Abstract
Although glutamine is considered the main precursor for citrulline synthesis, the current literature does not differentiate between the contribution of glutamine carbon skeleton vs. nonspecific nitrogen (i.e., ammonia) and carbon derived from glutamine oxidation. To elucidate the role of glutamine and nonspecific nitrogen in the synthesis of citrulline, l-[2-15N]- and l-[5-15N]glutamine and 15N-ammonium acetate were infused intragastrically in mice. The amino group of glutamine labeled the three nitrogen groups of citrulline almost equally. The amido group and ammonium acetate labeled the ureido and amino groups of citrulline, but not the δ-nitrogen. D5-glutamine also infused in this arm of the study, which traces the carbon skeleton of glutamine, was utilized poorly, accounting for only 0.2–0.4% of the circulating citrulline. Dietary glutamine nitrogen (both N groups) incorporation was 25-fold higher than the incorporation of its carbon skeleton into citrulline. To investigate the relative contributions of the carbon skeleton and nonspecific carbon of glutamine, arginine, and proline to citrulline synthesis, U-13Cn tracers of these amino acids were infused intragastrically. Dietary arginine was the main precursor for citrulline synthesis, accounting for ∼40% of the circulating citrulline. Proline contribution was minor (3.4%), and glutamine was negligible (0.4%). However, the glutamine tracer resulted in a higher enrichment in the ureido group, indicating incorporation of nonspecific carbon from glutamine oxidation into carbamylphosphate used for citrulline synthesis. In conclusion, dietary glutamine is a poor carbon skeleton precursor for the synthesis of citrulline, although it contributes both nonspecific nitrogen and carbon to citrulline synthesis.
Keywords: arginine, proline, urea cycle
the central role of the small intestine in the metabolism of glutamine was firmly established by the studies of Windmueller and Spaeth (56) using isolated perfused small intestinal preparations. Their work revealed that citrulline was one of the many compounds generated by the metabolism of glutamine in the small intestine. Since then, glutamine has been considered the main precursor for citrulline synthesis (10, 15, 36), and tracer studies utilizing l-[2-15N](amino) glutamine seem to indicate that 60–80% of citrulline originates from glutamine (4, 5, 23, 24). Furthermore, some researchers have shown that glutamine supplementation increases citrulline production or plasma concentration (20, 36, 40, 59) or, conversely, that glutamine depletion reduces citrulline concentration (39). For these reasons, the case for glutamine as the main precursor for citrulline synthesis has become accepted in the literature (10). Citrulline supplementation has been used to reduce blood pressure (13), as part of a full-spectrum antioxidant therapy (30), or to increase protein deposition in a model of aging (35). Although some of these effects may be due to a more efficient utilization of citrulline rather than arginine, recent reports suggest that citrulline may have some direct effects such as activation of the mTOR pathway (41, 49) or in regulating local availability for nitric oxide production (17). Furthermore, to increase the endogenous synthesis of citrulline, supplementation strategies (47), which have been dubbed immunonutrition or, more recently, pharmaconutrition (11), have been proposed.
However, some researchers have found that glutamine was a poor precursor for ornithine synthesis (16) or that no glutamine-derived carbon could be detected in citrulline in isolated rat enterocytes (52). A detailed examination of the pathway for citrulline synthesis shows that no direct precursor-product relationship can be inferred utilizing [15N]glutamine tracers (Fig. 1) (43a). Furthermore, the plasma citrulline increase observed during glutamine supplementation may be the consequence of the effect of glutamine on the overall function and metabolism of the enterocytes (37). Because the design of optimal supplementation strategies relies on a more clearly defined contribution of glutamine to the synthesis of citrulline, the present studies were designed to determine 1) the site-specific incorporation of the amino and amido nitrogen groups of glutamine into each of the three possible positions of the citrulline molecule and 2) the contribution of the carbon skeletons of glutamine, proline, and arginine to citrulline synthesis in a conscious murine model.
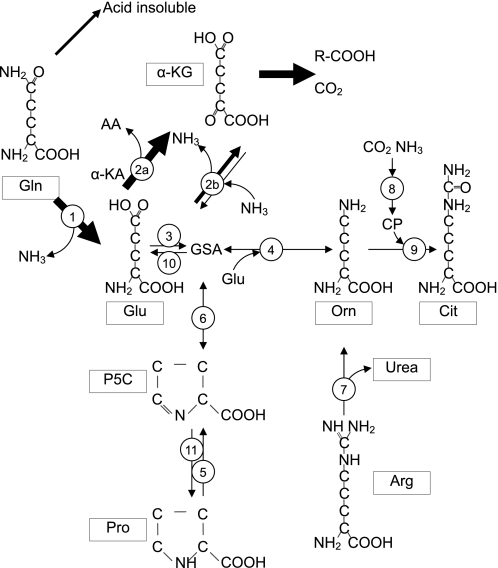
Fig. 1.
Fate of glutamine (Gln) and the origin of ornithine (Orn) in the enterocyte. A small portion (14%) of the Gln carbon is incorporated into protein or other acid-insoluble material, whereas most of the Gln is deamidated to glutamate (Glu) and ammonia by action of mitochondria glutaminase (1). Glutamate can be transaminated (2a) or oxidized by glutamate dehydrogenase (2b) to α-ketoglutarate (α-KG) and ammonia. Since this is a reversible reaction, glutamine may exchange its amino group. α-KG is further metabolized to ketoacids and CO2 and accounts for 18 and 57% of the glutamine carbon, respectively. Glu is converted to glutamate-semialdehyde (GSA) by pyrroline-5-carboxylate (P5C) synthase (3), which is the substrate for Orn transaminase (OAT; 4), generating Orn. Proline (Pro) can be oxidized to P5C by action of proline oxidase (5); P5C spontaneously interconverts to GSA (6). Ornithine can be generated directly by hydrolysis of arginine (Arg) by arginase (7). Carbamyl phosphate (CP), which is synthesized from CO2 and ammonia by action of carbomyl phosphate synthase (8), condenses with Orn by action of Orn carbomyl transferase (9) to generate citrulline (Cit). Cit accounts for 6% of glutamine carbon. Other enzymes involved in this pathway are P5C dehydrogenase (10) and P5C reductase (11). Based on Windmueller and Spaeth (56) and Smith et al. (43a).
MATERIALS AND METHODS
Animals and treatments.
Young adult male Institute of Cancer Research mice (6 wk old) were used for all the experiments. Mice were housed in an specific pathogen-free facility and had access to an irradiated pelleted feed (Rodent Diet 2920X; Harlan Teklad) with the following dietary proximate analysis: protein (185 g/kg), gross energy (14.1 MJ/kg), fat (60 g/kg), fiber (28 g/kg), and ash (46 g/kg). Autoclaved reverse osmosis water was available at all times. Mice were maintained under a 12-h light cycle (600–1800) in a temperature- (22 ± 2°C) and humidity-controlled (55 ± 5%) environment. All animal procedures were authorized by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Gastric catheterization.
After an overnight fast, a lateral (left flank) laparotomy was performed under general isofluorane anesthesia. The stomach was exteriorized and a purse string suture performed in the fundic region of the stomach adjacent to the glandular region. After a small incision was made with a 19-gauge needle in the stomach wall, a silicone catheter (0.5 mm ID, 0.9 mm OD) was introduced into the stomach. The stomach was kept moist by using a saline solution containing an antibiotic (cefazolin, 0.1 g/ml). The catheter had a small retention bead ∼2 mm from the tip, which was secured by tightening the purse suture. A second (single) suture secured the catheter to the glandular region of the stomach, and a third one secured the catheter to the abdominal wall. The catheter was exteriorized in the interscapular region, and mice were returned to their cages (single caged). Analgesics were given (buprenorphine, 0.1 mg/kg sc) right before the surgery and 12 and 24 h after the procedure. Mice recovered their presurgery body weight within 5 days, and infusions were conducted ≥7 days after surgery.
