Abstract
The family B G protein-coupled glucagon-like peptide 1 (GLP-1) receptor is an important drug target for treatment of type 2 diabetes. Like other family members, the GLP-1 receptor is a glycosylated membrane protein that contains three potential sites for N-linked glycosylation within the functionally important extracellular amino-terminal domain. However, the roles for each potential site of glycosylation in receptor biosynthesis, trafficking, and function are not known. In this work, we demonstrated that tunicamycin inhibition of glycosylation of the GLP-1 receptor expressed in CHO cells interfered with biosynthesis and intracellular trafficking, thereby eliminating natural ligand binding. To further investigate the roles of each of the glycosylation sites, site-directed mutagenesis was performed to eliminate these sites individually and in aggregate. Our results showed that mutation of each of the glycosylation sites individually did not interfere with receptor expression on the cell surface, ligand binding, and biological activity. However, simultaneous mutation of two or three glycosylation sites resulted in almost complete loss of GLP-1 binding and severely impaired biological activity. Immunostaining studies demonstrated receptor biosynthesis but aberrant trafficking, with most of the receptor trapped in the endoplasmic reticulum and golgi compartments and little of the receptor expressed on the cell surface. Interestingly, surface expression, ligand binding, and biological activity of these mutants improved significantly when biosynthesis was slowed using low temperature (30°C). These data suggest that N-linked glycosylation of the GLP-1 receptor is important for its normal folding and trafficking to the cell surface.
Keywords: G protein-coupled receptor, ligand binding, receptor biological activity, receptor cell surface expression
g protein-coupled receptors (GPCRs) constitute the largest family of membrane proteins that convert external stimuli into cellular activity. They regulate a variety of biological functions, and their activity can be modulated by posttranslational modifications such as phosphorylation, fatty acid acylation, and glycosylation. Most GPCRs are N-linked glycoproteins that utilize the Asn-X-Ser/Thr motif within extracellular domains. The carbohydrate moieties of glycoproteins can be important for normal protein folding, intracellular trafficking, cell surface expression, and protein-protein interactions (19, 25, 29). This posttranslational modification has even been demonstrated to be important for high-affinity binding and signal transduction at various members of the GPCR superfamily (4, 24).
The functional importance of glycosylation of family A GPCRs has been well described (18, 20), but its role in family B GPCRs has been less extensively studied. In this work, we focus on the family B glucagon-like peptide 1 (GLP-1) receptor. Its natural agonist, GLP-1, has multiple antidiabetic actions, being highly effective in reducing blood glucose by increasing β-cell proliferation and sensitivity to glucose, and currently holds promise for effective treatment of type 2 diabetes mellitus. Understanding the role of N-linked glycosylation in the function of the GLP-1 receptor may provide insights into our understanding of the role of this posttranslational modification for family B GPCRs.
In this study, we demonstrated that tunicamycin inhibition of N-linked glycosylation of the GLP-1 receptor abolished its binding and biological activity. We then studied the role of each of the sites of N-linked glycosylation within the extracellular amino-terminal domain of this receptor. We achieved this by utilizing site-directed mutagenesis of the receptor to explore the effects of elimination of single sites and the simultaneous elimination of multiple sites of glycosylation on receptor biosynthesis, trafficking, and function.
MATERIALS AND METHODS
Materials.
Human GLP-1-(7–36) amide (GLP-1) was purchased from Bachem (Torrance, CA). Tunicamycin was from Sigma (St. Louis, MO), and stock solutions of tunicamycin were made by dissolving it in 0.05 M NaOH and stored frozen in aliquots. The solid-phase oxidant N-chlorobenzenesulfonamide (iodo-bead) was purchased from Pierce Chemical (Rockford, IL). Soybean trypsin inhibitor, Fetal Clone II, and tissue culture medium were from Gibco (Grand Island, NY). Bovine serum albumin was from Serologicals (Norcross, GA). Monoclonal mouse anti-human GLP-1 receptor antibody was purchased from R & D Systems (Minneapolis, MN). Alexa 488-conjugated goat anti-mouse IgG was from Invitrogen (Carlsbad, CA). All other reagents were of analytical grade.
Site-directed mutagenesis.
GLP-1 receptor mutants were prepared to replace each of the asparagines that are sites of potential glycosylation (Asn63, Asn82, and Asn115) with leucines (N63L, N82L, and N115L). In addition, these residues were also replaced with leucines in groups of two (N63L/N82L, N63L/N115L, and N82L/N115L) and in groups of three (N63L/N82L/N115L). They were generated using the QuikChange Site-Directed Mutagenesis kit from Stratagene (La Jolla, CA), with all products verified by direct DNA sequencing.
