Abstract
Recent studies in animal and human models have revealed that free fatty acid (FFA) release from adipose tissue is oscillatory. We have shown in our laboratory that these oscillations are controlled by the sympathetic nervous system (SNS). Although FFAs have been shown to directly stimulate glucose production [endogenous glucose production (EGP)] by the liver and to reduce peripheral glucose utilization, whether the specific pattern of FFA release affects glucose metabolism is unknown. The aim of this study was to examine the effects of pulsatile vs. constant infusion of FFA on glucose homeostasis in the canine model. Euglycemic clamps with basal insulin replacement (0.1 mU·kg−1·min−1 insulin) were performed in dogs (n = 8) during infusion of saline (SAL) or the medium-chain fatty acid octanoate, which was given by either pulsatile infusion (PUL: 10 mmol over 2 min every 10 min) or continuous infusion (C-INF: 1 mmol/min) designed to achieve equivalent total FFA mass. Endogenous lipolytic pulses were suppressed with the β3-specific adrenergic receptor antagonist bupranolol. PUL infusion elicited a pulsatile pattern of FFA in circulation with average maximum pulse height of 0.82 ± 0.04 mM, whereas C-INF FFA levels reached 0.47 ± 0.03 mM (fasting levels) and were maintained throughout. Glucose uptake was not affected by PUL; however, C-INF significantly reduced glucose uptake compared with both SAL and PUL. Steady-state EGP increased by >90% from basal steady state during PUL but did not change during either SAL or C-INF. Thus, pulsatile FFA infusion led to an increase in EGP while preserving glucose disposal. These data suggest that the pattern of FFA may have a role in regulation of glucose homeostasis, which may have consequences in the obese or insulin-resistant state where the SNS is known to be altered.
Keywords: octanoate, oscillations, lipolysis, sympathetic nervous system
it is becoming increasingly clear that pulsatile secretion is a phenomenon that is of vital physiological importance. It is well known that the pulsatility of various hormones, such as insulin, growth hormone, luteinizing hormone, and others, is necessary to maintain normal physiology and that pulsatile delivery of these hormones is more effective in eliciting physiological responses than continuous delivery (16, 19, 23). In addition, defects in pulsatile secretion are associated with disease states; for example, pulsatility of insulin release from the pancreas is altered in type 2 diabetes, which contributes to the impaired insulin action seen in this disease (19, 29).
In addition to pulsatility of hormone release, free fatty acid (FFA) levels have also been shown to oscillate in the circulation in both animal and human models (10, 14). Arteriovenous difference studies performed in our laboratory have shown that the oscillatory FFAs can be accounted for at least in part by pulsatile FFA release from the visceral adipose depot (10). These oscillations in plasma FFA levels are not regulated by pulsatile insulin secretion (10) but are apparently controlled by the activation of the sympathetic branch of the central nervous system (13). Supporting a role for the sympathetic nervous system (SNS), administration of the high-affinity β3-adrenergic receptor antagonist bupranolol suppresses pulses of plasma FFAs (13). Although FFA release is pulsatile, it has not been examined whether pulsatile FFA release is of any physiological significance.
It is well known that FFAs have the ability to induce insulin resistance. In particular, increasing FFA reduces insulin's ability to suppress endogenous glucose production (EGP) as well as directly enhances glucose production by the liver by activation of the gluconeogenic pathway (11, 28). FFAs also have a profound effect on peripheral glucose uptake by reducing glucose oxidation and inhibiting glucose transport at skeletal muscle (2, 27). Previous studies examining the effects of FFAs use pharmacological agents to either suppress or raise FFA levels such that FFA levels are held constant. However, these latter studies did not take into account the pulsatile nature of plasma FFAs. Therefore, it is of interest whether the pattern of plasma FFAs in the circulation might contribute to the effects of FFA on glucose homeostasis.
In this study, we examined whether exogenous pulses of FFAs will lead to specific changes in glucose turnover in vivo and compared those changes with an equimolar constant increase in average FFA levels. The medium-chain FFA octanoate was intravenously infused in a pulsatile fashion, and its effect on glucose turnover was compared with that of a continuous infusion of octanoate during a euglycemic clamp.
