Abstract
Endothelial progenitor cells (EPCs) play an important role in angiogenesis, which is essential for numerous physiological processes as well as tumor growth. Several microRNAs (miRNAs) have been reported to be involved in angiogenesis. MiR-34a, recently reported as a tumor suppressor, has been found to target silent information regulator 1 (Sirt1), leading to cell cycle arrest or apoptosis. However, the role of miR-34a in EPC-mediated angiogenesis was unknown. The present study tested the hypothesis that miR-34a inhibits EPC-mediated angiogenesis by inducing senescence via suppressing Sirt1. Bone marrow-derived EPCs from adult male Spraque-Dawley rats were used. Results of flow cytometry showed that EPCs after 7 days of culture expressed both stem cell markers CD34 and CD133 and endothelial cell markers VEGFR-2 (flk-1) and VE-cadherin. MiR-34a was expressed in normal EPCs, and overexpression of miR-34a via its mimic transfection significantly increased its expression and impaired in vitro EPC angiogenesis. MiR-34a overexpression led to a significantly increased EPC senescence, paralleled with an ∼40% Sirt1 reduction. Furthermore, knockdown of Sirt1 by its siRNA resulted in diminished EPC angiogenesis and increased senescence. Finally, overexpression of miR-34a increased the level of Sirt1 effector-acetylated forkhead box O transcription factors 1 (FoxO1), an effect mimicked in EPCs following Sirt1 knockdown. In conclusion, miR-34a impairs EPC-mediated angiogenesis by induction of senescence via inhibiting Sirt1.
angiogenesis plays an essential role in the maintenance and repair of tissues. An imbalance of this process contributes to the pathogenesis of numerous disorders, such as ischemia and tumor growth (7). Endothelial progenitor cells (EPCs), first described by Asahara et al. (3) in 1997, are a population of bone marrow-derived cells that are home to sites of new blood vessel formation and play an important role in angiogenesis and maintenance of vascular integrity (19, 36).
MicroRNAs (miRNAs), a class of small noncoding RNAs that negatively regulate gene expression at the posttranscriptional level, are emerging as important regulators of angiogenesis (2, 4, 12). Recent studies have reported the involvement of miRNAs in angiogenesis, and several specific miRNAs targeting angiogenesis have been identified. For instance, miR-27b and let-7f were identified as proangiogenic miRNAs, whereas miR-221 and miR-222 were described as antiangiogenic miRNAs in endothelial cells (20, 32, 37). MiR-34a is a tumor suppressor, and its loss or reduced expression has been detected in a number of tumor tissues and cancer cell lines (13, 21, 22, 26, 33, 35, 41). In contrast, ectopic expression of miR-34a has been found to contribute to apoptosis, cell cycle arrest, or senescence (17, 38). However, the role of miR-34a in angiogenesis has not been reported to date.
Silent information regulator 1 (Sirt1), one of the potential targets of miR-34a (38), plays a critical role in regulating cell cycle, senescence, apoptosis, and metabolism (5, 14). MiR-34a was reported to directly bind to Sirt1 mRNA and regulate cell apoptosis via Sirt1-p53 pathway (38, 39). Initially identified as a longevity gene, Sirt1 has recently been implicated as a novel modulator of vascular endothelial cell homeostasis, playing a key role in angiogenesis through the deacetylation of forkhead box O transcription factor 1 (FoxO1) (29, 30). In endothelial cells, silencing of Sirt1 was accompanied by impaired angiogenic function in vitro (28). However, it is entirely unknown whether Sirt1 affects angiogenesis in EPCs by miR-34a regulation.