Radioiodination.
The natural GLP-1 ligand GLP-1-(7–36) peptide contains a naturally occurring tyrosine residue in position 19 as a site for radioiodination. It was radioiodinated using brief exposure to the iodobead solid-phase oxidant iodobead, as we described previously (6). The radioiodinated peptide was purified by reverse-phase HPLC to yield approximate specific radioactivity of 2,000 Ci/mmol (27).
Cell culture and tunicamycin treatment.
The GLP-1 receptor-bearing Chinese hamster ovary (CHO) cell line stably expressing the human GLP-1 receptor (CHO-GLP-1R) that was established previously (8) was used for tunicamycin treatment. Cells were cultured in Ham's F-12 medium supplemented with 5% Fetal Clone II in a humidified atmosphere containing 5% CO2 and incubated at 37°C for 40 h in the absence and presence of tunicamycin (0–1 μg/ml). Control cells received equivalent amounts of 0.05 M NaOH. The viability of the cells on the day of study was determined by trypan blue exclusion.
The GLP-1 receptor mutants described above were expressed transiently in COS-1 cells (American Type Culture Collection, Manassas, VA) after transfection using a modification of the DEAE-dextran method (15). The transfection efficiency was ∼60%, as determined by flow cytometry analysis of a yellow fluorescent protein-tagged GPCR (data not shown). Cells were cultured in DMEM supplemented with 5% Fetal Clone II in a humidified atmosphere containing 5% CO2 at 37 or 30°C for 48–72 h before being used in binding, cAMP, and immunostaining assays.
Receptor binding assay.
The binding activities of the COS-1 cells transiently expressing GLP-1 receptor mutant constructs were characterized by radioligand binding, using procedures that we have established previously (11). This assay was used to evaluate both the binding affinity and the density of binding sites per cell (maximal binding capacity). In brief, receptor-bearing COS-1 cells grown at 37 or 30°C were incubated with increasing concentrations of the nonradiolabeled GLP-1 in the presence of a constant amount of the radioligand 125I-GLP-1 (5–10 pM) in Krebs-Ringer-HEPES (KRH) medium (25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, 1.2 mM MgSO4) containing 0.01% soybean trypsin inhibitor and 0.2% bovine serum albumin for 1 h at room temperature (reaction volume 500 μl). After incubation, the receptor-bound radioligand was separated from free radioligand by washing the cells twice with ice-cold KRH medium containing 0.01% soybean trypsin inhibitor and 0.2% bovine serum albumin. Cells were lysed with 0.5 M NaOH, and receptor-bound radioactivity was quantified using a γ-counter. Data were calculated using the nonlinear, least-squares curve-fitting program LIGAND (22) and graphed using the Prism program version 3.02 (GraphPad Software, San Diego, CA). Data are reported as means ± SE of duplicate determinations from a minimum of three independent experiments.
Biological activity assay.
Approximately 8,000 COS-1 cells expressing the GLP-1 receptor or receptor mutants were placed in each well in a 96-well plate and cultured at 37 or 30°C for 24–30 h. Cells were washed twice with PBS and stimulated for 30 min at 37°C with increasing concentrations of GLP-1 (0–1 μM) in KRH medium containing 0.01% soybean trypsin inhibitor, 0.2% bovine serum albumin, 0.1% bacitracin, and 1 mM 3-isobutyl-1-methylxanthine. The reaction was terminated by removing the peptide solution and adding 6% ice-cold perchloric acid. After vigorous agitation for 15 min, the cell lysates were adjusted to pH 6.0 with 30% KHCO3. The supernatants were applied directly for cAMP quantification using a LANCE cAMP-384 kit from PerkinElmer (Boston, MA). The assay was performed according to the manufacturer's instructions and repeated in duplicate in at least three independent experiments.
Immunostaining.
For morphological assessment of the expression of the GLP-1 receptor constructs, COS-1 cells transiently transfected with each construct were replated on coverslips and allowed to grow at 37 or 30°C for 72 h after transfection. Cells were washed with PBS and fixed in 2% paraformaldehyde in PBS for 30 min. For selected experiments, cells were treated with 0.1% saponin in PBS for 10 min. Cells were washed with PBS and then blocked with 1% normal goat serum in PBS before being incubated with monoclonal anti-human GLP-1 receptor antibody (1:60) for 1 h. Cells were washed three times with 1% normal goat serum in PBS and were incubated with Alexa 488-conjugated goat anti-mouse IgG (1:200) for 1 h. Coverslips were then washed four times with PBS, mounted on slides, and examined with an LSM 510 confocal microscope (Zeiss, Thornwood, NY). All above procedures were performed at room temperature.