Infusions and sampling.
On the day of the infusion, feed was removed at 0700, and mice were weighed at 0930. After a 3-h feed deprivation, mice were restrained and a tail vein catheter was inserted, as described previously (27). The tail vein catheter and the gastric catheter were then connected to syringe infusion pumps (PHD2000; Harvard Apparatus, Holliston, MA). In addition to the different tracers described below (Cambridge Isotope Laboratories, Andover, MA), a liquid amino acid and glucose solution (Table 1) was infused intragastrically for 4 h at a rate of 20 ml·kg−1·h−1 to maintain a fed steady state. We have shown previously that it takes ∼3.2 h for urea to reach isotopic plateau enrichment (26). The primed continuous infused amino acid tracers and their products reached plateau enrichment ≥3 h after the initiation of the infusions (Fig. 2). The amino acid and glucose solution, lacking the amino acids of interest, was prepared the day before the infusions. Labeled and unlabeled glutamine, proline, and arginine were added immediately before the infusions were started. The infusion protocols were designed to determine the source of citrulline nitrogen and carbon. For this reason, two different sets of infusions were conducted (nitrogen and carbon arms). The tracers given intragastrically replaced isomolar quantities of the amino acids present in the amino acid solution infused (Table 2).
Table 1.
Rate of intragastric infusion of amino acid and glucose in mice
| Infusion Rate, μmol·kg−1·h−1 | |
|---|---|
| Tryptophan | 69 |
| Tyrosine | 77 |
| Phenylalanine | 374 |
| Leucine | 916 |
| Valine | 628 |
| Isoleucine | 515 |
| Lysine | 620 |
| Threonine | 617 |
| Histidine | 264 |
| Proline | 759 |
| Arginine | 301 |
| Alanine | 680 |
| Aspartate | 701 |
| Serine | 621 |
| Glutamate | 876 |
| Glutamine | 638 |
| Glycine | 606 |
| Methionine | 203 |
| Cysteine | 154 |
| NH4-acetate | 200 |
| Dextrose | 12,890 |
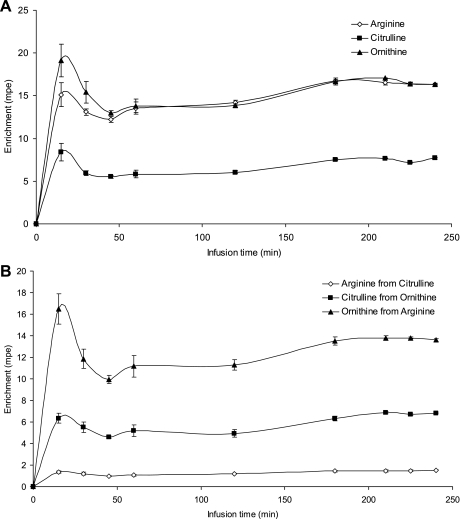
Fig. 2.
Time to reach plateau enrichment of primed, continuously infused tracers (A) and their products (B). Four mice were infused using a lateral vein catheter and samples collected from a carotid artery catheter. Sample size was ∼20 μl/time point. Tracers infused were [U13C6]arginine, [15N](ureido) citrulline, and [5,5]D2-ornithine; their products are [U13C5]ornithine, [15N](guanidino) arginine, and [5,5]D2-citrulline, respectively. Error bars denote SE.
Table 2.
Tracer infused continuously in mice for 4 h in the different infusion protocols followed to determine the origin of the nitrogen and carbon moieties of citrulline
| Intragastric | Intravenous | ||
|---|---|---|---|
| Nitrogen arm* | |||
| Amino glutamine | [2-15N]glutamine | D5-glutamine† | 13C18O urea |
| Amido glutamine | [5-15N]glutamine | D5-glutamine | 13C18O urea |
| Ammonium | 15NH4-acetate | D5-glutamine | 13C18O urea |
| Carbon arm | |||
| Glutamine | [U-13C5]glutamine | [15N](ureido) citrulline | |
| Arginine | [U-13C6]arginine | [15N](ureido) citrulline | |
| Proline | [U-13C5]proline | [15N](ureido) citrulline | |
Nitrogen arm also had a [5-13C-4,4,5,5]D4-citrulline intravenous infusion 1 wk prior to the infusion described in the table.
D5-glutamine is [2,3,3,4,4]D5-glutamine.
Nitrogen arm: incorporation of glutamine nitrogen into citrulline.
Two infusions took place 7 days apart in the same mice. In the first infusion, [5-13C 4,4,5,5]D4-citrulline (6.4 μmol·kg−1·h−1, prime 6.4 μmol/kg) was infused intravenously for 4 h to determine the plasma entry rate of citrulline. After a 7-day recovery period, one of the three following infusion protocols was performed to determine the contribution of intragastrically infused 1) amino and 2) amido glutamine N and 3) ammonium N to citrulline synthesis.
To determine the contribution of the amino nitrogen and carbon skeleton of glutamine to citrulline synthesis, l-[2-15N](amino) glutamine (200 μmol·kg−1·h−1, prime 200 μmol/kg) and [2,3,3,4,4-D5]glutamine (200 μmol·kg−1·h−1, prime 200 μmol/kg) were infused intragastrically (n = 10 mice).
To determine the contribution of the amido nitrogen and carbon skeleton of glutamine to citrulline synthesis, [5-15N](amido) glutamine (200 μmol·kg−1·h−1, prime 200 μmol/kg) and [2,3,3,4,4]D5-glutamine (200 μmol·kg−1·h−1, prime 200 μmol/kg) were infused intragastrically (n = 10 mice).
To determine the contribution of ammonium N to citrulline synthesis, [15N]ammonium acetate (200 μmol·kg−1·h−1, prime 200 μmol/kg) and [2,3,3,4,4]D5-glutamine (200 μmol·kg−1·h−1, prime 200 μmol/kg) were infused intragastrically (n = 10 mice).
In addition, 13C18O urea (160 μmol·kg−1·h−1, prime 160 μmol/kg) was infused intravenously for 4 h in all three nitrogen arm infusion protocols to determine the entry rate of urea and the contribution of ammonium N and glutamine amino and amido N to urea synthesis.
Carbon arm: utilization of the carbon skeleton of different precursors for the synthesis of citrulline.
To study the origin of citrulline carbon, the following carbon labeled precursors were infused intragastrically. 1) To determine the contribution of the carbon skeleton of glutamine to citrulline synthesis, [U-13C5]glutamine (200 μmol·kg−1·h−1, prime 200 μmol/kg) was infused intragastrically (n = 10 mice); 2) to determine the contribution of the carbon skeleton of arginine to citrulline synthesis, [U-13C6]arginine (95 μmol·kg−1·h−1, prime 95 μmol/kg) was infused intragastrically (n = 10 mice); and 3) to determine the contribution of the carbon skeleton of proline to citrulline synthesis, [U-13C5]proline (240 μmol·kg−1·h−1, prime 240 μmol/kg) was infused intragastrically (n = 10 mice). In addition, [15N](ureido) citrulline (7 μmol·kg−1·h−1, prime 7 μmol/kg) was infused intravenously in all three carbon arm infusion protocols to determine the rate of appearance of citrulline.
After a 4-h infusion, blood was drawn from the submaxibular bundle and centrifuged at 1,500 g for 10 min at 4°C, and plasma was kept frozen at −80°C until analysis. Blood samples from similar mice under identical housing conditions and infused with the amino acid and glucose mixture for 4 h were used to determine isotopic enrichment background.
Sample analysis.