METHODS
Animals.
Male mongrel dogs (n = 8) were housed in the University of Southern California (USC) Keck School of Medicine Vivarium under controlled kennel conditions with a 12:12-h light-dark cycle. Animals were maintained on an ad libitum standard chow diet [21% carbohydrate, 26% protein, 15% fat, and 2.8% fiber (Prolab Canine Diet and Laboratory High Density Canine Diet; LabDiet, St. Louis, MO)] for the duration of the study. Animals were accepted in the study after physical examination and comprehensive blood panel analysis by USC veterinarians. Chronic catheters were surgically implanted at least 7 days before the beginning of the study. Catheters inserted through the right jugular vein and advanced to the right atrium were used to sample mixed central venous blood. Catheters inserted in the left femoral vein and advanced to the inferior vena cava were used for infusion purposes only. All catheters were led to the neck and exteriorized. Chronic catheters were flushed weekly with heparinized saline (10 U/ml), and the exteriorization site was cleaned with hydrogen peroxide (4%). Animals were allowed to acclimate to laboratory procedures and were familiarized with Pavlov slings at least 1 wk before the beginning of experiments. Animals were used for experiments if they were judged in good health as determined by visual inspection, body temperature, and hematocrit. On the morning of each experiment, 19-gauge angiocatheters (Becton Dickinson, Sandy, UT) were inserted percutaneously in both saphenous veins for the infusion of glucose and octanoate. The experimental protocol was approved by the USC Institutional Animal Care and Use Committee.
Experimental protocol.
Euglycemic clamps were performed as follows: after an overnight fast, animals were brought to the laboratory at 6:00 A.M., placed in the Pavlov sling, and allowed to acclimate to the laboratory environment for 1 h before the start of experiments. Angiocatheters were placed at this time in both the left and right saphenous veins and secured. Baseline samples were taken at 7:00 A.M. after which somatostatin (1 mg·kg−1·min−1; Bachem California, Torrance, CA) was infused peripherally to suppress endogenous insulin and glucagon secretion. Insulin (0.1 mU·kg−1·min−1; Eli Lilly, Indianapolis, IN) was infused to replace fasting insulin levels. In addition, a primed continuous infusion of high-performance liquid chromatography, purified [3-3H]glucose (25 μCi bolus + 0.25 μCi/min; Dupont-NEN, Boston, MA) was started at this time. Glucose was clamped at baseline levels by a variable glucose infusion labeled with [3-3H]glucose (1.8 μCi/g) to minimize fluctuations in plasma specific activity. After a 90-min tracer equilibration period, four basal samples were taken every 10 min for 30 min after which an infusion of the high-affinity β3-adrenergic receptor antagonist bupranolol (1.5 μg·kg−1·min−1; donated by Schwarz Pharma, Mannheim, Germany) was started to suppress endogenous FFA pulses. Previous studies have shown that intravenous infusion of bupranolol suppresses endogenous pulsatile FFA release, whereas a constitutive component of FFA release remained intact (13). Bupranolol was allowed to exert its effect for a 30- to 60-min period before the following 60-min infusion protocols were begun: 1) 2-min pulsatile infusion of the medium-chain fatty acid (MCFA) octanoate (Sigma, St. Louis, MO) at a rate of 5 mM/min with a pulse interval of 10 min (PUL), 2) 1 mM/min constant infusion of octanoate (C-INF), a quantitatively equimolar infusion to protocol 1 designed to maintain basal, or fasting, levels of FFA in circulation, and 3) control group experiments performed under the same conditions as protocol 1 except that saline was infused (SAL). The three experimental protocols were performed in each animal on separate days at least 1 wk apart in random order. During the tracer equilibration, basal, and bupranolol infusion periods, blood samples were drawn from the jugular catheter every 10 min for the measurement of glucose, insulin, FFAs, and glycerol. During the 60-min infusion protocols, samples were taken at 1-min intervals for the measurement of FFAs and glycerol. In addition, the first sample for the measurement of glucose and insulin was taken 5 min after the start of the 60-min infusion protocols, and subsequent samples were taken every 10 min thereafter.
