitate improved staging, tumour subtyping, identification of disease recurrence, development of new therapies and assessment of therapy response, thus creating an opportunity to progress towards a more personalised approach to cancer care and help improve clinical outcomes.
Acknowledgements
We are very grateful to Dr Tim Smith for critical reading of this manuscript, Dr Helen Young for helpful comments on the use of surrogate response biomarkers to demonstrate drug efficacy, and Professor Sir Michael Peckham for suggesting breast cancer as a suitable paradigm to demonstrate opportunities for PET to deliver clinical benefit in cancer.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Turner NC, Jones AL. Management of breast cancer – part I. BMJ. 2008;337:a421. doi: 10.1136/bmj.a421. doi:10.1136/bmj.a421. PMid:18614462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadrmas DJ, Casey ME, Conti M, Jakoby BW, Lois C, Townsend DW. Impact of time-of-flight on PET tumor detection. J Nuclear Med. 2009;50:1315–23. doi: 10.2967/jnumed.109.063016. doi:10.2967/jnumed.109.063016. PMid:19617317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen SL, Wu Y, Chaudhari AJ, et al. Initial characterization of a dedicated breast PET/CT scanner during human imaging. J Nuclear Med. 2009;50:1401–8. doi: 10.2967/jnumed.109.064428. doi:10.2967/jnumed.109.064428. PMid:19690029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullan PB, Millikan RC. Molecular subtyping of breast cancer: opportunities for new therapeutic approaches. Cell Mol Life Sci. 2007;64:3219–32. doi: 10.1007/s00018-007-7389-z. doi:10.1007/s00018-007-7389-z. PMid:17957336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousseau C, Devillers A, Sagan C, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–72. doi: 10.1200/JCO.2006.05.7406. doi:10.1200/JCO.2006.05.7406. PMid:17088570. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz JD, Bader M, Jenicke L, Hemminger G, Jänicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J Nuclear Med. 2005;46:1144–50. [PubMed] [Google Scholar]
- 7.Basu S, Chen W, Tchou J, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer. 2008;112:995–1000. doi: 10.1002/cncr.23226. doi:10.1002/cncr.23226. PMid:18098228. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Hoeven JJM, Krak NC, Hoekstra OS, et al. 18F-2-Fluoro-2-deoxy-D-glucose positron emission tomography in staging of locally advanced breast cancer. J Clin Oncol. 2004;22:1253–59. doi: 10.1200/JCO.2004.07.058. doi:10.1200/JCO.2004.07.058. PMid:15051773. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull LW, Brown SR, Olivier C, et al. Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE) Health Technol Assess. 2010;14:1–182. doi: 10.3310/hta14010. [DOI] [PubMed] [Google Scholar]
- 10.Aukema TS, Rutgers EJT, Vogel WV, et al. The role of FDG PET/CT in patients with locoregional breast cancer recurrence: a comparison to conventional imaging techniques. Eur J Surg Oncol. 2010;36:387–92. doi: 10.1016/j.ejso.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Groheux D, Hindié E, Rubello D, et al. Should FDG PET/CT be used for the initial staging of breast cancer? Eur J Nuclear Med Mol Imaging. 2009;36:1539–42. doi: 10.1007/s00259-009-1159-0. doi:10.1007/s00259-009-1159-0. PMid:19449002. [DOI] [PubMed] [Google Scholar]
- 12.Lavayssière R, Cabée A, Filmont J. Positron emission tomography (PET) and breast cancer in clinical practice. Eur J Radiol. 2009;69:50–8. doi: 10.1016/j.ejrad.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Murray AD, Staff RT, Redpath TW, et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. Br J Radiol. 2002;75:220–8. doi: 10.1259/bjr.75.891.750220. [DOI] [PubMed] [Google Scholar]
- 14.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG pet: Differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–9. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 15.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenton JD, Carey LA, Ahmed A, Caldas C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. doi:10.1200/JCO.2005.03.3845. PMid:16145060. [DOI] [PubMed] [Google Scholar]