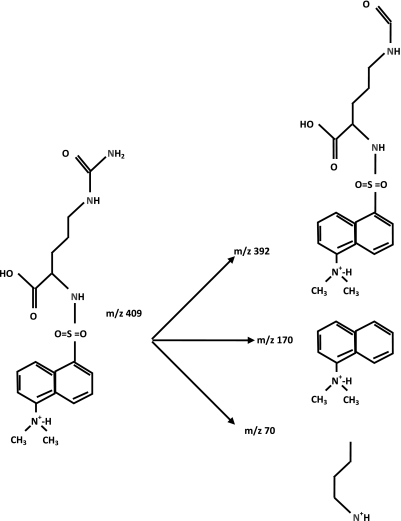
Plasma glutamine, citrulline, arginine, proline, and ornithine were determined as their dansyl derivatives by liquid chromatography-mass spectrometry, utilizing a TSQ Quantum Ultra System (Thermo Finnigan, San Jose, CA). Glutamine and citrulline isotopic enrichments were determined by multiple reactions monitoring the transitions mass-to-charge ratio (m/z) 380 to 84 and 363, 381 to 85 (amino nitrogen) and 363 (amido nitrogen) for glutamine, 409 to 70 and 392, and 410 to 71 (α-nitrogen), 392 (ureido nitrogen), and 393 (α- and δ-nitrogen, and ureido C) for citrulline (Fig. 3). Arginine, proline, and ornithine isotopic enrichments were determined by single reaction monitoring the transitions m/z 408 to 170, 413 to 170, and 414 to 170 for arginine, 349 to 170 and 354 to 170 for proline, and 599 to 170 and 604 to 170 for ornithine.
Fig. 3.
Fragmentation pattern of the dansyl derivative of citrulline. At low-collision energy (15 eV), the mass-to-charge ratio (m/z) 392 fragment predominates and is used to determine the enrichment of the ureido group. At higher-collision energy (26 eV) m/z 70 (carrying the α-nitrogen) and 170 fragment are more abundant.
Plasma urea isotopic enrichment was determined by electron impact GC-MS after the urea was derivatized to the tert-butyldimethylsilyl derivative. Plasma (20 μl) protein was precipitated with ice-cold acetone (100 μl), and the supernatant containing the urea was obtained after centrifugation at 1,500 g for 15 min at 4°C. The supernatant was evaporated under a gentle stream of nitrogen gas at 80°C, and the sample was derivatized with 25 μl of a 1:1 mixture of N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (Sigma-Aldrich, St. Louis, MO) and acetonitrile at 80°C for 20 min in tightly capped V-vials. The analysis was performed in a 5973 Agilent GC MSD (Agilent Technologies, Santa Clara, CA) in SIM mode, monitoring m/z ions 231, 232, and 234.
Calculations.
The rate of appearance of urea and citrulline was calculated from the isotopic dilution of the intravenously infused tracers {13C18O urea and [5-13C 4,4,5,5]D4-citrulline or [15N](ureido) citrulline} at plateau enrichment, as described by the equation
| (1) |
where Ra M is the rate of appearance (flux) of the unlabeled metabolite M (μmol·kg−1·h−1), iM is the intravenous infusion rate (μmol·kg−1·h−1), Ei is the enrichment of the infused tracer, and EM is the plasma enrichment of metabolite M at isotopic plateau enrichment (mpe).
When the rate of appearance of citrulline (Ra Cit) and the contribution of the different dietary precursors are determined at the same time (C Arm), this equation fails to take into account the contribution of the labeled dietary precursor. Note that in these experiments the dietary precursors were ∼30% enriched and that the tracers replaced isomolar quantities of the amino acid present in the amino acid solution infused intragastrically. For these reasons, the Ra Cit is adjusted to take into account the contribution of the dietary tracers, as described in Eq. 2.
The contribution of the different labeled precursors to the synthesis of citrulline was calculated from the recovery of the tracers in plasma citrulline as
| (2) |
where Citrec_p is the recovery of the dietary labeled precursor p as citrulline in peripheral plasma (μmol·kg−1·h−1), ECit_p is the isotopic plasma enrichment of citrulline due to the infusion of the labeled precursor p (mpe), and Ra Cit is the entry rate of unlabeled citrulline as calculated above (μmol·kg−1·h−1; Eq. 1).
The contribution of the labeled precursor can also be calculated as the percentage of the tracer recovered as citrulline as
| (3) |
where Citrec_p% is the percentage recovery of the infused tracer as citrulline (%) and ip is the infusion rate of the precursor p (μmol tracer·kg−1·h−1) and the other variables, as defined above. This equation allows for the comparison of the incorporation of different labeled molecules when the unlabeled molecules are not present in the diet (i.e., ammonia or ornithine) or the infusion rates are different.
The contribution of the intragastrically infused dietary precursors can then be calculated by dividing the recovery of the labeled precursor by the corresponding enrichment of the tracer in the infusate as
| (4) |
where dietary_P_contribution is the contribution of the dietary (labeled and unlabeled) precursor P to the synthesis of citrulline (μmol·kg−1·h−1), Citrec_p is the recovery of the labeled precursor p as citrulline in peripheral plasma (μmol·kg−1·h−1; Eq. 2), and Eip is the enrichment of the intragastrically infused precursor p (mpe).
Finally, the relative importance of the different dietary precursors to Ra Cit can be calculated as
| (5) |
where citorigen% is the contribution, as percentage, of the dietary precursor P to the production of citrulline (note that this equation is valid even in the event of hepatic extraction of citrulline) and Ra Cit is the adjusted entry rate of citrulline, as described above.
The contribution of the different 15N-labeled compounds to urea can be calculated by similarly applying the equations shown above.
Data analysis.
Data were analyzed statistically as a complete randomized design utilizing the Proc Mixed procedure of SAS (version 9.1; SAS Institute, Cary, NC) with tracer as a fixed effect. If a significant effect for the tracer infused was obtained (P ≤ 0.05), the post hoc Tukey procedure for multiple pairwise comparisons was also applied.
RESULTS
Nitrogen arm: incorporation of glutamine nitrogen into citrulline.
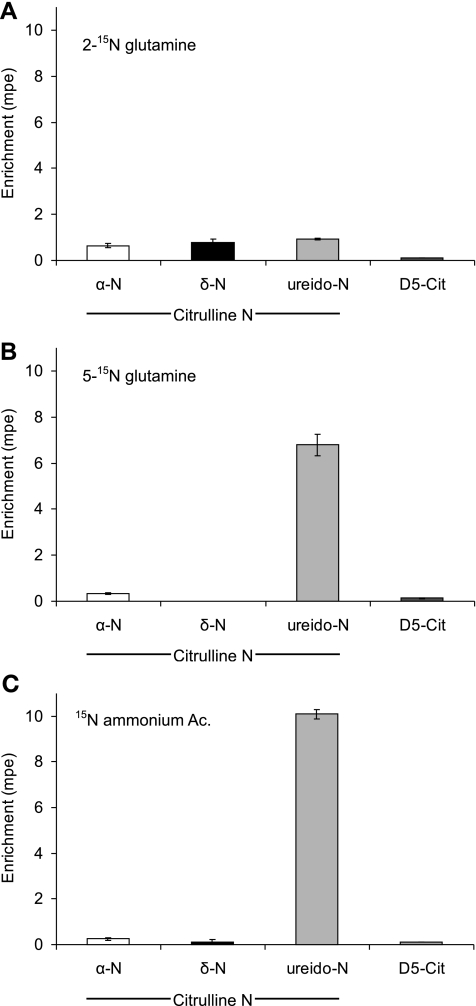
The intragastric infusion of the [15N]glutamine tracers, as well as [15N]ammonium acetate, resulted in measurable enrichments in both amino and amido groups of plasma glutamine (Table 3 and Fig. 4). The infusion of l-[2-15N]glutamine resulted in the incorporation of the 15N tracer in the three possible positions of citrulline (α, δ, and ureido). Both [5-15N]glutamine and [15N]ammonium infusions resulted in a higher enrichment of the ureido group, a smaller enrichment of the α-N, and no measurable labeling in the δ-position of citrulline (not different from zero, P > 0.15). The infusion of D5-glutamine resulted in a modest incorporation of the D5 label into citrulline (Table 3 and Fig. 4).
