Abstract
Background: Unmetabolized serum folic acid (UMFA) has been detected in adults. Previous research indicates that high folic acid intakes may be associated with risk of cancer.
Objective: The objective was to examine UMFA concentrations in relation to dietary and supplemental folate and status biomarkers in the US population aged ≥60 y.
Design: Surplus sera were analyzed with the use of data from the National Health and Nutrition Examination Survey (NHANES) 2001–2002, a cross-sectional, nationally representative survey (n = 1121).
Results: UMFA was detected in 38% of the population, with a mean concentration of 4.4 ± 0.6 nmol/L (median: 1.2± 0.2 nmol/L). The group with UMFA (UMFA+) had a significantly higher proportion of folic acid supplement users than did the group without UMFA (60% compared with 41%). UMFA+ men and women also had higher supplemental and total (food + supplements) folic acid intakes than did their counterparts without UMFA. Forty percent of the UMFA+ group was in the highest quartile of total folic acid intake, but total folic acid intake was only moderately related to UMFA concentrations (r2 = 0.07). Serum folate concentrations were significantly higher in the UMFA+ group and were predictive of UMFA concentrations (r2 = 0.15). Serum 5-methyltetrahydrofolate and vitamin B-12 concentrations were higher in the UMFA+ group, whereas there was no difference between the 2 UMFA groups in red blood cell folate, serum homocysteine, or methylmalonic acid concentrations.
Conclusions: Approximately 40% of older adults in the United States have UMFA that persists after a fast, and the presence of UMFA is not easily explained in NHANES by folic acid intakes alone. Given the possibility that excessive folic acid exposure may relate to cancer risk, monitoring of UMFA may be warranted.
INTRODUCTION
Folic acid is a synthetic compound that when ingested is converted by dihydrofolate reductase to the dihydrofolate and then to the tetrahydrofolate form of folate; these reduced compounds are identical to those that would arise from ingestion of natural folate. However, large oral doses of folic acid can overwhelm this mechanism, and conversion of folic acid to reduced folate is bypassed, which leads to a build-up of folic acid in the serum (1, 2). Unmetabolized serum folic acid (UMFA) does not arise after consumption of naturally occurring folate. Very little is known about the metabolism and biological effects of UMFA. Some have hypothesized that the presence of UMFA may be a contributing factor in safety concerns associated with high intakes of folic acid (3).
Folic acid fortification has increased dietary intakes of folic acid (4) and blood folate in the United States (5). Some (6–10) but not all (11–13) research suggests that high folic acid intakes may promote the growth of preexisting cancers or malignant lesions. Thus, from a public health perspective, continued monitoring of folate status in the US population is essential for ensuring the safety of folic acid fortification (14, 15). However, to date, biomarker data have not been available to determine what concentrations of UMFA are present in the population. In this article, serum concentrations of folic acid are presented and related to folic acid intake from diet and supplements and other relevant biomarkers in a nationally representative sample of older adults in the US population (aged ≥60 y) from the National Health and Nutrition Examination Survey (NHANES) 2001–2002. Currently, these are the only nationally representative data available from NHANES that have both information on dietary exposures to and serum concentrations of folic acid.
SUBJECTS AND METHODS
The NHANES is a nationally representative, cross-sectional survey of the noninstitutionalized US population that uses a complex, stratified, multistage probability cluster sampling design. Data are collected by the National Center for Health Statistics of the Centers for Disease Control and Prevention. Survey participants are first interviewed in their homes; at this household interview, demographic information, dietary supplement use, and some health-related data are collected. Participants then complete a physical examination, 24-h dietary recall, and blood draw in a mobile examination center (MEC) ≈1–2 wk after the household interview. A surplus sera project analyzed UMFA concentrations in NHANES 2001–2002 participants (n = 1330 individuals aged ≥60 y). The unweighted examination response rate for all participants aged ≥60 y, calculated as the number of participants divided by the total number selected in the sample, was 75% for the interview component and 65% for the examination component in NHANES 2000–2001. Written informed consent was obtained from all participants or proxies, and the survey protocol was approved by the Research Ethics Review Board of the National Center for Health Statistics (Hyattsville, MD).
