Abstract
In peripheral tissues, the link between obesity and insulin resistance involves low-grade inflammation induced by macrophage activation and proinflammatory cytokine signaling. Since proinflammatory cytokines are also induced in the hypothalamus of animals placed on a high-fat (HF) diet and can inhibit neuronal signal transduction pathways required for normal energy homeostasis, hypothalamic inflammation is hypothesized to contribute to the pathogenesis of diet-induced obesity (DIO). We addressed this hypothesis by perturbing the inflammatory milieu of the hypothalamus in adult male Wistar rats using intracerebroventricular (icv) administration of interleukin-4 (IL-4), a Th2 cytokine that promotes alternative activation (M2) of macrophages and microglia. During HF feeding, icv IL-4 administration increased hypothalamic proinflammatory cytokine gene expression and caused excess weight gain. Intracerebroventricular pretreatment with PS1145, an inhibitor of IKKβ (a key intracellular mediator of inflammatory signaling), blocked both IL-4 effects, suggesting a causal relationship between IL-4-induced weight gain and hypothalamic inflammation. These observations add to growing evidence linking hypothalamic inflammation to obesity pathogenesis.
Keywords: obesity, hypothalamus, high-fat diet, Iκβ kinase
obesity predisposes to type 2 diabetes and related metabolic disorders in part by reducing insulin sensitivity, an effect that involves low-grade inflammation in peripheral tissues such as adipose tissue (12, 34). Tissue inflammation during high-fat (HF) feeding results from the recruitment and activation of macrophages, which can cause insulin resistance through local or systemic release of proinflammatory cytokines (12, 19, 25, 34). Recent evidence that HF feeding increases the expression of proinflammatory cytokines, including TNFα, interleukin (IL)-1β, and IL-6, in the hypothalamus as well as in peripheral tissues (6) has raised the possibility that hypothalamic inflammation contributes to the pathogenesis of diet-induced obesity (DIO). One potential mechanism to explain this effect links inflammation in hypothalamic areas central to energy homeostasis, such as the arcuate nucleus, with resistance to input from key afferent signals, including the hormones insulin and leptin (33). Decreased neuronal insulin/leptin signaling, in turn, favors weight gain.
The IKKβ/NF-κB intracellular signaling pathway is a key mediator of the cellular response to inflammatory stimuli and also plays an important role in obesity-associated peripheral metabolic disorders (10). In the hypothalamus, activation of the IKKβ/NF-κB pathway is implicated in the pathogenesis of DIO during HF feeding (28, 39). This assertion is based on evidence that hypothalamic IKKβ activation causes leptin resistance and favors weight gain (39), whereas central administration of an IKKβ inhibitor acutely reverses hypothalamic insulin resistance and reduces food intake in rats after 8 wk of HF feeding (28).
Although a great deal has been learned about mechanisms underlying obesity-associated inflammation in peripheral tissues, much less is known about the pathogenesis of hypothalamic inflammation induced by HF feeding. In the current study, we used IL-4, a Th2 cytokine that favors induction of an anti-inflammatory “M2” phenotype in both peripheral macrophages and brain microglia (3, 8, 9, 16, 20, 22) as an immunomodulator in the brain to determine whether energy homeostasis is affected by altering the inflammatory milieu of the hypothalamus and whether this effect is sensitive to differences in diet composition. Unexpectedly, we observed that intracerebroventricular (icv) IL-4 administration increased hypothalamic expression of proinflammatory cytokines during HF diet (HFD) feeding (but not during consumption of standard chow) and that this hypothalamic proinflammatory response is required for the effect of central IL-4 treatment to promote weight gain.
METHODS
Experimental Animals
Weight-matched male Wistar rats (320–350 g; Harlan, Indianapolis, IN) were housed individually in a specific pathogen-free environment, maintained in a temperature-controlled room with a 12:12-h light-dark cycle, and provided with ad libitum access to water and either standard laboratory chow (3.34 kcal/g; PMI Nutrition International) or a diet containing 60% kcal fat (HF, 5.24 kcal/g, D12492; Research Diets, New Brunswick, NJ). All study protocols were approved by the Animal Care and Use Committee at the University of Washington and conducted in accordance with the National Institutes of Health guidelines for the care and use of animals.
