Abstract
Background: Choline is essential for infant nutrition, and breast milk is a rich source of this nutrient. Common single nucleotide polymorphisms (SNPs) change dietary requirements for choline intake.
Objective: The aim of this study was to determine whether total choline intake and/or SNPs influence concentrations of choline and its metabolites in human breast milk and plasma.
Design: We gave a total of 103 pregnant women supplemental choline or a placebo from 18 wk gestation to 45 d postpartum and genotyped the women for 370 common SNPs. At 45 d postpartum, we measured choline metabolite concentrations in breast milk and plasma and assessed the dietary intake of choline by using a 3-d food record.
Results: On average, lactating women in our study ate two-thirds of the recommended intake for choline (Adequate Intake = 550 mg choline/d). Dietary choline intake (no supplement) correlated with breast-milk phosphatidylcholine and plasma choline concentrations. A supplement further increased breast-milk choline, betaine, and phosphocholine concentrations and increased plasma choline and betaine concentrations. We identified 5 SNPs in MTHFR that altered the slope of the intake–metabolite concentration relations, and we identified 2 SNPs in PEMT that shifted these curves upward. Individuals who shared sets of common SNPs were outliers in plots of intake–metabolite concentration curves; we suggest that these SNPs should be further investigated to determine how they alter choline metabolism.
Conclusion: Total intake of choline and genotype can influence the concentrations of choline and its metabolites in the breast milk and blood of lactating women and thereby affect the amount of choline available to the developing infant. This study was registered at clinicaltrials.gov as NCT00678925.
INTRODUCTION
Breast milk is an important dietary source of choline for infants during a time when choline is critically needed for growth and development (1). Choline, or its metabolites, is needed for the structural integrity and signaling functions of cell membranes; choline is the major source of methyl groups in the diet (one of choline's metabolites, betaine, participates in the methylation of homocysteine to form methionine), and choline directly affects cholinergic neurotransmission, transmembrane signaling, and lipid transport/metabolism (2). Also, choline influences brain development and function (3–16). In humans, low choline intake during pregnancy is associated with an increased risk of birth defects in the fetus (17–19).
Choline can be derived from the diet (20) and from endogenous biosynthesis [catalyzed by the enzyme phosphatidylethanolamine-n-methyl transferase (PEMT)] (21–23). Many foods eaten by humans contain choline and choline esters (20). The Institute of Medicine (IOM) of the United States set an Adequate Intake (AI) for choline of 550 mg choline/d for men, 425 mg choline/d for women, and 550 mg choline/d for lactating women (24). Normal choline intake in pregnant women in California ranges from half the recommended intake (lowest quartile) to slightly more than the recommended intake (highest quartile) (17), and similar distributions for choline intake in pregnant women were reported in the National Health and Nutrition Examination Survey dataset (25). During pregnancy, choline is made available to the fetus across the placenta (26, 27), and after birth, human milk is a rich source of choline for the developing infant (28, 29).
People differ in dietary choline requirements because of, in part, common single nucleotide polymorphisms (SNPs) in genes of choline and folate metabolism (30, 31). We hypothesized that women with common SNPs that increase dietary requirements for choline may need to consume diets higher in choline while pregnant and lactating to optimally supply the choline needed to support the developing nervous system of their children. To investigate this, we enrolled 103 healthy pregnant women (at 18 wk gestation) who expressed the intention to breastfeed and followed them through 45 d postpartum. One-half of the participants received a choline supplement (as phosphatidylcholine), and one-half of the participants received a placebo. We genotyped these women for 370 SNPs in genes related to choline metabolism (such as the PEMT gene) and determined if the presence of these SNPs influenced breast-milk and plasma choline and choline metabolite concentrations at 45 d postpartum across a broad range of choline intake.
SUBJECTS AND METHODS
Participants
Healthy pregnant women at ≤18 wk gestation were recruited for a protocol approved by the Institutional Review Board (IRB) at the University of North Carolina (UNC) at Chapel Hill (informed consent was obtained from all participants). The study was advertised by using mass informational e-mails, IRB-approved flyers or pamphlets displayed in target locations, and advertisements in local newspapers. Of the initially recruited 140 participants, 103 participants completed the study per the protocol, whereas 37 participants voluntarily dropped out or were discharged for noncompliance. Participants ranged in age from 21 to 41 y. The ethnic heritages of the 103 participants were white American (89%), African American (3%), Asian (6%), American Indian (1%), and other (1%), which reflected the local population diversity of the greater Raleigh-Durham-Chapel Hill metropolitan area.
For inclusion in the study, participants had to have a good state of health, an uncomplicated, low-risk pregnancy, a prepregnancy body mass index (in kg/m2) between 18 and 35, expressed the intention to breastfeed for ≥90 d, received regular prenatal care, taken a prenatal vitamin, and had fluency in English. Women who used tobacco products, illicit drugs, alcohol, or were pregnant with multiple fetuses were excluded.
Study design
This study was conducted as part of a larger ongoing study aimed at investigating the effects of supplemental choline on brain development and memory function. Upon entry into this study, one-half of the participants was randomly assigned to receive a choline supplement (6 PhosChol gelcaps/d (Nutrasal, Westbrook, ME); each gelcap contained 900 mg phosphatidylcholine, which is equivalent to 125 mg choline; thus, 6 gelcaps delivered 750 mg choline/d), and one-half of the participants was randomly assigned to receive a placebo (6 gelcaps/d, each of which contained 900 mg corn oil) from 18 wk pregnancy (on the basis of their due date) through 90 d postpartum. This was done to obtain a broader range of choline intake and to determine whether choline intake above the highest normal dietary intake was needed to compensate for effects of SNPs. The choline supplement used in this study was administered as phosphatidylcholine, and there are differences in the bioavailability between free choline and phosphatidylcholine. Free choline is taken up quickly and cleared from plasma quickly (3 h), whereas phosphatidylcholine increases plasma choline concentrations for 8–12 h with no appreciable change in plasma phosphatidylcholine concentrations (32, 33). Most of the choline consumed by humans is in the form of phosphatidylcholine, but there are several forms of choline in the diet.
Subjects were instructed to take 3 gelcaps in the morning with breakfast and 3 capsules in the evening. Subjects were given small calendar cards in a plastic sleeve on which they were instructed to record their daily supplement or placebo intake. Subjects came in for follow-up visits at 20 and 30 wk pregnancy and 45 and 90 d postpartum. At each of these visits they were asked to bring in any unused portion of capsules as well as their supplement calendar. Capsule counts were done to assess compliance and results compared with the intake recorded on the calendars. Subjects were asked about any discrepancies. If a discrepancy existed, the capsule count was used to estimate compliance. Then, subjects were given a new supply of capsules to carry them through the next study interval (except at the 90-d postpartum visit). At each visit, a 3-d food record and a nonfasting blood sample were collected. Moreover, a first morning breast-milk sample was collected at the 45- and 90-d postpartum visits. Subjects continued to take supplements through 90 d postpartum and the infants were tested at 10 and 12 mo of age as part of the larger study. However, data from only the 45-d postpartum visit is presented in the current study. This time was chosen because breast milk has fully matured by this point, and a greater number of subjects were breastfeeding (relative to the 90-d postpartum time point).