Sample collection and assays.
Samples for the assay of FFAs and glycerol were taken in tubes containing EDTA and paraoxon to inhibit lipase activity. Samples for the determination of glucose and insulin were taken in tubes precoated with lithium fluoride and heparin and containing EDTA. All samples were immediately centrifuged, and the plasma was separated and stored at −20°C.
FFAs (NEFA C; Wako Pure Chemical Industries, Richmond, VA) and glycerol (Serum Glycerol Determination Kit; Sigma Chemical) were measured using commercially available colorimetric kits. Glucose was measured with a YSI 2300 autoanalyzer (Yellow Springs Instruments, Yellow Springs, OH). Insulin was measured by an enzyme-linked immunosorbent assay originally developed for human serum or plasma (Linco Research, St. Charles, MO) and adapted for dog plasma using a dog standard kindly provided by Novo-Nordisk. The method is based on two murine monoclonal antibodies that bind to different epitopes of insulin but do not bind to proinsulin. Samples for [3H]glucose tracer assay were deproteinized using barium hydroxide and zinc sulfate. The supernatants were then evaporated in a vacuum, reconstituted in water, and counted in Ready Safe scintillation fluid (Beckman liquid scintillation fluid; Beckman Instruments, Fullerton, CA). Tracer infusates were processed in an identical manner as plasma samples.
Calculations.
The time course of EGP and glucose disappearance was calculated using the modified Steele's equation with a labeled glucose infusion as previously described (9). The derivatives of all time course data were calculated using OOPSEG (5), an optimal segments data smoothing algorithm. Basal was defined as the average of four samples taken every 10 min after a 90-min tracer equilibration period. Steady state was defined as the average of four samples taken during the last 30 min of the infusion protocol.
Statistical analysis.
All experimental data are expressed as means ± SE. Statistical analyses were performed using one-way repeated-measures ANOVA with Bonferroni posttests (95% confidence intervals) for comparisons of octanoate pulse infusion or constant infusion with the saline control group. Paired Student's t-tests were used to compare specific time points within each experimental group. A P value of <0.05 was considered significant. ANOVAs were performed using GraphPad InStat version 3.0 (GraphPad Software, San Diego, CA), and Student's t-tests were performed using Microsoft Excel 2003.
RESULTS
Glucose and insulin.
Glucose levels were well clamped to baseline levels throughout each experimental protocol, and the average time course of plasma glucose during the clamp period did not differ between infusion protocols [P = nonsignificant (NS)] (Table 1). Fasting insulin levels were suppressed by infusion of somatostatin, and insulin was replaced with a 0.1 mU·kg−1·min−1 insulin infusion. Insulin levels throughout the experiment were not significantly different from preexperimental insulin values and were not significantly different between the infusion protocols (P = NS) (Table 1).
Table 1.
Average glucose and insulin values at fasting, during the basal period, and steady-state period for SAL, PUL, and C-INF infusion groups
| Glucose, mg/dl |
Insulin, pM |
|||||
|---|---|---|---|---|---|---|
| SAL | C-INF | PUL | SAL | C-INF | PUL | |
| Fasting | 95.8 ± 1.8 | 93.7 ± 1.8 | 93.7 ± 2.2 | 33.6 ± 3.9 | 34.6 ± 3.3 | 41.2 ± 13.2 |
| Basal | 95.8 ± 2.1 | 92.1 ± 3.2 | 94.9 ± 3.7 | 63.2 ± 0.9 | 57.6 ± 2.6 | 54.3 ± 2.5 |
| Steady state | 99.6 ± 2.3 | 95.2 ± 2.6 | 96.7 ± 2.5 | 56.2 ± 2.6 | 66.5 ± 3.5 | 65.9 ± 1.3 |
Data are means ± SE. SAL, saline infusion; C-INF, constant octanoate infusion (1 mmol/min); PUL, pulsatile octanoate infusion (10 mmol for 2 min every 10 min). There was no significant difference between groups.
FFA.