Stereotaxic Surgery
All rats underwent surgical implantation of an indwelling stainless steel cannula into the third cerebral ventricle (3V) under isoflurane anesthesia, as described previously (32). Animals were allowed to recover for ≥7 days while daily food intake and body weight were recorded. Cannula placement was verified through the measurement of a sympathetically mediated increase of plasma glucose 60 min after icv injection of 5-thio-d-glucose (210 mg) (30).
Study Protocols
Experiment 1: effect of icv IL-4 on body weight and hypothalamic inflammation.
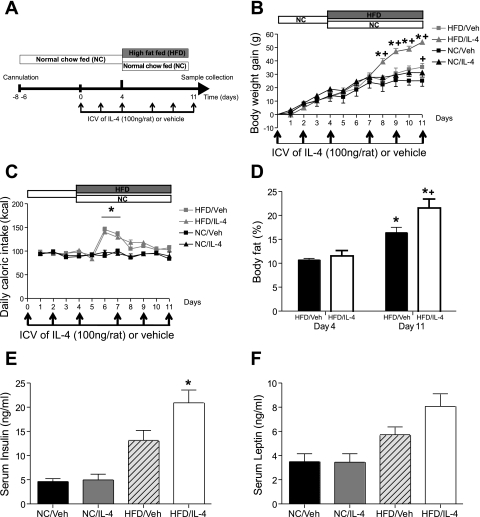
As shown in Fig. 1A, 3V-cannulated rats were separated into two groups (n = 18–20/group), one of which was switched from standard chow to the HFD on day 5, whereas the other was maintained on standard chow throughout the 11-day study period. On the basis of preliminary dose-finding studies (data not shown), rats in each group received a single 2-µl icv injection of either IL-4 (100 ng) dissolved in saline containing 0.1% BSA or saline-BSA vehicle three times/wk. Food intake and body weight were measured daily. Body composition analysis to determine lean and fat mass was conducted on days 4 and 11 in conscious rats, using quantitative magnetic resonance spectroscopy (EchoMRI 3-in-1 machine; Echo Medical Systems, Houston, TX). At study termination, rats were euthanized with blood and hypothalamic (mediobasal wedge) samples, collected as described previously (38) and stored at −80°C.
Fig. 1.
Effect of intracerebroventricular (icv) interleukin (IL)-4 on rats during high-fat (HF) feeding. A: diagram of experimental protocol used in experiment 1. Four groups of 3rd ventricle (3V)-cannulated rats (n = 9–10/group) were treated with icv vehicle (Veh) or IL-4 (100 ng) 3 times/wk over 11 days. All animals were maintained on normal chow (NC) during the surgical recovery phase (day −7 to day 0) and for the 1st 4 days after icv treatments were initiated. On day 4, 1 Veh group and 1 IL-4 group began the HF diet (HFD) and continued it for an additional 7 days. Body weight (B) and caloric intake (C) were monitored daily for NC/Veh (■), HFD/Veh (gray squares), NC/IL-4 (▲), and HFD/IL-4 (gray triangles). *P < 0.05 vs. HFD/Veh; +P < 0.05 vs. NC/Veh. D: %body fat was determined by quantitative magnetic resonance (MR) spectroscopy on days 4 and 11 for icv HFD/Veh (black bars) and HFD/IL-4 (open bars). *P < 0.05 vs. HFD/Veh on day 4; +P < 0.05 vs. HFD/Veh on day 11. Insulin (E) and leptin (F) levels were measured from sera obtained 4 h after icv injection on day 11. *P < 0.05 vs. all other groups.
Experiment 2: role of hypothalamic IKKβ signaling in the actions of icv IL-4.