All mothers and infants who participated in this study were monitored for adverse events as detailed in a safety monitoring plan. Specifically, upon enrollment, subjects were given an information packet to give to their obstetrician, which included a letter requesting that the obstetrician notify us in the event that any unusual or unexpected symptoms occur in the patient. Any clinically significant symptoms that were reported to us by the obstetrician were reported to the IRB as adverse events. In addition, a member of our study team contacted each subject by e-mail or telephone every 4 wk to inquire about her overall health and the progress of her pregnancy. A written questionnaire was used for this purpose to ensure that the same data were collected at each inquiry. All collected medical data were reviewed by the study physician on a monthly basis. Moreover, all subjects were advised to notify the study coordinator at any time should they experience any unexpected health problem or complication. At each follow-up visit, the subject's vital signs, including weight and blood pressure, were checked. Any clinically significant findings noted at follow-up visits were also reported to the IRB as adverse events. After subjects delivered, the study coordinator contacted them to inquire about the well being of the mother and the infant, and advised the subjects to notify the study team should any major problems arise pertaining to the health or development of their children. An interim safety analysis was conducted in all adverse events once the first 50 infants had been born and was reviewed by the UNC Data Safety and Monitoring Board. The UNC Biomedical IRB also conducted a final safety analysis of all adverse events once the study was complete.
Dietary analyses of choline intake
All participants were asked to keep a complete 3-d food record, which was reflective of their usual intake, immediately before their 45-d postpartum visit. Participants were asked to record everything that they ate and drank on 2 typical weekdays and 1 weekend day. Daily food-intake records were analyzed with the Esha Food Processor SQL program (Version 10.3; ESHA Research, Salem, OR). This nutrient-analysis software references the USDA National Nutrient Database and includes food values for choline from the USDA Database for the Choline Content of Common Foods (2008; http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choln02.pdf). The software does not include values for betaine, a metabolite of choline. Hence, all known-food betaine values (20) were manually entered into the database before conducting the analyses, and referenced according to the 5-digit Nutrient Databank Number. The total choline content of a food was calculated as the sum of the amounts of choline, phosphocholine, glycerophosphocholine, phosphatidylcholine, and sphingomyelin. Betaine was calculated independently. If the total choline or betaine content of a specific food item was unavailable, a nutritionally equivalent food was substituted in the analysis. Each day of the 3-d food record was analyzed individually and averaged for each subject. The amount of choline ingested on average per day was summed with the amount from supplementation (750 or 0 mg) to arrive at an estimate of total choline intake.
Determination of breast-milk and plasma choline concentrations
Participants were asked to completely empty one breast of milk upon waking in the morning (between 0500 and 1000) on the day of their 45-d postpartum visit with a mechanical or electric pump that they supplied. This sample was mixed, and a 10-mL aliquot was immediately frozen at −20°C. Frozen milk samples were transferred to our storage facility and stored at −80°C until assayed. At the 45-d postpartum visit, subjects were also asked to complete a breastfeeding questionnaire that included questions about the exclusivity of breastfeeding and formula use.
At the time of the participant's visit to our clinic (generally late morning), blood samples were collected by venipuncture in sodium heparin and immediately placed on ice. Within 30 min, samples were centrifuged (2000 × g for 10 min) at 4°C to isolate plasma, which was then aliquoted and stored at −80°C until assayed for concentrations of choline and its metabolites. Choline and its metabolites were extracted from the plasma or breast milk by using the method of Bligh and Dyer (34). Specifically, aqueous and organic compounds were separated, analyzed, and quantified directly by using liquid chromatography/electrospray ionization-isotope–dilution mass spectrometry after the addition of internal standards labeled with stable isotopes to correct for recovery (35). In plasma, concentrations of free choline and esterified choline (phosphatidylcholine and sphingomyelin), as well as betaine, were measured. In milk samples, free choline, phosphatidylcholine, sphingomyelin, betaine, phosphocholine, and glycerophosphocholine were measured, all of which were previously shown to be present in human milk (36).
Analyses of SNPs
Maternal blood samples were collected at the 45-d postpartum visit by venipuncture, and peripheral lymphocytes were isolated from blood by Ficoll-Hypaque gradient with evacuated cell preparation tubes with sodium citrate (Becton Dickinson, Franklin Lakes, NJ) (37, 38). Genomic DNA was extracted with a PureGene kit (Gentra Systems, Minneapolis, MN) according to the manufacturer's instructions. We genotyped participants with respect to 370 common SNPs in a defined set of 10 genes related to choline metabolism (30). DNA samples were genotyped for the SNPs of interest by the UNC Mammalian Genotyping Core with the Illumina GoldenGate oligo-specific extension–ligation assay (39). Briefly, 3 assay oligonucleotides—2 allele specific (ASO) and one locus specific (LSO)—were designed for each SNP locus and scored for likelihood of success. The ASOs for each allele and corresponding LSOs were hybridized to whole genomic DNA from each individual and washed to remove nonhybridized material. The ASOs and LSOs were extended across the 1–20-base pair gap between them and ligated to form the template for the polymerase chain reaction (PCR) with ASO fluorescent-labeled universal primers, the product of which was hybridized to oligonucleotides complementary to the unique address of the LSO anchored to the bead substrate. The relative fluorescence of each allele was quantified, and the genotype was determined.
For SNPs that failed the design score test for the bead array, forward primers specific to each allele were designed so that the SNP would be located at the 3′ end of the priming sequence, which allows for specific PCR products to be synthesized only if the primer is 100% complementary to its template DNA. Primers were designed with GeneFisher program (version 2.0)(http://bibiserv.techfak.uni-bielefeld.de/genefisher) and purchased from Qiagen Operon (Huntsville, AL). The PCRs were optimized for each pair of primers, and the products were visualized on a 1.5% agarose gel to determine the genotype.
Statistical methods
Complete data sets were not obtained from all participants. Specifically, a plasma sample was not obtained from 4 subjects, 4 subjects did not provide breast-milk samples, and 9 participants did not complete 3-d food records.
We used t tests to compare the placebo and supplement groups for dietary choline and betaine intakes as well as for total choline intake after including the amount from the supplement. We also used t tests to compare the placebo and supplement groups for the 6 breast-milk and 4 plasma choline metabolites.