Table 3.
Nitrogen arm results: plasma glutamine and citrulline enrichments after the intragastric infusion of [2,3,3,4,4-D5]glutamine and [2-15N]glutamine, l-[5-15N]glutamine, or l-[15N]ammonium acetate in mice
| Tracers |
||||
|---|---|---|---|---|
| l-[2-15N]glutamine and D5-glutamine | l-[5-15N]glutamine and D5-glutamine | 15NH4-acetate and D5-glutamine | P Value | |
| Plasma glutamine, mpe | ||||
| l-[2-15N]glutamine | 2.01 ± 0.17a | 0.39 ± 0.06b | 0.38 ± 0.04b | <0.0001 |
| l-[5-15N]glutamine | 0.73 ± 0.03c | 2.04 ±0.14a | 1.28 ± 0.06b | <0.0001 |
| D5-glutamine | 1.13 ± 0.21 | 0.85 ± 0.06 | 0.93 ± 0.05 | <0.3376 |
| Plasma citrulline, mpe | ||||
| α-N | 0.65 ± 0.10a | 0.33b ± 0.05 | 0.25 ± 0.05b | <0.0008 |
| δ-N | 0.78 ± 0.14a | −0.09 ± 0.05*b | 0.13 ± 0.09*b | <0.0001 |
| ureido-N | 0.94 ± 0.04c | 6.79 ± 0.46b | 10.09 ± 0.22a | <0.0001 |
| D5-citrulline‡ | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.01 | <0.1683 |
Values are means ± SE; n = 10. Identical infusion rate for all tracers, 200 μmol·kg−1·h−1.
D5-citrulline originates from D5-glutamine.
Values in the same row with different superscripted letters differ (P < 0.05).
Not different from zero.
Fig. 4.
Nitrogen arm results: plasma citrulline isotopic enrichments achieved in each of the 3 nitrogen positions of citrulline with identical infusion rates (200 μmol·kg−1·h−1) of [2-15N]glutamine (A), [5-15N]glutamine (B), and [15N]ammonium acetate (Ac.; C). The D5-citrulline isotopic enrichment in was achieved with a coinfusion of D5-glutamine (also a rate of 200 μmol·kg−1·h−1).
The rate of appearance of citrulline, determined by the intravenous infusion of [5-13C 4,4,5,5]D4-citrulline, was not different (P = 0.562) among the three infusions (Eq. 1 and Table 4). The recovery of the 15N label infused in the three N groups of citrulline was greater (P < 0.0001) for l-[5-15N]glutamine than for l-[2-15N]glutamine (10.17 vs. 3.48 μmol 15N·kg−1·h−1, respectively; Eq. 2 and Table 4) but several times higher (P < 0.0001) than the recovery of the D5 label (0.18 μmol·kg−1·h−1; Eq. 2 and Table 4). A higher incorporation (P < 0.0001) of 15N into citrulline resulted from the infusion of [15N]ammonium acetate than from any of the glutamine tracers (Eq. 2 and Table 4). Because the infusion rates of the glutamine (and ammonium) tracers were identical, the recovery of the tracer as percentage of the tracer infused followed the same pattern ([15N]ammonium acetate > l-[5-15N]glutamine > l-[2-15N]glutamine; Eq. 3 and Table 4).
Table 4.
Nitrogen arm results: 15N tracer recovery as citrulline, dietary contribution of glutamine, and dietary origin of citrulline after the intragastric infusion of [2,3,3,4,4-D5]glutamine and l-[2-15N]glutamine, l-[5-15N]glutamine, or [15N]ammonium acetate in mice
| Tracers |
||||
|---|---|---|---|---|
| l-[2-15N]glutamine and D5-glutamine | l-[5-15N]glutamine and D5-glutamine | 15NH4-acetate and D5-glutamine | P Value | |
| Ra Cit, μmol·kg−1·h−1§ (Eq. 1) | 146.7 ± 3.4 | 143.6 ± 3.1 | 149.4 ± 4.6 | <0.5618 |
| Label recovered as citrulline, μmol·kg−1·h−1 (Eq. 2) | ||||
| α-15N | 0.94 ± 0.14a | 0.48 ± 0.08b | 0.39 ± 0.09b | <0.0016 |
| δ-15N | 1.16 ± 0.19a | −0.13 ± 0.08*b | 0.17 ± 0.14*b | <0.0001 |
| Ureido-15N | 1.38 ± 0.07c | 9.69 ± 0.58b | 15.05 ± 0.48a | <0.0001 |
| D5-citrulline‡ | 0.17 ± 0.02 | 0.20 ± 0.01 | 0.18 ± 0.01 | <0.4220 |
| Label recovered as citrulline %infused tracer (Eq. 3) | ||||
| α-N | 0.46 ± 0.07a | 0.24b ± 0.04 | 0.19 ± 0.04b | <0.0022 |
| δ-N | 0.56 ± 0.09a | −0.06 ± 0.04*b | 0.08 ± 0.07*b | <0.0001 |
| Ureido-N | 0.67 ± 0.03c | 4.79b ± 0.29 | 7.32 ± 0.26a | <0.0001 |
| D5-citrulline‡ | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | <0.3383 |
| Dietary contribution, μmol·kg−1·h−1 (Eq. 4) | ||||
| α-N | 1.45 ± 0.21 | 0.77 ± 0.12 | <0.0120 | |
| δ-N | 1.79 ± 0.30 | −0.20 ± 0.11* | <0.0001 | |
| Ureido-N | 2.13 ± 0.10 | 15.35 ± 0.89 | <0.0001 | |
| D5-citrulline‡ | 0.28 ± 0.03 | 0.33 ± 0.02 | 0.29 | <0.3327 |
| Citrulline origin, %Ra Cit (Eq. 5) | ||||
| ϖα-N | 0.99 ± 0.15 | 0.53 ± 0.08 | <0.0129 | |
| δ-N | 1.20 ± 0.21 | −0.14 ± 0.08* | <0.0001 | |
| Ureido-N | 1.45 ± 0.06 | 10.74 ± 0.71 | <0.0001 | |
| D5-citrulline‡ | 0.19 ± 0.02 | 0.23 ± 0.01 | 0.19 ± 0.01 | <0.1894 |
Values are means ± SE; n = 10. Ra Cit, rate of appearance of citrulline.
Ra Cit determined with [5-13C-4,4,5,5-D4]citrulline. Identical infusion rate for all tracers, 200 μmol·kg−1·h−1.
D5-citrulline originates from D5-glutamine.
Values in the same row with different superscripted letters differ (P < 0.05).
Not different from zero.
The dietary contribution of glutamine to the synthesis of citrulline was approximately threefold greater for the amido group than for the amino group, but taken together glutamine contributed N for the synthesis of ∼10 μmol·kg−1·h−1 citrulline. This contrasts with the dietary contribution of the carbon moiety of glutamine to citrulline synthesis (0.30 μmol·kg−1·h−1; Eq. 4 and Table 4). Hence, whereas ∼5% of citrulline nitrogen originated from dietary glutamine, only ∼0.20% of the carbon had the same origin (Eq. 5 and Table 4).
The rate of appearance of urea was not different (P = 0.130) among the three infusions of the N arm (Eq. 1 and Table 5). A higher (P < 0.0001) label recovery and recovery as percentage of the tracer infused was observed for the infusion of [15N]ammonium acetate than for l-[2-15N]- and l-[5-15N]glutamine, with no difference (P = 0.88) between the two [15N]glutamine tracers (Eqs. 2 and 3 and Table 5). Both amino and amido groups of dietary glutamine contributed roughly equally (∼191 μmol·kg−1·h−1) to the synthesis of urea and represented 3.4% of the urea production (Eqs. 4 and 5 and Table 5).
Table 5.
