Abstract
Pancreatic islet β-cells contain synaptic-like microvesicles (SLMVs). The origin, trafficking, and role of these SLMVs are poorly understood. In neurons, synaptic vesicle (SV) biogenesis is mediated by two different cytosolic adaptor protein complexes, a ubiquitous AP-2 complex and the neuron-specific AP-3B complex. Mice lacking AP-3B subunits exhibit impaired GABAergic (inhibitory) neurotransmission and reduced neuronal vesicular GABA transporter (VGAT) content. Since β-cell maturation and exocytotic function seem to parallel that of the inhibitory synapse, we predicted that AP-3B-associated vesicles would be present in β-cells. Here, we test the hypothesis that AP-3B is expressed in islets and mediates β-cell SLMV biogenesis. A secondary aim was to test whether the sedimentation properties of INS-1 β-cell microvesicles are identical to those of bona fide SLMVs isolated from PC12 cells. Our results show that the two neuron-specific AP-3 subunits β3B and μ3B are expressed in β-cells, the first time these proteins have been found to be expressed outside the nervous system. We found that β-cell SLMVs share the same sedimentation properties as PC12 SLMVs and contain SV proteins that sort specifically to AP-3B-associated vesicles in the brain. Brefeldin A, a drug that interferes with AP-3-mediated SV biogenesis, inhibits the delivery of AP-3 cargoes to β-cell SLMVs. Consistent with a role for AP-3 in the biogenesis of GABAergic SLMV in β-cells, INS-1 cell VGAT content decreases upon inhibition of AP-3 δ-subunit expression. Our findings suggest that β-cells and neurons share molecules and mechanisms important for mediating the neuron-specific membrane trafficking pathways that underlie synaptic vesicle formation.
Keywords: adaptin, β-cells, adaptor protein-3, β-NAP, vesicular GABA transporter
the pancreatic islet β-cells, by virtue of the architecture of their secretory apparatus and their pathway of differentiation, have much in common with neurons (1, 36, 46, 49, 52). In neurons, the synaptic vesicles (SVs) store neurotransmitters in the presynaptic compartment. β-Cells contain small vesicles, termed synaptic-like microvesicles (SLMVs), that are distinct from the insulin granules and resemble SVs (35, 49).
The biogenesis and trafficking of the SLMVs in β-cells are poorly understood. Since β-cell SLMVs carry the inhibitory neurotransmitter GABA, one benefit of their better characterization will be an increased knowledge of the β-cell GABAergic signaling machinery (20, 35, 55). In autoimmune diseases of the nervous system, nerve terminal proteins are especially susceptible to autoimmune attack, and in type 1 diabetes, shared β-cell nerve terminal proteins are major early targets of autoimmunity (6, 27, 45, 54). Since an important characteristic of both nerve terminal and β-cell autoantigens is their tendency to be vesicle-associated proteins, increased knowledge of the β-cell SLMVs may provide new insights into the pathogenesis of islet autoimmunity (16, 24, 32, 45, 51).
Outside the central nervous system (CNS), the pancreatic β-cells are the only tissue with significant expression of the enzyme GAD65, which catalyzes the synthesis of GABA (8). GAD65 binds to the surface of SVs/SLMVs and is both a neural and β-cell autoantigen (27). We determined previously that β-cells also express the SV-associated vesicular GABA transporter VGAT (vesicular GABA transporter; also referred to as vesicular inhibitory amino acid transporter) (9, 47). Overall, β-cells express all of the machinery necessary for GABA synthesis, secretion, and signaling (8, 20, 47). More recently, we found that β-cells express cell-surface synaptic cleft proteins critical for the maturation and continuing function of inhibitory pre- and postsynaptic terminals (46). If, as we believe, the β-cell secretory machinery develops and functions in a manner parallel to that of the inhibitory synapse, a pathway of SV biogenesis that regulates the composition of GABAergic nerve terminals in the CNS would be predicted to be operative in the β-cell.
Adaptor protein (AP) complexes are involved in the biogenesis and cargo selection of a variety of transport vesicles in the secretory and endocytic pathways (3). Four adaptor complexes have been described (AP-1, AP-2, AP-3, and AP-4). Each is comprised of four subunits (called adaptins): a large α-, γ-, δ-, or ϵ-subunit, a large β-subunit, a medium μ-subunit, and a small σ-subunit (3). SV biogenesis is driven primarily by AP-2 complexes via clathrin-mediated endocytosis from the plasma membrane; however, SV formation also occurs at endosomes by means of an AP-3-mediated mechanism (2, 31). Several subunits of the AP-3 complex are encoded by alternative genes. The β3A-, μ3A-, and σ3-subunits are expressed ubiquitously and play a role in the biogenesis of lysosomes and other specialized secretory organelles. The β3B-subunit (also referred to as β-NAP or AP-3B2) and μ3B (AP-3M2), which are unique to the AP-3B adaptor protein complex, are expressed exclusively in neural tissue and are important for the formation and function of a distinct subset of synaptic vesicles, including GABAergic SVs (29, 31, 44).
Mice with brains lacking AP-3B have a neural phenotype consistent with defective SV assembly (29, 31, 43). Manifestations of synaptic dysfunction include epileptic seizures, increased locomotor activity, and electroencephalographic abnormalities. This neural phenotype has been observed in mice lacking expression of the β3B-, δ-, and μ3B-subunits (29, 31, 43). Defective GABAergic signaling is an important component of the underlying synaptic dysfunction, as has been revealed by investigations of the brain of the μ3B-knockout mouse (29). Of note, reduced levels of VGAT were observed in the hippocampus, likely due to impaired formation of GABAergic SVs (29, 43).
Because of the similarities between β-cells and neurons, most notably β-cell expression of VGAT and other synaptic proteins, we hypothesized that β-cells would express the neuronal AP-3B subunits and that AP-3B would participate in the biogenesis of SLMVs within β-cells. Here, we demonstrate β-cell-specific expression of the neuronal AP-3 subunits. We show that INS-1 β-cell SLMVs have sedimentation properties identical to PC12 SLMVs and that SLMV formation in INS-1 β-cells is sensitive to brefeldin A (BFA), much like the formation of SLMVs by AP-3-dependent mechanisms in PC12 cells. Reduced levels of VGAT are observed after treatment of INS-1 β-cells, with short hairpin (sh)RNA targeting an AP-3 subunit, consistent with the reductions previously observed in the hippocampus of AP-3-deficient mice. These data suggest that the GABAergic β-cell SLMVs may be generated primarily from endosomes via the AP-3B-mediated pathway. Further analysis of this pathway may yield new insights into the trafficking and role of GAD65, VGAT, and GABA in the pancreatic islets and into the mechanisms that render β-cells susceptible to autoimmune targeting.
MATERIALS AND METHODS
Antibodies.