For each of the 10 choline metabolites (6 breast milk and 4 plasma), a linear regression model was fitted with the metabolite as the response and total choline intake as the predictor. To determine outliers with undue influence, a model was fitted by using all observations, and to determine outliers with undue influence, Cook's D (40) was computed for each. Observations with a value of Cook's D >4/n, where n is the number of observations used in fitting the model, were deemed to be overly influential and were dropped. The functional form of the model (ie, linearity) was assessed by using aggregates of cumulative residuals (41). The normality assumption was tested by performing Shapiro-Wilk's test on the residuals generated from fitting the model. If the normality assumption was violated, square root and natural log transformations on the response variable were considered. The assumptions of homoskedasticity and independence of the residuals were tested by using the procedure of White (42) as implemented in the SAS software (version 9.2; SAS Institute, Chicago, IL). If the assumption of homoskedasticity was violated, a model with weighted least squares was used.
To test for possible confounding by age, body mass index, parity, prenatal vitamin use, exclusivity of breastfeeding, and supplement compliance (supplement group only), a forward-selection procedure was used according to the Schwarz Bayesian information criterion (43).
We used 2 exploratory approaches to identify SNPs that may influence choline and choline metabolite concentrations in breast milk or plasma. In the first approach, we considered 10 metabolites (free choline, phosphatidylcholine, sphingomyelin, betaine, phosphocholine, and glycerophosphocholine in breast milk and free choline, phosphatidylcholine, sphingomyelin, and betaine in plasma) and 370 SNPs. Each of the 370 SNPs was classified in 3 different ways (W-W compared with W-V compared with V-V, W-W + W-V compared with V-V, and W-W compared with W-V + V-V), where W is the wild-type allele, and V is the variant allele. For each of the 3 SNP classifications and each metabolite, a linear regression model was performed with the metabolite as the response, and total choline intake and SNPs as predictors. The resulting P values for all 3 possible SNP configurations were entered using the PROC MULTTEST procedure (SAS version 9.2; SAS Institute) simultaneously to adjust for multiple testing and determine the false discovery rate. Combinations of SNPs that had a false discovery rate < 0.05 and a sample size ≥5 for all groups compared were considered statistically significant, and consequently it was concluded that there was a difference in metabolite across SNPs after adjusting for total choline intake.
These procedures were performed with an interaction term for total choline intake and SNP in the model to determine which SNPs altered the relation of total choline intake to metabolite concentration. All analyses were done for placebo subjects, supplement subjects, and all subjects (placebo and supplement) combined.
Because the outliers that were discarded in the first set of analyses may have been of significance, a second exploratory approach was used to identify combinations of SNPs that were shared by outliers. These combinations of SNPs may influence the dose-response relation of total choline intake to breast-milk and plasma choline concentrations. Specifically, a regression analysis was performed to examine how choline intake affects breast-milk and plasma choline and choline metabolite concentrations within placebo subjects, supplement subjects, and across all subjects combined (supplement and placebo). Individual participants whose values did not fall within the 95% prediction limits were identified. We identified the variant alleles that these outliers had in common (considering only the homozygous V-V genotypes). For example, if all of the outliers above the 95% prediction limit in a particular regression analysis had V-V at SNP A and at SNP B, this combination of SNPs could be potentially interesting. We also identified all other participants who carried this same set of variant alleles. With the use of regression analysis, we calculated the main effect of this set of SNPs and the interaction effect between this set of SNPs and total choline intake to evaluate whether individuals with this set of variant alleles had a significantly different pattern of metabolites in response to total choline intake. P values were adjusted by using the false discovery rate in the PROC MULTTEST procedure (SAS version 9.2; SAS Institute). Combinations of SNPs that had a false discovery rate <0.01 in either the main effect or interaction effect were considered significant.
RESULTS
Adverse events
Because the study population comprised pregnant women and infants (both vulnerable populations), we erred on the side of caution with respect to adverse-event reporting, even if the events were unlikely to be related to study participation (eg, urinary tract infection). The majority of mothers participating in this study gave birth to healthy infants or infants with minor health concerns such as jaundice. The most frequent adverse event that took place in mothers was gestational diabetes (7 subjects; 4 subjects in the choline group, 3 subjects in the placebo group) followed by gastrointestinal disturbances (nausea, cramping, and diarrhea) that occurred in 5 subjects (2 in the choline group, 3 in the placebo group). The most frequent adverse event that occurred in infants was gastrointestinal reflux (6 subjects; 2 in the choline group, 4 in the placebo group). Results of the interim and final safety analyses showed that there was no difference in the frequency of adverse events between the supplemental choline and placebo groups, and there was no clear evidence of a trend or suggestion of causality of the study agent (PhosChol; Nutrasal). In addition, the study physician observed that adverse events did not occur more frequently among the study population relative to a normal obstetric population.
Dietary and supplemental choline intake
All subjects began a choline- or placebo-supplement regimen at 18 wk pregnancy (on the basis of their due date) per the protocol. The mean duration of treatment from that point until the 45-d postpartum visit was 195 d (range: 163–215 d) in the supplemented group and 196 d (range: 165–217 d) in the placebo group.
Daily intake of choline from the diet was estimated by analyzing 3-d food records kept by participants (46 participants in the placebo group, 48 participants in the supplement group) immediately before their 45-d postpartum study visit. In the placebo group, the dietary intake of choline ranged from 139 to 671 mg choline/d, with a mean intake of 364 mg choline/d (Table 1). In the supplement group, daily choline intake (excluding the supplement) ranged from 124 to 622 mg choline/d, with a mean intake of 338 mg choline/d. Mean intakes of choline and betaine did not differ between the 2 groups. In the placebo group, 4 participants met or exceeded the AI for lactating women (550 mg choline/d) and 3 other participants consumed an average intake that exceeded 500 mg choline/d (data not shown). In the supplement group, only one participant consumed choline at or above the AI for lactating women, and 2 other participants consumed >500 mg choline/d on average (data not shown).
TABLE 1.
Dietary intake of choline and betaine in women at 45 d postpartum1
| Intake | Placebo group (n = 46) | Supplement group (n = 48) |
| Dietary choline (mg/d) | 364 ± 18 (139–671)2 | 338 ± 14 (124–622) |
| Total choline for diet plus supplement groups (mg/d) | 364 ± 183 | 1088 ± 144 |
| Betaine (mg/d) | 295 ± 32 | 263 ± 23 |
Pregnant women at <18 wk gestation were enrolled and randomly assigned to receive either a choline supplement (750 mg choline/d) or a placebo (corn oil). Participants took these supplements from 18 wk pregnancy to 45 d postpartum. Participants kept 3-d food records immediately before the 45-d postpartum study visit. Participants were asked to record all food and beverage intakes on 2 typical weekdays and 1 weekend day. Food records were analyzed with Food Processor SQL nutrient analysis software (version 10.3; ESHA Research, Salem, OR). Daily choline and betaine intakes (mg/d) were estimated for each subject by taking an average of their 3-d intake. Groups were compared by t tests.
Mean concentration ± SE; range of dietary intake of choline (excluding the supplement) in parentheses (all such values).
Mean concentration ± SE (all such values).
Significantly different from placebo group, P < 0.0001.