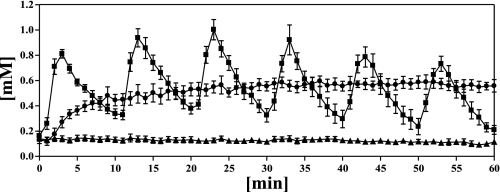
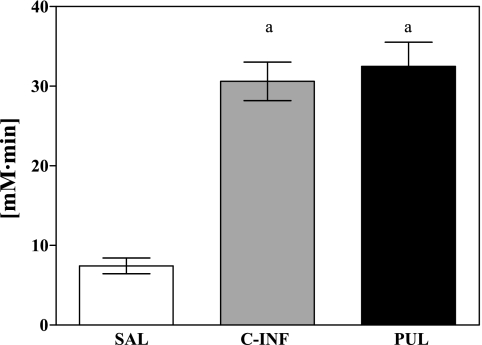
Reflecting the maintenance of baseline conditions during the 90-min tracer equilibration period, FFA levels were not affected by infusion of somatostatin with insulin replacement during this period of the clamp. However, infusion of the adrenergic receptor antagonist bupranolol suppressed SNS-induced pulsatile lipolysis such that plasma FFA concentrations were inhibited by ∼62% from basal steady state to 0.17 ± 0.02 mM before the start of the infusion protocols. As expected, the SAL control infusion did not further alter FFA levels. However, the continuous infusion of 1 mmol/min octanoate raised bupranolol-suppressed FFA levels to 0.51 ± 0.04 mM, which was not significantly different from baseline concentrations (0.55 ± 0.04 mM, P = NS). Pulsatile infusion of octanoate generated a dynamic pattern in plasma FFA levels, with peaks of FFAs of ∼0.8–1.0 mM (Fig. 1). Six pulses of 10 mmol of octanoate each were given over the course of the 1-h clamp period. The total amount of octanoate infused in this protocol was identical to the total mass of octanoate given during the 1-h continuous octanoate infusion protocol (60 mmol). Plasma levels of FFA reflected this mass equivalence, since areas under the curve of FFA concentrations over the 60-min octanoate infusion protocols were almost identical (PUL: 30.6 ± 2.4 vs. C-INF: 32.5 ± 3.0 mM, P = NS), whereas both groups were significantly different from saline control (7.4 ± 1.0 mM, P < 0.05 and P < 0.05, respectively) (Fig. 2).
Fig. 1.
Time course of free fatty acid (FFA) concentrations taken at 1-min intervals during the 60-min experimental infusion protocols: saline infusion (SAL, ▴), pulsatile octanoate infusion (10 mmol for 2 min every 10 min) (PUL, ■), and constant octanoate infusion (1 mmol/min) (C-INF, ●). Data are means ± SE.
Fig. 2.
Area under the curve (AUC) for FFA concentrations over the 60-min experimental infusion protocols (SAL, PUL, and C-INF). Data are means ± SE. aP < 0.05, C-INF, PUL vs. SAL.
Glucose uptake.
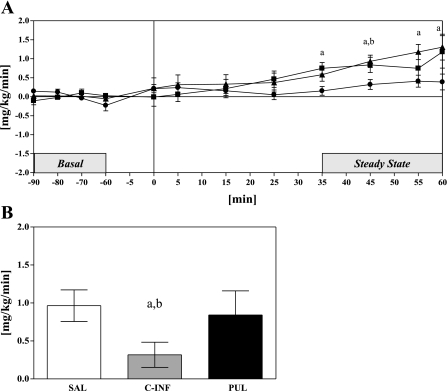
When comparing the effects of pulsatile octanoate infusion on glucose uptake measured during the clamp, we found that, although during pulsatile infusion of octanoate we observed a significant increase in glucose uptake from 2.4 ± 0.5 mg·kg−1·min−1 at basal to 3.3 ± 0.4 mg·kg−1·min−1 (P < 0.05) during the steady-state period, an increase of ∼49% above basal, this increase was similar to that observed during saline control experiments (SAL vs. PUL, P = NS), where basal glucose uptake increased from 2.1 ± 0.3 mg·kg−1·min−1 to steady-state values of 3.0 ± 0.3 mg·kg−1·min−1 (P < 0.001) (Fig. 3A). Interestingly, constant infusion of octanoate may have blunted this increase, since glucose disposal increased from 2.3 ± 0.3 to 2.6 ± 0.3 mg·kg−1·min−1 (P < 0.05). This 17% increase in glucose uptake was significantly lower than the ∼50% increase in glucose uptake in both saline and pulsatile octanoate infusion experiments (P < 0.05 vs. PUL, SAL) (Fig. 3B). Thus, constant infusion of octanoate is associated with impairments in peripheral glucose utilization, whereas pulsatile FFA administration preserves skeletal muscle functionality.