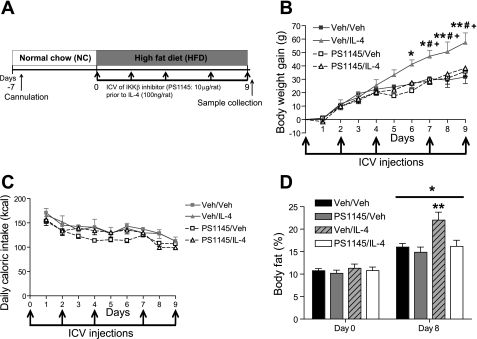
Four groups of rats (n = 6/group) consumed the HFD for a 9-day study period. Animals in each group received two consecutive icv injections three times/wk, as in experiment 1 and shown in Fig. 3A. Immediately prior to icv injection of IL-4 (100 ng) or vehicle, all animals received a pretreatment icv injection of either the IKKβ inhibitor PS1145 (10 μg) or its vehicle (saline). The dose of PS1145 used for pretreatment was selected on the basis of our previous publication (28) showing effective amelioration of HF feeding-induced hypothalamic inflammation. Food intake and body weight were measured daily. Body composition analysis was conducted as above on days 0 and 8. On day 9, animals were euthanized and samples collected as above.
Fig. 3.
Effect of icv IKKβ inhibitor pretreatment on icv IL-4 enhancement of weight gain during HF feeding. A: diagram of experimental protocol for experiment 2. Three times/wk, 3V-cannulated rats (n = 6/group) maintained on HFD received icv pretreatment with saline or PS1145 (10 μg) 1 h prior to icv Veh or IL-4 (100 ng). Body weight (B) and caloric intake (C) were monitored daily in Veh/Veh (gray squares), Veh/IL-4 (gray triangles), PS1145/Veh (☐), and PS1145/IL-4 (▵) animals. *P < 0.05, **P < 0.001: Veh/IL-4 vs. Veh/Veh; #P < 0.05: Veh/IL-4 vs. PS1145/Veh; and +P < 0.01: Veh/IL-4 vs. PS1145/IL-4. D: mean %body fat was determined by quantitative MR spectroscopy on days 0 and 8. *P < 0.05 vs. same groups on day 0; **P < 0.05 vs. all other groups on day 8. Black bars, Veh/Veh; gray bars, PS1145/Veh; hatched gray bars, Veh/IL-4; open bars, PS1145/IL-4.
Plasma Assays
Blood glucose was measured using a hand-held glucometer (Accu-Chek; Roche Diagnostics) on blood obtained from tail capillary samples. Plasma immunoreactive insulin and leptin levels were measured by ELISA (Crystal Chem, Chicago, IL).
Analysis of Gene Expression
Total RNA was extracted from hypothalamus using TRIzol B according to the manufacturer's instructions (MRC, Cincinnati, OH) and reverse-transcribed (1 mg) with AMV reverse transcriptase (Promega, Madison, WI). Real-time PCR was performed on an ABI Prism 7900 HT (Applied Biosystems, Foster City, CA), as described previously (38). Expression levels of each gene were normalized to a housekeeping gene (GAPDH mRNA) and expressed as % control. To ensure that outcomes were not affected by changes in GAPDH expression, mRNA data were also normalized to cyclophilin and 18S RNA levels. Because normalization of our mRNA data to these alternative housekeeping genes had no effect on any outcome, only data obtained by normalizing to GAPDH mRNA are reported. The following primer sequences were used: IL-1β: forward 5′-tacaaggagagacaagcaacgaca-3′, reverse 5′-gatccacactctccagctgca-3′; TNFα: forward 5′-catcttctcaaaactcgagtgacaa-3′, reverse 5′-tgggagtagataaggtacagccc-3′; IL-6: forward 5′-aatctgccctgcggagtgaag-3′, reverse 5′-ttcatgcccttggctacctga-3′; Arg-1: forward 5′-ctccaagccaaagtccttagag-3′, reverse 5′-aggagctgtcattagggacacatc-3′; and GAPDH: forward 5′-aacgaccccttcattgac-3′, reverse 5′-tccacgacatactcagcac-3′.
Statistical Analysis
All results are expressed as means ± SE. Statistical analyses were performed using Graph Pad PRISM (version 4.0b; Graph Pad Software, La Jolla, CA). Two-way factorial and repeated-measures ANOVA with least significant difference post hoc tests were used to compare mean values between multiple groups. In all instances, P values of <0.05 were considered significant.