Choline and its metabolites in breast milk and plasma
Breast milk samples (n = 48 in the placebo group, n = 51 in the supplement group) and plasma samples (n = 48 in the placebo group, n = 51 in the supplement group) were collected from participants at 45 d postpartum and analyzed for choline and individual choline metabolites (Table 2). Participants randomly assigned to the supplemental choline group had, on average, significantly higher concentrations of free choline, betaine. and phosphocholine in breast milk than did participants in the placebo group irrespective of their dietary intake or genotype. Moreover, participants in the supplement group had significantly higher concentrations of free choline and betaine in their plasma relative to participants in the placebo group irrespective of dietary intake and genotype. On the basis of a breastfeeding questionnaire collected at the 45-d postpartum visit, 84 of 103 subjects (82%) exclusively breastfed, whereas 19 subjects (18%) supplemented with formula.
TABLE 2.
Choline and choline metabolite concentrations in breast milk and plasma at 45 d postpartum1
| Metabolite | Placebo group (n = 48) | Supplement group (n = 51) |
| Breast milk (nmol/mL) | ||
| Free choline | 83 ± 8 | 106 ± 102 |
| Phosphatidylcholine | 107 ± 7 | 113 ± 5 |
| Betaine | 7.0 ± 0.5 | 12.3 ± 1.42 |
| Phosphocholine | 553 ± 27 | 722 ± 392 |
| Glycerophosphocholine | 388 ± 25 | 426 ± 23 |
| Sphingomyelin | 67 ± 4 | 67 ± 4 |
| Plasma (nmol/mL) | ||
| Free choline | 7.7 ± 0.3 | 13.7 ± 0.62 |
| Phosphatidylcholine | 2009 ± 57 | 1994 ± 46 |
| Betaine | 64 ± 5 | 126 ± 92 |
| Sphingomyelin | 514 ± 15 | 506 ± 11 |
All values are mean concentrations ± SEs. Pregnant women at <18 wk gestation were enrolled and randomly assigned to receive either a choline supplement (750 mg choline/d) or a placebo (corn oil) from 18 wk pregnancy to 45 d postpartum. Choline and choline metabolite concentrations were measured by using liquid chromatography/mass spectrometry in breast-milk and plasma samples collected at 45 d postpartum. Groups were compared by t tests.
Significantly different from placebo group, P < 0.001.
Influence of total choline intake on concentrations of choline and its metabolites in breast milk and plasma
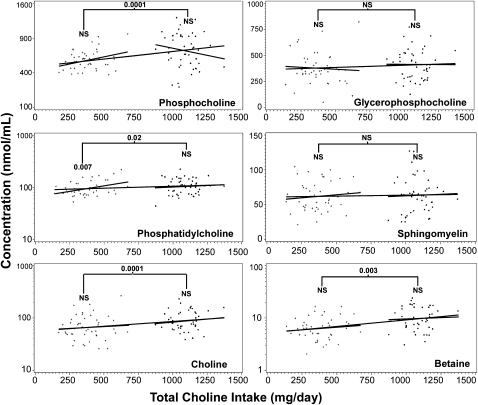
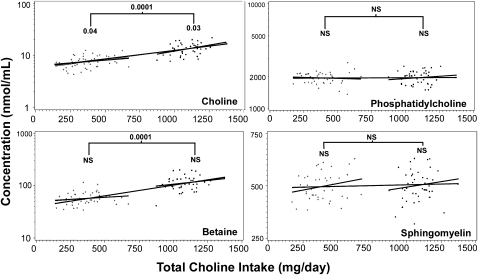
Linear regression analyses were carried out for all of the breast milk and plasma choline metabolites to determine whether choline intake was a significant predictor of metabolite concentrations. Graphs of all breast-milk metabolites are shown in Figure 1. Graphs for all of the plasma choline metabolites are shown in Figure 2.
FIGURE 1.
Influence of dietary choline intake on breast-milk choline metabolite concentrations. Pregnant women were enrolled and randomly assigned to receive either a choline supplement (750 mg choline/d) or a placebo (corn oil) from 18 wk gestation to 45 d postpartum. Choline and choline metabolite concentrations were measured by using liquid chromatography/mass spectrometry in breast milk collected at 45 d postpartum. Dietary intake was estimated by using 3-d food records and adding the intake from the supplement or placebo. Plots are shown for all 6 metabolites that were measured in breast milk. Regression models with metabolite as the response and total choline intake (diet plus supplement) as the predictor were fitted considering either placebo subjects, supplemented subjects, or all subjects combined (3 solid lines). The following numbers of outliers (defined in Subjects and Methods) for each metabolite were excluded: subjects who received placebo only—glycerophosphocholine (4), phosphocholine (4), phosphatidylcholine (4), sphingomyelin (3), choline (3), and betaine (2); subjects who received the supplement only—glycerophosphocholine (3), phosphocholine (1), phosphatidylcholine (2), sphingomyelin (3), choline (3), and betaine (3); and all subjects combined—glycerophosphocholine (5), phosphocholine (5), phosphatidylcholine (3), sphingomyelin (5), choline (5), and betaine (7). Weighted least-squares regression was performed in the model with breast-milk phosphocholine to overcome heteroskedasticity. To achieve normality, log transformations were made on breast-milk phosphatidylcholine, phosphocholine, betaine, and choline concentrations. Three statistical analyses were performed for each metabolite: effect of intake on concentrations for the placebo group (indicated above the line on the left), effect of intake plus supplement on concentrations for the supplemented group (indicated above the line on the right), and effect of intake plus supplement on concentrations for the combined groups (indicated above the bracket). P values are indicated when differences were significant. Group sizes for the placebo and supplement groups were 46 and 48 subjects, respectively. The R2 values for the placebo, supplement, and placebo plus supplement groups, respectively, are as follows: for phosphocholine—0.11, 0.02, and 0.16; for glycerophosphocholine—0.003, 0.0001, and 0.006; for phosphatidylcholine—0.11, 0.008, and 0.07; for sphingomyelin—0.006, 0.003, and 0.003; for choline—0.008, 0.009, and 0.08; and for betaine—0.005, 0.003, and 0.13. +, subjects randomly assigned to the placebo group; •, subjects randomly assigned to the choline supplement group.
FIGURE 2.