Fig. 3.
A: time course of glucose uptake during the 60-min experimental infusion protocol, shown as a change from basal: SAL (▴), PUL (■), and C-INF (●). Horizontal solid line represents basal glucose uptake. B: change in steady-state glucose uptake, shown as change from basal. Data are means ± SE. P < 0.05 SAL vs. C-INF (a) and C-INF vs. PUL (b).
Glucose production.
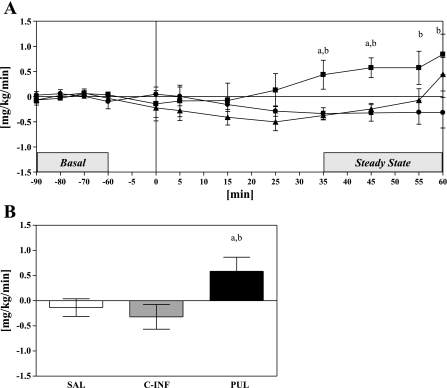
As expected, neither saline nor 1 mmol/min constant infusion of octanoate led to any changes in steady-state glucose production compared with basal. Glucose production had a tendency to decrease in these two groups, although not significantly (SAL: −0.14 mg·kg−1·min−1 vs. C-INF: −0.32 mg·kg−1·min−1, P = NS). In contrast, pulsatile octanoate infusion significantly increased steady-state glucose production; glucose production increased by from 0.9 ± 0.3 mg·kg−1·min−1 at basal to 1.5 ± 0.3 mg·kg−1·min−1 during the clamp steady-state period, an increase of >90% from basal steady state (Fig. 4A). This increase was significantly greater than both saline and constant infusion groups (P < 0.05) (Fig. 4B), demonstrating that a pulsatile pattern of octanoate stimulates glucose output, unlike constant octanoate for which glucose output is the same, or reduced.
Fig. 4.
A: time course of glucose production during the 60-min experimental infusion protocol, shown as a change from basal: SAL (▴), PUL (■), and C-INF (●). Horizontal solid line represents basal glucose production. B: change in steady-state glucose production, shown as change from basal. Data are means ± SE. P < 0.05, SAL vs. C-INF (a) and C-INF vs. PUL (b).
DISCUSSION
Studies demonstrating the role of FFAs in the regulation of glucose production and peripheral glucose uptake have used experimental protocols involving the tonic inhibition of endogenous lipolysis (6, 20) or a constant infusion of exogenous lipids (4, 28, 30). Although these studies are key in establishing a stimulatory effect of FFAs on glucose production by the liver, they did not take into account the pattern of endogenous FFAs in circulation. Studies performed in our laboratory and others (13) have revealed that FFAs are released in an oscillatory, or pulsatile, pattern and that this pulsatile release is controlled by the SNS. However, the physiological effects such a pattern of FFA release may have on glucose production have not been studied previously. Therefore, in the present study, we sought to determine whether an exogenous pulsatile infusion of a FFA would have differential effects on glucose production compared with a continuous infusion.
In this study, glucose turnover was examined while the MCFA octanoate was infused at a constant rate of 1 mmol/min or while a quantitatively identical dose of octanoate was delivered in a pulsatile fashion (10 mmol over 2 min at 10-min intervals). In addition, as a second control, saline was infused in a manner identical to the pulsatile octanoate group. In all experiments, insulin secretion was suppressed by somatostatin, and insulin was replaced at fasting concentrations to prevent FFA-induced stimulation of insulin secretion and resulting suppression of glucose production via hepatic autoregulation (3). The β3-antagonist bupranolol was used to suppress endogenous FFA pulses.