Nitrogen arm results: urea entry rate, 15N tracer recovery as urea, and dietary contribution of glutamine to urea synthesis after the intragastric infusion of [2,3,3,4,4-D5]glutamine and l-[2-15N]glutamine and l-[5-15N]glutamine or [15N]ammonium acetate in mice
| Tracers |
||||
|---|---|---|---|---|
| l-[2-15N]glutamine | l-[5-15N]glutamine | 15NH4-acetate | P Value | |
| Ra urea, μmol·kg−1·h−1 (Eq. 1)* | 5,553 ± 131 | 5,855 ± 230 | 5,336 ± 150 | <0.1298 |
| Label recovery as urea, μmol·kg−1·h−1 (Eq. 2) | 121.0 ± 8.2b | 123.9 ± 8.8b | 161.4 ± 7.0a | <0.0020 |
| Label recovery as urea, %tracer infused (Eq. 3) | 58.7 ± 3.7b | 61.1 ± 4.1b | 78.5 ± 3.3a | <0.0015 |
| Dietary glutamine contribution, μmol·kg−1·h−1 (Eq. 4) | 186.8 ± 11.8 | 195.9 ± 12.9 | <0.6093 | |
| Urea originated from glutamine, %Ra urea (Eq. 5) | 3.4 ± 0.24 | 3.4 ± 0.22 | <0.9456 | |
Values are means ± SE; n = 10. Identical infusion rate for all tracers, 200 μmol·kg−1·h−1.
Ra urea determined with 13C18O urea.
Values in the same row with different superscripted letters differ (P < 0.05).
Carbon arm: utilization of the carbon skeleton of different precursors for the synthesis of citrulline.
The infusion of the U-13Cn amino acid tracers resulted in the appearance of the infused tracers in peripheral plasma (Table 6). The infusion of [U 13C6]arginine resulted in higher enrichments (P < 0.0001) of plasma ornithine, citrulline, and arginine (M + 5) than either proline or glutamine tracers despite a reduced rate of infusion of the arginine tracer. The enrichment of citrulline in one carbon position, presumably the ureido carbon that originates from CO2, was higher for glutamine than for proline and arginine. However, the enrichment due to the infusion of the arginine tracer was not different from zero (P = 0.676).
Table 6.
Carbon arm results: plasma glutamine, proline, arginine, ornithine, and citrulline enrichments after the intragastric infusion of [U-13C5]glutamine, [U-13C5]proline, and [U-13C6]arginine in mice
| Tracers |
||||
|---|---|---|---|---|
| [U13C5]glutamine | [U13C5]proline | [U13C6]arginine | P Value | |
| Plasma enrichment of the infused tracers, mpe | ||||
| Glutamine M + 5 | 1.27 ± 0.12 | ND | ND | |
| Proline M + 5 | −0.02 ± 0.01c* | 20.38 ± 0.24a | 0.66 ± 0.04b | <0.0001 |
| Arginine M + 6 | 0.00 ± 0.00*b | 0.02 ± 0.01*b | 5.22 ± 0.49a | <0.0001 |
| Labeled products, mpe | ||||
| Argine M + 5 | 0.08 ± 0.01c | 0.71 ± 0.04b | 4.20 ± 0.11a | <0.0001 |
| Ornithine M +5 | 0.08 ± 0.02c | 1.71 ± 0.10b | 15.65 ± 0.38a | <0.0001 |
| Citrulline M +5 | 0.25 ± 0.02c | 2.50 ± 0.11b | 11.53 ± 0.15a | <0.0001 |
| Citrulline 13C ureido | 0.52 ± 0.05a | 0.23 ± 0.06b | 0.03 ± 0.06*c | <0.0001 |
Values are means ± SE, n = 10. ND, not determined.
Values with different superscripted letters differ (P < 0.05).
Not different from zero.
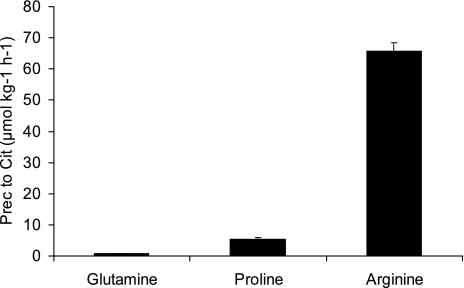
Citrulline entry rate did not differ (P = 0.8581) among the three infusion groups (Eq. 1 and Table 7). The recovery of arginine tracer (19.25 μmol·kg−1·h−1) in citrulline was higher (P < 0.0001) than proline (4.2 μmol·kg−1·h−1), which in turn was higher (P < 0.0001) than glutamine (0.4 μmol·kg−1·h−1) (Eq. 2 and Table 7). Despite the different amounts of dietary tracers infused, the recovery of the tracers as citrulline followed the same order (20.4% arginine, 1.7% proline, and 0.2% glutamine; Eq. 3 and Table 7). Dietary arginine was the main precursor that contributed to circulating citrulline (65.8 μmol·kg−1·h−1), followed by proline (5.5 μmol·kg−1·h−1) and glutamine (0.7 μmol·kg−1·h−1) (Eq. 4, Table 7, and Fig. 5). Dietary arginine was the precursor for ∼40% of the citrulline entry rate, whereas proline contributed only 3.3% and glutamine 0.4% (Eq. 5 and Table 7). The contribution of carbon skeleton glutamine to circulating citrulline in this arm of the study determined with [U-13C5]glutamine was statistically higher (P < 0.0001) than in the N arm determined with D5-glutamine (0.4 vs. 0.2% of Ra Cit).
Table 7.
Carbon arm results: 13C tracer recovery as citrulline, dietary contribution, and dietary origin of citrulline after the intragastric infusion of [U-13C5]glutamine, [U-13C5]proline, and [U-13C6]arginine in mice
| Tracers (n = 10) |
||||
|---|---|---|---|---|
| [U13C5]glutamine | [U13C5]proline | [U13C6]arginine | P Value | |
| RaCit, μmol·kg−1·h−1 (Eq. 1)* | 163.2 ± 3.99 | 166.1 ± 3.14 | 166.8 ± 5.63 | <0.8581 |
| Label recovery as citrulline, μmol·kg−1·h−1 (Eq. 2) | 0.4 ± 0.03c | 4.2 ± 0.22b | 19.25 ± 0.81a | <0.0001 |
| %Label recovery as citrulline (Eq. 3) | 0.2 ± 0.02c | 1.7 ± 0.10b | 20.4 ± 0.78a | <0.0001 |
| Dietary contribution, μmol·kg−1·h−1 (Eq. 4) | 0.7 ± 0.05c | 5.5 ± 0.33b | 65.8 ± 2.54a | <0.0001 |
| Citrulline origin, %Ra Cit (Eq. 5) | 0.4 ± 0.03c | 3.3 ± 0.16b | 39.4 ± 0.61a | <0.0001 |
Ra Cit determined with [15N](ureido) citrulline. Values are means ± SE; n = 10.
Values with different superscripted letters differ (P < 0.05).
Fig. 5.
Carbon arm results. Contribution of dietary glutamine, proline, and arginine to the appearance rate of citrulline. Prec, precursor.
DISCUSSION
Origin of circulating citrulline and model domain.
Since little or no citrulline is present in the diet [with the notable exception of watermelon (44)], circulating citrulline is almost entirely of endogenous origin. Although there are other sources of endogenous citrulline [e.g., from turnover of citrullinated proteins and by action of nitric oxide synthase on arginine (18) and dimethylarginine dimethylaminohydrolase on dimethylarginine (34)], it is believed that these sources are quantitatively minor compared with the synthesis of citrulline from ornithine. The enzyme that catalyzes the conversion of ornithine into citrulline, ornithine transcarbamylase, is present in only two cell types, hepatocytes and enterocytes. In hepatocytes ornithine transcarbamylase functions as part of the urea cycle, and under normal conditions there is no net synthesis of citrulline because urea production regenerates ornithine (7). However, the citrulline produced by the enterocytes is exported and enters the portal circulation. It has been shown in rodents that citrulline is neither removed nor released by the liver, and thus Ra Cit represents citrulline production (53). Recently, however, this concept has been challenged in humans undergoing surgery, mostly liver resection (46). It is not clear whether these human data are applicable to the other species or to humans in more physiological conditions. Nevertheless, the kinetic model employed estimates the contribution of the different precursors to citrulline appearing in the peripheral circulation (Ra Cit), which is the variable usually measured (4, 23, 25). Also, note that the small dilution in citrulline enrichment, due to citrulline production by nitric oxide synthase (and other minor sources), is compensated for by the increase in rate of appearance from the same source.