Influence of dietary choline intake on plasma choline metabolite concentrations. Pregnant women at <18 wk gestation were randomly assigned to receive either a choline supplement (750 mg choline/d) or a placebo (corn oil) from 18 wk pregnancy to 45 d postpartum. Choline and choline metabolite concentrations were measured by using liquid chromatography/mass spectrometry in plasma samples collected at 45 d postpartum. Values are plotted against total choline intake (diet plus supplement or placebo). Plots are shown for all metabolites measured in plasma. Regression models with metabolite as the response and total choline intake (diet plus supplement) as the predictor were fitted considering placebo subjects, supplemented subjects, or all subjects combined (3 solid lines). The following numbers of outliers (defined in Subjects and Methods) for each metabolite were excluded: subjects who received placebo only—choline (3), betaine (2), phosphatidylcholine (7), and sphingomyelin (4); subjects who received the supplement only—choline (2), betaine (4), phosphatidylcholine (4), and sphingomyelin (4); and all subjects combined—choline (3), betaine (7), phosphatidylcholine (7), and sphingomyelin (7). Weighted least-squares regression was performed in the model with plasma phosphatidylcholine to overcome heteroskedasticity. To achieve normality, log transformations were done on plasma betaine, choline, and phosphatidylcholine concentrations. Three statistical analyses were performed for each metabolite: the effect of intake on concentrations for the placebo group (indicated above the line on the left), the effect of intake plus the supplement on concentrations for the supplemented group (indicated above the line on the right), and the effect of intake plus the supplement on concentrations for the combined groups (indicated above the bracket). P values are indicated when differences were significant; group sizes for placebo and supplement groups were 46 and 48 subjects, respectively. The R2 values for placebo, supplement, and placebo plus supplement groups, respectively, are as follows: for choline—0.11, 0.15, and 0.55; for phosphatidylcholine—0.004, 0.01, and 0.0001; for betaine—0.09, 0.07, and 0.56; and for sphingomyelin—0.07, 0.02, and 0.02. +, subjects randomly assigned to the placebo group; •, subjects randomly assigned to the choline supplement group.
In the placebo group, breast-milk concentrations of phosphatidylcholine (P = 0.007) and plasma concentrations of choline (P = 0.0001) were significantly correlated with total intake of choline (diet alone). In the supplement group, plasma concentrations of choline (P = 0.03) were significantly correlated with total choline intake. Finally, in all subjects combined, breast milk concentrations of choline (P = 0.001), phosphatidylcholine (P = 0.02), betaine (P = 0.0003), and phosphocholine (P = 0.0001) were significantly correlated with the total intake of choline (diet plus supplement), as were plasma concentrations of choline (P = 0.0001) and betaine (P = 0.0001). For all of these significant associations, metabolite concentrations went up as the total choline intake increased.
Plasma choline concentrations were correlated with breast-milk choline concentrations in the placebo group (P = 0.04) and in all subjects combined (P = 0.0001). Plasma betaine concentrations were significantly associated with breast-milk betaine concentrations when all subjects were considered together (P = 0.0001) but not when placebo or supplemented subjects were examined alone.
Effects of SNPs on choline metabolite concentrations in breast milk and plasma
A list of SNPs in participants that significantly interacted (P < 0.05) with the total choline intake on breast-milk and plasma choline concentrations is provided in Table 3. These SNPs (either with 1 or 2 copies of the variant allele) changed the slope of the response curves of choline intake–breast-milk concentrations. These SNPs were revealed only when the placebo participants were considered alone, and the SNPs were all in the MTHFR gene. No significant interactions were seen in the supplement group or in all subjects combined.
TABLE 3.
Single nucleotide polymorphisms (SNPs) that influenced breast-milk choline metabolite concentration curves in participants given a placebo or choline supplement1
| Group and gene | SNP | W-W | W-V | V-V | Metabolite | Effect2 | Change in slope3 | Change in least-squares mean (95% CI)4 |
| Placebo | ||||||||
| MTHFR | rs1537516 | GG (34)5 | GA (8) | AA (0) | Free Cho | Interaction | −0.00714 | — |
| MTHFR | rs17367629 | GG (34) | GA (7) | AA (0) | Free Cho | Interaction | −0.00693 | — |
| MTHFR | rs3753582 | AA (34) | AC (8) | CC (0) | Free Cho | Interaction | 0.007137 | — |
| MTHFR | rs3753588 | GG (34) | GA (8) | AA (0) | Free Cho | Interaction | −0.00714 | — |
| MTHFR | rs6687229 | CC (34) | CT (8) | TT (0) | Free Cho | Interaction | −0.00714 | — |
| Supplement | ||||||||
| PEMT | rs711352 | GG (22) | GC (18) | CC (3) | Betaine | Main effect | — | 2.0 (1.5, 2.7) |
| Placebo plus supplement | ||||||||
| PEMT | rs711352 | GG (51) | GC (30) | CC (3) | Betaine | Main effect | — | 1.6 (1.3, 2.0) |
W, wild type; V, variant; Free Cho, unesterified choline. Pregnant women at <18 wk gestation were enrolled and randomly assigned to receive either a choline supplement (750 mg choline/d) or a placebo (corn oil) from 18 wk pregnancy to 45 d postpartum. Participants were genotyped for 370 SNPs by using lymphocyte DNA. Each SNP was individually tested for an interaction with dietary intake and a main effect on breast-milk and plasma choline and choline metabolite concentrations among placebo subjects, supplement subjects, and all participants combined. Each comparison was carried out via linear regression analysis in 3 different ways as follows: W-W compared with W-V compared with V-V, W-W + W-V compared with V-V, or W-W compared with W-V + V-V. Comparisons were only carried out for SNPs that had a sample size ≥5 for all groups compared. The results from all 3 SNP configurations were entered by using the PROC MULTTEST procedure (SAS version 9.2; SAS Institute, Chicago, IL) simultaneously.
SNPs with a significant interaction or main effect (P < 0.05). Note that the only significant interactions or main effects were in breast milk.
Change in slope indicates how much the slope of the line predicting the effect of total choline intake on metabolite changes when going from W-W to W-V or V-V. A minus sign indicates that the slope for W-V or V-V subjects was smaller than for W-W subjects.
Change in least-squares mean indicates how much the least-squares mean metabolite concentration (in μmol/L) changed when going from W-W to W-V or V-V.
Genotype; number of participants with the genotype in parentheses (all such values).
SNPs that had significant main effects in the placebo and supplement groups, separately and combined, are listed in Table 3. These SNPs (either as W-V or V-V) shifted the response curves of choline intake–breast-milk concentrations up but did not change the slope of the response curves. The 2 SNPs revealed were in the comparisons of the supplement group and of all subjects combined and all occurred in the PEMT gene.
Combinations of SNPs that influence breast-milk and plasma choline and choline metabolite concentrations
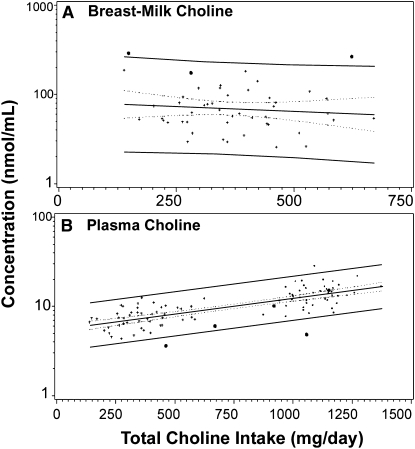
A second exploratory approach identified outliers with values that fell beyond the 95% prediction limits. Variant alleles that these outlier participants had in common (homozygous V-V genotype) were identified. Other participants homozygous for these alleles were also identified and plotted on the intake-concentration curve to visually evaluate their response to choline intake. Two sample plots that used this approach are shown in Figure 3. The first plot (Figure 3A) shows breast-milk choline concentrations compared with total choline intake in placebo subjects. Three participants, identified with bold dots, had 5 SNPs in common: rs1076991, rs2983733, rs2987981, rs8003379, and rs17824591. All of these occurred in the methylene tetrahydrofolate dehydrogenase 1 (MTHFD1) gene. All 3 of these subjects had very high breast-milk choline concentrations; 2 subjects were outliers and the third subject had high concentration-for-intake values. In the second plot of plasma choline (Figure 3B), 5 participants, identified with bold dots, had 2 SNPs in common: rs2461248 (BHMT) and rs7700970 (BHMT). Four of the 5 subjects had lower-than-average plasma choline concentrations, and 2 subjects were outliers. A list of all SNP groupings that have significant effects on choline metabolite concentrations is shown in Table 4.
