Abstract
Background: During early postnatal development, the nervous system accretes docosahexaenoic acid (DHA; 22:6n−3), a highly unsaturated n−3 (omega-3) fatty acid (FA) used in the formation of neural cell membranes. DHA, which is present in human breast milk, may also be biosynthesized from n−3 FAs such as 18:3n−3 [α-linolenic acid (ALA)] or 20:5n−3 [eicosapentaenoic acid (EPA)]. An important concern is to what extent these precursors can supply DHA to the developing infant.
Objective: We analyzed measurements of fractional percentages of plasma 2H5-ALA and 13C-U-EPA directed toward the synthesis of labeled 22:6n−3 in 11 newborn infants by using compartmental modeling procedures.
Design: One-week-old infants received doses of 2H5-ALA and 13C-U-EPA ethyl esters enterally. We drew blood from the infants periodically and analyzed the plasma for endogenous and labeled n−3 FAs. From the time-course concentrations of the labeled FAs, we determined rate constant coefficients, fractional synthetic rates, and plasma turnover rates of n−3 FAs.
Results: In infants, ≈0.04% of the 2H5-ALA dose converted to plasma 2H5-EPA. Plasma 2H5-EPA and 2H5-22:5n−3 [docosapentaenoic acid (DPA)] efficiently converted to 2H5-DPA and 2H5-DHA, respectively. The percentage of plasma 13C-U-EPA directed toward the synthesis of 13C-DHA was lower than the percentage of plasma 2H5-EPA that originated from 2H5-ALA.
Conclusions: Endogenously synthesized EPA was efficiently converted to DHA. In comparison, preformed EPA was less efficiently used for DHA biosynthesis, which suggests a differential metabolism of endogenous EPA compared with exogenous EPA. However, on a per mole basis, preformed EPA was 3.6 times more effective toward DHA synthesis than was ALA. Newborns required an intake of ≈5 mg preformed DHA · kg−1 · d−1 to maintain plasma DHA homeostasis.
INTRODUCTION
In recent years, some infant formulas marketed in North America and elsewhere have been fortified with arachidonic acid (20:4n−6) and docosahexaenoic acid (DHA; 22:6n−3), which are long-chain n−6 (omega-6) and n−3 (omega-3) polyunsaturated fatty acids (PUFAs) that are present in human breast milk. Evidence from human studies (1, 2) indicated that 20:4n−6 (3–5) and 22:6n−3 (6) play important roles in the development of the human nervous system, which is supported by experimental evidence from animal studies (7–9). DHA may be biosynthesized from n−3 fatty acids (FAs) such as 18:3n−3 [α-linolenic acid (ALA)] or 20:5n−3 [eicosapentaenoic acid (EPA)]. It is unclear to what degree infants have the capability to biosynthesize long-chain n−3 FAs from ALA during the early postnatal period. Consequently, no precise amounts or appropriate ratios of n−6 and n−3 FAs have been established for infant formulas on the basis of nutritional needs or the biosynthetic capabilities of the newborn. In addition, human adults have a relatively low capacity to convert ALA to DHA on the basis of evidence from analyses of the plasma time-course concentrations of isotopically labeled n−3 FAs (10–12). However, a recent study in adult men suggested that the liver has a greater biosynthetic capacity for production of long-chain n−3 PUFAs than that inferred from the plasma FA time-course data (13).
We and other authors carried out human studies that used stable isotopes to determine the conversion of ALA to DHA in infants (14–17). However, very little quantitative information is available on the effects of the nutritional supply of ALA and EPA toward synthesis and maintenance of plasma DHA in infants (17).
The purpose of this study was to assess plasma n−3 FA kinetic variables and the quantitative contributions of dietary ALA and EPA toward the maintenance of plasma DHA during the first week of life in term and late preterm neonates. The research strategy involved the development of 2 independent compartmental models to assess the biosynthetic capacity of ALA and EPA on the production and appearance of DHA in the plasma. Each of the models integrated the n−3 FA intake from infant formula and/or breast-milk feeding with the n−3 FA plasma concentrations and the time-course concentrations of the 13C-labeled n−3 FA (derived from 13C-U-EPA) and the 2H5-labeled n−3 FA (derived from 2H5-ALA) for each subject.
SUBJECTS AND METHODS
Study subjects and clinical procedures
Neonates with gestational ages >34 wk were admitted to the neonatal intensive care unit (NICU) and had an umbilical line in place on the basis of clinical indication. Infants who were small for gestational age, with major malformations, or in evaluation for necrotizing enterocolitis or feeding intolerance were excluded from the study. Written informed consent was obtained from mothers, and the study protocol was approved by the National Institutes of Health Institutional Review Board (under protocol OH93-AA-N027), the Institute of Nutrition and Food Technology's ethics committee, and the research committee of the participating NICU (Hospital Sótero del Rio and Clínica Presbiteriana Madre-Hijo) in metropolitan Santiago, Chile. The gestational age of each infant was assessed from the mother's last menstrual period or by using the date of conception on the basis of early ultrasound and confirmed by the modified Ballard neonatal physical evaluation. Infants were classified as small for gestational age or appropriate for gestational age according to the Lubchenco standard (18). Birth weights of infants were between 2.35 and 4.65 kg (mean ± SE: 3.19 ± 0.66 kg), and gestational ages of infants were 34–41 wk (Table 1). Most subjects presented with mild forms of hypoxia or transient respiratory problems. Feeding was started generally ≤2 d after birth, and the type of feeding varied. If breast milk was unavailable, infants received a commercial brand infant formula (Ross Laboratories, Columbus, OH) that contained 18:3n−3 (77 mg 18:3n−3/100 mL) but was devoid of 20- and 22-carbon PUFAs. Subjects received a mixture of 2H5-18:3n−3 (20 mg 2H5-18:3n−3/kg) and deuterated 13C-U-20:5n−3 (2 mg 3C-U-20:5n−3/kg) with an enteral gastric tube. Blood was drawn (0.5 mL) from an umbilical catheter and a peripheral vein after the catheter had been removed into a tube containing EDTA. Blood was drawn at 0, 4, 8, 24, and 48 h and on days 4 and 7 after dosing; if possible, the time of drawing was made to coincide with the timing of sampling for clinical needs. Plasma was separated by centrifugation shortly after sampling and frozen at –80°C.
TABLE 1.
Subject weight, age, and feeding variables1
| ID | Birth weight | GA | Sex | Age at entrance | Weight at entrance | Weight at end | Enteral feeding | Formula | Breast milk |
| g | wk | wk | g | g | d | mL/d | mL/d | ||
| 81 | 3310 | 39 | M | 2 | 3172 | 3245 | 3 | 118 | 45 |
| 82 | 3250 | 38 | F | 1 | 3400 | 3550 | 4 | 136 | 143 |
| 83 | 3890 | 42 | M | 1 | 3930 | 3920 | 2 | 38 | 390 |
| 84 | 3070 | 39 | M | 3 | 3080 | 3250 | 3 | 110 | 405 |
| 86 | 2350 | 36 | M | 2 | 2390 | 2170 | 4 | 0 | 70 |
| 87 | 3070 | 37 | M | 3 | 3060 | 2940 | 3 | 165 | 136 |
| 88 | 3310 | 39 | M | 4 | 3510 | 3510 | 4 | 180 | 0 |
| 89 | 3160 | 37 | M | 2 | 3410 | 3480 | 3 | 234 | 10 |
| 90 | 2540 | 35 | M | 1 | 2550 | 2270 | 2 | 0 | 60 |
| 91 | 4650 | 41 | F | 2 | 4580 | 4680 | 3 | 169 | 44 |
| 92 | 2490 | 37 | M | 2 | 2440 | 2350 | 3 | 0 | 130 |
ID, subject identification number; GA, gestational age.