15N-labeled glutamine is a poor tracer to determine precursor-product relationships between glutamine and citrulline.
The extensive catabolism of glutamine in the small intestine generates large quantities of ammonia and CO2 (56). The current literature does not differentiate between these nonspecific contributions (ammonia and CO2) from the specific contribution (the carbon skeleton) of the intact glutamine to the synthesis of citrulline. Glutamine supplementation should increase citrulline production by mass action only if the carbon skeleton of glutamine is utilized for the synthesis of the ornithine used for citrulline synthesis. With this premise in mind, the l-[2-15N]glutamine used as tracer by others to follow the fate of the glutamine molecule (4, 10, 23) can be utilized to describe a precursor-product relationship between glutamine and citrulline only if 1) the 15N tracer follows the carbon skeleton and 2) the 15N tracer is only incorporated into citrulline in the amino position.
The intragastric infusion of 15N-labeled glutamine in the current experiment resulted in the appearance of labeled glutamine in peripheral plasma. However, the 15N label did not remain in the original position. This indicates that the label can be lost and reincorporated, labeling other parts of the molecule. Furthermore, the plasma glutamine enrichments achieved with an identical infusion rate of D5-glutamine, which follows the carbon skeleton of the molecule, were less than one-half than the ones achieved with 15N. This indicates that there is an extensive recycling of the N moiety of glutamine, a conclusion further supported by the labeling of glutamine from the [15N]ammonium acetate infusion.
Phosphate-dependent glutaminase, a mitochondrial enzyme highly expressed in enterocytes (8), is responsible for the first step in the hydrolysis of glutamine to ammonia and glutamate. The ammonia generated by the action of glutaminase on the intragastrically infused l-[5-15N]glutamine contributed to the mitochondrial ammonia pool utilized in the synthesis of carbomyl phosphate and was eventually incorporated as the ureido group of citrulline. However, the intragastric infusion of the ammonium tracer was more effective in the labeling of the ureido position of citrulline, presumably because some glutamine can escape first-pass utilization and also because glutamine is used by the small intestine for protein synthesis (56). Dietary glutamine also acted as a source of nitrogen for the synthesis of urea, with both amino and amido groups contributing equally. Although urea was the main fate for dietary glutamine nitrogen (∼60%), it accounted for only a small fraction of the urea appearance rate (3.4%).
The disposal of glutamate, which contains the amino group of glutamine, follows two possible routes (Fig. 1): 1) reductive deamination by glutamate dehydrogenase to generate α-ketoglutarate and ammonia or 2) transamination that results in α-ketoglutarate and an amino acid. Although glutamate dehydrogenase is thought to be a minor pathway for glutamate disposal in the small intestine (22), our present results indicate that it is at least as important as transamination since the amino group of glutamine contributed similarly to the ureido and δ-groups of citrulline. The similarity between the enrichments achieved by the amino and δ-nitrogen of citrulline is consistent with glutamate being the nitrogen donor in the transamination of glutamate-semialdehyde (GSA) to produce ornithine (Fig. 1) (28). These results show that the amino group of glutamine can be incorporated into any of the three nitrogen positions of citrulline.
Our findings on the recycling of the 15N label within the glutamine molecule and the similar enrichments achieved by the three nitrogen groups of citrulline when l-[2-15N]glutamine was infused indicate that 15N-labeled glutamine is a poorly suited tracer to determine a precursor-product relationship between glutamine and citrulline (4, 10, 23).
Glutamine oxidation provides nonspecific carbon for the ureido group of citrulline.
The incorporation of a single labeled carbon into citrulline indicated the labeling of carbamyl phosphate by 13CO2 generated from the oxidation of the intragastrically infused tracers. Whereas glutamine and proline seemed to be oxidized to some extent in the small intestine, arginine was poorly oxidized, which is consistent with in vitro data (3). In fact, any substrate oxidized in enterocytes, such as aspartate (54) or glucose (Marini JC, unpublished observations), can be a source of nonspecific carbon for the synthesis of citrulline. This is likely the reason for the 14C labeling of citrulline by glutamine in Windmueller and Spaeth's experiments (56). In fact, it has been shown in neonatal piglets that [U-14C]- but not l-[3,4-3H]glutamate is able to label citrulline (33, 53). This incorporation of a single C has to be taken into account if 13C-labeled tracers are coinfused with 15N tracers to determine citrulline metabolism, since both traces may yield M + 1 enrichments. For these reasons, we did not infuse any [13C]glutamine tracers in the N arm of the study, but we chose instead a deuterated glutamine tracer.
The utilization of glutamine for citrulline synthesis depends on the synthesis of ornithine by ornithine aminotransferase, a bidirectional enzyme.
In vitro studies have shown that enterocytes were able to synthesize citrulline when glutamine, but not ornithine or proline, was the sole substrate (58). However, the addition of ammonium chloride to ornithine (58) or glutamine to proline (57) was able to trigger an increase in the synthesis of citrulline from ornithine and proline, respectively. Likewise, the addition of glutamine increased the production of citrulline from arginine (16). This interaction between possible precursors for citrulline synthesis highlights the difficulty of providing the appropriate mix of precursors and energy substrates to mimic the in vivo situation. This is especially true because not only may glutamine act as a precursor for citrulline synthesis, it is also an important respiratory fuel for the small intestine (56). Additionally, glutamine also provides glutamate, which can be used for the synthesis of N-acetylglutamate, the allosteric activator of carbamylphosphate synthase (21), and ammonia for the synthesis of carbamylphosphate (32).
Although ornithine aminotransferase (OAT) can function in both directions, toward the synthesis of ornithine or its disposal, it seems that in adults the catabolic route predominates (31) since the equilibrium strongly favors the formation of GSA (43). However, the accumulation of glutamate in the medium during in vitro culture of enterocytes (51, 58) might tilt the OAT balance toward the synthesis of ornithine instead of its degradation (43) and thus not reflect in vivo metabolism. It has been shown in vivo that OAT inhibition in adult mice (1, 42) increased plasma ornithine concentration, whereas in suckling piglets OAT inhibition decreased ornithine levels (14). The inhibition of OAT in transgenic mice results in a paradoxical neonatal hypo-ornithemia and hyperornithemia after weaning, which mimics similar findings reported in humans with gyrate atrophy (50). This is due to developmental changes in OAT activity that have been reported in the small intestine of rats and mice (19, 38). OAT activity is high while the pups are nursing, but there is a decline, concomitant with the ingestion of solid feed, toward weaning. Other related enzymes such as pyrroline-5-carboxylate (P5C) synthase and proline oxidase also decline together with OAT, whereas arginase activity increases. Furthermore, ornithine itself is an inhibitor of P5C synthase (48). These ontological changes seem to result in a reduction in the de novo synthesis of ornithine from GSA (i.e., from glutamine and proline) and an increased dependence on ornithine generated by arginase.
Moreover, the isolation of enterocytes for in vitro studies results in the loss of the architecture of the intestinal epithelium. In situ hybridization studies in neonatal small intestine have shown that the enzymes that metabolize citrulline into arginine are present only in the upper part of the villi, whereas the mRNA of the enzymes involved in the synthesis of citrulline were concentrated in the crypts (9). In adult rats, it has been shown inmunohistochemically that OAT is localized in villi but not in the crypts (28). This intraepithelial localization of enzyme activity adds an extra layer of complexity to the already complex interorgan trafficking of intermediates for citrulline synthesis.