The SV2 monoclonal antibody developed by K. M. Buckley was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) (19). Synaptophysin (SY38) and vacuolar ATPase antibodies were purchased from Chemicon (Temecula, CA) and Synaptic Systems (Göttingen, Germany), respectively. Carboxypeptidase E, tubulin, and β3B antibodies (for Western blotting) were purchased from BD Pharmingen (San Diego, CA). One of us (V. Faundez) has previously confirmed the specificity of the monoclonal β3B antibody in immunoblot experiments using brain tissue lysates from β3B-knockout mice and control mice (43). We also found that the monoclonal antibody yields a band corresponding to β3B in Western blots with extracts from human brain but not human liver. A previously characterized rabbit polyclonal anti-β3B antibody (used for immunostaining) was generously donated by Robert Darnell of The Rockefeller University (32). In preliminary experiments, this polyclonal antibody also yielded a band at the expected molecular weight in Western blots with protein extracts from brain and not with extracts from liver. Phosphatidylinositol 4-kinase type IIα (PI4KIIα) antibodies were described previously (23). An affinity-purified antibody to the carboxyl terminus of VGAT was purchased from Alpha Diagnostic International (San Antonio, TX) and has been characterized by us in prior studies (sometimes referred to as “VT/510”) (9, 47). Rabbit antibodies against μ3B were made by injection of the peptides TVTSQMPKGVLN and YFGVCSEPVIKD after conjugation to Keyhole Limpet Hemocyanin. Antiserum was validated for affinity and specificity for μ3B by ELISA, immunoprecipitation of recombinant μ3B, and Western blotting. Recombinant μ3B was made by cloning the full-length mouse cDNA (IMAGE Consortium/Open Biosystems, Huntsville, AL) into the plasmid vector pTNT (Promega, Madison, WI) and then performing in vitro transcription and translation in a reticulocyte lystate-based system (TnT SP6 Quick Coupled Transcription/Translation System, Promega) exactly per the manufacturer's instructions.
RT-PCR/quantitative RT-PCR.
Total RNA from human islets was prepared as described previously (46). Gene-specific primers were designed over exon-exon boundaries to the neuronal AP-3 subunits β3B (5′-AGCCAAGCTCTACCTGACCA-3′ and 5′-TCCAAGACTGGAGCTGGTTT-3′) and μ3B (5′-TGTCAGCTTCCATCCTTGTG-3′ and 5′-TCCCACCGTTATTTCAAAGC-3′), and PCR was performed using human islet and brain cDNA (OriGene Technologies, Rockville, MD). Amplification was performed with HotStarTaq enzyme. cDNA was denatured at 92°C, annealed at 60°C, and extended at 72°C for 35 cycles. PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining. Controls with no template (no reverse transcriptase added to the reverse transcription reaction) were used to ensure that there was no genomic DNA contamination.
For quantitative RT-PCR experiments, total RNA was isolated from INS-1 cells using TRIzol (Invitrogen) and then treated with Turbo DNAse (Ambion) to remove any contaminating genomic DNA. RNA was reverse transcribed with Superscript II reverse transcriptase (Invitrogen) and random hexamers. Using gene-specific primers, quantitative PCR analysis was performed with the cDNA product from 10 ng RNA/well on an ABI Prism 7700 (Applied Biosystems), using the SYBR green system. The following primers were used: AP-3δ1 primer set A (5′-CCCAGACCCAGGGGTGCAGT-3′ and 5′-GGGCTCCAGGGGAGTCAGGG-3′), AP-3δ1 primer set B (5′-ATCCCAAGTCTGTGCAATCC-3′ and 5′-AGCAGCTCGTCACGATAGGT-3′), AP-3δ1 primer set C (5′-TGTGGAGCTGACAAGACTGG-3′ and 5′-ACCAGGTGGGCACTATCAAG-3′), and 18S rRNA (5′-CGCCGCTAGAGGTGAAATTC-3′ and 5′-TTGGCAAATGCTTTCGCTC-3′). Each sample was analyzed in duplicate along with a corresponding sample to which no reverse transcriptase was added (no RT control). AP-3δ1 gene expression was normalized to the expression of 18S rRNA, and knockdown was analyzed using the following formula: 2^[(CT AP3D1 in INS-1 cells treated with RNAi − CT 18s in same cells) − (CT AP3D1 in INS-1 cells treated with control RNAi − CT 18S in same cells)]. AP-3δ1 knockdown was confirmed using three distinct primer sets.
Cell culture/lentivirus treatment.
INS-1 cells (provided by the cell culture facility at the University of Washington Diabetes and Endocrinology Research Center, Seattle, WA) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, San Diego, CA) in a humidified 37°C chamber with 5% CO2. PC12 cells were grown in DME H21 medium supplemented with 10% horse serum, 5% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified 37°C chamber with 10% CO2. Brefeldin A (Epicentre Biotechnologies, Madison, WI), when used, was added to the tissue culture medium for 2 h at 10 μg/ml (final concentration; stock solution: 5 mg/ml in methanol).
Lentiviral particles encoding AP-3δ1 shRNA and a nontargeting control shRNA, both also containing a puromycin resistance gene, were purchased from Sigma-Aldrich. Two premade lentiviral constructs encoding AP-3δ1 shRNAs were tested initially, but only one of the two was found to result in AP-3δ1 gene silencing (Supplemental Fig. S1; Supplemental Material for this article can be found on the AJP-Endocrinology and Metabolism web site). The shRNA employed herein was designed against the target sequence: 5′-TCCATGTACAGCCGCTCTATCC-3′. Available anti-AP-3δ1 antibodies either do not work for immunoblotting or are relatively insensitive in this application and require application of large amounts of protein for Western blotting experiments, frequently yielding high backgrounds that interfere with blot interpretation (Suckow AT and Faundez V, unpublished observations). For this reason, and because preliminary testing revealed that the level of AP-3δ1 protein knockdown approximated the reduction in AP-3δ1 mRNA (Supplemental Fig. S1), quantitative RT-PCR was employed in subsequent experiments to monitor AP-3δ1 gene silencing. INS-1 cells were treated with control or AP-3δ1 lentivirus overnight at an multiplicity of infection of 1. Virus was removed and replaced with normal INS-1 medium for 24 h, and then INS-1 medium containing 2 μg/ml puromycin was used to select for cells in which the shRNA integrated. Cells remained under selection for 2 wk before colonies were harvested and pooled. Prior to expansion in normal INS-1 medium and analysis, the pooled colonies were then grown under selection for an additional 2 wk.
Subcellular fractionation.
PC12 and INS-1 cells were fractionated according to the method of Clift-O'Grady et al. (11). Briefly, cells were removed from culture dishes by rinsing with calcium- and magnesium-free phosphate-buffered saline (PBS). They were pelleted at 800 g for 5 min, resuspended in budding buffer (38 mM potassium glutamate, 38 mM potassium gluconate, 20 mM 4-morpholinepropanesulfonic acid, pH 7.2, 5 mM reduced glutathione, 5 mM sodium carbonate, and 2.5 mM magnesium sulfate), and pelleted again at 800 g for 5 min. Cells were resuspended in budding buffer with protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN) and homogenized using a Balch-designed cell cracker (European Molecular Biology Laboratory) prerinsed with budding buffer. INS-1 cells were passed 24 times and PC12 cells 16 times through the cell cracker. Trypan blue exclusion was used to ensure efficient homogenization.
The resulting homogenate was sedimented in an SS34 rotor at 1,000 g for 5 min to obtain S1 supernatant. For sucrose sedimentation, S1 supernatants were loaded onto a 10–45% continuous sucrose gradient. Sucrose gradients were centrifuged for 2.5 h at 183,440 g. For glycerol sedimentation, S1 supernatants were sedimented at 27,000 g for 35 min to generate S2 supernatant. S2 supernatant was loaded onto a 5–25% glycerol gradient and spun in an SW55 rotor at 218,000 g for 75 min. For both types of gradients, 400-μl fractions were collected from the bottom and stored at −80°C.
Insulin content analysis.
The insulin content of the crude fractions P2 and S2 and the glycerol gradient fractions was measured using a rat insulin radioimmunoassay (Linco-Millipore, Billerica, MA). Insulin content is expressed as a percentage of the total fractional protein concentration that was determined using the Bio-Rad (Hercules, CA) DC Protein Assay kit.