RESULTS
Effects of tunicamycin on GLP-1 receptor binding.
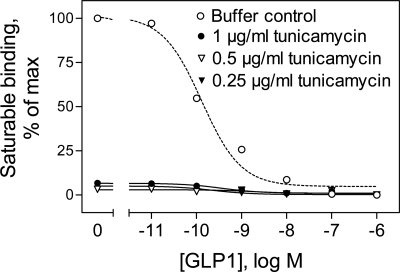
The effects of tunicamycin treatment were examined with the GLP-1R-CHO cells stably expressing the GLP-1 receptor. Tunicamycin is an antibiotic that blocks N-linked glycosylation by preventing the transfer of N-acetyl-glucosamine residue to dolichol phosphate, thus inhibiting the synthesis of all N-linked glycoproteins. It has been reported that tunicamycin treatment of natural GLP-1 receptor-bearing rat RINm5F insulinoma cells decreases GLP-1 binding, with the affinity of receptors on the cell surface being unchanged and the number of surface receptors being reduced in a concentration-dependent manner (10). Figure 1 shows that GLP-1R-CHO cells treated with 0.25–1 μg/ml tunicamycin did not show any detectable radioligand binding (under conditions in which cell viability assessed using trypan blue exclusion was >99%). The intracellular cAMP accumulation in response to GLP-1 treatment in tunicamycin-treated cells also decreased dramatically compared with that of control cells (data not shown). These data indicate that glycosylation of the GLP-1 receptor is important for normal receptor function.
Fig. 1.
Effects of tunicamycin on glucagon-like peptide-1 (GLP-1) binding to human GLP-1 receptor-bearing Chinese hamster ovary (GLP-1R-CHO) cells. The GLP-1R-CHO cells were incubated for 40 h at 37°C with a range of concentrations of tunicamycin before being incubated for 1 h with the radioligand 125I-GLP-1 in the presence of increasing concentrations of unlabeled GLP-1. A representative competition-binding curve is shown for each condition.
Effects of single mutations of the putative glycosylation sites on the function of the GLP-1 receptor.
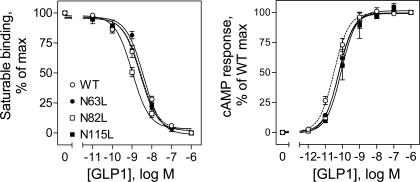
There are three consensus sites for potential N-linked glycosylation (Asn-X-Ser/Thr) within the human GLP-1 receptor, and they are located within the extracellular amino terminus. Mutation of either the Asn or Ser/Thr residue would eliminate the glycosylation on this asparagine. To evaluate the functional importance of the glycosylation sites, we mutated each of the potential glycosylation sites, Asn63, Asn82, and Asn115, to a leucine and characterized these constructs by radioligand binding and GLP-1-induced cAMP responses in COS-1 cells. As shown in Fig. 2 and Table 1, COS-1 cells expressing each of the three single-site mutants (N63L, N82L, and N115L) bound the natural GLP-1 ligand saturably and specifically, with affinities not significantly different from that of the wild-type receptor. In addition, GLP-1 stimulated cAMP responses in COS-1 cells expressing these mutants in a concentration-dependent manner, with similar efficacy and potency to that of the wild-type receptor.
Fig. 2.
Effects of single mutations of the GLP-1 receptor glycosylation sites on ligand binding and biological activity. Left: binding curves for increasing concentrations of the natural GLP-1 ligand to displace binding of the radioligand 125I-GLP-1 to COS-1 cells transfected with the wild-type (WT) and single Asn-to-Leu mutant receptors (N63L, N82L, and N115L). Cells were grown at 37°C after transfection. Values are calculated as percentages of maximal saturable binding observed in the absence of the competitor. They are expressed as means ± SE of duplicate data from 3 independent experiments. Right: intracellular cAMP responses in these cells (grown at 37°C) to stimulation by increasing concentrations of GLP-1. Values are expressed as the means ± SE of at least 3 independent experiments, with data normalized relative to maximal responses. Basal and maximal cAMP levels were similar for each of the GLP-1 receptor constructs tested (4.5 ± 0.9 and 191 ± 49 pmol/1,000,000 cells, respectively).
Table 1.