A data dictionary of all NHANES variables that were used in this analysis and more detail on exclusion criteria are provided in Supplementary Table 1 under “Supplemental data” in the online issue. Individuals were excluded who had high creatinine concentrations (>131 μmol/L for men, >115 μmol/L for women; n = 29), high alanine amino transferase concentrations >40 units/L (n = 59), self-reported anemia therapy within the past 3 mo (n = 39), or who reported the use of folate-blocking cancer therapies (Drug Class 20, Level 23 medications; n = 4). From this sample, we removed individuals with incomplete dietary recall information (n = 54), and then excluded those who reported taking a folic acid–containing dietary supplement in the fasting time period before the blood draw (n = 24). The final analytic sample was 1121.
A single 24-h dietary recall was administered to each participant by trained NHANES staff in the MEC. Dietary variables that were estimated included dietary folic acid (in μg) and dietary folate (expressed as dietary folate equivalents) (16). Dietary supplement information was collected via the NHANES Dietary Supplement Questionnaire, which was used to determine a sample person's use of vitamins, minerals, herbs, and other dietary supplements over the past 30 d. Detailed information about name and type, consumption frequency, duration of use, and amount taken was collected for each reported dietary supplement. The average daily intake of folic acid from all dietary supplements was calculated for individuals by using the number of days that consumption of the supplement was reported, the reported amount taken per day, and the serving size unit from the product label (17). The average folic acid from supplemental sources was combined with folic acid intake from fortified foods in the diet to reflect total folic acid exposure.
Biochemical methods
Details on fasting before blood draw were collected via questionnaire at the MEC before blood draw. The length of time reported for fasting from food and dietary supplements and session of blood draw (morning, afternoon, or evening) were used in this analysis. UMFA and 5-methyltetrahydrofolate (5-methylTHF) concentrations were determined as part of a surplus sera project in participants aged ≥60 y by using a revised affinity/HPLC method with electrochemical (coulometric) detection (J Sehlub, personal communication, 18 February 2010; 18, 19). The level of detection (LOD) for UMFA was 0.18 and 0.034 nmol/L for 5-methylTHF; values below the LOD are replaced with a zero value before public release of the data. The sum of UMFA and 5-methylTHF represents total serum folate from the HPLC analysis (serum folate-HPLC).
Other biochemical variables determined as part of the full NHANES 2001–2002 survey period were as follows: serum folate, red blood cell (RBC) folate, and serum vitamin B-12 (by using the Quantaphase II radioassay from BioRad, Hercules, CA); plasma homocysteine (by using a fluorescence polarization immunoassay reagent set from Abbott Laboratories, Abbott Park, IL); and plasma methylmalonic acid (by using gas chromatography–mass spectrometry). Serum cotinine was measured by an HPLC/atmospheric-pressure ionization tandem mass spectrometry method. Complete details and documentation for each of these methods are publically available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm).
Statistical analysis
All statistical analyses were performed by using SAS (version 9; SAS Institute Inc, Cary, NC) and SAS-callable SUDAAN (version 9; Research Triangle Institute, Research Triangle Park, NC) software. Sample weights were used to account for differential nonresponse and noncoverage and to adjust for the planned oversampling of some groups. Any variables with a skewness >4 were log-transformed before group comparison analysis. The range of UMFA was quite large (0–273 nmol/L); the highest 2 data points (273 and 185 nmol/L) were extreme outliers and were winsorized to the next highest value of 85 nmol/L to remove the influence that these points would exert (20).
Descriptive statistics (means and medians) were estimated for all variables by using PROC REG and PROC DESCRIPT in SAS-callable Sudaan. Small sample sizes precluded examination by racial-ethnic groups. Two groups were constructed: those without (UMFA−) and those with (UMFA+) detectable concentrations of UMFA in the serum. The UMFA− and UMFA+ groups were examined by contrasts and by sex and age subgroup characteristics between groups. The linear trend for the percentage of each UMFA group per quartile of intakes of 5-methylTHF, RBC folate, and total folic acid and serum folate were examined. To achieve this, the percentage of each UMFA group per quartile was estimated by using SUDAAN's CROSSTAB procedure, along with the covariance matrix of the estimates. The contrast to test the linear trend was then computed, along with its associated SE, by using the SAS/IML matrix manipulation procedure. All statistical comparisons were controlled for sex, age, and race-ethnicity and presented as least-squares means. All group comparisons of biochemical measures were also controlled for duration of food fasting (in h) before blood draw and session of sample collection. SEs for all statistics of interest were approximated by Taylor series linearization, and significance was set at a Bonferroni-adjusted P value ≤ 0.006 to account for multiple comparisons.