Western blot analysis.
Human brain and human and rat liver protein extracts were obtained commercially (Biochain, Hayward, CA, and ProSci, Poway, CA). INS-1 and human islet extracts (ICR Basic Science Islet Distribution Program; City of Hope, Duarte, CA) were prepared by homogenization in RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 45 μg/ml aprotinin, 100 μg/ml phenylmethylsulfonyl fluoride, and 1 mM sodium orthovanadate). Cell extracts were quantified using the Bio-Rad DC Protein Assay. Fractions and lysates diluted 1:4 with reducing sample buffer were electrophoresed on either 10% SDS-PAGE gels, 4–12% Bis-Tris NuPage gels, or 3–8% Tris-Acetate NuPage gels according to the molecular weight of the protein of interest (Invitrogen). The protein was transferred to a polyvinylidene difluoride membrane (Millipore) and blocked for 1 h in 5% nonfat dry milk and TBS-Tween (20 mM Tris·HCl, 137 mM NaCl, 0.1% Tween-20, pH 7.4). The membrane was incubated in primary antibody at 4°C overnight and washed three times in TBS-Tween for 10 min. It was then incubated with HRP-conjugated anti-mouse IgG or anti-rabbit IgG (Jackson Laboratories, Bar Harbor, ME) for 1 h and washed three times in TBS-Tween for 10 min. The membrane was immersed in ECL (Amersham Biosciences, Piscataway, NJ) for 5 min and exposed to X-ray film (Kodak, Rochester, NY).
For quantitative Western blot analysis, membranes were treated with IRDye-conjugated anti-mouse IgG or anti-rabbit IgG for 1 h and washed three times in PBS-Tween for 10 min. The membrane was then washed in PBS, and detection was performed using a LiCor Odyssey infrared imager.
Immunofluorescence.
Flash-frozen rat pancreas (Rockland Immunochemicals, Gilbertsville, PA) was fixed for 24 h in Pen-Fix (Richard Allan Scientific, Kalamazoo, MI) and embedded in paraffin, and sections were cut at 6 μm by the Moores Cancer Center histology core at the University of California San Diego. Tissue sections were deparaffinized in d-limonene (Fisher Scientific, Fairlawn, NJ) and rehydrated through a graded ethanol series, and antigen retrieval was performed on sections for 10 min in boiling 1× citrate buffer (10 mM sodium citrate, 0.05% Tween-20, pH 6.0). Sections were blocked in a 3% bovine serum albumin-1% goat or donkey serum-PBS solution for 1 h and then incubated with primary antibody in the same solution for 1 h. They were washed twice in PBS for 10 min each and then incubated with Alexa Flour-conjugated anti-mouse and anti-rabbit IgG conjugates (Invitrogen). Sections were washed twice in PBS and mounted with coverslips. Images were captured using an Olympus FV1000 spectral confocal microscope configured with an Argon/Krypton laser (488- and 568-nm excitation lines) and either ×40 or ×60 oil immersion lenses. Controls included tissue either stained exclusively with secondary antibody or treated with preimmune serum followed by secondary antibody.
Immunohistochemistry.
Paraffin-embedded rat pancreas sections were treated as described above through the first wash step. Sections were then incubated with biotinylated anti-mouse or anti-rabbit IgG in blocking buffer for 1 h and washed twice with PBS. They were then incubated for 30 min in a 1:200 dilution of alkaline phosphatase streptavidin (Vector Laboratories, Burlingame, CA). Vector Red (Vector Laboratories) was used to visualize the binding of the primary antibodies. Sections were counterstained with hematoxylin (Fisher Scientific) using standard methods. Images were captured on a Nikon Optiphot microscope with a Nikon Coolpix 5400 using a ×20 lens. Controls included tissue treated with preimmune serum, followed by secondary antibody, or stained exclusively with secondary antibody.
Statistical analysis.
Data are presented as means ± SE. Differences between quantitative data sets were analyzed by the t-test. P < 0.05 was considered significant.
RESULTS
Neuronal AP-3 subunits are expressed in pancreatic islets.
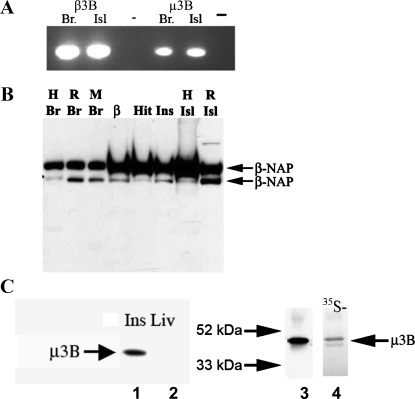
An expressed sequence tag search suggested the expression of the neuronal AP-3 subunits β3B and μ3B in human and mouse islets (data not shown). To confirm expression of these genes, primers for β3B and μ3B were designed over exon-exon boundaries, and PCR analysis was performed using human brain and islet cDNA. Amplicons of the expected size were detected in human islets and, as expected, in human brain (Fig. 1A).
Fig. 1.
Expression of neuronal adaptor protein (AP)-3 mRNA and protein in INS-1 β-cells and pancreatic islet extracts. A: PCR analysis was performed to determine whether the neuronal AP-3 subunits β3B and μ3B are expressed in human islets (Isl). Human brain (Br) cDNA was used as a positive control. PCR products of the expected size were detected in brain and islets for both β3B and μ3B. A negative control (−) in which no reverse transcriptase was added during the reverse transcription reaction (no RT control) is shown to the right of the brain and islet lanes. cDNA from human tissues (shown) and from rat tissues (not shown) yielded identical results. B: to determine whether β3B protein is expressed in islets, Western blot analysis was performed using a β3B-specific monoclonal antibody. As expected, β3B was detected in human (H), rat (R), and mouse (M) Br. β3B protein was also present in cell lysates from the insulin-secreting β-cell lines β-TC3 (β), HIT-T15 (Hit), and INS-1 (Ins) and in tissue lysates from human and rat Isl. β3B was not detected in human liver or in brain from β3B-knockout mice (Ref. 43 and data not shown). As observed previously, immunoblot detection of β3B yielded 2 bands (Mr of ∼140 K). C, left, lanes 1 and 2: μ3B was detected in INS-1 cell lysate (Ins) but not in rat liver lysate (Liv). C, right: μ3B produced by in vitro transcription and translation (lane 4) and analyzed by immunoblotting with the μ3B antibody ran at the expected Mr of ∼47,000. This band comigrated precisely with the band that was observed in INS-1 cells (lane 3). 35S-met was included in the in vitro translation reaction. Autoradiographic detection of the radiolabeled μ3B (lane 4) yielded a band that precisely aligned with the band detected by immunoblot analysis. This confirms that the μ3B antibody detected μ3B synthesized de novo in the in vitro transcription and translation reaction.
To confirm β3B protein expression in islets, Western blot analysis was performed using a β3B-specific monoclonal antibody. As expected, β3B was detected in lysates from human, rat, and mouse brain (Fig. 1B). β3B protein was also detected in cell lysates from the insulin-secreting β-cell lines β-TC3, HIT-T15, and INS-1 and in tissue lysates from isolated human and rat islets (Fig. 1B) (25, 53). The monoclonal antibody is specific for the neuronal protein; it does not yield a band with extracts from β3B-knockout mouse brain (43) or from human liver (not shown). β3B has previously been observed to form a doublet band, as is seen here. The reason the protein migrates as a doublet is unknown.