Binding and biological activity characteristics of WT and mutant GLP-1 receptors expressed in COS-1 cells grown at 37 or 30°C
| Binding |
|||
|---|---|---|---|
| GLP-1 Receptor Constructs | Ki, nM | Bmax, × 103 sites/cell | Intracellular cAMP Response, EC50, pM |
| 37°C | |||
| WT | 2.8 ± 0.1 | 144 ± 35 | 26 ± 5 |
| N63L | 2.9 ± 0.1 | 114 ± 31 | 63 ± 6† |
| N82L | 1.7 ± 0.6 | 122 ± 25 | 61 ± 12† |
| N115L | 2.8 ± 0.3 | 125 ± 30 | 56 ± 4† |
| N63L/N82L | ND | ND | ND |
| N63L/N115L | ND | ND | ND |
| N82L/N115L | ND | ND | ND |
| N63L/N82L/N115L | ND | ND | ND |
| 30°C | |||
| WT | 3.0 ± 0.1 | 96 ± 28 | 12 ± 5 |
| N63L/N82L | 5.6 ± 0.9*† | 85 ± 19* | 20 ± 5* |
| N63L/N115L | 45 ± 7*†‡ | 89 ± 20* | 57 ± 8*†‡ |
| N82L/N115L | 32 ± 6*†‡ | 82 ± 34* | 50 ± 8*† |
| N63L/N82L/N115L | 69 ± 9*†‡ | 72 ± 22* | 51 ± 7*† |
Values are means ± SE. WT, wild type; GLP-1, glucagon-like peptide 1; Ki, binding inhibition constant; Bmax, maximal binding capacity; ND, not detectable. Two-tailed P value tests were performed to determine the significance of differences using InStat3 (GraphPad Software, San Diego, CA).
Value at 30°C is significantly different from that of the same construct at 37°C (P < 0.01; see Figs. 3 and 5).
Value is significantly different from that of the WT receptor at the same temperature (P < 0.05,
P < 0.01).
Effects of multiple mutations of the putative glycosylation sites on the function of the GLP receptor.
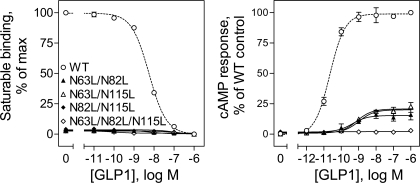
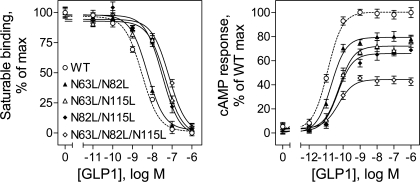
Since individual mutation of glycolsylation sites did not have significant impact on either ligand binding or biological activity of the GLP-1 receptor, we examined whether simultaneous mutation of these sites in groups of two and three sites could result in significant impact on the function of the GLP-1 receptor. As shown in Fig. 3, COS-1 cells expressing each of the double mutants (N63L/N82L, N63L/N115L, and N82L/N115L) and the triple mutant (N63L/N82L/N115L) did not show detectable GLP-1 binding, and only minimal cAMP responses to GLP-1 stimulation were detected in these cells. This was felt to most likely reflect interference of receptor trafficking to the cell surface.
Fig. 3.
Effects of multiple mutations of the GLP-1 receptor glycosylation sites on ligand binding and biological activity. Left: binding curves for increasing concentrations of the natural GLP-1 ligand to displace binding of the radioligand 125I-GLP-1 to COS-1 cells transfected with the WT and multiple Asn-to-Leu mutant receptors (N63L/N82L, N61L/N115L, N82L/N115L, and N63L/N82L/N115L). Cells were grown at 37°C after transfection. Values are calculated as percentages of maximal saturable binding observed in the absence of the competitor. They are expressed as means ± SE of duplicate data from 3 independent experiments. Right: intracellular cAMP responses in these cells (grown at 37°C) to stimulation by increasing concentrations of GLP-1. Values are expressed as means ± SE of at least 3 independent experiments, with data normalized relative to maximal responses.
Effects of mutations of the putative glycosylation sites on the expression of the GLP-1 receptor.