RESULTS
The mean age of the study population was 70 y, and 58% were women (Table 1). Use of a dietary supplement containing folic acid was reported by 47% of the population and was significantly higher in women (50%) than in men (43%) (P ≤ 0.006, data not shown). UMFA was detected in 38% of the population, with a median of 1.2 ± 0.2 nmol/L and a mean concentration of 4.4 ± 0.6 nmol/L (Table 1). The predominant circulating form of folate in the serum was 5-methylTHF. UMFA represented ≈2.25% of serum folate in the population (ie, UMFA+ and UMFA− combined); however, in the UMFA+ group, UMFA represented ≈6% of serum folate–HPLC.
TABLE 1.
Unmetabolized serum folic acid (UMFA) and its relation to selected demographic, dietary, and biomarker variables presented by participants without (UMFA−) and with (UMFA+) UMFA for adults aged ≥60 y in the United States (2001–2002)1
| Variables | All subjects (n = 1121) | UMFA− (n = 756) | UMFA+ (n = 365) |
| Demographic | |||
| Age (y) | 70 ± 0.5 | 71 ± 0.3 | 70 ± 0.5 |
| Male (%) | 42 ± 2 | 44 ± 2 | 38 ± 2 |
| Non-Hispanic white (%) | 82 ± 1 | 80 ± 1 | 86 ± 1 |
| Folic acid supplement users (%) | 47 ± 2 | 40 ± 2 | 61 ± 3* |
| Dietary | |||
| Dietary folic acid (μg) | 178 ± 8 | 164 ± 11 | 202 ± 10 |
| Dietary total folate (DFEs) | 517 ± 16 | 489 ± 21 | 564 ± 18 |
| Supplemental folic acid (μg) | 175 ± 10 | 140 ± 11 | 235 ± 21* |
| Total folic acid (μg)2 | 353 ± 14 | 303 + 17 | 437 ± 20* |
| Total energy-adjusted folic acid (μg) | 105 ± 5 | 99 ± 7 | 113 ± 6 |
| Biomarker3 | |||
| Serum UMFA (nmol/L) | 1.7 ± 0.2 | — | 4.4 ± 0.6 |
| Serum 5-methyltetrahydrafolate (nmol/L) | 49.3 ± 1.0 | 45.7 ± 1.3 | 55.2 ± 1.6* |
| Red blood cell folate (nmol/L) | 831 ± 16 | 810 ± 24 | 866 ± 17 |
| Serum folate (nmol/L) | 42 ± 0.9 | 37 ± 0.9 | 51 ± 2.6* |
| Serum vitamin B-12 (pmol/L) | 402 ± 14 | 384 ± 17 | 432 ± 12* |
| Serum methylmalonic acid (μmol/L) | 0.19 ± 0.1 | 0.20 ± 0.01 | 0.18 ± 0.01 |
| Serum homocysteine (μmol/L) | 10.4 ± 0.2 | 10.6 ± 0.1 | 10.2 ± 0.4 |
| Serum cotinine (ng/mL) | 33.1 ± 3.6 | 34.3 ± 4.2 | 31.0 ± 6.5 |
All values are means or percentages ± SEs. DFEs, dietary folate equivalents. For all variables other than those presented under Demographics, the results were controlled for sex, age, and race-ethnicity. Reasons for exclusion were elevated concentrations of serum creatinine or alanine amino transferase, self-reported anemia, use of folate-blocking cancer therapy, missing or incomplete dietary data, or self-reported folic acid supplement use in the fasting period before blood draw. *Group differences were significant at P ≤ 0.006 (Bonferroni-adjusted for multiple comparisons).
Total folic acid represents the sum of dietary and supplemental consumption.
All values were controlled for session of blood collection and length of fast before blood draw. Significance level was obtained from variables in the log-transformed scale. UMFA and 5-methyltetrahydrafolate in serum were measured by using affinity/HPLC with electrochemical (coulometric) detection. Serum folate, red blood cell folate, and serum vitamin B-12 were assessed by radioassay, and plasma homocysteine was assessed by immunoassay. Plasma methylmalonic acid was measured by gas chromatography–mass spectrometry. Serum cotinine was analyzed by an HPLC/atmospheric-pressure ionization tandem mass spectrometry method.