- 17.Bertucci F, Finetti P, Cervera N, et al. How different are luminal A and basal breast cancers? Int J Cancer. 2009;124:1338–48. doi: 10.1002/ijc.24055. doi:10.1002/ijc.24055. PMid:19058218. [DOI] [PubMed] [Google Scholar]
- 18.Di Cosimo S, Baselga J. Targeted therapies in breast cancer: where are we now? Eur J Cancer. 2008;44:2781–90. doi: 10.1016/j.ejca.2008.09.026. doi:10.1016/j.ejca.2008.09.026. PMid:19013786. [DOI] [PubMed] [Google Scholar]
- 19.Ocaña A, Pandiella A. Identifying breast cancer druggable oncogenic alterations: lessons learned and future targeted options. Clin Cancer Res. 2008;14:961–70. doi: 10.1158/1078-0432.CCR-07-1630. [DOI] [PubMed] [Google Scholar]
- 20.Abd El-Rehim DM, Ball G, Finder SE, et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer. 2005;116:340–50. doi: 10.1002/ijc.21004. doi:10.1002/ijc.21004. PMid:15818618. [DOI] [PubMed] [Google Scholar]
- 21.Kucab JE, Dunn SE. Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Dis. 2003;17:41–7. doi: 10.3233/bd-2003-17105. [DOI] [PubMed] [Google Scholar]
- 22.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials – early lessons. J Mammary Gland Biol Neoplasia. 2008;13:471–83. doi: 10.1007/s10911-008-9104-6. doi:10.1007/s10911-008-9104-6. PMid:19023648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finn RS. Targeting Src in breast cancer. Ann Oncol. 2008;19:1379–86. doi: 10.1093/annonc/mdn291. doi:10.1093/annonc/mdn291. PMid:18487549. [DOI] [PubMed] [Google Scholar]
- 24.Huang F, Reeves K, Han X, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. doi:10.1158/0008-5472.CAN-06-3633. PMid:17332353. [DOI] [PubMed] [Google Scholar]
- 25.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–17. [PubMed] [Google Scholar]
- 26.Farmer H, McCabe H, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. doi:10.1038/nature03445. PMid:15829967. [DOI] [PubMed] [Google Scholar]
- 27.Turner NC, Jones AL. Management of breast cancer – part II. BMJ. 2008;337:164–9. doi: 10.1136/bmj.a421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin Nuclear Med. 2007;37:451–61. doi: 10.1053/j.semnuclmed.2007.07.001. doi:10.1053/j.semnuclmed.2007.07.001. PMid:17920352. [DOI] [PubMed] [Google Scholar]
- 29.Tan EY, Yan M, Campo L, et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer. 2009;100:405–11. doi: 10.1038/sj.bjc.6604844. doi:10.1038/sj.bjc.6604844. PMid:19165203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajendran JG, Mankoff DA, O'Sullivan F, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245–52. doi: 10.1158/1078-0432.ccr-0688-3. doi:10.1158/1078-0432.CCR-0688-3. PMid:15073099. [DOI] [PubMed] [Google Scholar]
- 31.Kurdziel KA, Kalen JD, Hirsch JI, et al. Imaging multidrug resistance with 4-[18F]fluoropaclitaxel. Nuclear Med Biol. 2007;34:823–31. doi: 10.1016/j.nucmedbio.2007.04.011. doi:10.1016/j.nucmedbio.2007.04.011. PMid:17921033. [DOI] [PubMed] [Google Scholar]
- 32.Beer AJ, Niemeyer M, Carlsen J, et al. Patterns of αvβ3 expression in primary and metastatic human breast cancer as shown by 18F-galacto-RGD PET. J Nuclear Med. 2008;49:255–9. doi: 10.2967/jnumed.107.045526. doi:10.2967/jnumed.107.045526. PMid:18199623. [DOI] [PubMed] [Google Scholar]
- 33.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: Indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 34.Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009;113:509–17. doi: 10.1007/s10549-008-9953-0. doi:10.1007/s10549-008-9953-0. PMid:18327670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mankoff DA, Dunnwald LK, Gralow JR, et al. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nuclear Med. 2003;44:1806–14. [PubMed] [Google Scholar]
- 36.Groves AM, Wishart GC, Shastry M, et al. Metabolic-flow relationships in primary breast cancer: Feasibility of combined PET/dynamic contrast-enhanced CT. Eur J Nuclear Med Mol Imaging. 2009;36:416–21. doi: 10.1007/s00259-008-0948-1. doi:10.1007/s00259-008-0948-1. PMid:18818917. [DOI] [PubMed] [Google Scholar]
- 37.Tu Z, Chu W, Zhang J, Dence CS, Welch MJ, Mach RH. Synthesis and in vivo evaluation of [11C]PJ34, a potential radiotracer for imaging the role of PARP-1 in necrosis. Nuclear Med Biol. 2005;32:437–43. doi: 10.1016/j.nucmedbio.2005.03.001. doi:10.1016/j.nucmedbio.2005.03.001. PMid:15982573. [DOI] [PubMed] [Google Scholar]