FIGURE 3.
Outlier approach: participants with breast-milk choline metabolite concentrations that were beyond the 95% prediction limits for the mean shared common single nucleotide polymorphisms (SNPs). A: Plot of breast-milk choline concentrations compared with total choline intake in all participants. Three participants, identified with bold dots, had 5 SNPs in common: rs1076991, rs2983733, rs2987981, rs8003379, and rs17824591. All of these SNPs occurred in the methylene tetrahydrofolate dehydrogenase 1 (MTHFD1) gene. All 3 of these subjects had very high breast-milk choline concentrations, and 2 of these subjects were outliers. A linear regression model was used to compare these 3 subjects with the rest of the subjects. B: Plot of plasma choline concentrations compared with total choline intake in all participants. Five participants, identified with bold dots, had 2 SNPs in common: rs2461248 and rs7700970, both of which were in the BHMT gene. Four of the 5 subjects had lower than average plasma choline concentrations, and 2 of the subjects were outliers. A linear regression model was used to compare these 5 subjects with the rest of the subjects. +, represents participants who did not carry the common set of SNPs.
TABLE 4.
Single nucleotide polymorphisms (SNPs) shared by subjects who were outliers1
| Metabolite | Group | Location of outliers (n) | SNPs shared by outliers (V-V only) | Total number of subjects V-V for set of SNPs | Main effect of this set of SNPs |
| Breast milk | |||||
| Phosphocholine | Placebo | Low (2) | rs1076991 (MTHFD1) | P = 0.0053 | |
| Choline | Placebo | High (2) | rs17824591 (MTHFD1) | P = 0.0032 | |
| rs2983733 (MTHFD1) | |||||
| rs2987981 (MTHFD1) | |||||
| rs8003379 (MTHFD1) | 3 | ||||
| Choline | Supplement | High (2) | rs1275103 (BHMT) | 3 | P = 0.0008 |
| rs955897 (BHMT) | |||||
| rs131759 (CHKB) | |||||
| rs131766 (CHKB) | |||||
| rs140514 (CHKB) | |||||
| rs470117 (CHKB) | |||||
| Plasma | |||||
| Choline | Placebo plus supplement | Low (2) | rs2461248 (BHMT) | 5 | P = 0.0008 |
| rs7700970 (BHMT) | |||||
| Betaine | Placebo | High (2) | rs10131416 (MTHFD1) | 2 | P = 0.0008 |
| rs10145013 (MTHFD1) | |||||
| rs745686 (MTHFD1) | |||||
| rs8016556 (MTHFD1) | |||||
| rs9840079 (CHDH) | |||||
| rs2276840 (CHDH) | |||||
| rs3017 (CHDH) | |||||
| rs4687753 (CHDH) | |||||
| rs1202283 (ABCB4) |
Pregnant women at <18 wk gestation were randomly assigned to receive either a choline supplement (750 mg choline/d) or a placebo (corn oil) from 18 wk pregnancy to 45 d postpartum and were genotyped for 370 SNPs by using lymphocyte DNA. Regression analyses were performed to determine how breast-milk and plasma choline and choline metabolite concentrations were affected by total choline intake within placebo subjects, supplement subjects, and across all subjects combined. Individual participants whose values did not fall within the 95% prediction limits were identified as outliers. We identified the set of SNPs that these upper or lower outliers had in common (considering only the homozygous variant V-V genotype). We identified all subjects who had this set of SNPs (including the outliers) and calculated the main effect of this set of SNPs and the interaction effect between this set of SNPs and total choline intake. The sets of SNPs that had P < 0.01 in a main effect are listed. No set of SNPs had a significant interaction effect.
DISCUSSION
The purpose of this study was to refine our understanding of dietary requirements for choline in women who are lactating, which is a developmental period when adequate choline is critical for optimal infant brain development and mothers themselves are at greater risk of choline deficiency (44). We observed that breast-milk concentrations of choline and its metabolites were influenced by maternal diet and maternal genotype. Perhaps this explains why Zeisel et al (29) and Ilcol et al (45) reported that lactating women have a wide range of concentrations of choline and choline metabolites in their breast milk.
We collected and analyzed 3-d food records at 20 and 30 wk pregnancy and 45 and 90 d postpartum and saw very little intraindividual variation in these estimates (data not shown); thus, the estimate of choline intake measured at 45 d postpartum was a reasonable representation of habitual intake. In our participants, the mean maternal dietary intake of choline (excluding the supplement) was >33% lower than the daily recommended AI for lactating women (24). A number of studies previously reported that dietary choline intake during pregnancy was below the recommended AI (25, 46).
Choline intake from the diet in the placebo group was directly correlated with breast-milk phosphatidylcholine concentrations; phosphatidylcholine in milk is part of the milk fat globule (28). Dietary choline was also directly correlated with plasma choline concentrations. A dietary supplement that contained phosphatidylcholine (delivering 750 mg choline/d) increased choline, betaine, and phosphocholine concentrations in breast milk (Table 2, Figure 1) and increased choline and betaine concentrations in maternal plasma (Table 2, Figure 2). Phosphocholine is the major water-soluble form of choline in milk because choline, once transported into mammary epithelium, is rapidly phosphorylated and, thereby, prevents diffusion back into maternal blood (47). Betaine is formed from choline in the maternal liver and kidney (48). The supplement achieved what appears to be the maximal concentrations of choline metabolites in milk and plasma because the slopes of the intake-concentration curves for these metabolites in the supplemented group were flat (additional choline from the diet did not further increase the metabolite concentrations). The intake-concentration response curves for choline and betaine in milk and plasma had similar slopes for placebo and supplemented groups, and the correlations between intakes and concentrations for these metabolites was most significant when the placebo and supplemented groups were combined (larger n). With the use of this approach, there was a significant correlation between intakes and milk concentrations for phosphocholine, phosphatidylcholine, choline, and betaine. We suggest that the response range for these metabolites is linear from 150 to ≥750 mg intake. Thus, a variation in dietary intake and diet supplementation can alter breast-milk composition.