Infant feeding
Infants were nursed and/or fed expressed breast milk when available and/or an infant formula (Ross Laboratories) on demand. The quantity of expressed milk and the amount of formula consumed were recorded for each subject. Subjects that could not be initially fed enterally received parenteral glucose until they were capable of receiving enteral nutrition (100 mL · kg−1 · d−1). Infants received no intravenous lipids during the study period.
Stable isotopes
Carbon-13 uniformly labeled eicosapentaenoate ethyl ester (13C-U-20:5n−3; 13C >95%) was obtained from Martek Biosciences Corp (Columbia, MD), and deuterium-labeled α-linolenate (17, 17, 18, 18, 18-2H5-18:3n−3; 2H >95%) ethyl esters were obtained from Cambridge Isotope Laboratories (Andover, MA).
Lipid extraction and FA methyl esters
Plasma lipids were extracted by using a modified Folch procedure (19). Plasma (200 μl) was added to methanol (1 mL) that contained ethyl tricosanoate (0.13 nmol) as an internal standard and vigorously extracted twice with chloroform. One-half of the lipid extract was derivatized to methyl esters by using 14% boron trifluoride in methanol (20) and dissolved in hexane.
Gas chromatographic analyses
Gas chromatographic analyses were made with an Agilent 6890 system (Agilent Technologies, Santa Clara, CA) with flame ionization detection. Two microliters of the sample was injected on to a Durabond Free Fatty Acid Phase capillary column (30-m × 0.25-mm inside diameter and 0.25-μm film thickness; J&W Scientific, Folsom, CA) with hydrogen carrier gas. The inlet and detector temperatures were set at 250°C. The oven was programmed from 130°C to 175°C at 4°C/min and to 210°C at 1°C/min and then increased to 245°C at 30°C/min.
Pentafluorobenzyl derivatization
The lipid extract (100 μL) was evaporated and saponified with 5% methanolic KOH. Free FAs were extracted into hexane and derivatized to pentafluorobenzyl esters as described previously (21). Reagents were evaporated under a steam of nitrogen and resuspended in 100 μL hexane.
Gas chromatographyndashmass spectrometry analyses
Gas chromatography–mass spectrometry was carried out on an Agilent 6890 GC-5973 Mass Selective Detector system (Agilent Technologies) in the negative chemical ionization mode as previously described (21). Samples (1 μL) were injected in the splitless mode onto a Durabond Free Fatty Acid Phase column (J&W Scientific), and the oven was programmed from 125°C to 245°C at 8°C/min. Data were acquired by monitoring the M-pentafluorobenzyl anion of each analyte and converted to the absolute quantity with reference to the internal standard by using an appropriate response factor. Analyses for n−3 and n−6 FAs (23) were carried out at the same time.
Compartmental models
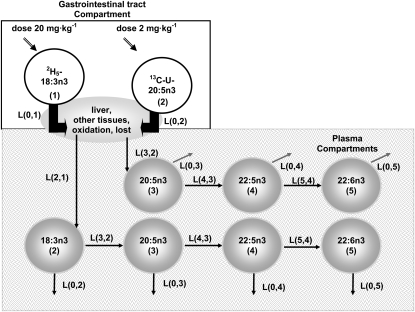
The n−3 FA compartmental model has been described previously and is only briefly detailed here (11). The hepatocyte is a main site for biosynthesis of desaturated and chain elongated PUFAs from 18:3n−3 and for the formation of lipoproteins. Because liver specimens were not available, rate constants represent kinetics of labeled FAs from their plasma pool and may only indirectly reflect liver metabolism. Two independent compartmental models of n−3 FA metabolism were developed by using the concentration-time courses of the labeled FAs and concentrations of endogenous FAs in plasma (Figure 1) with WinSAAM modeling software (version 3.0.7; http://www.winsaam.com).
FIGURE 1.
Diagram of the 18:3n−3 compartmental model and 20:5n−3 compartmental models. Open circles represent plasma and gastrointestinal compartments for n−3 fatty acids. LI,J values denote rate constant coefficients. L0,J values indicate the loss of isotopes from the pathway. Numbers in parentheses represent individual compartments.
The fractional transfer rate constant coefficient LI,J is the fraction of substrate transferred from substrate-compartment J to product-compartment I (and L0,J represents the isotopes lost from the path). The units are in hours. LI,J represents an assemblage of several independent enzymatic and transport processes, each having a separate rate constant, for which no intermediates were isolated. The rate of flow (RI,J) from substrate-compartment J to product-compartment I is obtained by multiplying the mass (MJ) of endogenous FAs in compartment J by LI,J and is given in micrograms per hour. The FA concentrations from the total lipid extract represent the endogenous amounts. The percentage of isotopes transferred from J to I is given as PI,J and is a percentage of the total flux of FAs leaving J. PI,J is the fraction of the isotopes remaining in the metabolic pathway as opposed to the isotopes taken up by tissues or in other ways irreversibly lost from the compartment. Variances for the measured variables are reported as either the SE or SD.
Model illustration and rate equations
Diagrams of the compartmental models for 18:3n−3 and 20:5n−3 are presented in Figure 1. The initial compartment represents the dose of the labeled FAs absorbed from the gastrointestinal tract into the system. Other compartments denote plasma pools of 18:3n−3, 20:5n−3, 22:5n−3, and 22:6n−3. Arrows connecting the 6 compartments indicate the transfer of isotopes along the path. The rate equations were defined by a set of differential equations that corresponded to the flux of the labeled FAs through each respective compartment and those that exit the system.
Approximating endogenous nminus3 FA masses, predicting nminus3 FA intake and the influence of nminus6 FA intake on nminus3 FA rate variables, and statistical comparisons
Mean concentrations of plasma n−3 FAs (over 168 h) for each subject were used to represent the mass of endogenous substrates (MJ) available for biosynthesis (Table 2). For purposes of this study, both deuterium- and 13C-labeled FAs, which elute within the gas chromatographic peak envelope, were considered part of the endogenous pool because it was assumed that these FAs are biochemically equivalent to the unlabeled n−3 essential fatty acids (EFAs). These values were held constant. When the daily upper and lower n−3 FA intake limits were estimated, the FA content of the formula, availability of breast milk, and frequency of feeding for each subject were entered into the model. Intakes of n−3 FAs (Ui) by breastfed infants and/or those who received expressed breast milk were approximated from breast-milk samples obtained from mothers who lived in metropolitan Santiago, Chile (22). Initial n−3 FA intake approximations were further refined through successive iterations while constraining plasma n−3 EFA concentrations. In addition, the model was adjusted to compensate for low intake volumes during the first 48 h after birth with gradual increases in volume through the week. Because most subjects had low intakes during the first 24–48 h period, initial Ui estimates tended to be overestimated. On the basis of each subject's unique feeding regimen, the estimates were reduced by a similar factor. For instance, an ≈30% reduction of the initial Ui estimate would be applied to this value if an infant only began nursing on day 2 (≈48/168 h = ≈29%) to compensate for a 48-h period. There was no direct control incorporated in the modeling procedure to account for n−6 FA intake, and variable n−6 FA intake may have had an effect on n−3 FA rate variables. Differences between rate variables and the efficacy of each of the 2 precursors (18:3n−3 and 20:5n−3) toward synthesis of 22:6n−3 were compared by using a paired Student's t test analysis with each subject serving as its own control. P ≤ 0.05 was considered significant.