In the present study, we hypothesized that miR-34a impairs EPC-mediated angiogenesis by induction of senescence via inhibiting Sirt1. MiR-34a level in normal EPCs was detected for the first time. MiR-34a was overexpressed in EPCs to investigate its effect on angiogenic function. The interplay of EPC senescence and Sirt1 level was then determined following miR-34a upregulation, and Sirt1 was silenced to further ascertain its effects on EPC angiogenesis and senescence. Finally, expression of Sirt1 downstream target-acetylated FoxO1 was demonstrated upon miR-34a overexpression or Sirt1 knockdown in EPCs. Our study demonstrates for the first time that miR-34a impairs EPC-mediated angiogenesis by induction of senescence via inhibiting Sirt1. These findings provide a mechanistic insight on miRNA-34a regulation of EPC function.
MATERIALS AND METHODS
Animals.
Adult male Spraque-Dawley rats (201–225 g) were obtained from Charles River Laboratories (Wilmington, MA). The animals were maintained under controlled environments (12:12-h light-dark cycle, temperature ∼25°C) and provided with standard laboratory food and water ad libitum. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Isolation and in vitro culture of bone marrow-derived EPCs.
EPCs were isolated from bone marrow and cultured afterward on the basis of published protocols, as described recently with some minor modifications (10). Briefly, bone marrow mononuclear cells were isolated from the femurs and tibias of rats by density gradient centrifugation using Histopaque-1083 (Sigma) according to the manufacturer's protocol. After two washing steps, mononuclear cells were plated onto rat plasma vitronectin (Sigma-Aldrich)-coated six-well plates at a density of 2 × 107 cells/well and maintained in endothelial basal medium-2 (Lonza) supplemented with EGM-2MV single aliquots (Lonza) containing vascular endothelial growth factor (VEGF), fibroblast growth factor-2, epidermal growth factor, insulin-like growth factor, ascorbic acid, hydrocortisone, gentamycin, amphotericin-B, and fetal bovine serum. Cells were cultured at 37°C with 5% CO2 in a humidified atmosphere. After 4 days of culture, nonadherent cells were removed by washing with PBS, and fresh medium was added. EPCs after 7 days of culture were used for further analysis.
EPC characterization.
After 7 days of culture, the expression of stem cell markers CD34 and CD133, endothelial lineage markers VEGF receptor-2 (VEGFR-2, flk-1) and VE-cadherin, and hematopoietic cell marker CD45 was analyzed by flow cytometry and compared with those of freshly isolated bone marrow mononuclear cells. Briefly, EPCs were gently detached using 5 mmol/l EDTA-PBS at 37°C and then washed with PBS. To detect CD133, VEGFR-2, and VE-cadherin, cells were first incubated with rabbit anti-rat CD133 antibody, rabbit anti-rat VEGFR-2 antibody (2 μg/1 × 106 cells; Abcam), or mouse anti-rat VE-cadherin antibody (1 μg/1 × 106 cells; Santa Cruz Biotechnology) on ice for 1 h after permeabilization with 50% methanol in PBS for 15 min at room temperature and then incubated with FITC-conjugated goat anti-rabbit secondary antibody (2 μg/1 × 106 cells; Abcam) or PE-conjugated goat anti-mouse secondary antibody (2 μg/1 × 106 cells; Santa Cruz Biotechnology) on ice for another hour. As staining controls, 0.5% rabbit serum and 0.5% mouse serum were used for goat anti-rabbit antibody and goat anti-mouse antibody, respectively. To detect CD34 and CD45, cells were incubated with PE-conjugated anti-rat CD34 antibody (2 μg/1 × 106 cells; Santa Cruz Biotechnology) or PE-Cy5-conjugated mouse anti-rat CD45 antibody (1 μg/1 × 106 cells; BD Bioscience) on ice for 1 h. PE-labeled isotype control antibody (2 μg/1 × 106 cells; Santa Cruz Biotechnology) and PE-Cy5-labeled isotype control antibody (1 μg/1 × 106 cells; BD Bioscience) were used for PE-CD34 antibody and PE-Cy5-CD45 antibody, respectively. For flow cytometry analysis, ≥10,000 cells were acquired and scored using a FACScan analyzer (Becton Dickinson). Data were processed using the CellQuest software program (Becton Dickinson).