The contribution of glutamine carbon skeleton in the present study, determined with the 2,3,3,4,4-D5 and U-13C5 tracers, accounted for a modest fraction (∼0.2–0.4%) of the circulating citrulline that contrasts with previous determination in in vitro systems (58). For the reasons mentioned above, the localization of the different enzymes within different enterocyte subpopulations, the concentration of precursors, and the removal of products (i.e., glutamate), we suggest that whole animal experiments are the only means to fully understand the complex interorgan trafficking of precursors utilized in the synthesis of citrulline.
“Preformed” ornithine is used preferentially for citrulline synthesis.
As discussed above, ornithine utilized for the synthesis of citrulline can originate not only from the transamination of GSA by OAT (“de novo”; Fig. 1) but also by the hydrolysis of arginine by arginase (i.e., “preformed”). In the present study, the predominant dietary precursor for the synthesis of citrulline was arginine, which contributed ∼40% to the circulating citrulline, whereas proline participation was minor (3.3%) and the contribution of glutamine negligible (0.2–0.4%). The remaining ∼55% of the circulating citrulline originated, presumably, from endogenous sources. In a multitracer study in piglets, Urschel et al. (45) concluded that proline was the main precursor (50%) in the synthesis of citrulline. In this study tracers were given orally, but plasma enrichments were utilized in their calculations as the enrichment of the precursors (45). The implicit assumption of this approach is that amino acids are absorbed but not metabolized by the enterocytes, appear in peripheral plasma, and then return to the small intestine, where they are metabolized. Applying Eqs. 2 and 3, which do not rely on plasma enrichments to evaluate the contribution of oral tracers to the synthesis of citrulline, to the data of Urschel et al. (45) indicates that the recovery of the ornithine tracer used was ∼37 and 74% for the arginine-sufficient and -deficient diets, respectively, whereas the recovery of the proline tracer was only 0.4 and 2.4%, respectively. This reanalysis of the data in piglets supports our finding that preformed ornithine is the preferred precursor for the synthesis of citrulline rather than de novo ornithine synthesized by means of OAT.
The supply of preformed ornithine to the enterocytes seems to be guaranteed due to the ∼40% first-pass extraction of dietary arginine that, at least in piglets, is due entirely to small intestinal metabolism (2). Similar first-pass arginine disappearance has been shown in humans (33%) (6) and rats (33%) (54). However, the contribution of dietary arginine to the synthesis of citrulline in the present study was not necessarily a first-pass phenomenon since plasma ornithine enrichment was higher than the enrichment of citrulline. This means that arginine escaping first-pass extraction and metabolized somewhere else in the body to ornithine could have returned to the small intestine to be substrate for citrulline synthesis. In fact, we have previously seen substantial incorporation of plasma ornithine into citrulline (25).
Conclusions.
In conclusion, we have been able to dissect the contribution of dietary arginine, proline, and glutamine to the synthesis of citrulline and for the first time trace the incorporation of glutamine amino and amido nitrogen into the three possible positions of citrulline in vivo in a whole conscious animal model. We were also able to determine that glutamine oxidation provides ammonia and CO2 that can be utilized for the synthesis of citrulline. This nonspecific contribution of glutamine cannot be employed to derive precursor-product relationships, since any substrate oxidized by the enterocyte will behave in the same manner. Thus [15N]glutamine tracers are not appropriate to determine the conversion of glutamine into citrulline. The use of tracers that follow the carbon skeleton of glutamine showed that this amino acid is a minor contributor for the synthesis of citrulline. These observations, together with our finding that arginine is the main dietary precursor for circulating citrulline, will hopefully lead to the reevaluation of glutamine supplementation and further work to elucidate the mechanism by which glutamine supplementation increases citrulline availability.
GRANTS
The work was supported by the US Department of Agriculture (6250-51000-044) and the National Institutes of Health (K01-RR-024173, RO1-DK-54450).
DISCLOSURES
The authors have no disclosures to report.
Supplementary Material
ACKNOWLEDGMENTS
We thank Farook Jahoor, Doug Burrin, and Marta Fiorotto for their useful comments on this article.
REFERENCES
- 1.Alonso E, Rubio V. Participation of ornithine aminotransferase in the synthesis and catabolism of ornithine in mice. Studies using gabaculine and arginine deprivation. Biochem J 259: 131–138, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertolo RF, Brunton JA, Pencharz PB, Ball RO. Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab 284: E915–E922, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Blachier F, Darcyvrillon B, Sener A, Duee PH, Malaisse WJ. Arginine metabolism in rat enterocytes. Biochim Biophys Acta 1092: 304–310, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Boelens PG, Melis GC, van Leeuwen PA, ten Have GA, Deutz NE. Route of administration (enteral or parenteral) affects the contribution of l-glutamine to de novo l-arginine synthesis in mice: a stable-isotope study. Am J Physiol Endocrinol Metab 291: E683–E690, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Boelens PG, van Leeuwen PA, Dejong CH, Deutz NE. Intestinal renal metabolism of l-citrulline and l-arginine following enteral or parenteral infusion of l-alanyl-l-[2,15N]glutamine or l-[2,15N]glutamine in mice. Am J Physiol Gastrointest Liver Physiol 289: G679–G685, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR. Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol Endocrinol Metab 265: E532–E539, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Cheung CW, Cohen NS, Raijman L. Channeling of urea cycle intermediates insitu in permeabilized hepatocytes. J Biol Chem 264: 4038–4044, 1989 [PubMed] [Google Scholar]
- 8.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 15: 133–159, 1995 [DOI] [PubMed] [Google Scholar]
- 9.De Jonge WJ, Dingemanse MA, de Boer PA, Lamers WH, Moorman AF. Arginine-metabolizing enzymes in the developing rat small intestine. Pediatr Res 43: 442–451, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Deutz NE. The 2007 ESPEN Sir David Cuthbertson Lecture: amino acids between and within organs. The glutamate-glutamine-citrulline-arginine pathway. Clin Nutr 27: 321–327, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Dupertuis YM, Meguid MM, Pichard C. Advancing from immunonutrition to a pharmaconutrition: a gigantic challenge. Curr Opin Clin Nutr 12: 398–403, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Figueroa A, Trivino JA, Sanchez-Gonzalez MA, Vicil F. Oral l-citrulline supplementation attenuates blood pressure response to cold pressor test in young men. Am J Hypertens 23: 12–16, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Flynn NE, Wu G. An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am J Physiol Regul Integr Comp Physiol 271: R1149–R1155, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Fujita T, Yanaga K. Association between glutamine extraction and release of citrulline and glycine by the human small intestine. Life Sci 80: 1846–1850, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Guihot G, Blachier F, Colomb V, Morel MT, Raynal P, Corriol O, Ricour C, Duée PH. Effect of an elemental vs a complex diet on l-citrulline production from l-arginine in rat isolated enterocytes. JPEN J Parenter Enteral Nutr 21: 316–323, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Juliet PA, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. l-Citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc Natl Acad Sci USA 102: 13681–13686, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of l-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle l-citrulline to l-arginine. Proc Natl Acad Sci USA 87: 8612–8616, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzfeld A, Raper SM. Enzymes of ornithine metabolism in adult and developing rat intestine. Biochim Biophys Acta 428: 600–610, 1976 [DOI] [PubMed] [Google Scholar]