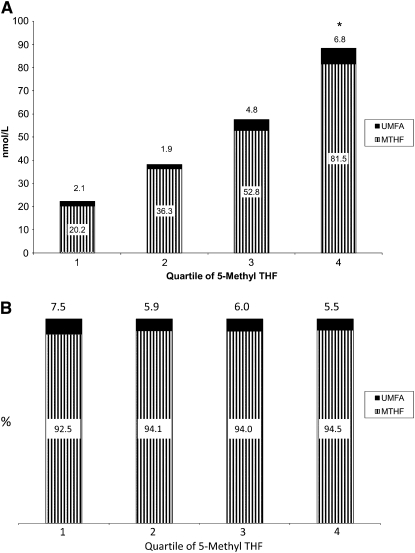
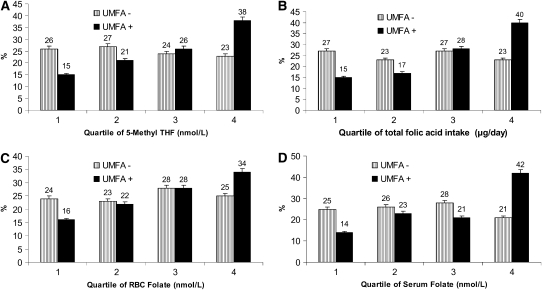
In the UMFA+ group, the percentage contribution of folic acid in the serum did not vary by quartile of 5-methylTHF concentration. UMFA was between 5.5% and 7.5% of the serum folate–HPLC; however, those in the highest quartile of 5-methylTHF had a significantly higher mean UMFA concentration (Figure 1, A and B). Those who had no detectable UMFA in their serum were approximately equally distributed across quartiles of 5-methylTHF concentrations (Figure 2A; quartile 1, 26%; quartile 2, 27%; quartile 3, 24%; and quartile 4, 23%). However, the distribution of those having detectable UMFA in their serum increased with increasing quartiles of 5-methylTHF concentrations (quartile 1, 15%; quartile 2, 21%; quartile 3, 26%; and quartile 4, 38%; P for trend < 0.006). We observed the same pattern when we analyzed the distribution of those in the UMFA− and UMFA+ groups across quartiles of the remaining variables, with increasing trends in the UMFA+ groups [total folate intake: quartile 1, 15%; quartile 2, 17%; quartile 3, 28%; and quartile 4, 40%; P for trend < 0.006 (Figure 2B); RBC folate concentrations: quartile 1, 16%; quartile 2, 22%; quartile 3, 28%; and quartile 4, 34%; P for trend < 0.006 (Figure 2C); and serum folate concentrations: quartile 1, 11%; quartile 2, 23%; quartile 3, 21%; and quartile 4, 42%; P for trend < 0.006 (Figure 2D)].
FIGURE 1.
Mean (A) and percentage concentrations (B) of unmetabolized serum folic acid (UMFA) and serum 5-methyltetrahydrofolate (MTHF, 5-methylTHF) in US adults aged ≥60 y with detectable UMFA by 5-methylTHF quartile. Data are from the National Health and Nutrition Examination Survey (http://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Reasons for exclusion were elevated concentrations of serum creatinine or alanine amino transferase, self-reported anemia, use of folate-blocking cancer therapy, missing or incomplete dietary data, or self-reported folic acid supplement use in the fasting period before blood draw. UMFA and 5-methylTHF in serum were measured by using affinity/HPLC with electrochemical (coulometric) detection. *Quartile 4 was significantly different from other quartiles, P ≤ 0.006 (Bonferroni-adjusted for multiple comparisons). Numbers above each bar represent the black portion of that bar.
FIGURE 2.