As with β3B protein, INS-1 cell lysates also contained μ3B protein (Fig. 1C). As expected, μ3B was not detected in liver extract or, as will be discussed later, in immunostained pancreatic exocrine tissue. The observed μ3B band comigrated precisely with μ3B synthesized de novo by in vitro translation, providing further confirmation of the specificity of the μ3B antibody (Fig. 1C).
β-Cell-specific expression of neuronal AP-3B subunits in rat islets.
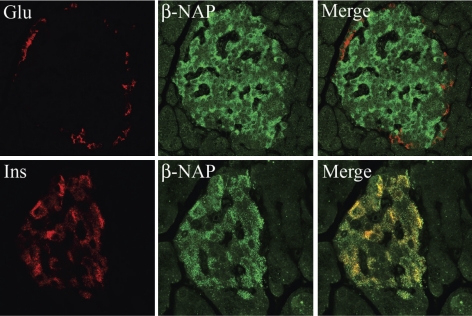
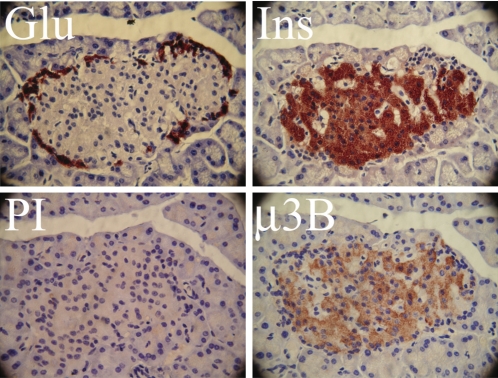
To determine the localization of the β3B and μ3B proteins in islets, immunolocalization studies were performed on rat pancreas sections using antibodies to β3B and μ3B. β3B was detected exclusively in cells expressing insulin (i.e., β-cells) but not in those expressing glucagon (α-cells) (Fig. 2). β3B was absent from the surrounding pancreatic exocrine tissue. Islet-specific expression of β3B was also observed in mouse and human pancreas sections (not shown). A similar pattern of expression was observed for μ3B. Analysis of serial sections of rat pancreas revealed μ3B protein expression in β-cells but not in α-cells (Fig. 3). As expected, there was no immunostaining of the exocrine pancreas. The latter result provides further confirmation that the μ3B polyclonal antibody does not cross-react with the ubiquitously expressed μ3A isoform.
Fig. 2.
β3B is detected in the pancreatic islet β-cells. Immunofluorescent localization was performed to determine the localization of β3B (β-NAP; green) in rat pancreas sections. Tissues were costained for either insulin (Ins; bottom, red) or glucagon (Glu; top, red). β3B was detected in cells expressing insulin (colocalization yields orange-yellow in the merged images) but not in cells expressing glucagon. As expected, β3B was also not present in the surrounding pancreatic exocrine tissue. Islet-specific expression of β3B was also observed by immunostaining using the same antibody in mouse and human pancreas sections (not shown). Magnification, ×60.
Fig. 3.
Localization of μ3B in pancreatic islets. Immunohistochemical localization was performed to determine the localization of μ3B in rat pancreas sections. Serial sections were stained for either Ins, Glu, or, as a control, preimmune serum (PI). Four serial sections containing the same representative islet are shown. As is typical in rodent islets, the glucagon-expressing α-cells are found in the islet periphery, surrounding the insulin-producing β-cells. μ3B was observed in the β-cells but not in cells that stained positive for glucagon. As expected, μ3B was also not present in the surrounding pancreatic exocrine tissue. PI yielded very little background. Magnification, ×60.
Resemblance of INS-1 β-cell SLMVs to PC12 cell SLMVs.
The expression of AP-3B in β-cells, along with its role in the biogenesis of SV in neurons, led us to next ask whether AP-3B is important for the formation of SLMVs in β-cells. A well-characterized β-cell line, INS-1, was chosen to test for AP-3B function. Utilization of this cultured cell line model in subsequent experiments was done for two reasons. First, the fractionation methods that were necessary for analyzing isolated SLMVs required large numbers of cells, more than could practically be obtained in sufficient quantities from isolated rodent islets (25, 34). Second, preliminary studies of β-cell function in whole body β3B-knockout mice were complicated by the increased activity level of these mice, their propensity for seizures, their lower average weight and greater adiposity, and uncertainties regarding whether altered CNS functioning would affect efferent neural signaling to the β-cells (Ref. 43 and Suckow AT and Waldrop M, unpublished obervations). We thus determined that in vivo studies of the effect of loss of AP-3B activity on islet function and glucose homeostasis would need to await the future development of a mouse model with islet- or β-cell-specific AP-3B knockout.
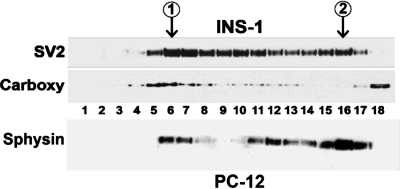
To confirm that INS-1 cells are a suitable model for the study of β-cell SLMV biogenesis, INS-1 cell postnuclear supernatant (S1) was sedimented in a sucrose velocity gradient to resolve both secretory granules and SLMVs. The larger secretory granules and endosomes migrate faster through the gradient than SLMVs (11). Fractions were collected from the bottom of the gradient and analyzed by immunoblotting with a monoclonal antibody to the SV protein SV2, which in β-cells is present in both the microvesicles and secretory granules, or with an antibody to the secretory granule protein carboxypeptidase E (Fig. 4) (22, 26).
Fig. 4.
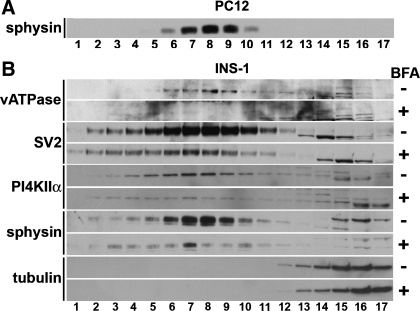
Detection of synaptic-like microvesicles (SLMVs) in INS-1 cell homogenates by sucrose gradient sedimentation. Postnuclear supernatant (S1) from INS-1 cells was sedimented in 5–45% sucrose gradients, and the resulting fractions were analyzed by immunoblotting. When fractions were stained with synaptic vesicle-2 (SV2) antibody, 2 distinct peaks [labeled peak 1 and peak 2 (inside circles)] were observed at fractions 6 and 16. Larger vesicles sediment to the lower-numbered fractions, and peak 1 results from the presence of SV2 in larger endosomes and secretory granules. The presence of secretory granule proteins in peak 1 was confirmed by immunoblotting for a specific marker of the secretory granules, carboxypeptidase E (carboxy). The presence of a 2nd peak centered at fraction 16 is consistent with the presence of SLMVs in INS-1 cells and with the association of SV2 with the INS-1 cell SLMVs. The absence of carboxy from the 2nd peak demonstrates that the population of microvesicles comprising peak 2 was not contaminated with secretory granules. Carboxy released from broken secretory granules was, as expected, present in the pool of soluble proteins in fraction 18. An immunoblot from PC12 cells fractionated in parallel (bottom row) was probed for synaptophysin (sphysin), which localizes primarily to SLMV. Sphysin content formed a peak around fraction 16, demonstrating that the pattern of PC12 SLMV sedimentation in the sucrose gradient matches that of INS-1 SLMV. Lesser amounts of sphysin are also present in larger PC12 cell compartments, including secretory granules, which explains the presence of bands in PC12 fractions 6 and 7 (40, 41).