- 20.Houdijk AP, van Leeuwen PA, Teerlink T, Flinkerbusch EL, Boermeester MA, Sauerwein HP, Wesdorp RI. Glutamine-enriched enteral diet increases renal arginine production. JPEN J Parenter Enteral Nutr 18: 422–426, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto S, Sonoda T, Ohtake A, Tatibana M. Stimulatory effect of arginine on acetylglutamate synthesis in isolated mitochondria of mouse and rat liver. Biochem J 232: 329–334, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kight CE, Fleming SE. Transamination processes promote incomplete glutamine oxidation in small-intestine epithelial cells. J Nutr Biochem 6: 27–37, 1995 [Google Scholar]
- 23.Ligthart-Melis GC, van de Poll MC, Boelens PG, Dejong CH, Deutz NE, van Leeuwen PA. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr 87: 1282–1289, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Ligthart-Melis GC, van de Poll MC, Dejong CH, Boelens PG, Deutz NE, van Leeuwen PA. The route of administration (enteral or parenteral) affects the conversion of isotopically labeled l-[2–15N]glutamine into citrulline and arginine in humans. JPEN J Parenter Enteral Nutr 31: 343–348, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Marini JC, Erez A, Castillo L, Lee B. Interaction between murine spf-ash mutation and genetic background yields different metabolic phenotypes. Am J Physiol Endocrinol Metab 293: E1764–E1771, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Marini JC, Lee B, Garlick PJ. In vivo urea kinetic studies in conscious mice. J Nutr 136: 202–206, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Marini JC, Lee B, Garlick PJ. Non-surgical alternatives to invasive procedures in mice. Lab Anim 40: 275–281, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Markova M, Peneff C, Hewlins MJ, Schirmer T, John RA. Determinants of substrate specificity in omega-aminotransferases. J Biol Chem 280: 36409–36416, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Matsuzawa T, Kobayashi T, Tashiro K, Kasahara M. Changes in ornithine metabolic enzymes induced by dietary protein in small intestine and liver: intestine-liver relationship in ornithine supply to liver. J Biochem 116: 721–727, 1994 [DOI] [PubMed] [Google Scholar]
- 30.McCarty MF. Potential utility of full-spectrum antioxidant therapy, citrulline, and dietary nitrate in the management of sickle cell disease. Med Hypotheses 74: 1055–1058, 2010 [DOI] [PubMed] [Google Scholar]
- 31.McGivan JD, Bradford NM, Beavis AD. Factors influencing the activity of ornithine aminotransferase in isolated rat liver mitochondria. Biochem J 162: 147–156, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev 70: 701–748, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Murphy JM, Murch SJ, Ball RO. Proline is synthesized from glutamate during intragastric infusion but not during intravenous infusion in neonatal piglets. J Nutr 126: 878–886, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Ogawa T, Kimoto M, Sasaoka K. Purification and Properties of a New Enzyme, Ng,Ng-Dimethylarginine Dimethylaminohydrolase, from Rat-Kidney. J Biol Chem 264: 10205–10209, 1989 [PubMed] [Google Scholar]
- 35.Osowska S, Duchemann T, Walrand S, Paillard A, Boirie Y, Cynober L, Moinard C. Citrulline modulates muscle protein metabolism in old malnourished rats. Am J Physiol Endocrinol Metab 291: E582–E586, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Peters JH, Wierdsma NJ, Teerlink T, Van Leeuwen PA, Mulder CJ, Van Bodegraven AA. The citrulline generation test: proposal for a new enterocyte function test. Aliment Pharmacol Ther 27: 1300–1310, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Plauth M, Raible A, Vieillard-Baron D, Bauder-Gross D, Hartmann F. Is glutamine essential for the maintenance of intestinal function? A study in the isolated perfused rat small intestine. Int J Colorectal Dis 14: 86–94, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Riby JE, Hurwitz RE, Kretchmer N. Development of ornithine metabolism in the mouse intestine. Pediatr Res 28: 261–265, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Rougé C, Des Robert C, Robins A, Le Bacquer O, Volteau C, De La Cochetière MF, Darmaun D. Manipulation of citrulline availability in humans. Am J Physiol Gastrointest Liver Physiol 293: G1061–G1067, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Rutten EP, Engelen M, Wouters EF, Schols A, Deutz NE. Metabolic effects of glutamine and glutamate ingestion in healthy subjects and in persons with chronic obstructive pulmonary disease. Am J Clin Nutr 83: 115–123, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Schneider SM, Boirie Y, Zeanandin G, Mothe-Satney I, Habuterne X. Metabolisme et apports en acides amines chez le sujet age (amino acid metabolism and intake in the elderly). Nutr Clin Metab 22: 183–188, 2008 [Google Scholar]
- 42.Seiler N, Grauffel C, Dauneanglard G, Sarhan S, Knodgen B. Decreased hyperammonemia and orotic aciduria due to inactivation of ornithine aminotransferase in mice with a hereditary abnormal ornithine carbamoyltransferase. J Inherit Metab Dis 17: 691–703, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Smith AD, Benziman M, Strecker HJ. The formation of ornithine from proline in animal tissues. Biochem J 104: 557–563, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Smith RJ, Downing SJ, Phang JM, Lodato RF, Aoki TT. Pyrroline-5-carboxylate synthase activity in mammalian cells. Proc Natl Acad Sci USA 77: 5221–5225, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tedesco TA, Benford SA, Foster RC, Barness LA. Free amino acids in Citrullus vulgaris (watermelon). Pediatrics 73: 879, 1984 [PubMed] [Google Scholar]
- 45.Urschel KL, Rafii M, Pencharz PB, Ball RO. A multitracer stable isotope quantification of the effects of arginine intake on whole body arginine metabolism in neonatal piglets. Am J Physiol Endocrinol Metab 293: E811–E818, 2007 [DOI] [PubMed] [Google Scholar]
- 46.van de Poll MC, Siroen MP, van Leeuwen PAM, Soeters PB, Melis GC, Boelens PG, Deutz NE, Dejong CH. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr 85: 167–172, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Vermeulen MA, van de Poll MC, Ligthart-Melis GC, Dejong CH, van den Tol MP, Boelens PG, van Leeuwen PA. Specific amino acids in the critically ill patient–exogenous glutamine/arginine: a common denominator? Crit Care Med 35: S568–S576, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Wakabayashi Y, Yamada E, Hasegawa T, Yamada R. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. I. Pyrroline-5-carboxylate synthase. Arch Biochem Biophys 291: 1–8, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Walrand S, Guillet C, Salles J, Tardif N, Maurin AC, Fafournoux P, Cano N, Boirie Y. Acides amines et signalisation cellulaire (amino acids as regulators of cell signalling). Nutr Clin Metab 22: 161–167, 2008 [Google Scholar]
- 50.Wang T, Lawler AM, Steel G, Sipila I, Milam AH, Valle D. Mice lacking ornithine-delta-aminotransferase have paradoxical neonatal hypoornithinaemia and retinal degeneration. Nat Genet 11: 185–190, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Watford M. Glutamine metabolism in rat small intestine: synthesis of three-carbon products in isolated enterocytes. Biochim Biophys Acta 1200: 73–78, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Watford M, Lund P, Krebs HA. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J 178: 589–596, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkinson DL, Bertolo RF, Brunton JA, Shoveller AK, Pencharz PB, Ball RO. Arginine synthesis is regulated by dietary arginine intake in the enterally fed neonatal piglet. Am J Physiol Endocrinol Metab 287: E454–E462, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Windmueller HG, Spaeth AE. Metabolism of absorbed aspartate, asparagine, and arginine by rat small intestine in vivo. Arch Biochem Biophys 175: 670–676, 1976 [DOI] [PubMed] [Google Scholar]
- 55.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol Endocrinol Metab 241: E473–E480, 1981 [DOI] [PubMed] [Google Scholar]
- 56.Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem 249: 5070–5079, 1974 [PubMed] [Google Scholar]
- 57.Wu G. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol 272: G1382–G1390, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Wu GY, Knabe DA, Flynn NE. Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299: 115–121, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziegler TR, Benfell K, Smith RJ, Young LS, Brown E, Ferrari-Baliviera E, Lowe DK, Wilmore DW. Safety and metabolic effects of l-glutamine administration in humans. JPEN J Parenter Enteral Nutr 14: 137S–146S, 1990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.