TABLE 2.
Plasma n−3 fatty acid values (in μg) for compartments (MJ) 2, 3, 4, and 5 in newborn infants1
| Subject identification number |
||||||||||||
| n−3 Fatty acid | 81 | 82 | 83 | 84 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | Mean ± SE |
| M2/18:3 | 1137 | 466 | 3473 | 1926 | 186 | 1566 | 370 | 988 | 196 | 1041 | 298 | 1059 ± 299 |
| M3/20:5 | 2026 | 2996 | 1446 | 1044 | 1152 | 1028 | 667 | 1081 | 1073 | 1760 | 768 | 1367 ± 204 |
| M4/22:5 | 1589 | 1901 | 1047 | 644 | 959 | 657 | 717 | 633 | 549 | 1672 | 681 | 1004 ± 148 |
| M5/22:6 | 19,219 | 22,192 | 14,335 | 8680 | 13,904 | 8749 | 7787 | 8993 | 9275 | 30,907 | 8811 | 13,896 ± 2245 |
Mean values were measured from plasma concentrations of fatty acids over 6 d and multiplied by the total plasma volume from each subject.
Calculations, errors, and predicting dietary nminus3 FA intake
Initial LI,J and PI,J estimates, which were derived from the concentration-time curves, were adjusted to compensate for individual variances in plasma data until the model prediction gave the best fit to the experimental data. Final values were measured by using an iterative nonlinear least-squares routine. The error model included assumptions of independence, constant variance, and normal distribution of about zero. Consistent with the precision of analytic methods, data points were weighted by assigning a fractional SD of 0.1 to each measurement in the WinSAAM software (version 3.0.7; http://www.winsaam.com). The use of a fractional SD weighting factor was considered appropriate for this study because it assigns the highest weight to the lowest observation values and the lowest weight to the highest observation values.
RESULTS
Subject characteristics
Eleven infants (9 boys and 2 girls) completed the study protocol. Their sex, weights, gestational ages, and feeding regimens at birth and during the study are given in Table 1. Infants had mild forms of respiratory distress syndrome secondary either to meconium aspiration or perinatal asphyxia. Subjects were monitored in the NICU until respiratory function was normalized. Infants received supplemental feeding with breast milk and/or infant formula in increasing volumes during the study. Mean (±SE) plasma concentrations (in μg/mL) of the n−3 FA 18:3n−3, 20:5n−3, 22:5n−3, and 22:6n−3 for infants in this group were 6.55 ± 1.03, 9.76 ± 0.71, 7.17 ± 0.57, and 99.26 ± 8.00, respectively. Generally, plasma FA masses for 20:5n−3, 22:5n−3, and 22:6n−3 varied over a narrow range (<20% from the mean value) through the study period (Table 3). However, plasma 18:3n−3 was more variable, and its concentration in the plasma increased with greater volume intake through the period.
TABLE 3.
Concentrations of plasma fatty acids and variances in 11 infants over 168 h
| Subject identification number |
|||||||||||
| n−3 Fatty acid | 81 | 82 | 83 | 84 | 86 | 87 | 88 | 89 | 90 | 91 | 92 |
| 18:3 (nmol/mL) | 10.6 ± 0.761 | 3.9 ± 0.23 | 14.8 ± 1.16 | 10.1 ± 0.62 | 2.1 ± 0.20 | 8.3 ± 0.71 | 3.0 ± 0.28 | 6.0 ± 0.54 | 3.4 ± 0.33 | 5.0 ± 0.20 | 2.7 ± 0.19 |
| CV2 | 0.36 | 0.30 | 0.40 | 0.31 | 0.19 | 0.46 | 0.49 | 0.45 | 0.49 | 0.52 | 0.38 |
| 20:5 (nmol/mL) | 12.4 ± 0.54 | 11.7 ± 0.73 | 6.5 ± 0.28 | 6.7 ± 0.14 | 7.1 ± 0.26 | 6.0 ± 0.17 | 4.6 ± 0.07 | 7.8 ± 0.21 | 8.8 ± 0.40 | 6.4 ± 0.26 | 6.1 ± 0.19 |
| CV2 | 0.22 | 0.34 | 0.22 | 0.11 | 0.18 | 0.14 | 0.08 | 0.14 | 0.23 | 0.20 | 0.16 |
| 22:5 (nmol/mL) | 11.7 ± 0.22 | 10.7 ± 0.26 | 7.2 ± 0.21 | 4.3 ± 0.05 | 5.9 ± 0.27 | 4.3 ± 0.21 | 5.1 ± 0.13 | 4.0 ± 0.07 | 4.8 ± 0.31 | 10.0 ± 0.22 | 5.6 ± 0.15 |
| CV2 | 0.10 | 0.12 | 0.14 | 0.05 | 0.23 | 0.25 | 0.13 | 0.08 | 0.32 | 0.11 | 0.14 |
| 22:6 (nmol/mL) | 148 ± 1.56 | 116 ± 3.94 | 91 ± 1.59 | 64 ± 0.78 | 93 ± 4.57 | 77 ± 1.19 | 61 ± 1.46 | 77 ± 2.60 | 73 ± 2.34 | 134 ± 2.37 | 83 ± 1.56 |
| CV2 | 0.05 | 0.17 | 0.09 | 0.06 | 0.25 | 0.08 | 0.12 | 0.17 | 0.16 | 0.9 | 0.10 |
Mean ± SE (all such values).
CVs of fatty acid concentrations across the trial period.
Appearance of 2H5-18:3nminus3 and 13C-U-20:5nminus3 in plasma
From previous analyses of isotopes recovered from feces, it was estimated that infants absorbed ≈94% of the labeled EFAs (23). This value, similar to findings in other infants (24), was used to estimate the absorption of labeled-FA and dietary-EFA components. Mean (±SE) values for area under the concentration curve for 2H5-18:3n−3 and 13C-U-20:5n−3 in plasma were 26.2 ± 2.2 nmol 2H5-18:3n−3 ∙ h/mL and 6.5 ± 0.7 nmol 13C-U-20:5n−3 ∙ h/mL. When calculated as the percentage of dose of the 2 labeled FAs by using isotope values from the gastrointestinal and plasma compartments (Figure 1) only 0.13 ± 0.02% and 0.28 ± 0.01%, of the dosages of 2H5-18:3n−3 and 13C-U-20:5n−3, respectively, appeared in the plasma compartment.