Two peaks were observed when fractions were stained for SV2, one consistent with the presence of endosomes/secretory granules (centered at fractions 6–7; Fig. 4, top) and one consistent with the presence of SLMVs (centered at fraction 16; Fig. 4, top). The location of the peak resulting from sedimentation of INS-1 SLMVs in the sucrose gradient was identical to the location of the PC12 cell SLMV peak (Fig. 4, bottom). This demonstrates that INS-1 SLMVs and PC12 SLMVs have the same sedimentation properties in sucrose velocity gradients. To confirm that the first peak contained secretory granules, INS-1 fractions were stained for carboxypeptidase E, a marker of secretory granules but not SLMV, and only one peak, which corresponded to the earlier of the two SV2 peaks, was observed (fractions 6–7; Fig. 4) (22). Carboxypeptidase E can be processed from a membrane-bound to a soluble form that is released from broken secretory granules, and the protein was, as expected, also present in the top fractions (fractions 17 and 18; Fig. 4) containing the pool of soluble proteins (21).
INS-1 and PC12 SLMVs cosediment and share similar SV markers.
A defining feature of SLMVs and SVs is their distinctive size of ∼40 nm (14, 48). Past studies to determine the size of the β-cell SLMVs by electron microscopy have produced conflicting results. This may be due to the difficulty of discriminating sectioned tubules and endosomes from SLMVs in whole cells (12, 13). A recent study, for example, reported the average size of the β-cell SLMV to be 90 nm, with SLMVs ranging up to ∼150 nm (5).
Glycerol velocity gradient sedimentation allows discrimination of organelles based on size (11). In these gradients, brain SVs and PC12 SLMVs cosediment in the middle of the gradient as a symmetric peak. Moreover, the size of these organelles has been confirmed independently by electron microscopy (14, 38, 42). To corroborate that the SV2 peak observed in Fig. 4 corresponded to SLMV, we performed glycerol velocity sedimentation to determine whether INS-1 cell microvesicles cosediment with PC12 cell SLMVs (12). PC12 cells contain SLMVs that are virtually indistinguishable from neuronal SVs, and the PC12 cell line is commonly used for the study of SV/SLMV biogenesis (12).
INS-1 and PC12 postnuclear supernatants (S1) were spun in parallel at 27,000 g for 35 min to generate high-speed supernatants (S2) lacking larger membranes such as endosomes and secretory granules. S2 high-speed supernatants from both cell lines were then sedimented in glycerol velocity gradients to resolve SLMV according to size. Fractions were collected from the bottom of the gradients and analyzed by immunoblotting with antibodies to markers of PC12 cell SLMVs, including synaptophysin, SV2, and vacuolar ATPase (Fig. 5). PC12 fractions were analyzed by immunoblot with synaptophysin antibodies (Fig. 5A). Maximal PC12 cell synaptophysin immunoreactivity was observed in fraction 8. When INS-1 glycerol gradient fractions were stained with synaptophysin, maximal immunoreactivity was also observed in fraction 8 (sphysin; see Fig. 5B, 7th row down from top). This indicates that INS-1 SLMVs cosediment with PC12 SLMVs, as would be predicted if β-cell SLMVs were ∼40 nm. Furthermore, INS-1 fractions enriched in SLMVs were also enriched in other characteristic markers of SVs and SLMVs such as SV2 and vacuolar ATPase (SV2 and vATPase; Fig. 5B). Importantly, INS-1 SLMVs also contained an SV/SLMV protein whose sorting to these organelles is regulated by AP-3: PI4KIIα (PI4KIIa; Fig. 5B) (15, 30, 39). These results indicate that INS-1 β-cell SLMVs closely resemble PC12 SLMVs and suggest that neuronal AP-3 subunits may contribute to the biogenesis of INS-1 β-cell SLMVs.
Fig. 5.
SLMV biogenesis in INS-1 β-cells is sensitive to brefeldin A (BFA) inhibition. PC12 cells produce SLMVs with biophysical properties indistinguishable from those of neuronal SVs. A: high-speed supernatants (S2) obtained from PC12 cells were fractionated by glycerol gradient centrifugation, a technique especially well suited for resolving the ∼40-nm SVs and SLMVs. Fractions containing SLMVs were identified using an antibody to the SV marker sphysin, a known PC12 SLMV protein. Maximal immunoreactivity to sphysin was observed in fraction 8. B: high-speed S2 supernatants obtained from INS-1 cells that had been incubated for 2 h in the presence (+ sign in column on the far right) or absence (−) of BFA were sedimented in 5–25% glycerol velocity gradients to resolve SLMV. Fractions (numbered 1–17) were analyzed by immunoblotting using antibodies to proteins known to be associated with SVs and PC12 SLMVs. As in PC12 cells, which were analyzed in parallel, maximal immunoreactivity to the SV marker sphysin peaked at fraction 8, demonstrating that the SLMVs in the 2 cell types are the same size. All other SLMV markers tested also peaked at fraction 8, including phosphatidylinositol 4-kinase type IIα (PI4KIIα), an SV protein sorted specifically by an AP-3B-dependent mechanism. Treatment with BFA markedly reduced the content of vacuolar ATPase (vATPase), SV2, PI4KIIα, and sphysin in the INS-1 SLMV fractions (compare fractions 6–9 in the + rows with the same fractions in the − rows directly above). The observed depletion of protein content in the INS-1 SLMV fractions during BFA treatment is identical to previous results demonstrating AP-3-mediated SLMV biogenesis using BFA in PC12 cells (18, 37, 38). Probing for tubulin (bottom 2 rows, fractions 14–17) confirmed that the glycerol gradients were loaded with extracts with equal protein content. Cytosolic proteins like tubulin are expected to localize to the upper fractions, as is seen here. + and − blots were run, transferred, and exposed to film for equal lengths of time in parallel.
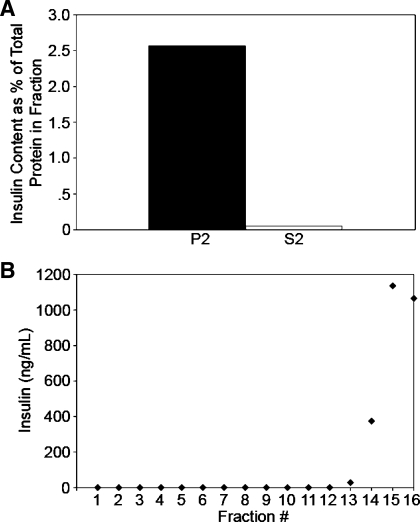
To further confirm that the vesicles analyzed in Fig. 5 via glycerol gradient sedimentation represented β-cell SLMV and demonstrate that these vesicles were distinct from the insulin-containing secretory granules, the insulin content of the SLMV-containing S2 fraction was compared with the secretory granule-containing P2 fraction. Consistent with the S2 fraction being devoid of secretory granules, only 0.06% of the protein in the S2 fraction represented insulin, whereas 2.57% of the total protein in the P2 fraction represented insulin (Fig. 6A). Because the S2 fraction contained some insulin and S2 is used as the input for glycerol velocity sedimentation, S2 was sedimented in glycerol velocity gradients, and the resulting fractions were analyzed for insulin content. The small amount of insulin present in S2 cosedimented with glycerol fractions where soluble proteins equilibrate (fractions 13 and above; Fig. 6B). Importantly, insulin was undetectable in all of the fractions containing SLMVs (fractions 3–12; Fig. 6B). These results confirm that SLMVs isolated by glycerol velocity sedimentation are devoid of β-cell secretory granules.