RESULTS
Effect of icv IL-4 Administration on Body Weight, Body Composition, and Energy Balance
To examine the effect of central IL-4 administration on energy balance, a series of studies was performed in which either IL-4 (100 ng icv) or vehicle was administered three times/wk directly into the 3V of rats fed standard chow. On day 5 one group was switched to the HFD, whereas the other was maintained on standard chow (Fig. 1A). In rats fed standard chow throughout the study, icv injection of IL-4 had no effect on body weight over the 11-day protocol relative to icv vehicle-treated controls (Fig. 1B). By comparison, consumption of the HFD increased body weight in both vehicle and IL-4-treated groups relative to chow-fed animals [main effect of diet: F(1,25) = 9.267, P < 0.01], but weight gain among icv IL-4-injected rats during HF feeding was 54% greater than was observed in icv vehicle-treated controls [main effect of time × diet × IL-4: F(6,150) = 4.46, P < 0.05]. This additional body weight gain reached statistical significance by the 5th day after the switch to the HFD and remained significant on each subsequent day (P < 0.05 for HFD/IL-4 vs. HFD/vehicle on days 8–11; Fig. 1B). As expected, animals placed on the HFD were transiently hyperphagic relative to chow for the first several days, irrespective of icv treatment condition (P < 0.01 on day 6; Fig. 1C). Relative to icv vehicle, however, icv IL-4 had no significant effect on intake of either diet (Fig. 1C). Thus, centrally administered IL-4 increased weight gain selectively during HF feeding through a mechanism independent of changes in caloric intake.
The effects of both the HFD and icv IL-4 to induce weight gain were attributable to increased body fat rather than increased lean mass. HF feeding increased percent body fat among icv vehicle-injected rats by ∼50% (measured 8 days after the switch to HF feeding) but nearly doubled percent body fat among IL-4-injected rats over the same time interval [main effect of diet: F(1,9) = 178.5, P < 0.0001; main effect of IL-4: F(1,9) = 5.493, P < 0.05; diet × IL-4: F(1,9) = 17.46, P < 0.01] (Fig. 1D). As expected, HF feeding increased plasma insulin [F(1,18) = 37.06, P < 0.0001; Fig. 1E] and leptin levels [F(1,20) = 25.96, P < 0.0001; Fig. 1F] above chow-fed controls. The effect of icv IL-4 to increase body fat content during HF feeding was associated with corresponding near-significant increases in plasma concentrations of insulin [60% increase; F(1,18) = 4.203, P = 0.0552] and leptin [58% increase; F(1,20) = 4.058, P = 0.0576] (Fig. 1, E and F). These observations suggest that excess fat accumulation induced by central IL-4 administration during HF feeding was associated with increased insulin and leptin resistance.
Effect of HF Feeding and icv IL-4 on Hypothalamic Proinflammatory Gene Expression
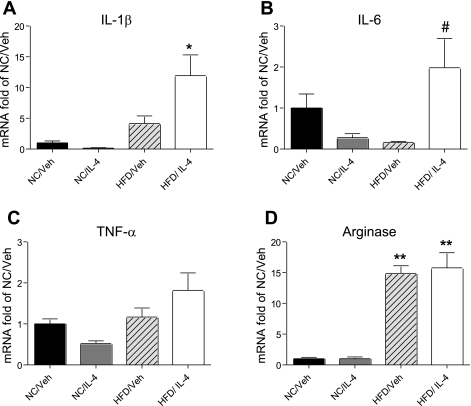
To evaluate the effect of repeated central IL-4 administration on hypothalamic inflammatory status during HF feeding, we used RT-PCR to measure proinflammatory cytokine and macrophage activation marker mRNA levels in samples of mediobasal hypothalamus obtained 4 h after the last IL-4 injection (day 11; Fig. 2, A–D). Consistent with previous reports (4), the level of IL-1β mRNA in mediobasal hypothalamus was increased fourfold in rats fed the HFD vs. standard chow [main effect of diet: F(1,31) = 21.433, P = 6.2 × 10−5], and icv IL-4 administration further increased this value >10-fold [P = 4.4 × 10−5 vs. chow/vehicle; P = 1.7 × 10−3 vs. HFD/vehicle] (Fig. 2A). Similarly, icv IL-4 treatment increased hypothalamic levels of TNFα and IL-6 mRNA twofold compared with vehicle in rats fed a HFD, although this effect was not statistically significant (Fig. 2, B and C). The same outcome was obtained whether cytokine mRNA species were normalized to GAPDH, cyclophilin, or 18S mRNA. Although HF feeding substantially increased hypothalamic arginase mRNA content [a marker of the macrophage M2 activation phenotype; F(1,33) = 146.36, P = 1.12 × 10−13], there was no further enhancement by icv IL-4 treatment (Fig. 2D). Thus, proinflammatory cytokine gene expression was induced in the hypothalamus of HFD-fed rats relative to chow-fed controls, as reported previously (6, 28, 38, 39), and this effect was augmented by IL-4.