Concentration-time curves for nminus3 FAs and average weighted residuals
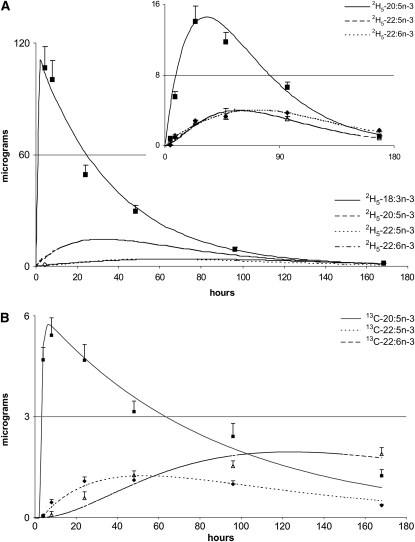
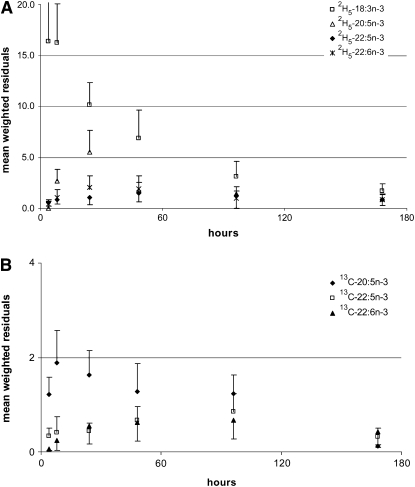
The mean best-fit model-derived concentration-time curves to the experimentally measured plasma concentrations for 2H5-18:3n−3, 2H5-20:5n−3, 2H5-22:5n−3, and 2H5-22:6n−3 from the 11 subjects is illustrated in Figure 2A. The best-fit curves for 13C-20:5n−3, 13C-22:5n−3, and 13C-22:6n−3 are illustrated in Figure 2B. Changes in the mean values of the concentrations of each of the labeled FA in the plasma and the approximate concentrations at their peak appearance times throughout 168 h for all subjects are depicted in the figures. Model-derived kinetic variables were determined for each subject uniquely by using individual dosing regimens, plasma volumes, and the unique time-course concentrations for 13C- and 2H-labeled FAs. Mean values of the weighted residuals determined from differences between the experimentally measured deuterium-labeled (Figure 3A) and 13C-labeled (Figure 3B) plasma n−3 EFAs and the best-fit values across the time course are presented in Figure 3. These values give an indication of the variability between the experimentally measured isotope data and the best-fit model-derived values.
FIGURE 2.
A: Mean (±SD) values for the experimentally measured plasma fatty acids 2H5-18:3n−3, 2H5-20:5n−3, 2H5-22:5n−3, and 2H5-22:6n−3 from 11 newborn infants who received an oral dose of 2H5-18:3n−3 (20 mg 2H5-18:3n−3/kg) ethyl ester. Time-course curves are model-determined best fits to the data by the modeling procedure developed from WinSAAM software (version 3.0.7; http://www.winsaam.com). B: Mean (±SD) values for the experimentally determined plasma fatty acids 13C-20:5n−3, 13C-22:5n−3, and 13C-22:6n−3 from 11 newborn infants who received an oral dose of 13C-20:5n−3 (2 mg 13C-20:5n−3/kg) ethyl ester. Time-course curves are model-determined best fits to the data.
FIGURE 3.
Mean (±SD) values for 11 subjects of weighted residuals calculated from the difference between the experimentally determined deuterium-labeled (A) and 13C-labeled (B) n−3 essential fatty acids in plasma and compartmental model assigned values at various time points. Infants received oral doses of 2H5-18:3n−3 (20 mg 2H5-18:3n−3/kg) and 13C-20:5n−3 (2 mg 13C-20:5n−3/kg) ethyl esters, and plasma was sampled at 4, 8, 24, 48, 96, and 168 h. Along the ordinate, differences between experimental and calculated values are given for 2H5-18:3n−3, 2H5-20:5n−3, 2H5-22:5n−3, and 2H5-22:6n−3 (A) and for 13C-20:5n−3, 13C-22:5n−3, and 13C-22:6n−3 (B).
Rate constant coefficients and R values
Fractional rate constant coefficient estimates (LI,J) for in vivo metabolism of 2H5-18:3n−3 and 13C-20:5n−3 were optimized for each subject, and final values are given in Tables 4 and 5, respectively. The appearance and disappearance rates of an n−3 FA in the plasma [RI,J (in μg/h)] (Table 6) are presented as the mass per unit of time for a given FA as it exits a substrate compartment J and is either transferred to product compartment Ior exits the biosynthetic pathway (0). From these calculations, the predicted daily mean whole-body turnover of 18:3n−3 in subjects (R0,1) was 187 ± 56 mg 18:3n−3 · kg−1· d−1, whereas the mean turnover of 18:3n−3 in plasma was 1.37 ± 0.33 mg 18:3n−3/d for the group (R0,2). The mean (±SE) turnovers for other n−3 FAs in the plasma were 0.67 ± 0.03 mg 20:5n−3/d, 1.49 ± 0.21 22:5n−3 mg/d, and 16.6 ± 1.6 mg 22:6n−3/d. The mean (±SE) replacement of plasma 22:6n−3 via its synthesis from 22:5n−3 (R5,4) was 1.5 ± 0.2 mg 22:6n−3/d (Table 6).
TABLE 4.
Fractional transfer rate constant coefficients1
| Subject identification number |
||||||||||||
| 81 | 82 | 83 | 84 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | Mean ± SE | |
| L2,1 | 0.195 | 1.899 | 0.618 | 0.618 | 0.115 | 0.872 | 1.295 | 2.913 | 0.340 | 0.791 | 0.857 | 0.956 ± 0.263 |
| Precision of estimates | 0.012 | 0.023 | 0.052 | 0.363 | 0.133 | 0.151 | 0.077 | 0.036 | 0.295 | 0.128 | 0.109 | — |
| L3,2 | 8.0 | 4.7 | 4.4 | 4.2 | 25.5 | 14.3 | 4.5 | 9.4 | 6.6 | 11.4 | 2.6 | 8.7 ± 2.0 |
| Precision of estimates | 0.011 | 0.052 | 0.097 | 0.546 | 0.198 | 0.572 | 0.094 | 0.062 | 0.215 | 0.027 | 0.218 | — |
| L4,3 | 38.7 | 22.7 | 32.9 | 32.0 | 17.6 | 37.5 | 18.2 | 18.2 | 22.5 | 22.5 | 34.9 | 27.1 ± 2.5 |
| Precision of estimates | 0.012 | 0.153 | 0.197 | 0.101 | 0.124 | 0.113 | 0.124 | 0.124 | 0.114 | 0.124 | 0.145 | — |
| L5,4 | 120.0 | 41.4 | 56.6 | 107.8 | 62.4 | 108.3 | 20.4 | 53.5 | 71.1 | 50.2 | 36.4 | 66.2 ± 9.9 |
| Precision of estimates | 0.033 | 0.287 | 0.312 | 0.220 | 0.241 | 0.123 | 0.458 | 0.254 | 0.174 | 0.214 | 0.224 | — |
| L0,1 | 0.618 | 0.195 | 1.899 | 0.618 | 1.295 | 0.872 | 2.913 | 0.791 | 0.857 | 0.115 | 0.340 | 0.956 ± 0.255 |
| Precision of estimates | 0.015 | 0.129 | 0.1599 | 0.045 | 0.128 | 0.036 | 0.208 | 0.109 | 0.162 | 0.117 | 0.139 | — |
| L0,2 | 36.8 | 45.9 | 12.0 | 109.1 | 4.5 | 6.6 | 28.6 | 38.0 | 8.8 | 12.7 | 35.7 | 30.8 ± 9.5 |
| Precision of estimates | 0.048 | 0.031 | 0.048 | 0.478 | 0.064 | 0.155 | 0.038 | 0.027 | 0.082 | 0.052 | 0.028 | — |
| L0,3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.40 | 0.00 | 0.22 ± 0.23 |
| Precision of estimates | 0.023 | 0.092 | 0.115 | 0.038 | 0.049 | 0.085 | 0.056 | 0.038 | 0.045 | 0.046 | 0.027 | — |
| L0,4 | 0.98 | 0.11 | 0.35 | 0.61 | 4.97 | 0.98 | 4.86 | 4.56 | 0.33 | 0.63 | 6.47 | 2.26 ± 0.62 |
| Precision of estimates | 0.005 | 0.154 | 0.212 | 0.103 | 0.120 | 0.105 | 0.082 | 0.125 | 0.088 | 0.111 | 0.210 | — |
| L0,5 | 85.2 | 60.2 | 39.1 | 59.1 | 68.0 | 36.3 | 25.9 | 25.9 | 53.1 | 55.1 | 55.1 | 51.4 ± 5.8 |
| Precision of estimates | 0.036 | 0.244 | 0.246 | 0.119 | 0.158 | 0.091 | 0.237 | 0.211 | 0.112 | 0.177 | 0.122 | — |
values represent the fractional transfer rate constant coefficients for the labeled n−3 fatty acid that was transferred between 2 adjoining compartments. For example, L3,2 is the rate constant coefficient for the transfer of 2H5-18:3n−3 from plasma compartment 2 to plasma compartment 3 (20:5n−3). Precision estimates are given as SDs of the estimates normalized to the values of the estimates expressed as percentages determined from the modeling procedure.