Fig. 6.
SLMVs do not contain detectable amounts of insulin. To confirm that the analyzed vesicles identified as being SLMV were devoid of insulin-containing secretory granules, the insulin content of the crude SLMV-containing S2 fraction was compared with the secretory granule-containing P2 fraction. A: bars represent insulin content as %total protein content in the fraction; 0.06% of the total protein in the S2 fraction represented insulin, whereas 2.57% percent of the protein in the P2 fraction represented insulin. B: the sedimentation of residual insulin in the S2 fraction was determined by glycerol velocity sedimentation and analysis of the resulting fractions for insulin content; the concentration of insulin in each fraction is shown. Insulin was detected in the cytosolic fractions where tubulin is found (fractions 13 and above) and was absent from all of the fractions containing the SLMVs (fractions 3–12).
An AP-3-dependent pathway drives SLMV biogenesis in INS-1 β-cells.
BFA inhibits AP-3-mediated SV/SLMV biogenesis from endosomes but does not affect AP-2-mediated SV/SLMV biogenesis from the plasma membrane (33, 44). The drug is thus an important tool for discriminating between AP-2- and AP-3-mediated SV biogenesis and for identification of the cargo proteins of AP-3-associated SVs (37, 38, 50). To determine whether SLMV biogenesis in β-cells is dependent on AP-3, INS-1 cells were incubated in the presence or absence of BFA, and high-speed supernatants were sedimented in 5–25% glycerol gradients to resolve SLMV. SLMV content was analyzed using antibodies to proteins known to be associated with rat brain SV and PC12 SLMV. Equal loading of proteins from untreated (−) and BFA-treated cells (+) into the gradients was confirmed using an antibody to tubulin (Fig. 5B). Relative to control cells (− signs in Fig. 5), treatment with BFA (+ signs) markedly reduced the content of SV2, synaptophysin, and vacuolar ATPase in INS-1 SLMVs (fractions 6–10; Fig. 5B). The content of PI4KIIα, a protein targeted specifically to AP-3-derived SLMV, was similarly reduced (fractions 5–11; Fig. 5B) (15, 30, 39). These data show that INS-1 SLMV biogenesis is sensitive to BFA. The depletion of SLMV proteins from the INS-1 SLMV fractions by BFA parallels results from earlier studies in which the presence of an AP-3-mediated pathway of SLMV biogenesis in PC12 cells was demonstrated using BFA in the same manner as depicted here (18, 37, 38). These results are consistent with a model where AP-3 mediates the bulk of SLMV biogenesis in INS-1 β-cells.
AP-3 downregulation decreases VGAT levels in INS-1 β-cells.
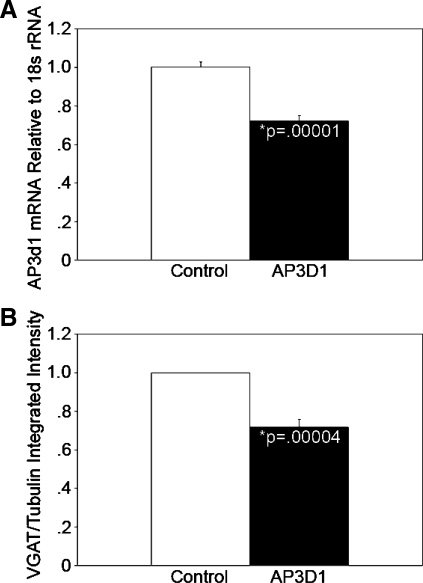
Mice lacking the μ3B subunit of the AP-3B complex possess reduced levels of VGAT in both total hippocampal extracts and SVs (29). We have demonstrated that β-cells express high levels of VGAT in SLMVs (9, 47). Thus, we hypothesized that AP-3 loss-of-function in pancreatic β-cells would phenocopy the reduced VGAT level phenotype observed in AP-3-null brain. To test this hypothesis, INS-1 cells were transduced with lentiviral particles expressing shRNA against the δ-subunit (AP-3δ1) of the AP-3 complex, and cells stably expressing the virus were selected with puromycin. Knockdown was confirmed by quantitative RT-PCR, which indicated that there was a 28% decrease in AP-3δ1 gene expression compared with cells stably expressing a nontargeting shRNA (n = 3, P < 0.00002; Fig. 7A). Quantitative Western blot analysis of protein lysates revealed that the partial downregulation of the AP-3 subunit transcript in INS-1 cells resulted in a 28% reduction in VGAT content relative to levels in control shRNA-treated INS-1 cells (n = 5, P < 0.00004; Fig. 7B). These data suggest that, as occurs in the brain, AP-3 regulates the subcellular fate of VGAT in pancreatic β-cells.
Fig. 7.
Knockdown of the AP-3 δ-subunit reduces the expression of the GABAergic SV marker vesicular GABA transporter (VGAT). A: INS-1 cells were transduced with lentiviral particles expressing short hairpin (sh)RNA against the AP-3δ1 subunit of the AP-3 complex or with control nontargeting shRNA. After selection for cells stably expressing the viral constructs, knockdown was confirmed by quantitative RT-PCR. AP-3δ1 mRNA levels are shown relative to the level in cells expressing the control shRNA and were 28% less in AP-3δ1 shRNA-expressing INS-1 cells (right) than in INS-1 cells stably expressing the nontargeting shRNA (left; n = 3, P < 0.00002). B: quantitative Western blot analysis of protein lysates revealed a 28% reduction in VGAT content in AP-3δ1 shRNA-expressing INS-1 cells (right) relative to nontargeting shRNA control INS-1 cells (left; n = 5, P < 0.00004). Five separate control and AP-3-knockdown lysate samples with equal protein content were analyzed in duplicate or triplicate on separate immunoblots. VGAT and tubulin signal intensities were measured using an Odyssey infrared imaging system. VGAT signal intensities were normalized to tubulin. The anti-VGAT antibody has previously been characterized by us in detail (9, 47).
DISCUSSION
The pancreatic islet β-cells express much of the machinery that is important for the differentiation and maintenance of GABAergic (inhibitory) synapses in CNS (46). The neuronal AP-3B adaptor complex is important for normal synaptic function and plays an important role in GABAergic signaling (29, 43). In μ3B-knockout mice, Nakatsu et al. (29) observed impairment of K+-evoked release of GABA, which they attributed, at least in part, to reductions in synaptic VGAT protein levels. Islet β-cells express VGAT and other components of the neuronal GABAergic signaling machinery (9, 47). Because of more recent findings also suggesting that β-cells mature in a manner similar to that of GABAergic synapses, we hypothesized that AP-3B would be important for the biogenesis of SLMVs in β-cells (46). Here, we demonstrate the expression of β3B and μ3B in human and rodent pancreatic islets. This is the first time that β3B and μ3B have been shown to be expressed outside of neural tissue.
Our results indicate that rat pancreatic expression of AP-3B is islet β-cell specific. We show that, as in neurons, AP-3 protein complexes in INS-1 β-cells are involved in the biogenesis of SLMVs, as suggested by the strong inhibition of SLMVs after brefeldin A treatment. Because AP-3-mediated microvesicle formation occurs at endosomes, β-cell SLMVs may originate primarily from endosomal compartments (2). Our results confirm that the protein content and sedimentation properties of INS-1 β-cell SLMVs closely resemble those of PC12 and neuronal SVs/SLMVs and that, much like PC12 cells, INS-1 β-cell SLMVs are essentially devoid of secretory granule components, as indicated by the absence of carboxypeptidase E (Fig. 4) and insulin (Fig. 6).