Fig. 2.
Effect of icv IL-4 on hypothalamic expression of mRNA encoding proinflammatory cytokines during HF feeding. 3V-cannulated rats (n = 9–10/group) from experiment 1 were euthanized 4 h after the final icv injection of either Veh or IL-4 (100 ng) for collection of hypothalamic tissue for measurement of proinflammatory cytokine mRNA encoding IL-1β (A), IL-6 (B), TNFα (C), and arginase (D) by real-time PCR. Data are normalized to GAPDH levels and then represented as fold elevation over NC/Veh. *P < 0.05 vs. all others; #P < 0.05 vs. HFD/Veh; **P < 0.001 vs. NC/Veh.
Unlike in rats fed a HFD, chow-fed rats receiving icv IL-4 injection showed no significant changes in proinflammatory or arginase gene expression (Fig. 2, A–D) relative to vehicle-treated controls. Thus, IL-4 affected markers of hypothalamic inflammation in a diet-dependent manner, having a stimulatory effect only during HFD feeding. This differential effect on hypothalamic inflammation indicates that the unexpectedly proinflammatory effect of icv IL-4 in the HF setting was not simply a nonspecific byproduct of central nervous system (CNS) injections.
Role of Hypothalamic Inflammation in Weight Gain Induced by icv IL-4
The above findings suggested that the effect of central IL-4 administration to increase weight gain during HF feeding was causally linked to the increase in hypothalamic proinflammatory gene expression. To test this hypothesis, we used a previously published strategy (28) in which the IKKβ/NF-κB pathway inhibitor PS1145 was injected icv as a pretreatment to block hypothalamic inflammation induced by IL-4 in adult male Wistar rats consuming a HFD over an 11-day period (Fig. 3A). The four groups of rats according to icv pretreatment/treatment condition were Veh/Veh, Veh/IL-4, PS1145/Veh, and PS1145/IL-4. Consistent with our earlier findings, rats in the Veh/IL-4 group displayed increased weight gain on the HFD compared with the Veh/Veh group (P < 0.05 on days 6–9; Fig. 3B). Furthermore, whereas icv pretreatment with the IKKβ inhibitor had no effect on body weight gain when given prior to icv vehicle (Fig. 3B), it fully blocked the ability of IL-4 to increase weight gain during HF feeding (P < 0.05 on days 6–9 vs. Veh/IL-4; Fig. 3B). Interestingly, these body weight changes again occurred despite the lack of any significant change of caloric intake between the four treatment groups (Fig. 3C). Thus, both the effect of IL-4 to favor weight gain and the effect of the IKKβ inhibitor to block this outcome were likely due to changes in energy metabolism rather than food intake.
Analysis of body composition across the four groups revealed a similar pattern of results. Specifically, consumption of the HFD was associated with significant increases of mean percent body fat over time in each of the four groups [main effect of time F(1,18) = 265.157, P < 0.0001; Fig. 3D], and this effect was greater in the Veh/IL-4 than in the other groups (P < 0.001, day 8 Veh/IL-4 vs. all other groups on days 0 and 8; Fig. 3D). Importantly, the effect of icv IL-4 administration to increase body fat mass during HF feeding was completely blocked by icv PS1145 pretreatment at a dose that had no independent effect on body composition (on day 8: P < 0.001, PS1145/Veh vs. PS1145/IL-4; P = not significant, PS1145/Veh vs. Veh/Veh; Fig. 3D). These findings collectively indicate that central inhibition of IKKβ blocks the increase of body weight and body fat content induced by icv IL-4 during HF feeding.