TABLE 5.
Fractional transfer rates (P) and rate constant coefficients (in h × 1000) of 13C-labeled n−3 fatty acids from 11 infants1
| Subject identification number |
||||||||||||
| 81 | 82 | 83 | 84 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | Mean ± SE | |
| P3,2 | 0.0004 | 0.002 | 0.002 | 0.008 | 0.004 | 0.002 | 0.002 | 0.008 | 0.001 | 0.001 | 0.002 | 0.003 ± 0.001 |
| P4,3 | 0.22 | 0.94 | 0.33 | 0.11 | 0.69 | 1.00 | 0.26 | 0.42 | 1.00 | 1.00 | 0.59 | 0.60 ± 0.11 |
| P5,4 | 1.00 | 0.92 | 1.00 | 0.77 | 0.55 | 1.00 | 0.05 | 0.99 | 1.00 | 0.82 | 0.12 | 0.75 ± 0.11 |
| L3,2 | 0.42 | 2.32 | 1.54 | 7.30 | 0.41 | 1.77 | 1.44 | 7.13 | 0.63 | 1.05 | 1.66 | 2.33 ± 0.75 |
| Precision of estimates | 0.066 | 0.008 | 0.503 | 0.059 | 0.051 | 0.008 | 0.010 | 0.002 | 0.024 | 0.014 | 0.009 | — |
| L4,3 | 5.1 | 12.8 | 10.6 | 6.4 | 10.4 | 13.6 | 6.0 | 11.8 | 10.0 | 12.7 | 14.4 | 10.3 ± 0.97 |
| Precision of estimates | 0.062 | 0.021 | 0.014 | 0.063 | 0.182 | 0.172 | 0.714 | 0.434 | 0.182 | 0.282 | 0.311 | — |
| L5,4 | 40.5 | 40.4 | 9.5 | 23.0 | 19.8 | 31.7 | 0.9 | 35.6 | 16.3 | 17.7 | 3.1 | 21.7 ± 4.3 |
| Precision of estimates | 0.203 | 0.219 | 0.098 | 0.046 | 0.136 | 0.136 | 0.265 | 0.137 | 0.131 | 0.165 | 0.211 | — |
| L0,3 | 17.6 | 0.8 | 21.3 | 52.5 | 4.7 | 0.0 | 17.5 | 16.2 | 0.0 | 0.0 | 10.1 | 12.8 ± 4.7 |
| Precision of estimates | 0.062 | 0.069 | 0.051 | 0.024 | 0.130 | 0.080 | 0.046 | 0.039 | 0.109 | 0.086 | 0.044 | — |
| L0,4 | 0.10 | 3.28 | 0.10 | 6.75 | 16.20 | 0.10 | 16.15 | 0.36 | 0.00 | 3.84 | 23.62 | 6.41 ± 2.54 |
| Precision of estimates | 0.072 | 0.068 | 0.085 | 0.040 | 0.277 | 0.213 | 0.397 | 0.187 | 0.413 | 0.086 | 0.252 | — |
| L0,5 | 72.5 | 119.5 | 45.1 | 53.2 | 29.0 | 8.4 | 0.0 | 38.0 | 38.3 | 31.4 | 29.4 | 42.2 ± 9.791 |
| Precision of estimates | 0.126 | 0.085 | 0.129 | 0.087 | 0.117 | 0.754 | 0.124 | 0.166 | 0.125 | 0.202 | 0.215 | — |
values represent percentages of labeled fatty acid transferred between 2 adjoining compartments. LI,J values represent fractional rate constant coefficients for transfer of fatty acid between 2 adjoining compartments. Precision estimates for individual LI,J values are given as SDs of the estimates normalized to the values of the estimates expressed as percentages determined from the modeling procedure.
TABLE 6.
Plasma appearance (from biosynthesis) and disappearance rates [RJ (in μg/h)] for n−3 fatty acids in newborn infants1
| Subject identification number |
||||||||||||
| 81 | 82 | 83 | 84 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | Mean ± SE | |
| R0,1 | 25,115 | 12,144 | 25,305 | 53,173 | 46,494 | 36,744 | 9066 | 15,393 | 8612 | 31,067 | 13,017 | 25,103 ± 4649 |
| R0,2 | 42 | 21 | 269 | 210 | 1 | 10 | 11 | 38 | 2 | 13 | 11 | 57 ± 28 |
| R3,2 | 9.1 | 2.2 | 10.6 | 8.0 | 4.7 | 22.4 | 1.7 | 9.3 | 1.3 | 11.9 | 0.8 | 7.5 ± 1.9 |
| R0,3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 ± 0 |
| R4,3 | 78 | 71 | 18 | 33 | 20 | 39 | 12 | 29 | 24 | 39 | 27 | 36 ± 6.4 |
| R0,4 | 11 | 7.2 | 2 | 6 | 1 | 6 | 36 | 3 | 4 | 8 | 2 | 7.8 ± 3.0 |
| R5,4 | 191 | 79 | 14 | 69 | 60 | 71 | 15 | 34 | 39 | 84 | 25 | 62 ± 15.1 |
| R0,5 | 1638 | 1336 | 213 | 513 | 945 | 318 | 202 | 279 | 511 | 1074 | 579 | 692 ± 148 |
values represent the rates of disappearance (not involved in synthesis) and appearance (synthesized from its precursor) of the n−3 fatty acids. Values were determined from the fractional rate constant coefficients and plasma masses for each fatty acid. For example, R3,2 is the hourly amount of plasma 18:3n−3 involved in synthesis of 20:5n−3, and R0,1 is the turnover of 18:3n−3 in each subject.