The percentage of US adults (aged ≥60 y) without detectable concentrations of unmetabolized serum folic acid (UMFA−) and with detectable concentrations of UMFA (UMFA+) by quartiles of serum 5-methyltetrahydrofolate (5-Methyl THF) concentration (A), quartiles of total folic acid intake (B), red blood cell (RBC) folate concentration (C), and serum folate concentration (D). Data are from the National Health and Nutrition Examination Survey (http://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Reasons for exclusion were elevated serum creatinine or alanine amino transferase concentration, self-reported anemia, use of folate-blocking cancer therapy, missing or incomplete dietary data, or self-reported folic acid supplement use in the fasting period before blood draw. UMFA and 5-methylTHF in serum were measured by using affinity/HPLC with electrochemical (coulometric) detection. Serum folate and RBC folate were assessed by radioassay. There was a significant P for trend across quartiles of 5-methylTHF (A), total folic acid intake (B), RBC folate (C), and serum folate (D). P ≤ 0.006 across quartiles for the UMFA+ group (Bonferroni-adjusted).
As shown in Table 1, the UMFA+ group contained a significantly higher proportion of folic acid dietary supplement users than did the UMFA− group. However, intakes of folic acid and folate from foods alone did not significantly differ between the 2 UMFA groups. Dietary supplement intakes and total folic acid intakes (food + supplements) were significantly higher in the UMFA+ group. However, the relation between UMFA and total folic acid intake was not strong when the adjusted log-log plot was examined in the entire population (ie, UMFA+ and UMFA− combined; r2 = 0.07) or in the UMFA+ group (r2 = 0.08). The UMFA+ group did not differ from the UMFA− group with regard to RBC folate concentrations, and there was no significant relation between UMFA and RBC folate concentration among the UMFA+ group (log-log plot, adjusted r2 = 0.03). Serum folate concentrations were significantly higher in the UMFA+ group (Table 1), and serum folate concentrations were predictive of UMFA concentrations (adjusted r2 = 0.15) (see Supplementary Figure 1 under “Supplemental data” in the online issue). Serum 5-methylTHF and vitamin B-12 concentrations were higher in the UMFA+ group, whereas there was no difference between the 2 UMFA groups for plasma homocysteine or methylmalonic acid. Smoking status as assessed by serum cotinine did not differ between the UMFA groups.
We evaluated selected characteristics of the UMFA− and UMFA+ groups within sex and age groups (Table 2). No significant differences were observed between UMFA+ and UMFA− men with regard to their folic acid intakes or serum 5-methylTHF concentrations. UMFA+ women had significantly higher dietary supplement and total folic acid intakes than UMFA− women. Among those aged ≤70 y, those with UMFA had higher supplemental and total folic acid intakes and higher 5-methylTHF concentrations than did UMFA− individuals aged ≤70 y. Among those aged >70 y with UMFA, higher dietary, supplemental, and total folic acid intakes were observed, and higher 5-methylTHF concentrations were noted than in those without UMFA.
TABLE 2.
Folic acid intake from diet, dietary supplements, and diet and supplements combined (total); serum 5-methyltetrahydrofolate (5-methylTHF) and unmetabolized serum folic acid (UMFA) concentrations; and detection rates of UMFA stratified by the presence (+) and absence (−) of UMFA by sex and age in US adults aged ≥60 y (2001–2002)1
| Folic acid intake |
Serum2 |
||||||
| n | Diet | Supplements | Total3 | 5-methylTHF | UMFA4 | Detection | |
| μg | nmol/L | nmol/L | % | ||||
| Entire group | 1120 | 178 ± 8 | 175 ± 10 | 353 ± 14 | 49.2 ± 1.0 | 1.2 ± 0.2 | 38 ± 2 |
| UMFA− | |||||||
| Men | 379 | 181 ± 11 | 134 ± 13 | 315 ± 20 | 43.2 ± 1.4 | — | — |
| Women | 376 | 150 ± 13 | 141 ± 14 | 291 ± 17 | 47.5 ± 1.7 | — | — |
| Age | |||||||
| ≤70 y | 385 | 172 ± 16 | 135 ± 13 | 308 ± 23 | 44.0 ± 1.4 | — | — |
| >70 y | 370 | 153 ± 10 | 140 ± 15 | 293 ± 17 | 47.8 ± 1.9 | — | — |
| UMFA+ | |||||||
| Men | 163 | 225 ± 21 | 211 ± 38 | 437 ± 39 | 49.5 ± 2.1 | 1.23 ± 0.14 | 34 ± 3 |
| Women | 202 | 190 ± 16 | 256 ± 16* | 446 ± 20* | 59.2 ± 2.3* | 1.18 ± 0.18 | 39 ± 3 |
| Age | |||||||
| ≤70 y | 154 | 189 ± 11 | 256 ± 36* | 444 ± 36* | 51.9 ± 2.2* | 1.07 ± 0.16 | 36 ± 3 |
| >70 y | 211 | 221 ± 21* | 221 ± 25* | 441 ± 27* | 59.5 ± 1.5* | 1.51 ± 0.19 | 41 ± 3 |
All values are means ± SEs unless otherwise noted. Reasons for exclusion were elevated concentrations of serum creatinine or alanine amino transferase, self-reported anemia, use of folate-blocking cancer therapy, missing or incomplete dietary data, or self-reported folic acid supplement use in the fasting period before blood draw. UMFA− and UMFA+ groups were compared within sex and age groups by using contrasts; sex comparisons were controlled for race-ethnicity and age, and age comparisons were controlled for sex and race-ethnicity. *Group differences were significant at P ≤ 0.006 (Bonferroni-adjusted for multiple comparisons).