Expression of AP-3B subunits in islets and β-cells.
Transcripts of both neuron-specific AP-3 subunits β3B and μ3B were detected in islets. Immunolocalization studies demonstrated that both of these subunits are expressed exclusively by the islet β-cells in rat pancreas sections.
The expression of β3B by β-cells, the propensity for β-cell autoantigens to be vesicle associated, and the prior observation that β3B functions as an autoantigen in cerebellar degeneration suggested that β3B itself could be a β-cell autoantigen (24, 32, 45). Alternatively, β3B and μ3B may contribute to the triggering of anti-β-cell autoimmunity by mediating the formation of nerve terminal-like vesicles that carry potential autoantigens in a way that allows them to leak or otherwise be released outside the cell under certain circumstances (45, 51). The SLMV-associated enzyme GAD65, for example, is a major autoantigen in type 1 diabetes and is released into the circulation during β-cell injury (51). We conducted a preliminary search for evidence of autoreactivity against β3B and μ3B using radioligand binding assays similar to a proven assay for GAD65 and other autoantibodies (7, 17). Plasma samples from controls and from 10 children newly diagnosed with type 1 diabetes were tested for β3B and μ3B antibodies. These 10 well-characterized samples have been used previously by the Immunology of Diabetes Workshops to standardize measurements of diabetes-associated autoantibodies (4, 28). Results from this pilot study were negative for the presence of autoantibodies against either of the two proteins in the sera of subjects with type 1 diabetes and controls.
AP-3-mediated SLMV biogenesis in β-cells.
The biophysical properties of SVs and SLMVs are virtually identical (12). Both are characterized by their homogenous size, the presence of unique markers, and the absence of markers of larger endosomes and secretory granules. It was unclear how the biophysical properties of islet β-cell SLMVs compare with SVs and PC12 SLMVs. Previous characterization of these vesicles has yielded conflicting results and suggested that the size of these microvesicles differs from true SVs/SLMVs, which have a remarkably constant diameter of about 40 nm (14, 48). Using electron microscopy, Braun et al. (5) reported that the β-cell SLMVs are on average 90 nm in diameter and can be as large as 150 nm, whereas Christgau et al. (10) reported that these same vesicles are between 30 and 60 nm. Such electron microscopy-based analysis performed in intact cells cannot discriminate between sectioned tubules and endosomes and SLMVs unless proper markers are used. To circumvent this problem, SLMVs can be separated from endosomes and larger vesicles with standard cell fractionation techniques followed by sedimentation through glycerol velocity gradients (12, 38).
The INS-1 line is one of the best-characterized β-cell lines (25, 34). Sucrose gradient sedimentation of INS-1 β-cell homogenates yielded a peak due to the presence of endosomes/secretory granules and a second peak consistent with the presence of SLMVs that cosediment with PC12 SLMVs. Glycerol gradient sedimentation confirmed that the INS-1 β-cells contain bona fide SLMVs. Our results show that INS-1 SLMVs carry SV/SLMV markers, including SV2, synaptophysin, PI4KIIα, and vacuolar ATPase. INS-1 SLMVs sedimented in the same glycerol gradient position as PC12 SLMVs, indicating that INS-1 SLMVs are the same size as PC12 SLMVs and neuronal SVs, with a diameter of around 40 nm. Further evidence that the population of microvesicles analyzed in these experiments are bona fide SLMVs and are distinct and devoid of contamination from larger vesicles such as the insulin secretory granules is provided through analyses of insulin content. The crude S2 fraction contained only minimal insulin, and the SLMV fractions collected after glycerol velocity sedimentation did not contain detectable amounts of insulin. These results validate INS-1 cells as a model for investigations of SLMV biogenesis and SLMV function in β-cells.
Treatment of INS-1 β-cells with brefeldin A markedly inhibited the formation of SLMVs, suggesting that AP-3 mediates the biogenesis of a significant portion of β-cell SLMVs. These studies do not exclude the possibility that there is also a population of SLMVs formed via an AP-2-dependent mechanism. Both pathways are used for the biogenesis of SLMVs in PC12 cells (38).
Analysis of INS-1 β-cells with decreased expression of the AP-3 δ-subunit revealed a decrease in the levels of VGAT protein in parallel with the level of δ-subunit knockdown. These results are further evidence of AP-3-mediated SLMV biogenesis and specifically of AP-3 mediating the biogenesis of GABAergic vesicles in islet β-cells. Further characterization of the function of β-cell SLMVs formed as a result of AP-3 action may lead to novel insights into the GABAergic autocrine/paracrine signaling within the islet.
Taken collectively, our data demonstrate expression of the neuronal AP-3 subunits outside of the CNS and neurosecretory chromaffin cells and suggest that AP-3-mediated SLMV biogenesis occurs in β-cells. Experiments utilizing glycerol velocity gradients confirm that the biophysical properties of β-cell SLMVs match those of the bona fide SLMVs produced by PC12 cells. Future studies of AP-3-derived SLMV may provide insights into important aspects of pancreatic β-cell biology, including the function of GABA within the islet, β-cell zinc metabolism, and the mechanisms that render neuronal vesicle-associated β-cell proteins particularly susceptible to autoimmune targeting.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK-063491–4S1 and DK-080971 (to S. D. Chessler), by a graduate research fellowship from the National Science Foundation (to A. T. Suckow), and by National Institutes of Health Grants NS-42599 and GM-077569 (to V. Faundez). Islet isolations and provision of cells by the University of Washington Diabetes and Endocrinology Research Center were enabled by NIDDK Grant DK-17047.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Åke Lernmark and members of Lernmark's laboratory for their assistance with setting up radioligand binding assays and autoantibody testing. We thank Sonya Egodage, Challise Cox, and Megan Waldrop for their excellent technical assistance and Merrie Mosedale for critical reading of the manuscript.