- 38.Murphy PS, Bergström M. Radiopharmaceuticals for oncology drug development: a pharmaceutical industry perspective. Curr Pharm Design. 2009;15:957–65. doi: 10.2174/138161209787581977. doi:10.2174/138161209787581977. PMid:19275660. [DOI] [PubMed] [Google Scholar]
- 39.Kissel J, Brix G, Bellemann ME, et al. Pharmacokinetic analysis of 5-[18F]fluorouracil tissue concentrations measured with positron emission tomography in patients with liver metastases from colorectal adenocarcinoma. Cancer Res. 1997;57:3415–23. [PubMed] [Google Scholar]
- 40.Zijlstra JM, Boellaard R, Hoekstra OS. Interim positron emission tomography scan in multi-center studies: optimization of visual and quantitative assessments. Leukemia Lymphoma. 2009;50:1748–9. doi: 10.3109/10428190903308049. [DOI] [PubMed] [Google Scholar]
- 41.MacManus MP, Seymour JF, Hicks RJ. Overview of early response assessment in lymphoma with FDG-PET. Cancer Imaging. 2007;7:10–18. doi: 10.1102/1470-7330.2007.0004. doi:10.1102/1470-7330.2007.0004. PMid:17766210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schelling M, Avril N, Nährig J, et al. Positron emission tomography using [18F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–95. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- 43.Smith IC, Welch AE, Hutcheon AW, et al. Positron emission tomography using [18F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676–88. doi: 10.1200/JCO.2000.18.8.1676. [DOI] [PubMed] [Google Scholar]
- 44.Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation. J Clin Oncol. 1993;11:2101–11. doi: 10.1200/JCO.1993.11.11.2101. [DOI] [PubMed] [Google Scholar]
- 45.Pio BS, Park CK, Pietras R, et al. Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol. 2006;8:36–42. doi: 10.1007/s11307-005-0029-9. doi:10.1007/s11307-005-0029-9. PMid:16362149. [DOI] [PubMed] [Google Scholar]
- 46.Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nuclear Med Mol Imaging. 2007;34:1339–47. doi: 10.1007/s00259-007-0379-4. doi:10.1007/s00259-007-0379-4. PMid:17333178. [DOI] [PubMed] [Google Scholar]
- 47.Kelloff GJ, Krohn KA, Larson SM, et al. The progress and promise of molecular imaging probes in oncologic drug development. Clin Cancer Res. 2005;11:7967–85. doi: 10.1158/1078-0432.CCR-05-1302. doi:10.1158/1078-0432.CCR-05-1302. PMid:16299226. [DOI] [PubMed] [Google Scholar]
- 48.Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–7. doi: 10.1016/s0960-9776(03)00106-1. doi:10.1016/S0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 49.McDermott GM, Welch A, Staff RT, et al. Monitoring primary breast cancer throughout chemotherapy using FDG-PET. Breast Cancer Res Treat. 2007;102:75–84. doi: 10.1007/s10549-006-9316-7. doi:10.1007/s10549-006-9316-7. PMid:16897427. [DOI] [PubMed] [Google Scholar]
- 50.Sohn HJ, Yang YJ, Ryu JS, et al. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res. 2008;14:7423–9. doi: 10.1158/1078-0432.CCR-08-0312. doi:10.1158/1078-0432.CCR-08-0312. PMid:19010859. [DOI] [PubMed] [Google Scholar]
- 51.BlueCross BlueShield Association (BCBSA) Magnetic resonance imaging of the breast for preoperative evaluation in patients with localized breast cancer. Chicago, IL: BCBSA; 2004; vol 18, p. 8. [Google Scholar]
- 52.Goerres GW, Michel SCA, Fehr MK, et al. Follow-up of women with breast cancer: comparison between MRI and FDG PET. Eur Radiol. 2003;13:1635–44. doi: 10.1007/s00330-002-1720-8. doi:10.1007/s00330-002-1720-8. PMid:12835979. [DOI] [PubMed] [Google Scholar]
- 53.Grahek D, Montravers F, Kerrou K, Aide N, Lotz J, Talbot J. [18F]FDG in recurrent breast cancer: diagnostic performances, clinical impact and relevance of induced changes in management. Eur J Nuclear Med Mol Imaging. 2004;31:179–88. doi: 10.1007/s00259-003-1348-1. doi:10.1007/s00259-003-1348-1. PMid:15129699. [DOI] [PubMed] [Google Scholar]
- 54.Pan L, Han Y, Sun X, Liu J, Gang H. FDG-PET and other imaging modalities for the evaluation of breast cancer recurrence and metastases: a meta-analysis. J Cancer Res Clin Oncol. 2010;136:1007–22. doi: 10.1007/s00432-009-0746-6. doi:10.1007/s00432-009-0746-6. PMid:20091186. [DOI] [PMC free article] [PubMed] [Google Scholar]