FFA concentrations were reduced by the infusion of bupranolol and remained low with saline control infusions, whereas both constant and pulsatile infusions of octanoate increased average plasma FFA concentrations. However, whereas constant infusion led to stable levels of FFAs in the range of normal fasting concentrations, pulsatile delivery of the FFA led to peaks of FFAs of 0.8–1.0 mM in height. As expected, neither saline nor constant infusion of octanoate increased glucose production. However, when octanoate was infused in a pulsatile manner, steady-state glucose production nearly doubled compared with the basal period. These data show for the first time that pulsatility of FFA may indeed have an important role in the regulation of glucose production.
FFAs are known to increase hepatic glucose production via stimulation of gluconeogenesis (4, 28, 30). It is thought that hepatic oxidation of FFA leads to the production of several key substrates, including NADH, ATP, and acetyl-coA, which may activate the gluconeogenic pathway or inhibit glycolysis. In addition, FFAs may directly increase glucose production by preventing glucose 6-phosphate formation through allosteric inhibition of glucokinase and concomitant stimulation of glucose-6-phosphatase activity (17). It is possible that pulsatile FFAs may be a more efficient means of stimulating glucose production, since peaks of pulsatile FFAs may lead to greater substrate formation and gluconeogenic enzyme activity while minimizing total lipolytic activity to achieve this state.
Pulsatile hormone secretion has been shown to elicit greater physiological effects in many areas. Of particular interest are the results of studies examining the role of pulsatile glucagon and pulsatile insulin delivery on hepatic glucose production. Komjati et al. (16) reported that pulsatile exposure of isolated perfused rat livers to glucagon required only half of the total dose to achieve the same effect on hepatic glucose production as a continuous infusion of glucagon. In this same study, giving insulin in a pulsatile pattern also reduced the total amount of insulin needed to suppress glucose production in vitro. In vivo studies have produced mixed results depending upon the dose and duration of hormone given. When given at supraphysiological levels, pulsatile glucagon and insulin delivery have the same effect on glucose production as continuous delivery in vivo (24, 25). However, studies in which both glucagon and insulin were infused to achieve more physiological conditions showed that pulsatile delivery induced a greater effect on glucose production than continuous infusion (21–23). That pulses in FFAs are more effective than continuous delivery supports the finding that the liver is sensitive to the pattern of effectors in circulation in the regulation of glucose homeostasis.
Glucose production had a tendency to decrease during saline infusion. This effect also occurred during constant infusion of octanoate. This decrease in glucose production may be due to several factors. The suppression of FFAs by bupranolol may lead to a reduction in glucose production, similar to the suppression of hepatic glucose production seen in studies using other lipolytic inhibitors, such as nicotinic acid or cylcohexyladenosine (3, 20). In addition, β-adrenergic receptor antagonism may also have a direct suppressive effect on the liver to inhibit glucose production (7). Moreover, glucagon was not replaced in this protocol, which may also add to the reduction in glucose production (18).
Studies examining the effect of FFAs on glucose production use the exogenous infusion of a lipid emulsion, typically Liposyn accompanied by heparin, to induce intravascular lipase activity. However, the emulsion was not appropriate for the present experiments because of varying rates of lipolysis induced by heparin administration, which may have interfered with the dynamic nature of the pulsatile delivery. In addition, the use of long-chain fatty acids (LCFAs), which are the most abundant of the FFAs in circulation, was impractical because of the high amounts of albumin or alkalinity necessary to solubilize LCFAs in plasma. The MCFA octanoate was chosen for these experiments because of its aqueous solubility. Because plasma FFAs mainly consist of LCFAs, there may be some question as to the generalization of the effects of octanoate to all FFAs. In addition to chain length, LCFAs and MCFAs also differ in that MCFAs cross the inner mitochondrial membrane independent of the carnitine transport system for β-oxidation, such that MCFA may be preferentially oxidized. This suggests that the kinetics of the effect of MCFA on glucose production may differ. In addition, there is some evidence that LCFAs may have a greater stimulatory effect on glucose production than MCFAs, although the effect on glucose uptake was similar (15). It would be of interest to compare the effect of the pulsatile delivery of various chain-length fatty acids on glucose production. However, these studies may be impractical, since extremely large volumes must be used to infuse LCFAs in such a fashion.