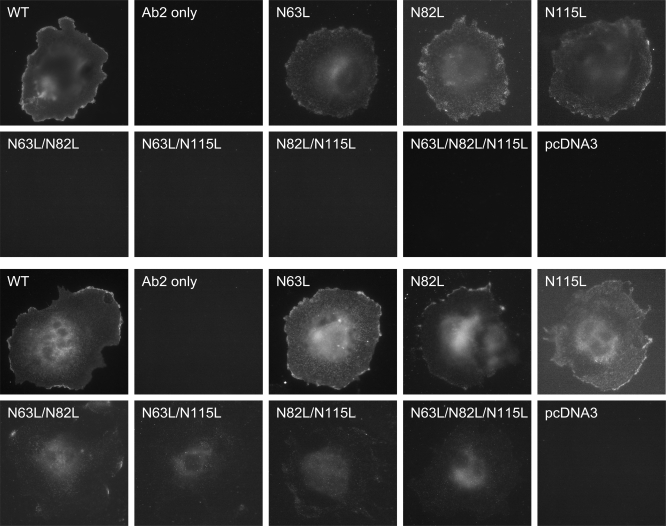
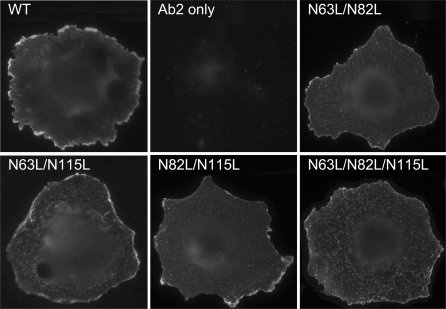
Wild-type and Asn-to-Leu mutant receptors were transiently transfected into COS-1 cells, and their expression was evaluated by immunostaining assays (Fig. 4). Whereas the wild-type receptor and all single Asn-to-Leu mutants were able to traffic normally to the cell surface, little or no cell surface receptor expression was observed for COS-1 cells transfected with the double or triple Asn-to-Leu mutants. It did not matter which two of the sites were mutated, with each pair yielding similar results. COS-1 cells transfected with double and triple Asn-to-Leu GLP-1 receptor mutants did show fluorescence inside the cells after being treated with saponin, further supporting that glycosylation was important for GLP-1 receptor trafficking to the plasma membrane.
Fig. 4.
Morphological localization of GLP-1 receptor constructs. Shown are representative examples of anti-GLP-1 receptor antibody immunostaining of COS-1 cells transfected with the WT and Asn-to-Leu mutant GLP-1 receptors with (top and upper middle rows) and without (lower middle and bottom rows) saponin treatment and cells transfected with the empty pcDNA3 eukaryotic expression vector. Also shown are COS-1 cells transfected with the WT GLP-1 receptor but immunostained only with Alexa 488-conjugated goat anti-mouse IgG. Cells used in this figure were grown at 37°C after transfection. Images are representative of 3 repetitions.
Rescue of surface expression of the misfolded Asn-to-Leu GLP-1 receptor mutants.
Efforts have been made to rescue the surface expression of above double and triple Asn-to-Leu GLP-1 receptor mutants. We first tested whether coexpression of another family B GPCR, the secretin receptor, known to form a heterodimer with the GLP-1 receptor (12), in the absence and presence of the interacting receptor activity-modifying protein 3 (13) was able to help transport these misfolded Asn-to-Leu GLP-1 receptor mutants to the cell surface. Unfortunately, this did not improve the biological activity in COS-1 cells stimulated by GLP-1 (data not shown). Furthermore, coexpression of these misfolded GLP-1 mutants with the chaperone protein heat shock protein 90 (HSP90) also failed to increase receptor surface expression as determined by biological activity assays (data not shown).
Interestingly, growing COS-1 cells transfected with each of the double and triple GLP-1 glycosylation site mutants at low temperature resulted in significant increases in their cell surface expression. Shown in Fig. 5 are the binding and biological activity data for these mutants expressed in COS-1 cells grown at 30°C. The maximal saturable binding of the N63L/N82L, N63L/N115L, N82L/N115L, and N63L/N82L/N115L constructs improved to 58, 60, 61, and 43%, respectively, of that of the wild-type GLP-1 receptor. The affinity of the N63L/N82L construct was similar to that of the wild-type receptor, whereas that of each of the N63L/N115L, N82L/N115L, and N63L/N82L/N115L constructs was >10-fold lower (Fig. 5 and Table 1). Similarly, the maximal intracellular cAMP responses of the N63L/N82L, N63L/N115L, N82L/N115L, and N63L/N82L/N115L constructs stimulated by GLP-1 improved to 78, 76, 68, and 43%, respectively, of that of the wild-type GLP-1 receptor. The potency of each of these mutant constructs was slightly lower than that of the wild-type receptor (Table 1). However, coexpression of HSP90 with these mutant GLP-1 receptors under these growth conditions did not further improve their surface expression (data not shown). Figure 6 shows images that confirm the expression of these GLP-1 receptor mutants on the surface of COS-1 cells grown at 30°C. Parameters for binding affinity and biological activity of these constructs grown under these conditions are also shown in Table 1.
Fig. 5.
Functional characterization of multiple Asn-to-Leu mutant receptors in COS-1 cells grown at low temperature. Left: binding curves for increasing concentrations of the natural GLP-1 ligand to displace binding of the radioligand 125I-GLP-1 to COS-1 cells transfected with the WT and the indicated multiple Asn-to-Leu mutant receptors. Cells were grown at 30°C after transfection. Values are calculated as percentages of maximal saturable binding for each of the receptor constructs observed in the absence of the competitor. They are expressed as means ± SE of duplicate data from 3 independent experiments. Right: intracellular cAMP responses in these cells (grown at 30°C) to stimulation by increasing concentrations of GLP-1. Values are expressed as means ± SE of at least 3 independent experiments, with data normalized relative to maximal responses observed for the WT GLP-1 receptor. Basal and maximal cAMP levels for the WT GLP-1 receptor were 5.1 ± 1.1 and 204 ± 53 pmol/1,000,000 cells, respectively.