Percentages of the labeled plasma n−3 FA (PI,J) directed toward biosynthesis were determined from the rate constant coefficients in the transfer of the label into and out of each FA compartment. These values for the 2H- and 13C-labeled FAs are given in Table 5 and Table 7, respectively. In most subjects, only ≈0.1% of 2H5-18:3n−3 appeared in the plasma; however, a substantial percentage of plasma 2H5-18:3n−3 (mean: 31 ± 4%; range: 3.7–85%) was directed toward the synthesis of 2H5-20:5n−3. Consequently, the net mean daily biosynthetic output of 20:5n−3 in plasma was ≈200 μg. This amount, which was equivalent to ≈30% of the total plasma EPA (Table 4), was equal to approximately one-third of the daily plasma EPA turnover (Table 6). The mean percentages of plasma 2H5-20:5n−3 and of 2H5-22:5n−3 directed toward the synthesis of 2H5-22:5n−3 and 2H5-22:6n−3, respectively, were both >95% of the isotope use (Table 7). These highly efficient biosynthetic processes were consistent with the 10-fold difference in the amount of plasma DHA compared with that of EPA and docosapentaenoic acid (Table 2).
TABLE 7.
Fractional transfer of labeled n−3 fatty acids (P)1
| Subject identification number |
||||||||||||
| 81 | 82 | 83 | 84 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | Mean ± SE | |
| P2,1 | 0.0002 | 0.0019 | 0.0006 | 0.0041 | 0.0001 | 0.0009 | 0.0013 | 0.0030 | 0.0004 | 0.0008 | 0.0009 | 0.0013 ± 0.0004 |
| P3,2 | 0.179 | 0.093 | 0.270 | 0.037 | 0.850 | 0.685 | 0.136 | 0.199 | 0.429 | 0.474 | 0.067 | 0.311 ± 0.081 |
| P4,3 | 1.00 | 0.60 | 1.00 | 1.00 | 0.88 | 0.95 | 1.00 | 1.00 | 0.97 | 1.00 | 1.00 | 0.945 ± 0.036 |
| P5,4 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 0.29 | 1.00 | 1.00 | 1.00 | 1.00 | 0.935 ± 0.065 |
values represent the percentages of labeled fatty acid transferred between 2 adjoining compartments. For example, P3,2 is the percentage of 2H5-18:3n−3 transferred from plasma compartment number 2 (18:3n−3) to plasma compartment number 3 (20:5n−3).
A comparatively smaller percentage of 13C-U-20:5n−3 was used for synthesis of 13C-22:5n−3 (mean: 59.6%) (Table 5) than 2H5-20:5n−3, which suggests that preformed EPA may be somewhat less efficient for DHA biosynthesis than that derived from ALA. This appears to be analogous to our previous observations on the metabolism of n−6 FA in the production of 20:4n−6 from 20:3n−6 (23). However, on a per mole basis, preformed EPA was 3.6 times more effective that ALA in DHA production (Tables 5 and 7). A direct side-by-side comparison of the n−6 from a previous report (23) and n−3 EFA metabolic variables in this group of infants is given in Table 8. P values for both n−6 and n−3 FA indicated uniformly high fractional synthetic rates for long-chain PUFAs in this group of infants.
TABLE 8.
Use and synthetic rates, plasma turnover, and intake amounts of n−6 and n−3 essential fatty acids in newborn infants1
| Mean ± SE | |
| n−6 EFA2 | |
| Plasma (% of use) | |
| P% dose in plasma | 0.54 ± 0.22 |
| P%18:2→20:3 | 3.83 ± 1.99 |
| P%18:2→18:3 | 1.24 ± 0.50 |
| P%18:2→20:2 | 3.02 ± 1.19 |
| P%20:3→20:4 | 71 ± 11 |
| Plasma synthesis (mg/d) | |
| R18:2→20:3 | 0.29 ± 0.05 |
| R18:2→18:3 | 0.52 ± 0.08 |
| R18:2→20:2 | 0.25 ± 0.04 |
| R20:3→20:4 | 0.94 ± 0.11 |
| Turnover (mg/d) | |
| Whole body | |
| R18:2 | 9471 ± 1746 |
| Plasma (Σ) | |
| R18:2 | 44 ± 9 |
| R18:3 | 0.41 ± 0.06 |
| R20:2 | 2.39 ± 0.34 |
| R20:3 | 1.67 ± 0.27 |
| R20:4 | 10.2 ± 2.4 |
| Estimated intake (mg/d) | |
| U18:2 | 7111 ± 1216 |
| U18:3 | 0.12 ± 0.02 |
| U20:2 | 1.36 ± 0.23 |
| U20:3 | 0.87 ± 0.11 |
| U20:4 | 7.43 ± 1.68 |
| n−3 EFA3 | |
| Plasma (% of use) | |
| P% dose in plasma | 0.13 ± 0.04 |
| P%18:3→20:5 | 31 ± 9 |
| P%20:5→22:5 | 93 ± 10 |
| P%22:5→22:6 | 94 ± 11 |
| Plasma synthesis (mg/d) | |
| R18:3→20:5 | 0.18 ± 0.05 |
| R20:5→22:5 | 0.85 ± 0.15 |
| R22:5→22:6 | 1.48 ± 0.36 |
| Turnover (mg/d) | |
| Whole body | |
| R18:3 | 602 ± 112 |
| Plasma (Σ) | |
| R18:3 | 1.55 ± 0.67 |
| R20:5 | 0.86 ± 0.17 |
| R22:5 | 1.67 ± 0.36 |
| R22:6 | 16.6 ± 3.5 |
| Estimated intake (mg/d) | |
| U18:3 | 482 ± 184 |
| U20:5 | 0.21 ± 0.05 |
| U22:5 | 0.21 ± 0.07 |
| U22:6 | 7.43 ± 1.68 |
EFA, essential fatty acid.
n = 10.
n = 11.
nminus3 FA intake
When integrating each subject's feeding regimen (Table 1) into each of the 2 compartmental models, an adjustment to n−3 FA intake values was necessary to account for the initial low intake volumes during the first 24–48 h after birth. FA intake for several subjects was negligible during this period. It was presumed that plasma EFA concentrations were maintained through mobilization of body-stored reserves. Individual and mean intake values for 18:3n−3, 20:5n−3, 22:5n−3, and 22:6n−3 are given in micrograms per hour (Table 9). The mean (±SE)predicted daily intakes of 18:3n−3 and 22:6n−3 were 150 ± 92 mg 18:3n−3 · kg−1 · d−1 and 2.2 ±0.5 mg 22:6n−3 · kg−1 · d−1. This amount of DHA intake was not sufficient to replace the plasma 22:6n−3 turnover. The combined input from synthesis (1.5 ± 0.2 mg DHA/d) and intake (≈7.08 ± 0.6 mg DHA/d) of DHA provided only about one-half of the 22:6n−3 needed to maintain plasma DHA homeostasis during the first week of life. In contrast, the predicted 18:3n−3 intake was well matched to its turnover in the system (187 ± 56 mg 18:3n−3 · kg−1 · d−1).