Interestingly, steady-state glucose uptake increased during saline control experiments. It has been shown that bupranolol maintained glucose tolerance during an intravenous glucose tolerance test despite a significant decrease in plasma insulin, suggesting that peripheral glucose uptake may be enhanced by the β3-specific adrenergic antagonist (8). Although glucose uptake also increased during constant infusion of octanoate compared with basal, this increase was significantly less than during saline control experiments, suggesting that constant infusion of octanoate partially impaired peripheral glucose disposal. FFAs are known to inhibit basal and insulin-stimulated glucose disposal in skeletal muscle in a dose-dependent manner, with effects observed even at low levels (12). FFA-induced insulin resistance is associated with several possible mechanisms, including reduction in glucose oxidation, impairment of insulin receptor signaling, and/or inhibition of glucose transporter activity (1, 26, 27). Pulsatile octanoate infusion had no specific effect on glucose uptake, since steady-state values were similar to those achieved during saline controls. It is possible that, whereas the “peaks” of FFA may stimulate glucose production, the reciprocal “valleys” seen with pulsatile FFA infusion may serve to relieve alterations in enzyme activity or receptor signaling induced by continuous infusion. These data taken together suggest that FFA pulsatility may spare skeletal muscle from impairments in glucose uptake, which are induced by a constant infusion of FFA.
Previous studies by Hucking et al. (13) found that FFAs oscillate in circulation at ∼9 pulses/h with an amplitude of ∼0.11 mM. In this study, a pulse frequency of 6 pulses/h was chosen to capture distinct differences in each pulse given, and the continuous infusion rate was chosen to maintain basal FFA levels. The infusion rates used in this study are quantitatively greater than what our previous studies showed in vivo. It is possible that differences between the in vivo state and the infusion protocol infusion used in this study may have had an effect on the current results. Further studies that more closely mimic in vivo conditions must be performed to confirm the results of this study.
In summary, we have shown that pulsatile delivery of the MCFA octanoate is able to stimulate glucose production in contrast to the neutral effect of a quantitatively identical constant infusion. In addition, pulsatile FFA may protect muscle from reductions in glucose uptake as seen with continuous infusion. These data suggest that the pattern of FFA release may have differential effects on hepatic glucose production and peripheral glucose uptake. More importantly, these data indicate that the central nervous system may be involved in the regulation of glucose homeostasis through SNS control of pulsatile lipolysis.
Because obesity is associated with alterations in lipolysis and elevated FFA levels, it is possible that changes in the pattern of FFA release may contribute to the development of obesity-induced insulin resistance. Therefore, further studies examining pulsatile FFA release and its role in glucose homeostasis in obesity may be important in elucidating the link between FFA and insulin resistance.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-27619 and by an American Diabetes Association Physician Scientist Training Award to I. R. Hsu.
DISCLOSURES
The authors do not have any conflicts of interest to disclose.
ACKNOWLEDGMENTS
We are grateful to Rita Thomas for expert technical assistance.