UMFA and 5-methylTHF in serum were measured by using affinity/HPLC with electrochemical (coulometric) detection. All values were controlled for session of blood collection and length of fast before blood draw.
Values are for users and nonusers of folic acid dietary supplements combined.
Values are medians ± SEs.
DISCUSSION
UMFA was detected in 38% of older adults in this analysis of a nationally representative sample of older adults in the United States who had fasted for a mean of 10 h. Little is known about how UMFA might alter folate pathways or what other effects it has on organs whose receptors transport folic acid without it being metabolized to natural forms of folate. It is currently unknown if UMFA can be used as a meaningful biomarker of folic acid intake or if it is related to any functional outcomes.
Some small, acute-dosing, short-term experimental studies raise several issues (2, 21, 22). First, Kelly et al (2) suggested that the appearance of UMFA is affected by the size of the dose (with a threshold of ≈200 μg). Our data do not identify a specific level or range of dietary intake of folic acid for which UMFA begins to appear in the serum. Although total folic acid intakes were, on average, higher in the UMFA+ group (437 ± 20 μg/d) than in the UMFA− group (303 ± 17 μg/d), our data showed that 15% of the UMFA+ group were in the lowest quartile of total folic acid intake (0–100 μg) and 23% of the UMFA− group were in the highest quartile of total folic acid intake. Thus, there is considerable overlap in folic acid intakes between the 2 groups.
A second finding of the small experimental studies is that there is a dose-frequency interaction—ie, smaller doses consumed more frequently result in higher UMFA concentrations than larger doses consumed less frequently (21). The second issue is whether the supplements with their generally high-dose, single-exposure pattern of exposure are associated with an UMFA pattern that differs between supplement users and nonusers. Our data show that although supplement use and supplemental folic acid intakes were higher in UMFA+ group (235 ± 21 μg/d) than in the UMFA− group (140 ± 11 μg/d), 40% of the UMFA− group were supplement users and 39% of the UMFA+ group were not supplement users. Thus, there is a trend for supplements to be associated with higher UMFA concentrations, but supplement use alone is an insufficient factor in determining UMFA concentrations.
Third, although the experimental studies observed a Tmax (the time after administration when the maximum plasma concentration is reached) for concentrations of UMFA at 2.25 h for fortified bread and at 80 min for folic acid in saline solutions (2), population-based studies also observed a persistence of UMFA in a high percentage of persons even after ≥8 h of fasting (22, 23). Although our study population had fasted for a mean of 10 h, UMFA was detected in 38%. A clear pattern for the length of fasting period from foods and the amount of UMFA was not evident (r = 0.09). However, among those who were excluded for consuming folic acid dietary supplements in the fasting period before blood draw (n = 32), the mean (±SE) UMFA was 9.6 ± 2.9 nmol/L.
Higher detection rates have been observed in other studies of UMFA. Cohorts in the Framingham Offspring study (aged 29–86 y) showed an UMFA+ detection rate of 67% in subjects who fasted ≥10 h, with higher detection rates in supplement users than in non–supplement users (22). Troen et al (23) reported a 78% detection rate in US postmenopausal women participating in a study after an overnight fast. Sweeney et al (2009) reported UMFA in 90% (18/20) of fasted, unsupplemented Irish mothers before cesarean surgery, and 85% (17/20) of their infants had UMFA in cord blood (3).