As discussed earlier, common genetic variations in humans in many of the genes that code for the enzymes of choline and folate metabolism influence the dietary requirement for choline (30, 31). Some of these genetic variations are extremely common and are present in the majority of the population (30). To our knowledge, we are the first to examine whether SNPs in choline or folate metabolism genes influence the composition of breast milk. There have been no published methods for analyzing the effects of SNPs on breast-milk composition. For this reason, we report on 2 different approaches. The first characterized the (response) curves of the diet intake (dose)–breast-milk concentrations and determines whether a given SNP altered the slope of the curve or whether the curve was shifted up or down at all intakes. The second approach identified outliers who fell outside of the 95% CI for the mean dose-response curve and asked whether these individuals shared SNPs in common. Our analyses were complicated by linkage disequilibrium; many SNPs are inherited together and form a haplotype (49, 50). Although we considered individual SNPs in our analyses, the effects on breast-milk metabolites may be the result of interactions between SNPs.
SNPs can have functional effects on metabolism in a number of ways. An exonic SNP that results in a nonsynonymous amino acid substitution could alter the substrate binding site of an enzyme and result in reduced affinity; this SNP would be associated with a decrease in the dose-response curves. An exonic SNP that results in a nonsynonymous amino acid substitution also could result in a reduction in the amount of functioning enzyme protein and reduce the total capacity of the enzyme such that it is saturated at low substrate concentrations and, again, cause a decrease in the slope of the dose-response curves. An SNP in a transcription factor binding site in the gene's promoter region could also reduce the amount of functioning enzyme protein and, thus, decrease the slope of the dose-response curve. An SNP in an inhibitory site in the promoter would have the opposite effect. It would increase the amount of functioning enzyme protein and increase capacity and, thereby, increase the slope of the dose-response curve. If an SNP altered an inhibitory binding site on the protein itself (eg, one that mediates product inhibition of the enzyme), the dose-response curve might shift at all points in the dose-response curve. An SNP that altered the binding of an activating cofactor would likely shift the curves in the opposite direction. We observed that a number of SNPs altered the slope of the diet intake compared with breast-milk concentration curves (Table 3), whereas other SNPs shifted these curves up but did not alter their slope (Table 3).
The effects of some SNPs might be apparent in the placebo group, whereas others might be apparent only in the supplemented group, and combining both groups in analyses might sometimes obscure these effects. For example, some SNPs could alter the Michaelis constant of enzymes and change the shape of the Michaelis-Menten curve such that differences in enzyme activity between wild-type and variant alleles would only be apparent at lower substrate concentrations. These SNPs (enzymes) would look very different at low choline intakes (placebo) but would look the same at high concentrations (supplemented). In contrast, if there are SNPs that affect the maximum enzyme velocity of enzymes and not the Michaelis constant, differences in enzyme activity between wild-type and variant alleles would only be apparent at higher substrate concentrations. These SNPs (enzymes) would look the same at low choline intakes (placebo) but would look very different at high choline intakes (supplemented). For example, such an SNP could occur in a gene that encodes a choline transporter enzyme. If the SNP reduced the function of the enzyme, little difference may be evident between individuals when choline intake is low because the transporter would not be saturated. However, as intake increases and exceeds the capacity of the transporter, the effect of the SNP would become apparent. Combining the placebo and supplement groups for either type of SNP would result in a loss of sensitivity to detect these types of genetic variants.
We only identified one gene, MTHFR, for which SNPs altered the slope of the intake-concentration curve for choline (Table 3). Genetically modified mice with defective methylenetetrahydrofolate reductase (MTHFR) activity become choline deficient (51), and 15–30% of humans have genetic polymorphisms that alter the activity of this enzyme (52, 53). We identified only one gene, PEMT, for which SNPs shifted the intake-concentration curve for breast-milk betaine concentrations upward (Table 3). PEMT catalyzes the de novo biosynthesis of choline in the liver. The PEMT gene is very polymorphic, but only a few functional SNPs have been identified (30, 54, 55). We expected that a functional SNP in PEMT would shift the intake-concentration curve for breast-milk betaine concentrations in the opposite direction from what was observed because individuals with defective PEMT should become choline deficient and make less betaine.
The outlier approach generated groupings of a number of SNPs that were shared by a small number of subjects who fell outside the 95% CI for the mean. For some of these groupings, most or all individuals had changes in a similar direction. We suggest that this approach can be used to generate testable hypotheses about SNP-metabolism relations. Because the number of subjects in the current study was small, further studies that selectively recruit such subjects need to be performed to achieve the needed power to detect significant effects.
This study had a number of limitations. We examined the relation between choline intake and milk metabolite concentrations at only 45 d postpartum, which is when women should be producing mature breast milk. Most, but not all, of the women were exclusively breastfeeding at this time. As discussed, we estimated habitual dietary intake from a series of 3-d food-intake records. The universal use of prenatal vitamins (with folic acid) may have obscured some effects/findings. All breast-milk samples were collected first thing in the morning upon rising (between 0500 and 1000); however, blood was sampled when the participant visited our facility that day, which was generally in the late morning, and the sample was not a fasting sample. We may have detected additional differences with a more stringent protocol. Similarly, if a larger number of subjects were included in the study, we may have identified additional SNPs of interest. Despite these limitations, we made a number of observations that can guide future studies.
In conclusion, we observed that breast-milk concentrations of choline and metabolites can be influenced by diet and dietary supplements. This is important because dietary intake of choline is low relative to the recommended AI in pregnant and lactating women (only 5% of our subjects consumed diets that met or exceeded the AI for choline). We observed that a dietary supplement of phosphatidylcholine (that contained 750 mg choline) had no adverse effects and was well tolerated by pregnant and lactating women. Also, we conducted exploratory studies on the effects that genetic variations had on breast-milk compositions. We observed that the effects of identified SNPs in genes of choline and folate metabolism are complex, but these effects alter the dose (total choline intake)-response (breast-milk or plasma concentration) relation and can significantly change the composition of breast milk. Because these gene variations are extremely common, it may be appropriate to consider them when developing individualized diets or population guidelines.
Acknowledgments
We thank Elena Pop and Zhong Guo for their assistance with the choline analyses and Corneliu Craciunescu for his help with the figures. We also thank the Solae Company, which provided the choline and corn-oil supplements used in this study.
The authors’ responsibilities were as follows—LMF: participated in the supervision of the human study; KAdC: supervised analyses of choline and metabolites, DNA extraction, and SNP assays; BS: oversaw recruitment of subjects into the study, compliance, safety monitoring, and collection of3-d food records; JV: analyzed the 3-d food records; JG and WS: performed statistical computations for data analyses; and SHZ: was responsible for the conceptualization, implementation, and design of the human study, participated in statistical analyses and data interpretation, and provided major input in the writing of the manuscript. SHZ received grant support from Mead Johnson Nutritionals, Balchem, and the Egg Nutrition Research Center for studies other than those described in this study. None of the authors declared a conflict of interest related to this study.