TABLE 9.
Predicted n−3 fatty acid intake [UJ (in μg/h)] in 11 infants during the first week of life1
| Subject identification number |
||||||||||||
| 81 | 82 | 83 | 84 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | Mean ± SE | |
| U1/18:3 | 69,834 | 3382 | 70,426 | 14,843 | 12,927 | 10,224 | 2524 | 4292 | 20,092 | 8644 | 3622 | 20,073 ± 7680 |
| U2/20:5 | 21.2 | 21.0 | 2.2 | 7.8 | 5.6 | 4.9 | 3.2 | 6.0 | 8.8 | 8.1 | 8.0 | 8.8 ± 1.9 |
| U3/22:5 | 31.1 | 0.2 | 0.0 | 9.7 | 13.2 | 8.6 | 12.5 | 0.6 | 8.5 | 12.4 | 0.0 | 8.8 ± 2.8 |
| U4/22:6 | 661 | 92 | 574 | 202 | 404 | 113 | 85 | 112 | 297 | 452 | 253 | 295 ± 61 |
values were calculated for each subject and reflect low intake values in most subjects during the first 48 h after birth.
DISCUSSION
Two compartmental models that used 2H and 13C isotopically labeled substrates were advanced to assess the contributions of dietary 18:3n−3 and 20:5n−3 toward the synthesis and maintenance of plasma 22:6n−3 in newborn infants. The current study continued our previous work that investigated n−6 EFA metabolism in the same group of subjects and, therefore, offered a unique opportunity to compare intrasubject n−3 and n−6 EFA metabolism during the first week of life (23). Clinical investigations that are intended to assess EFA use and metabolism in newborns, especially infants who present with variable levels of postnatal metabolic stress, have limitations. In particular, this group of neonates had delayed onset feeding, and the initial nutrient intake volumes were low, which may have contributed to nonideal plasma steady-state conditions for 18:3n−3. In addition, 6 infants lost some body weight (mean: 5%; range: 0.25–11%), however, 5 infants had an increase in body mass (mean: 2.8%; range: 2.1–4.4%) during the first week. Also, compartmental models that are based on plasma kinetics alone are somewhat limited in assessing true liver synthetic values (13). Further, because some infants received complex diets that contained both breast milk and infant formula, the variable n−3 and n−6 FA intake may have influenced the metabolism of labeled n−3 FA and n−3 FA rate variables. The modeling procedures provided a sufficient level of control to account for the varying intake of n−3 EFAs. However, the current version of this model does not extend similar control to a varying intake n−6 EFAs or n−6 long-chain PUFAs.
The efficiency of each biosynthetic step, which was calculated as a percentage of the isotope flux remaining in the biosynthetic pathway (P value), was measured from the rate constant coefficients for the transfer of label into and out of individual FA compartments. In infants there was about a 0.04% conversion of dietary ALA to plasma EPA (Table 7), whereas in adult subjects this value was 0.011% or a ≈4:1 ratio of infants to adults (11). Overall, the percentage conversion of dietary ALA to DHA was ≈14 times greater in infants than in adult humans, which signifies that DHA production was highly active during early developmental processes (11). The total amount of DHA supplied to plasma was 1.5 mg DHA/d. A high percentage of plasma 18:3n−3 was used for the biosynthesis of 20:5n−3 in most subjects (Table 7), which was of a similar magnitude as the percentage use of 18:2n−6 for the biosynthesis of PUFAs (≈10%) in the same group of infants (Table 8) (23). With the use of a similar isotope procedure in 3-wk-old infants, Sauerwald et al (17) estimated that the fractional rate of conversion (FRC) of plasma 18:3n−3–22:6n−3 (FRC is equivalent to the P value) varied from 1.5% to 4.2% and depended on the ALA content of the formula. Their analysis did not include isolation of any intermediates in the synthesis of DHA, and therefore, it is not possible to determine the efficiency associated with any individual biosynthetic processes. In the current study, the net mean FRC [net mean FRC = (P5,4) × (P4,3) × (P3,2)] for the conversion of plasma 18:3n−3–22:6n−3 was on the order of 28%. This higher FRC value may be due to several factors, including the somewhat earlier postnatal stage of our subjects and perhaps differences in each study's duration (24 compared with 168 h). Notably, the FRC from a bolus dose of labeled 18:3n−3–22:6n−3 in adult men and women who consumed their diets ad libitum was <1% (25). As characteristic of early life, it is supposed that the higher rate of biosynthesis may be related to the demand of the system for long-chain PUFAs during development. However, caution should be used when making cross comparisons (16).
In another earlier attempt to determine the conversion of 18-carbon EFA to long-chain PUFAs in infants, Demmelmair et al (26), who used changes in ratios of the 13C:12C isotopes of plasma FAs in subjects who received a corn-oil-based formula (a C-4 plant), took advantage of natural 13C:12C-isotope abundance differences that occur between C-3 and C-4 plant species. Carnielli et al (27) later used a modified version of this method in a long-term feeding study in premature infants. The former study (27) is more comparable with the current study design because it tracked changes in the time-course concentration ratios of plasma FA when the percentage conversion of 18:2n−6–20:4n−6 was computed. The black-box approach favored by Carnielli et al (27) inferred values for the percentage conversion of 18:3n−3 to DHA from single time-point assessments, lacked kinetic determinants, isolated no pathway intermediates, and required inclusion of several uncorroborated assumptions. Interestingly, both studies (26, 27) inferred a high percentage conversion of 18-carbon EFA to long-chain PUFAs from their data during the first few weeks of life. In the current study, we estimated that ≈28% of plasma ALA was converted to DHA by using a modeling paradigm that also accounted for FA turnover, dietary EFA intake, and the conversion of individual substrates to intermediates and final products. However, as the appearance of labeled ALA into the plasma was a very inefficient process, the overall conversion of dietary ALA to plasma DHA was only 0.04%.
The percentage conversion of 13C-20:5n−3 to 13C-22:6n−3 was significantly less than the percentage conversion of 2H-20:5n−3 to 2H-22:6n−3 (45% compared with 94%; P < 0.004, Student's t test). However, when compared on a per mole basis, dietary 20:5n−3 was ≈3.5 times more effective toward the synthesis of 22:6n−3 than was 18:3n−3.
The predicted mean intake of 18:3n−3 from the ALA compartmental model was ≈150 mg 18:3n−3 · kg−1 · d−1, with a whole-body turnover of 187 mg 18:3n−3 · kg−1 · d−1. This amount of ALA intake appeared to be sufficient to meet the newborn ALA requirements in contrast with a need for a greater amount of 18:2n−6 intake in the same group of subjects (23). The mean daily rates of synthesis and turnover of 22:6n−3 in the plasma of infants were estimated to be ≈1.5 mg 22:6n−3/d and 16.7 mg 22:6n−3/d, respectively. Although liver biosynthesis was not assessed directly on the basis of previous assessments of DHA biosynthesis in adults, it may be supposed that liver DHA biosynthesis was greater than that inferred from plasma analysis (13). From the predicted rates of turnover and synthesis of 22:6n−3 for the entire group, it was estimated that an additional intake of ≈2.5 mg 22:6n−3 · kg−1 · d−1 (or ≈8.1 mg 22:6n−3/d) was needed to maintain plasma DHA concentrations during the first week of life.