Fig. 6.
Morphological localization of multiple Asn-to-Leu mutant receptors in COS-1 cells grown at low temperature. Shown are representative examples of anti-GLP-1 receptor antibody immunostaining of COS-1 cells transfected with the WT and the indicated multiple Asn-to-Leu mutant GLP-1 receptors. Shown also are COS-1 cells transfected with the WT GLP-1 receptor but immunostained only with Alexa 488-conjugated goat anti-mouse IgG. Cells used in this experiment were grown at 30°C after transfection.
DISCUSSION
N-linked glycosylation is a common posttranslational modification of GPCRs and is known to play an important role in receptor folding, trafficking, and function of various members of this superfamily. Family B GPCRs consist of a number of important drug targets, including secretin, vasoactive intestinal polypeptide, parathyroid hormone (PTH), GLP-1, corticotropin-releasing factor (CRF), calcitonin, and calcitonin receptor-like (CRL) receptors. The role of glycosylation for members in this family is less well understood than that of the family A GPCRs. We are interested in understanding the molecular basis of ligand binding and activation of the GLP-1 receptor. Like all other family B members, the GLP-1 receptor has a large and structurally complex extracellular amino-terminal domain that functions as the predominant domain for natural ligand binding. Multiple critical sites for ligand binding within this domain have been identified by mutagenesis (9), photoaffinity labeling (6), and crystallography (26, 28). The human GLP-1 receptor contains three potential N-linked glycosylation sites (Asn63, Asn82, and Asn115), all being within this important domain. Understanding the roles of these glycosylation sites in folding, trafficking, and function should ultimately add to our understanding of the interactions between GLP-1 and its receptor.
An overall role for glycosylation of the GLP-1 receptor has previously been suggested by two studies in which tunicamycin treatment reduced or abolished GLP-1 binding and function (10, 16). In one study, Goke et al. (10) showed that tunicamycin treatment of natural GLP-1 receptor-bearing RINm5F rat insulinoma cells decreases GLP-1 binding and reduces GLP-1-stimulated cAMP production due to a decreased number of receptors on the cell surface. More recently, Huang et al. (16) showed that only the mature, fully glycosylated form of the GLP-1 receptor is present in the plasma membrane and that inhibition of glycosylation using tunicamycin prevents processing and cell surface expression. However, neither of these studies established that each site of glycosylation was utilized and functionally important. Consistent with those studies, we showed that tunicamycin treatment of the GLP-1R-CHO cells abolished ligand binding and significantly decreased the biological response to GLP-1. Our photoaffinity labeling studies with GLP-1R-CHO cell membranes have also confirmed that the GLP-1 receptor is a glycoprotein (6).
In the current study, we show that mutation of each of the three Asn glycosylation sites of the GLP-1 receptor had no effects on ligand binding and biological activity. However, simultaneous mutation of any two or three of the Asn glycosylation sites resulted in almost complete loss of binding and biological activity. This effect appears to be due to reduced cell surface expression of these mutant receptors. The observation that all single-site mutations were fully functional and that any combination of two sites had profound impact establishes the glycosylation of all three potential sites. The net effect of glycosylation of these sites on electrophoretic migration on 10% SDS-polyacrylamide gels was ∼24 kDa (6).
Many human diseases are caused by loss-of-function mutations in GPCRs that can result in receptor misfolding and retention in intracellular biosynthetic compartments. Reversal of this phenomenon with restoration of cell surface expression of these receptors and restoration of their function has become an area of great interest. Successful rescue of misfolded GPCRs can be achieved by a variety of methods, including chemical chaperones, pharmacological chaperones (ligands), lowering temperature, and interacting with receptor activity-modifying proteins. Chaperone interactions with GPCRs can play a critical role in the quality control of folding in the endoplasmic reticulum and can thereby modulate delivery of functional receptor to the plasma membrane. Heat shock proteins have been shown to interact with rhodopsin and luteinizing hormone receptor, whereby the interactions with the misfolded mutants are more stable than with the wild-type receptors (1, 21). Unfortunately, in the current study, attempts to improve trafficking and surface expression of the aberrantly glycosylated receptors by coexpression with a dimerization partner, such as the secretin receptor, or with a molecular chaperone, such as HSP90, were unsuccessful. It is noteworthy that we were able to rescue the surface expression by slowing down the biosynthesis at low temperature. This manipulation would be expected to slow the rate of biosynthesis, likely providing more time for correct folding of the receptor during transit through the endoplasmic reticulum, allowing some receptor molecules to escape the endoplasmic reticulum quality control system and traffic through the Golgi complex to the plasma membrane. At 30°C, the N63L/N82L construct expressed on the COS-1 cells had similar affinity to that of the wild-type GLP-1 receptor. The decreased affinities for N63L/N115L, N82L/N115L, and N63L/N82L/N115L constructs and reduced efficacy for all of these double and triple Asn mutants likely reflect the fact that they were neither fully folded nor completely trafficked to the cell surface. Different mutant receptors may have different requirements for folding and cell surface expression, and the conditions for folding and trafficking were likely not optimal for each of these mutants.