REFERENCES
- 1.Abderrahmani A, Niederhauser G, Plaisance V, Haefliger JA, Regazzi R, Waeber G. Neuronal traits are required for glucose-induced insulin secretion. FEBS Lett 565: 133–138, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Blumstein J, Faundez V, Nakatsu F, Saito T, Ohno H, Kelly RB. The neuronal form of adaptor protein-3 is required for synaptic vesicle formation from endosomes. J Neurosci 21: 8034–8042, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm M, Bonifacino JS. Adaptins: the final recount. Mol Biol Cell 12: 2907–2920, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifacio E, Lernmark A, Dawkins RL. Serum exchange and use of dilutions have improved precision of measurement of islet cell antibodies. J Immunol Methods 106: 83–88, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Braun M, Wendt A, Birnir B, Broman J, Eliasson L, Galvanovskis J, Gromada J, Mulder H, Rorsman P. Regulated exocytosis of GABA-containing synaptic-like microvesicles in pancreatic beta-cells. J Gen Physiol 123: 191–204, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrillo J, Puertas MC, Alba A, Ampudia RM, Pastor X, Planas R, Riutort N, Alonso N, Pujol-Borrell R, Santamaria P, Vives-Pi M, Verdaguer J. Islet-infiltrating B-cells in nonobese diabetic mice predominantly target nervous system elements. Diabetes 54: 69–77, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chessler SD, Hampe CS, Ortqvist E, Simonson WT, Bekris L. Immune reactivity to GAD25 in type 1 diabetes mellitus. Autoimmunity 35: 335–341, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chessler SD, Lernmark A. The role of glutamic acid decarboxylase and GABA in the pancreas and diabetes. In: GABA in the Nervous System: The View at Fifty Years, edited by Martin DL, Olson RW. Philadelphia, PA: Lippincott Williams & Wilkins, 2000, p. 471–484 [Google Scholar]
- 9.Chessler SD, Simonson WT, Sweet IR, Hammerle LP. Expression of the vesicular inhibitory amino acid transporter in pancreatic islet cells: distribution of the transporter within rat islets. Diabetes 51: 1763–1771, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Christgau S, Aanstoot HJ, Schierbeck H, Begley K, Tullin S, Hejnaes K, Baekkeskov S. Membrane anchoring of the autoantigen GAD65 to microvesicles in pancreatic beta-cells by palmitoylation in the NH2-terminal domain. J Cell Biol 118: 309–320, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clift-O'Grady L, Desnos C, Lichtenstein Y, Faundez V, Horng JT, Kelly RB. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods 16: 150–159, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Clift-O'Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol 110: 1693–1703, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci 22: 2215–2224, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craige B, Salazar G, Faundez V. Isolation of synaptic vesicles. In: Current Protocols in Cell Biology, edited by Bonifacino J. New York: John Wiley, 2004, p. 12.11–12.17 [DOI] [PubMed] [Google Scholar]
- 15.Craige B, Salazar G, Faundez V. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell 19: 1415–1426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Camilli P, Thomas A, Cofiell R, Folli F, Lichte B, Piccolo G, Meinck HM, Austoni M, Fassetta G, Bottazzo G, Bates D, Cartlidge N, Solimena M, Kilimann MW. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. J Exp Med 178: 2219–2223, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falorni A, Ortqvist E, Persson B, Lernmark A. Radioimmunoassays for glutamic acid decarboxylase (GAD65) and GAD65 autoantibodies using 35S or 3H recombinant human ligands. J Immunol Methods 186: 89–99, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Faundez V, Horng JT, Kelly RB. ADP ribosylation factor 1 is required for synaptic vesicle budding in PC12 cells. J Cell Biol 138: 505–515, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 70: 861–867, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Franklin IK, Wollheim CB. GABA in the endocrine pancreas: its putative role as an islet cell paracrine-signalling molecule. J Gen Physiol 123: 185–190, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fricker LD. Carboxypeptidase E. Annu Rev Physiol 50: 309–321, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Guest PC, Pipeleers D, Rossier J, Rhodes CJ, Hutton JC. Co-secretion of carboxypeptidase H and insulin from isolated rat islets of Langerhans. Biochem J 264: 503–508, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J, Wenk MR, Pellegrini L, Onofri F, Benfenati F, De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci USA 100: 3995–4000, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirai H, Miura J, Hu Y, Larsson H, Larsson K, Lernmark A, Ivarsson SA, Wu T, Kingman A, Tzioufas AG, Notkins AL. Selective screening of secretory vesicle-associated proteins for autoantigens in type 1 diabetes: VAMP2 and NPY are new minor autoantigens. Clin Immunol 127: 366–374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol 228: 121–128, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Iezzi M, Theander S, Janz R, Loze C, Wollheim CB. SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Sci 118: 5647–5660, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lernmark A. Glutamic acid decarboxylase—gene to antigen to disease. J Intern Med 240: 259–277, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Mire-Sluis AR, Das RG, Lernmark The development of a World Health Organisation international standard for islet cell antibodies: the aims and design of an international collaborative study. Diabetes Metab Res Rev 15: 72–77, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Nakatsu F, Okada M, Mori F, Kumazawa N, Iwasa H, Zhu G, Kasagi Y, Kamiya H, Harada A, Nishimura K, Takeuchi A, Miyazaki T, Watanabe M, Yuasa S, Manabe T, Wakabayashi K, Kaneko S, Saito T, Ohno H. Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J Cell Biol 167: 293–302, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newell-Litwa K, Salazar G, Smith Y, Faundez V. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell 20: 1441–1453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newell-Litwa K, Seong E, Burmeister M, Faundez V. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci 120: 531–541, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Newman LS, McKeever MO, Okano HJ, Darnell RB. Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell 82: 773–783, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Ooi CE, Dell'Angelica EC, Bonifacino JS. ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol 142: 391–402, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poitout V, Olson LK, Robertson RP. Insulin-secreting cell lines: classification, characteristics and potential applications. Diabetes Metab 22: 7–14, 1996 [PubMed] [Google Scholar]
- 35.Reetz A, Solimena M, Matteoli M, Folli F, Takei K, De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J 10: 1275–1284, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science: 1118–1120, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Salazar G, Craige B, Wainer BH, Guo J, De Camilli P, Faundez V. Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles. Mol Biol Cell 16: 3692–3704, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell 15: 575–587, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salazar G, Zlatic S, Craige B, Peden AA, Pohl J, Faundez V. Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol 4-kinase type II alpha in neuronal and non-neuronal cells. J Biol Chem 284: 1790–1802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilling K, Gratzl M. Quantification of p38/synaptophysin in highly purified adrenal medullary chromaffin vesicles. FEBS Lett 233: 22–24, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Schmidle T, Weiler R, Desnos C, Scherman D, Fischer-Colbrie R, Floor E, Winkler H. Synaptin/synaptophysin, p65 and SV2: their presence in adrenal chromaffin granules and sympathetic large dense core vesicles. Biochim Biophys Acta 1060: 251–256, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol 137: 445–458, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seong E, Wainer BH, Hughes ED, Saunders TL, Burmeister M, Faundez V. Genetic analysis of the neuronal and ubiquitous AP-3 adaptor complexes reveals divergent functions in brain. Mol Biol Cell 16: 128–140, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi G, Faundez V, Roos J, Dell'Angelica EC, Kelly RB. Neuroendocrine synaptic vesicles are formed in vitro by both clathrin-dependent and clathrin-independent pathways. J Cell Biol 143: 947–955, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solimena M. Vesicular autoantigens of type 1 diabetes. Diabetes Metab Rev 14: 227–240, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Suckow AT, Comoletti D, Waldrop MA, Mosedale M, Egodage S, Taylor P, Chessler SD. Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic beta-cells and the involvement of neuroligin in insulin secretion. Endocrinology 149: 6006–6017, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suckow AT, Sweet IR, Van Yserloo B, Rutledge EA, Hall TR, Waldrop M, Chessler SD. Identification and characterization of a novel isoform of the vesicular gamma-aminobutyric acid transporter with glucose-regulated expression in rat islets. J Mol Endocrinol 36: 187–199, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell 127: 831–846, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Thomas-Reetz AC, De Camilli P. A role for synaptic vesicles in non-neuronal cells: clues from pancreatic β-cells and from chromaffin cells. FASEB J 8: 209–216, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51: 71–84, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Waldrop MA, Suckow AT, Marcovina SM, Chessler SD. Release of glutamate decarboxylase-65 into the circulation by injured pancreatic islet beta-cells. Endocrinology 148: 4572–4578, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Tulina N, Carlin DL, Rulifson EJ. The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proc Natl Acad Sci USA 104: 19873–19878, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weaver CD, Yao TL, Powers AC, Verdoorn TA. Differential expression of glutamate receptor subtypes in rat pancreatic islets. J Biol Chem 271: 12977–12984, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Winer S, Tsui H, Lau A, Song A, Li X, Cheung RK, Sampson A, Afifiyan F, Elford A, Jackowski G, Becker DJ, Santamaria P, Ohashi P, Dosch HM. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat Med 9: 198–205, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3: 47–58, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.