REFERENCES
- 1.Zeisel SH.Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr 1994;14:269–96 [DOI] [PubMed] [Google Scholar]
- 3.Albright CD, Mar MH, Craciunescu CN, Song J, Zeisel SH. Maternal dietary choline availability alters the balance of netrin-1 and DCC neuronal migration proteins in fetal mouse brain hippocampus. Brain Res Dev Brain Res 2005;159:149–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albright CD, Siwek DF, Craciunescu CN, et al. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr Neurosci 2003;6:129–34 [DOI] [PubMed] [Google Scholar]
- 5.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr 2003;133:3614–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res 1999;115:123–9 [DOI] [PubMed] [Google Scholar]
- 7.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res 1999;113:13–20 [DOI] [PubMed] [Google Scholar]
- 8.Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol 1998;79:1790–6 [DOI] [PubMed] [Google Scholar]
- 9.Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res 2000;123:25–32 [DOI] [PubMed] [Google Scholar]
- 10.Jones JP, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res 1999;118:159–67 [DOI] [PubMed] [Google Scholar]
- 11.Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport 1997;8:3053–9 [DOI] [PubMed] [Google Scholar]
- 12.Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 1997;8:2831–5 [DOI] [PubMed] [Google Scholar]
- 13.Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport 1997;8:3045–51 [DOI] [PubMed] [Google Scholar]
- 14.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci 1989;103:1234–41 [DOI] [PubMed] [Google Scholar]
- 15.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol 1988;21:339–53 [DOI] [PubMed] [Google Scholar]
- 16.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res Dev Brain Res 1999;118:51–9 [DOI] [PubMed] [Google Scholar]
- 17.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol 2004;160:102–9 [DOI] [PubMed] [Google Scholar]
- 18.Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology 2006;17:285–91 [DOI] [PubMed] [Google Scholar]
- 19.Shaw GM, Finnell RH, Blom HJ, et al. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology 2009;20;714–9 [DOI] [PubMed] [Google Scholar]
- 20.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 2003;133:1302–7 (Published erratum appears in J Nutr 2003;133:2918–9.) [DOI] [PubMed] [Google Scholar]
- 21.Ridgway ND, Vance DE. Purification of phosphatidylethanolamine N-methyltransferase from rat liver. J Biol Chem 1987;262:17231–9 [PubMed] [Google Scholar]
- 22.Fischer LM, daCosta K, Kwock L, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr 2007;85:1275–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J 2007;21:2622–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine, National Academy of Sciences USA. Choline. : Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients, Institute of Medicine, eds Washington, DC: National Academy Press, 1998:390–422 [PubMed] [Google Scholar]
- 25.Jensen H.Choline in the diets of the US population: NHANES, 2003–2004. FASEB J 2007;21:lb219 [Google Scholar]
- 26.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J Dev Physiol 1986;8:435–45 [PubMed] [Google Scholar]
- 27.Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J Physiol 1985;366:251–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and infant formulas. Am J Clin Nutr 1996;64:572–6 [DOI] [PubMed] [Google Scholar]
- 29.Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr 1986;116:50–8 [DOI] [PubMed] [Google Scholar]
- 30.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J 2006;20:1336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci USA 2005;102:16025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeisel SH, Growdon JH, Wurtman RJ, Magil SG, Logue M. Normal plasma choline responses to ingested lecithin. Neurology 1980;30:1226–9 [DOI] [PubMed] [Google Scholar]
- 33.Jope RS, Domino EF, Mathews BN, Sitaram N, Jenden DJ, Ortez A. Free and bound choline blood levels after phosphatidylcholine. Clin Pharmacol Ther 1982;31:483–7 [DOI] [PubMed] [Google Scholar]
- 34.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7 [DOI] [PubMed] [Google Scholar]
- 35.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74:4734–40 [DOI] [PubMed] [Google Scholar]
- 36.Holmes HC, Snodgrass GJ, Iles RA. The choline content of human breast milk expressed during the first few weeks of lactation. Biochem Soc Trans 1996;24:350S. [DOI] [PubMed] [Google Scholar]
- 37.Fotino M, Merson E, Allen F. Micromethod for rapid separation of lymphocytes from peripheral blood. Ann Clin Lab Sci 1971;1:131–3 [PubMed] [Google Scholar]
- 38.Ting A, Morris P. A technique for lymphocyte preparation from stored heparinized blood. Vox Sang 1971;20:561–3 [DOI] [PubMed] [Google Scholar]
- 39.Shen R, Fan JB, Campbell D, et al. High-throughput SNP genotyping on universal bead arrays. Mutat Res 2005;573:70–82 [DOI] [PubMed] [Google Scholar]
- 40.Cook RD. Detection of influential observations in linear regression. Technometrics 1977;19:15–8 [Google Scholar]
- 41.Lin DY, Wei LJ, Ying Z. Model-checking techniques based on cumulative residuals. Biometrics 2002;58:1–12 [DOI] [PubMed] [Google Scholar]
- 42.White H.A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrics 1980;48:817–38 [Google Scholar]
- 43.Schwarz G.Estimating the dimension of a model. Ann Statist 1978;6:461–4 [Google Scholar]
- 44.Zeisel SH, Mar M-H, Zhou Z-W, da Costa K-A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr 1995;125:3049–54 [DOI] [PubMed] [Google Scholar]
- 45.Ilcol YO, Ozbek R, Hamurtekin E, Ulus IH. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem 2005;16:489–99 [DOI] [PubMed] [Google Scholar]
- 46.Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S. Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J 2005;54:355–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao CK, Pomfret EA, Zeisel SH. Uptake of choline by rat mammary-gland epithelial cells. Biochem J 1988;254:33–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crawford DC, Nickerson DA. Definition and clinical importance of haplotypes. Annu Rev Med 2005;56:303–20 [DOI] [PubMed] [Google Scholar]
- 50.Hinds DA, Stuve LL, Nilsen GB, et al. Whole-genome patterns of common DNA variation in three human populations. Science 2005;307:1072–9 [DOI] [PubMed] [Google Scholar]
- 51.Schwahn BC, Chen Z, Laryea MD, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J 2003;17:512–4 [DOI] [PubMed] [Google Scholar]
- 52.Rozen R.Molecular genetic aspects of hyperhomocysteinemia and its relation to folic acid. Clin Invest Med 1996;19:171–8 [PubMed] [Google Scholar]
- 53.Wilcken DE, Wang XL, Sim AS, McCredie RM. Distribution in healthy and coronary populations of the methylenetetrahydrofolate reductase (MTHFR) C677T mutation. Arterioscler Thromb Vasc Biol 1996;16:878–82 [DOI] [PubMed] [Google Scholar]
- 54.Dong H, Wang J, Li C, et al. The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population. J Hepatol 2007;46:915–20 [DOI] [PubMed] [Google Scholar]
- 55.Song J, da Costa KA, Fischer LM, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J 2005;19:1266–71 [DOI] [PMC free article] [PubMed] [Google Scholar]