The available studies on glycosylation of GPCRs reveal that the role of this posttranslational modification is quite variable and that no consistent pattern has emerged to define the role of receptor carbohydrates in regulating GPCR function. If the GPCR contains several potential N-linked glycosylation sites, these sites may not be glycosylated to the same degree and may not have the same importance for receptor function. This is particularly true for the family B GPCRs in which the role of glycosylation varies from one member to another. Nevertheless, the roles of glycosylation for members in this family can be roughly separated into two groups, the secretin/calcitonin/CRL receptor group and the PTH/VPAC1/CRF1 receptor group.
In the secretin/calcitonin/CRL receptor group, single mutations significantly affect receptor ligand binding and biological activity without affecting surface expression. Asn72 and Asn128 of the secretin receptor (23), Asn78 and Asn83 of the calcitonin receptor (14), and Asn123 as well as Asn66 and Asn118 in the CLR receptor (5, 17) have likely been identified to be implicated in ligand binding and biological activity. In the PTH/VPAC1/CRF1 receptor group, single mutations have not been shown to affect receptor binding and biological activity, but multiple mutations may result in reduced surface expression that leads to impaired binding and biological activity. This group includes the PTH receptor that requires maintaining at least one of these for cell surface expression (3, 30), the VPAC1 receptor that requires glycosylation at Asn58 and Asn69 for correct delivery of the receptor to the cell surface (7), and the type 1 CRF receptor that requires maintaining at least two of the four glycosylation sites for normal surface expression, binding, and biological activity (2). The current work establishes that the GLP-1 receptor behaves like the latter group of receptors.
In conclusion, we have shown in this work that mutation of each of the three glycosylation sites of the GLP-1 receptor individually had no effects on receptor expression on the cell surface, ligand binding, and biological activity. However, simultaneous mutation of two or three of these sites resulted in almost complete loss of GLP-1 binding and severely impaired biological activity due to aberrant trafficking of the correctly folded receptor to the cell surface. Of great interest, surface expression, ligand binding, and biological activity of these mutants improved significantly when biosynthesis was slowed using low temperature. These data suggest the importance of N-linked glycosylation for normal folding and trafficking of the GLP-1 receptor to the cell surface.
GRANTS
This work is supported by grants from the American Diabetes Association (1-08-RA-42 to M. Dong) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-46577 to L. J. Miller).
DISCLOSURES
No conflicts of interests are reported by the author(s).
ACKNOWLEDGMENTS
We thank Alicja M. Ball and Mary L. Augustine for their technical assistance. We acknowledge Dr. Leonard M. Neckers from the National Cancer Institute for providing us with the HSP90 construct.