REFERENCES
- 1.Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, DeFronzo RA, Cusi K. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 54: 1640–1648, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Boden G. Free fatty acids (FFA), a link between obesity and insulin resistance. Front Biosci 3: D169–D175, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Chen X, Capulong E, Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes 50: 810–816, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Boden G, Jadali F. Effects of lipid on basal carbohydrate metabolism in normal men. Diabetes 40: 686–692, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Bradley DC, Steil GM, Bergman RN. OOPSEG: a data smoothing program for quantitation and isolation of random measurement error. Comput Methods Programs Biomed 46: 67–77, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest 103: 365–372, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu CA, Sindelar DK, Igawa K, Sherck S, Neal DW, Emshwiller M, Cherrington AD. The direct effects of catecholamines on hepatic glucose production occur via α1- and β2-receptors in the dog. Am J Physiol Endocrinol Metab 279: E463–E473, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Conca W, Beck A, Bacher S, Raberger G. Plasma insulin levels and beta-adrenoceptor antagonists. The effects of cardioselective and non-cardioselective beta-adrenoceptor antagonists with and without intrinsic sympathomimetic activity on basal insulin level and insulin level after glucose stimulation in normoglycemic dogs. Naunyn Schmiedebergs Arch Pharmacol 320: 63–66, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36: 914–924, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Getty L, Panteleon AE, Mittelman SD, Dea MK, Bergman RN. Rapid oscillations in omental lipolysis are independent of changing insulin levels in vivo. J Clin Invest 106: 421–430, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Manchon C, Ayuso MS, Parrilla R. Control of hepatic gluconeogenesis: role of fatty acid oxidation. Arch Biochem Biophys 271: 1–9, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Gormsen LC, Jessen N, Gjedsted J, Gjedde S, Norrelund H, Lund S, Christiansen JS, Nielsen S, Schmitz O, Moller N. Dose-response effects of free fatty acids on glucose and lipid metabolism during somatostatin blockade of growth hormone and insulin in humans. J Clin Endocrinol Metab 92: 1834–1842, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hucking K, Hamilton-Wessler M, Ellmerer M, Bergman RN. Burst-like control of lipolysis by the sympathetic nervous system in vivo. J Clin Invest 111: 257–264, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karpe F, Fielding BA, Coppack SW, Lawrence VJ, Macdonald IA, Frayn KN. Oscillations of fatty acid and glycerol release from human subcutaneous adipose tissue in vivo. Diabetes 54: 1297–1303, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Keller U, Turkalj I, Laager R, Bloesch D, Bilz S. Effects of medium- and long-chain fatty acids on whole body leucine and glucose kinetics in man. Metabolism 51: 754–760, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Komjati M, Bratusch-Marrain P, Waldhausl W. Superior efficacy of pulsatile versus continuous hormone exposure on hepatic glucose production in vitro. Endocrinology 118: 312–319, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Lam TKT, Van de Werve G, Giacca A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am J Physiol Endocrinol Metab 284: E281–E290, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes 44: 185–189, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes 32: 617–621, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Mittelman SD, Bergman RN. Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab 279: E630–E637, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Paolisso G, Buonocore S, Gentile S, Sgambato S, Varricchio M, Scheen A, D'Onofrio F, Lefebvre PJ. Pulsatile glucagon has greater hyperglycaemic, lipolytic and ketogenic effects than continuous hormone delivery in man: effect of age. Diabetologia 33: 272–277, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Paolisso G, Scheen AJ, Albert A, Lefebvre PJ. Effects of pulsatile delivery of insulin and glucagon in humans. Am J Physiol Endocrinol Metab 257: E686–E696, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Paolisso G, Scheen AJ, Giugliano D, Sgambato S, Albert A, Varricchio M, D'Onofrio F, Lefebvre PJ. Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in man: importance of pulse frequency. J Clin Endocrinol Metab 72: 607–615, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Paolisso G, Scheen AJ, Luyckx AS, Lefebvre PJ. Pulsatile hyperglucagonemia fails to increase hepatic glucose production in normal man. Am J Physiol Endocrinol Metab 252: E1–E7, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Paolisso G, Scheen AJ, Verdin EM, Luyckx AS, Lefebvre PJ. Insulin oscillations per se do not affect glucose turnover parameters in normal man. J Clin Endocrinol Metab 63: 520–525, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14: 263–283, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97: 2859–2865, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, Nowotny P, Waldhausl W, Shulman GI. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 49: 701–707, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Schmitz O, Brock B, Hollingdal M, Juhl CB, Porksen N. High-frequency insulin pulsatility and type 2 diabetes: from physiology and pathophysiology to clinical pharmacology. Diabetes Metab 28: 4S14–4S20, 2002 [PubMed] [Google Scholar]
- 30.Staehr P, Hother-Nielsen O, Landau BR, Chandramouli V, Holst JJ, Beck-Nielsen H. Effects of Free Fatty Acids Per Se on Glucose Production, Gluconeogenesis, and Glycogenolysis. Diabetes 52: 260–267, 2003 [DOI] [PubMed] [Google Scholar]