Role of IKKβ in the Effect of IL-4 on Hypothalamic Cytokine Gene Expression
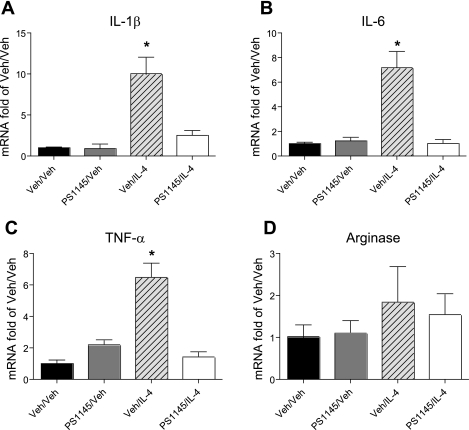
To determine whether the effect of PS1145 to prevent IL-4-induced weight gain was associated with reduced hypothalamic inflammation, we measured proinflammatory cytokine gene expression in the hypothalamus according to the protocol used in previous studies (specifically, 4 h after the last double injection on day 9). In PS1145/IL-4 injected rats, IL-1β mRNA content was decreased by ∼75% compared with that of Veh/IL-4-injected rats, and pretreatment with the IKKβ inhibitor similarly prevented IL-4-mediated increases of hypothalamic TNFα and IL-6 gene expression (P < 0.05 vs. all other groups; Fig. 4, A–C). By comparison, hypothalamic expression of arginase mRNA did not differ significantly across the four treatment groups. These findings indicate that the effect of icv IL-4 to augment HFD-induced hypothalamic proinflammatory cytokine gene expression and weight gain required intact IKKβ/NF-κB signaling, suggesting that hypothalamic inflammation induced by central IL-4 treatment caused excess body fat accumulation during HF feeding.
Fig. 4.
PCR analysis of hypothalamic gene expression in icv IKKβ inhibitor/IL-4-treated rats during HF feeding. 3V-cannulated rats (n = 6/group) from experiment 2 were euthanized 4 h after the final icv injection for collection of hypothalamic tissue. Real-time PCR analysis of IL-1β (A), IL-6 (B), TNFα (C), and arginase (D) gene expression was performed. Data are represented as in Fig. 2. *P < 0.05 vs. all other groups.
DISCUSSION
Despite steady progress in our understanding of hypothalamic neuronal systems that govern energy balance, the mechanisms that underlie the defense of elevated body fat mass in common forms of obesity remain incompletely characterized. Recent evidence that low-grade inflammation occurs not only in peripheral tissues (4, 12, 19, 34) but also in the hypothalamus (6, 28, 38, 39) of animals with DIO has raised the possibility that hypothalamic inflammation plays an etiological role in this disorder, and this hypothesis has received recent experimental support (7, 24, 28, 29, 37, 39). Here, we report that an intervention that increases hypothalamic inflammation, icv administration of IL-4, simultaneously enhances the effect of HF feeding to increase body fat accumulation when administered immediately upon the switch to a HF diet. Moreover, prevention of this inflammatory process using an icv pretreatment with PS1145, an inhibitor of IKKβ, reverses the increased susceptibility to DIO conferred by central IL-4. Thus, inhibition of IKKβ in the brain may be of benefit in efforts to treat or prevent other forms of obesity (28, 39).