REFERENCES
- 1.Anukanth A, Khorana HG. Structure and function in rhodopsin. Requirements of a specific structure for the intradiscal domain. J Biol Chem 269: 19738–19744, 1994 [PubMed] [Google Scholar]
- 2.Assil IQ, Abou-Samra AB. N-glycosylation of CRF receptor type 1 is important for its ligand-specific interaction. Am J Physiol Endocrinol Metab 281: E1015–E1021, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bisello A, Greenberg Z, Behar V, Rosenblatt M, Suva LJ, Chorev M. Role of glycosylation in expression and function of the human parathyroid hormone/parathyroid hormone-related protein receptor. Biochemistry 35: 15890–15895, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Breitfeld PP, Rup D, Schwartz AL. Influence of the N-linked oligosaccharides on the biosynthesis, intracellular routing, and function of the human asialoglycoprotein receptor. J Biol Chem 259: 10414–10421, 1984 [PubMed] [Google Scholar]
- 5.Buhlmann N, Aldecoa A, Leuthauser K, Gujer R, Muff R, Fischer JA, Born W. Glycosylation of the calcitonin receptor-like receptor at Asn(60) or Asn(112) is important for cell surface expression. FEBS Lett 486: 320–324, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Pinon DI, Miller LJ, Dong M. Molecular basis of glucagon-like peptide 1 docking to its intact receptor studied with carboxyl-terminal photolabile probes. J Biol Chem 284: 34135–34144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couvineau A, Fabre C, Gaudin P, Maoret JJ, Laburthe M. Mutagenesis of N-glycosylation sites in the human vasoactive intestinal peptide 1 receptor. Evidence that asparagine 58 or 69 is crucial for correct delivery of the receptor to plasma membrane. Biochemistry 35: 1745–1752, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Dong M, Gao F, Pinon DI, Miller LJ. Insights into the structural basis of endogenous agonist activation of family B G protein-coupled receptors. Mol Endocrinol 22: 1489–1499, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113: 546–593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goke R, Just R, Lankat-Buttgereit B, Goke B. Glycosylation of the GLP-1 receptor is a prerequisite for regular receptor function. Peptides 15: 675–681, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Hadac EM, Ghanekar DV, Holicky EL, Pinon DI, Dougherty RW, Miller LJ. Relationship between native and recombinant cholecystokinin receptors: role of differential glycosylation. Pancreas 13: 130–139, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Harikumar KG, Morfis MM, Sexton PM, Miller LJ. Pattern of intra-family hetero-oligomerization involving the G-protein-coupled secretin receptor. J Mol Neurosci 36: 279–285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harikumar KG, Simms J, Christopoulos G, Sexton PM, Miller LJ. Molecular basis of association of receptor activity-modifying protein 3 with the family B G protein-coupled secretin receptor. Biochemistry 48: 11773–11785, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho HH, Gilbert MT, Nussenzveig DR, Gershengorn MC. Glycosylation is important for binding to human calcitonin receptors. Biochemistry 38: 1866–1872, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Holtmann MH, Ganguli S, Hadac EM, Dolu V, Miller LJ. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J Biol Chem 271: 14944–14949, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Wilkinson G, Willars G. Role of the signal peptide in the synthesis and processing of the glucagon-like peptide-1 receptor. Br J Pharmacol 159: 237–251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamitani S, Sakata T. Glycosylation of human CRLR at Asn123 is required for ligand binding and signaling. Biochim Biophys Acta 1539: 131–139, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Lanctot PM, Leclerc PC, Escher E, Guillemette G, Leduc R. Role of N-glycan-dependent quality control in the cell-surface expression of the AT1 receptor. Biochem Biophys Res Commun 340: 395–402, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lanctot PM, Leclerc PC, Escher E, Leduc R, Guillemette G. Role of N-glycosylation in the expression and functional properties of human AT1 receptor. Biochemistry 38: 8621–8627, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Michineau S, Muller L, Pizard A, Alhenc-Gelas F, Rajerison RM. N-linked glycosylation of the human bradykinin B2 receptor is required for optimal cell-surface expression and coupling. Biol Chem 385: 49–57, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Mizrachi D, Segaloff DL. Intracellularly located misfolded glycoprotein hormone receptors associate with different chaperone proteins than their cognate wild-type receptors. Mol Endocrinol 18: 1768–1777, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem 107: 220–239, 1980 [DOI] [PubMed] [Google Scholar]
- 23.Pang RT, Ng SS, Cheng CH, Holtmann MH, Miller LJ, Chow BK. Role of N-linked glycosylation on the function and expression of the human secretin receptor. Endocrinology 140: 5102–5111, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem 57: 785–838, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Ray K, Clapp P, Goldsmith PK, Spiegel AM. Identification of the sites of N-linked glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J Biol Chem 273: 34558–34567, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Runge S, Thogersen H, Madsen K, Lau J, Rudolph R. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem 283: 11340–11347, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Ulrich CD, 2nd, Pinon DI, Hadac EM, Holicky EL, Chang-Miller A, Gates LK, Miller LJ. Intrinsic photoaffinity labeling of native and recombinant rat pancreatic secretin receptors. Gastroenterology 105: 1534–1543, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Underwood CR, Garibay P, Knudsen LB, Hastrup S, Peters GH, Rudolph R, Reedtz-Runge S. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J Biol Chem 285: 723–730, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheatley M, Hawtin SR. Glycosylation of G-protein-coupled receptors for hormones central to normal reproductive functioning: its occurrence and role. Hum Reprod Update 5: 356–364, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Zhou AT, Assil I, Abou-Samra AB. Role of asparagine-linked oligosaccharides in the function of the rat PTH/PTHrP receptor. Biochemistry 39: 6514–6520, 2000 [DOI] [PubMed] [Google Scholar]