The reasons for these highly variable detection rates are difficult to ascertain. The study groups differed in age and sex. The NHANES population is selected via a complex sampling plan to represent the US population; these individuals likely differ in many ways from the participants in the observational and experimental studies. The studies also differ in the analytic measurement procedures used to assay the UMFA and vary in the LODs that were used. The Irish studies with detection rates of 85% and 90% used an assay method with an LOD of 0.71 nmol/L. The LOD for the Troen et al (23) and Kalmbach et al (22) studies was 0.18 nmol/L, and thee authors reported 78% and 67% detection rates, respectively. The 38% detection rate for the current study was obtained by using a method with an LOD of 0.18 nmol/L. Thus, LODs and detection rates for the cited studies do not show an inverse relation; therefore, differences in the assay LOD appear not to be a likely reason for differences in the UMFA detection rates.
A potentially important reason for these differences between detection rates and the absence of a threshold intake effect may be that the UMFA+ group represents a sensitive subpopulation who may have altered metabolism of folic acid. When comparing the percentage distribution of persons from the UMFA− and UMFA+ groups by quartile of serum folate biomarkers and total folic acid intakes, there was a significant linear trend only for the UMFA+ group but not the UMFA− group. However, there was also considerable overlap between the 2 groups across quartiles of these variables. This suggests that the UMFA+ group may be responding differently than the UMFA− group to ingested folic acid and therefore differs in these indicators of folate status. Genetic differences are likely a critical factor in determining how folic acid is metabolized. Bailey and Ayling (24) suggested that humans have an extremely slow and variable activity of liver dihydrofolate reductase; therefore, individuals who possess lower than average activity may have difficulty converting high intakes of folic acid to biologically active forms. Kalmbach et al (22) suggested that the ability to handle high and low intakes of folic acid is affected by a 19-bp deletion polymorphism in dihydrofolate reductase (25). Because the NHANES is representative of the US population, there is likely greater heterogeneity in our study population with respect to race-ethnicity, health status, and other factors that may influence UMFA concentrations than would be found in the studies published to date. Thus, our results may differ from previous reports because our population is more diverse genetically and differs in other factors affecting folate status.
A report on UMFA and its associations with anemia, macrocytosis, and cognition using 4 y of combined NHANES data (1999–2002) is now available (26). UMFA data are also available for NHANES 1999–2000, but only data on dietary folate (not folic acid) are available for these years. Thus, a limitation of our analysis is that it only reports on a 2-y cycle of NHANES 2001–2002 because this is the only available data set with folic acid intakes from the diet. For NHANES 2001–2002, only one 24-h recall was available; the 1-d mean of nutrient intake from a 24-h recall is not an ideal measure of usual intake (27). An additional potential shortcoming is the limited information on the performance of the HPLC method to determine UMFA. Information on how this modified method—or any other method measuring UMFA—performs against the Standard Reference Material 1955 (28, 29) from the National Institute of Standards and Technology would be of great value to better assess how comparable data from different methods are. UMFA is a novel biomarker, and critical examination of this and other studies assay techniques is necessary before UMFA concentrations can be meaningfully interpreted. No standard reference method for quantifying folic acid in the serum or plasma exists; and we were unable to find documentation of the accuracy of currently available methods against the Standard Reference Material 1955. Without such quality controls, it is difficult to compare the results of different methods of analysis between laboratories or over time. Finally, whereas folic acid is generally regarded as stable, little is known regarding the effect of long-term storage of folic acid.
This is the first report of UMFA in a nationally representative US sample; ≈40% of older adults in the United States have UMFA that persists after a fast. The variability in the presence of UMFA is not entirely explained by folic acid intakes in this population, suggesting that genetic differences may be a contributing determinant. Clearly, more controlled studies are needed to determine the factors associated with circulating UMFA in fortified populations. Given the possibility that excessive folic acid exposure may relate to adverse effects such as cancer events for some individuals, understanding the association between intake (dietary and supplemental) and serum UMFA is important, particularly in vulnerable population groups such as the elderly.