Given its anti-inflammatory effects in other model systems, IL-4 administered centrally was expected to decrease hypothalamic proinflammatory cytokine expression induced by HF feeding. Indeed, this is the first example that we are aware of in which IL-4 exerts a proinflammatory effect in the CNS, and the diet-dependent nature of this effect is also novel. Since a large amount of literature documents the effect of IL-4 signaling to block immune-mediated inflammation in both peripheral tissues and brain (3, 8, 9, 16, 25, 34), our findings suggest that the pathogenesis of hypothalamic inflammation during HF feeding may differ fundamentally from that in other models of brain inflammation. One such example of the anti-inflammatory effect of IL-4 involves its action in mouse models of neuronal inflammation such as experimental autoimmune encephalomyelitis (a model of multiple sclerosis) (3) and Alzheimer's dementia (5). In these models, proinflammatory “classical” (M1) activation of microglia exacerbates neuronal injury; central IL-4 administration exerts anti-inflammatory effects and promotes tissue repair, whereas interventions that disrupt IL-4 signaling have the opposite effect (2, 3, 5). Similarly, systemic IL-4 administration was recently reported to reduce liver inflammation and insulin resistance in mice with DIO via a mechanism involving “alternative activation” of Kupffer cells (the resident macrophage of the liver) toward the M2 phenotype (26). Therefore, whether hypothalamic inflammation induced by HF feeding and exacerbated by IL-4 originates in neurons, microglia, or some other cell type is an important unanswered question. These considerations highlight the importance of ongoing efforts to delineate the primary cellular targets of hypothalamic inflammation induced by HF feeding, the time course over which this response occurs, and the mechanisms that underlie these responses.
Although there remains little doubt as to whether DIO is associated with hypothalamic inflammation, the consequences of this effect for the control of body weight remain to be fully elucidated. Of particular interest is the hypothesis that, in key hypothalamic neurons, inflammation causes resistance to input from negative feedback signals such as the hormones insulin and leptin (29, 38). This hypothesis is based in part on clear evidence that inflammation causes cellular insulin resistance in muscle (31), liver (4), adipose tissue (13, 19), and endothelial cells (35). One mechanism implicated in inflammation-induced insulin resistance involves serine phosphorylation of both the insulin receptor and insulin receptor substrate proteins that couple the insulin receptor to downstream cellular targets, including the enzyme phosphatidylinositol 3-OH kinase (PI3K) (14). Indeed, activation of the IKKβ/NF-κB inflammatory pathway potently inhibits insulin-mediated PI3K signaling in multiple cell types (17). In addition, IKKβ/NF-κB activation induces suppressor of cytokine signaling-3 (SOCS3), a protein that blocks cellular signaling by both insulin (11) and leptin (23, 36). Since food intake inhibition by both hormones depends upon intact hypothalamic PI3K signaling (15, 21), and since neuron-specific SOCS3 deletion protects against DIO (24), neuronal inflammation can reasonably be predicted to favor the defense of elevated body weight through its effects on these signal transduction pathways. Indeed, both neuron-specific deletion of IKKβ (39) and icv infusion of the IKKβ inhibitor PS1145 (28) have recently been shown to reduce food intake and weight gain selectively during HF feeding.
On the basis of these considerations, we sought to determine whether the effect of central IL-4 to enhance body fat accumulation during HF feeding was causally linked to increased hypothalamic IKKβ signaling. Our finding that icv pretreatment with PS1145 blocked the effect of icv IL-4 to increase both hypothalamic proinflammatory cytokine expression and weight gain constitutes direct evidence that, in this paradigm, IL-4 acted centrally to increase body fat deposition via a mechanism involving hypothalamic inflammation. Unlike in our previous study (28), icv PS1145 pretreatment did not independently reduce food intake during HF feeding. This discrepancy may reflect differences in study design, since the current study involved icv PS1145 administration during the first few days of HF feeding, whereas in the work by Posey, et al. (28), PS1145 was administered after 8 wk of HF diet when DIO was well established. These considerations highlight the need for future studies that clarify the dose-response and time course characteristics of the effects of IL-4 on energy balance and hypothalamic inflammatory signals during HF feeding. Studies in other more commonly employed rodent models of obesity may also prove illuminating.