The results of this study, together with findings of n−6 EFA metabolism (23) in the same group of subjects, form a basis on which to hypothesize the effects of feeding a particular infant formulation on the metabolism and maintenance of plasma 18–20- and 22-carbon n−3 and n−6 essential FAs in newborn infants. Even with relatively high rates of conversion of plasma 18:3n−3 and 18:2n−6 (23) to long-chain PUFAs, overall low rates of utilization of dietary 18:2n−6 and 18:3n−3 necessitate the fortification of infant formula with preformed 20:4n−6 and 22:6n−3 to ensure the proper maintenance of plasma homeostatic concentrations of these FAs when breast milk is not available. Three infants (82, 84, and 92) appeared to have low conversions of plasma 18:3n−3–20:5n−3 (Table 7) compared with other individuals, and in such cases a formula with preformed 22:6n−3 is particularly critical for the maintenance of DHA status.
Acknowledgments
The collaboration of the clinical staff of the Neonatal Unit at the Hospital Sotero del Rio in Santiago, Chile, is gratefully acknowledged.
The authors’ responsibilities were as follows—YHL: sample handling, data collection, analysis, and interpretation of compartment-modeled data; AL and PM: clinical care of the infants and administration of the labeled FAs; RU and NS: study design; RJP: overall responsibility for compartmental modeling and writing of the manuscript; and YHL, AL, RU, and NS: editing of the manuscript. None of the authors had a financial or personal conflict of interest.
REFERENCES
- 1.Gibson RA, Makrides M. n− 3 Polyunsaturated fatty acid requirements of term infants. Am J Clin Nutr 2000;71:251S–5S [DOI] [PubMed] [Google Scholar]
- 2.Birch E, Birch D, Hoffman D, Hale L, Everett M, Uauy R. Breast-feeding and optimal visual development. J Pediatr Ophthalmol Strabismus 1993;30:33–8 [DOI] [PubMed] [Google Scholar]
- 3.Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res 1998;44:201–9 [DOI] [PubMed] [Google Scholar]
- 4.Carlson SE, Ford AJ, Werkman SH, Peeples JM, Koo WW. Visual acuity and FA status of term infants fed human milk and formulas with and without docosahexaenoate and arachidonate from egg yolk lecithin. Pediatr Res 1996;39:882–8 [DOI] [PubMed] [Google Scholar]
- 5.Carlson SE, Ford AJ, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA 1993;90:1073–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koletzko B.Lipid supply and metabolism in infancy. Curr Opin Clin Nutr Metab Care 1998;1:171–7 [DOI] [PubMed] [Google Scholar]
- 7.Neuringer M, Connor WE, Petten CV, Barstad L. 1984 dietary omega-3-FA deficiency and visual loss in infant rhesus monkeys. J Clin Invest 1984;73:272–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champoux M, Hibbeln JR, Shannon C, et al. FA formula supplementation and neuromotor development in rhesus monkey neonates. Pediatr Res 2002;51:273–81 [DOI] [PubMed] [Google Scholar]
- 9.Diau GY, Loew ER, Wijendran V, Sarkadi-Nagy E, Nathanielsz PW, Brenna JT. Docosahexaenoic and arachidonic acid influence on preterm baboon retinal composition and function. Invest Ophthalmol Vis Sci 2003;44:4559–66 [DOI] [PubMed] [Google Scholar]
- 10.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr 2002;88:355–64 [DOI] [PubMed] [Google Scholar]
- 11.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. J Lipid Res 2001;42:1257–65 [PubMed] [Google Scholar]
- 12.Pawlosky RJ, Hibbeln JR, Salem N., Jr Compartmental analyses of plasma n−3 essential fatty acids among male and female smokers and nonsmokers. J Lipid Res 2007;48:935–43 [DOI] [PubMed] [Google Scholar]
- 13.Pawlosky RJ, Hibbeln JR, Herion D, Kleiner DE, Salem N., Jr Compartmental analysis of plasma and liver n−3 essential fatty acids in alcohol-dependent men during withdrawal. J Lipid Res 2009;50:154–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnielli VP, Wattimena DJ, Luijendijk IH, Boerlage A, Degenhart HJ, Sauer PJ. The very low birth weight premature infant is capable of synthesizing arachidonic and docosahexaenoic acids from linoleic and linolenic acids. Pediatr Res 1996;40:169–74 [DOI] [PubMed] [Google Scholar]
- 15.Salem N, Jr, Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA 1996;93:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koletzko B, Demmelmair H, Hartl W, et al. The use of stable isotope techniques for nutritional and metabolic research in paediatrics. Early Hum Dev 1998;53(suppl):S77–97 [DOI] [PubMed] [Google Scholar]
- 17.Sauerwald TU, Hachey DL, Jensen CL, Chen H, Anderson RE, Heird WC. Effect of dietary α-linolenic acid intake on incorporation of docosahexaenoic and arachidonic acids into plasma phospholipids of term infants. Lipids 1996;31(suppl):S131–5 [DOI] [PubMed] [Google Scholar]
- 18.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics 1963;32:793–800 [PubMed] [Google Scholar]
- 19.Folch J, Lees A, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 20.Morrison WR, Smith LM. Preparation of FA methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 1964;5:600–8 [PubMed] [Google Scholar]
- 21.Lin YH, Salem N., Jr In vivo conversion of 18- and 20-C essential fatty acids in rats using the multiple simultaneous stable isotope method. J Lipid Res 2005;46:1962–73 [DOI] [PubMed] [Google Scholar]
- 22.Milad M, Mena P, Nieto S, Uauy R. Fatty acid composition of human milk lipids in Chilean women. Acta Paediatr 2004;93:855–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawlosky RJ, Lin YH, Llanos A, Mena P, Uauy R, Salem N., Jr Compartmental analyses of plasma 13C- and 2H-labeled n−6 fatty acids arising from oral administrations of 13C-U-18:2 n−6 and 2H5-20:3 n− 6 in newborn infants. Pediatr Res 2006;60:327–33 [DOI] [PubMed] [Google Scholar]
- 24.Moya M, Cortes E, Juste M, De Dios JG, Vera A. Fatty acid absorption in preterms on formulas with and without long-chain polyunsaturated fatty acids in terms on formulas with there added. Eur J Clin Nutr 2001;55:755–62 [DOI] [PubMed] [Google Scholar]
- 25.Pawlosky RJ, Hibbeln JR, Lin Y, et al. Effects of beef- and fish-based diets on the kinetics of n− 3 fatty acid metabolism in human subjects. Am J Clin Nutr 2003;77:565–72 [DOI] [PubMed] [Google Scholar]
- 26.Demmelmair H, von Schenck U, Behrendt E, Sauerwald T, Koletzko B. Estimation of arachidonic acid synthesis in full term neonates using natural variation of 13C content. J Pediatr Gastroenterol Nutr 1995;21:31–6 [DOI] [PubMed] [Google Scholar]
- 27.Carnielli VP, Simonato M, Verlato G, et al. Synthesis of long-chain polyunsaturated fatty acids in premature newborns fed formula with long-chain polyunsaturated fatty acids. Am J Clin Nutr 2007;86:1323–30 [DOI] [PubMed] [Google Scholar]