Supplementary Material
Acknowledgments
The authors' responsibilities were as follows—RLB, JLM, EAY, JTD, CTS, and MFP: contributed to concept development and manuscript preparation; KWD and JJG: contributed to methodologic and statistical aspects of the work; and CMP and JMB: contributed to the analytic methods interpretations of this work. All authors reviewed the manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Lucock M, Wild J, Smithells R, Hartley R. Biotransformation of pteroylmonoglutamic acid during absorption: implications of Michaelis-Menten kinetics. Eur J Clin Nutr 1989;43:631–5 [PubMed] [Google Scholar]
- 2.Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr 1997;65:1790–5 [DOI] [PubMed] [Google Scholar]
- 3.Sweeney MR, Staines A, Daly L, et al. Persistent circulating unmetabolised folic acid in a setting of liberal voluntary folic acid fortification. Implications for further mandatory fortification? BMC Public Health 2009;9:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr 2005;24:266–74 [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988 2004. Am J Clin Nutr 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 6.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297:2351–9 [DOI] [PubMed] [Google Scholar]
- 7.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev 2007;16:1325–9 [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst 2009;101:432–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch S, Sanchez H, Albala C, et al. Colon cancer in Chile before and after the start of the flour fortification program with folic acid. Eur J Gastroenterol Hepatol 2009;21:436–9 [DOI] [PubMed] [Google Scholar]
- 10.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 2004;80:1123–8 [DOI] [PubMed] [Google Scholar]
- 11.Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA 2008:2012–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med 1998;129:517–24 [DOI] [PubMed] [Google Scholar]
- 13.Oaks BM, Dodd KW, Meinhold CL, Jiao L, Church TR, Stolzenberg-Solomon RZ. Folate intake, post-folic acid grain fortification, and pancreatic cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr 2010;91:449–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rader JI, Yetley EA. Nationwide folate fortification has complex ramifications and requires careful monitoring over time. Arch Intern Med 2002;162:608–9 [DOI] [PubMed] [Google Scholar]
- 15.Yetley EA, Rader JI. Modeling the level of fortification and post-fortification assessments: U.S. experience. Nutr Rev 2004;62:S50–9; discussion, S60–1 [DOI] [PubMed] [Google Scholar]
- 16.Food and Nutrition Board Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 17.Bailey RL, Dodd KW, Gahche JJ, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr 2010;91:231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagley PJ, Selhub J. Analysis of folate form distribution by affinity followed by reversed-phase chromatography with electrical detection. Clin Chem 2000;46:404–11 [PubMed] [Google Scholar]
- 19.National Center for Health Statistics National Health and Nutrition Examination Survey. Laboratory methods, 2001–2002. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2001-2002/nhanes01_02.htm (cited 19 March 2010)
- 20.Afifi AA, Clark V. Computer-aided multivariate analysis. 3rd ed New York, NY: Chapman & Hall/CRC, 1996 [Google Scholar]
- 21.Sweeney MR, McPartlin J, Weir DG, Daly L, Scott JM. Postprandial serum folic acid response to multiple doses of folic acid in fortified bread. Br J Nutr 2006;95:145–51 [DOI] [PubMed] [Google Scholar]
- 22.Kalmbach RD, Choumenkovitch SF, Troen AM, D'Agostino R, Jacques PF, Selhub J. Circulating folic acid in plasma: relation to folic acid fortification. Am J Clin Nutr 2008;88:763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr 2006;136:189–94 [DOI] [PubMed] [Google Scholar]
- 24.Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci USA 2009;106:15424–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalmbach RD, Choumenkovitch SF, Troen AP, Jacques PF, D'Agostino R, Selhub J. A 19-base pair deletion polymorphism in dihydrofolate reductase is associated with increased unmetabolized folic acid in plasma and decreased red blood cell folate. J Nutr 2008;138:2323–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr 2010;91:1733–44 [DOI] [PubMed] [Google Scholar]
- 27.Dodd KW, Guenther PM, Freedman LS, et al. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc 2006;106:1640–50 [DOI] [PubMed] [Google Scholar]
- 28.Nelson BC, Satterfield MB, Sniegoski LT, Pfeiffer CM, Welch MJ. Simultaneous quantification of homocysteine and folate in human serum or plasma using liquid chromatography/tandem mass spectrometry. Anal Chem 2005;77:3586–93 [DOI] [PubMed] [Google Scholar]
- 29.Nelson BC, Pfeiffer CM, Margolis SA, Nelson CP. Solid phase extraction-electrospray ionization mass spectrometry for the quantification of folate in human plasma or serum. Anal Biochem 2004;325:41–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.