Another interesting aspect of the current work is that the effect of icv IL-4 to increase body weight and body fat content was mediated independently of changes of food consumption. This lack of a feeding effect of IL-4 contrasts with the potent hyperphagia that occurs immediately upon the switch to a HF diet and suggests that icv IL-4-mediated hypothalamic inflammation augments weight gain by decreasing metabolic rate and/or fat oxidation. Studies are currently underway to explore this hypothesis. One additional point is that icv PS1145 pretreatment did not block the effect of HF feeding on expression of IL-1β mRNA (in icv vehicle-treated rats), although it did block the effect of icv IL-4 to further increase hypothalamic IL-1β gene expression. This observation raises the possibility that the effect of a HF diet to increase hypothalamic IL-1β mRNA may involve mechanisms additional to activation of IKKβ, whereas the response to IL-4 does not.
In conclusion, we report that, early in the course of HF feeding, paradoxical activation of hypothalamic IKKβ signaling by IL-4 amplifies the effect of a HF diet to promote weight gain and fat accumulation. These findings add to evidence implicating hypothalamic inflammation in the effect of a HF diet to promote obesity and support the hypothesis that inhibition of hypothalamic IKKβ activity may ultimately prove to be of benefit in efforts to treat or prevent obesity.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-068384, DK-052989, and DK-083042 (to M. W. Schwartz), by fellowships from the American Diabetes Association (to S. Oh-I and J. P. Thaler), by the Clinical Nutrition Research Unit (DK-035816) at the University of Washington, and by the American Heart Association Scientist Development Grant and Naomi Berrie Investigator in Diabetes Research Award from Columbia University (to G. J. Morton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the pilot experiments of Miles Matsen and the expert technical assistance of Alex Cubelo, J. D. Fischer, Charles Davis, and Iaela David in conducting this investigation. We thank members of the Schwartz laboratory for helpful discussions regarding these data and this article.
REFERENCES
- 1.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10: 1538–1543, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Butovsky O, Landa G, Kunis G, Ziv Y, Avidan H, Greenberg N, Schwartz A, Smirnov I, Pollack A, Jung S, Schwartz M. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest 116: 905–915, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butti E, Bergami A, Recchia A, Brambilla E, Del Carro U, Amadio S, Cattalini A, Esposito M, Stornaiuolo A, Comi G, Pluchino S, Mavilio F, Martino G, Furlan R. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene Ther 15: 504–515, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci 19: 928–939, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146: 4192–4199, 2005 [DOI] [PubMed] [Google Scholar]
- 7.García MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahrén B, Enerback S, Ohlsson C, Wallenius V, Jansson JO. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes 55: 1205–1213, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10: 1387–1394, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell 132: 344–362, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665–668, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10: 734–738, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hulshof S, Montagne L, De Groot CJ, Van Der Valk P. Cellular localization and expression patterns of interleukin-10, interleukin-4, and their receptors in multiple sclerosis lesions. Glia 38: 24–35, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4: 123–132, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW. Macrophage chemoattractant protein-1 deficiency fails to restrain macrophage infiltration into adipose tissue. Diabetes 57: 1254–1261, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 10: 1544–1553, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab 4: 619–626, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 7: 496–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park KW, Lee DY, Joe EH, Kim SU, Jin BK. Neuroprotective role of microglia expressing interleukin-4. J Neurosci Res 81: 397–402, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296: E1003–E1012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, Seeley RJ. GM-CSF action in the CNS decreases food intake and body weight. J Clin Invest 115: 3035–3044, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 24: 451–452, 1981 [DOI] [PubMed] [Google Scholar]
- 31.Röhl M, Pasparakis M, Baudler S, Baumgartl J, Gautam D, Huth M, De Lorenzi R, Krone W, Rajewsky K, Brüning JC. Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest 113: 474–481, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, Porte D., Jr Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130: 3608–3616, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science 307: 375–379, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweet IR, Gilbert M, Maloney E, Hockenbery DM, Schwartz MW, Kim F. Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phosphate but not increased mitochondrial respiration. Diabetologia 52: 921–931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 24: 5434–5446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Wisse BE, Ogimoto K, Morton GJ, Wilkinson CW, Frayo RS, Cummings DE, Schwartz MW. Physiological regulation of hypothalamic IL-1β gene expression by leptin and glucocorticoids: implications for energy homeostasis. Am J Physiol Endocrinol Metab 287: E1107–E1113, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]