Small interfering RNA and miRNA transfection.
Before transfection, EPCs after 7 days of culture were replated onto six-well plates at a density of 2.5 × 105 cells/well and incubated overnight. For small interfering RNA (siRNA)-mediated gene knockdown, 100 nmol/l of Sirt1 siRNA SMARTpool or scramble siRNA (Dharmacon) was transfected into cells. For overexpression of miR-34a, cells were transfected with 100 nmol/l of miRIDIAN miR-34a mimic or scramble miRNA mimic (Dharmacon). All siRNAs and miRNAs were transfected into EPCs with DharmaFECT Transfection Reagent I (Dharmacon) according to the manufacturer's protocol. After 72 h of transfection, cells were harvested for further analysis.
Isolation of miRNAs and real-time quantitative reverse transcription-polymerase chain reaction analysis.
Enriched miRNAs were isolated from untreated EPCs and EPCs transfected with miR-34a mimic or scramble miRNA mimic with the mirVana miRNA Isolation Kit (Ambion). Quantitative (q)RT-PCR was performed with the mirVana qRT-PCR miRNA Detection Kit (Ambion). Amplification and detection of specific products were performed according to the manufacturer's protocol with the ABI PRISM 7300 system (Applied Biosystems). The U6 small nucleolar RNA was used as the housekeeping small RNA reference gene. The relative gene expression was normalized to U6 small nucleolar RNA. Each reaction was performed in triplicate, and analysis was performed by the 2−ΔΔCT method, as described previously (31). Primer identification numbers (Applied Biosystems) were as follows: has-miR-34a, 000426; U6, 001973.
Western blot analysis.
Western blot analysis was performed as we described previously (9). Briefly, EPCs were lysed in Cell Lytic MT lysis buffer (Sigma) with Protease Inhibitor Cocktail (Sigma) for 15 min on a shaker. After centrifugation for 10 min at 12,000 g (4°C), the protein concentration of the samples was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific). Equal amounts of protein (50 μg) were loaded onto SDS-polyacrylamide gels and blotted onto Hybond ECL nitrocellulose membranes (Amersham Biosciences). Western blots were performed by using antibodies against Sirt1 (rabbit polyclonal anti-Sirt1, 1:1,000; Upstate Biotechnology), FoxO1 (rabbit polyclonal anti-FoxO1, 1:1,000; Cell Signaling Technology), and acetyl-FoxO1 (rabbit polyclonal anti-acetyl-FoxO1, 1:1,000; Santa Cruz Biotechnology). Antibody against actin (mouse monoclonal anti-β-actin, 1:2,500; Sigma) was used as a loading control. For detection, secondary antibodies (Rockland) were IR Dye 700-conjugated anti-rabbit (1:4,000) antibodies or IR Dye 800-conjugated anti-mouse (1:5,000) antibodies. Bands were visualized with an Odyssey Imager (Li-Cor) and quantified with Quantity One System (Bio-Rad).
In vitro angiogenesis assay.
The in vitro angiogenic activity of EPCs was determined by Matrigel tube formation assay, as described previously (15), with minor modifications. Briefly, EPCs after transfection were replated at a density of 5 × 104 cells/well in 48-well plates precoated with 150 μl/well growth factor-reduced Matrigel (BD Biosciences). After 15 h of incubation, tube formation was observed with a computer-assisted microscope (Nikon). Tube formation was defined as a tube-like structure exhibiting a length four times its width (15). Images of tube morphology were taken in 10 random microscopic fields per sample at ×100 magnification, and the cumulative tube lengths were measured by Image-Pro Plus (Media Cybernetics).
Senescence-associated β-galactosidase staining.
EPC senescence was determined by in situ staining for senescence-associated β-galactosidase (SA-β-gal) using a senescence cell histochemical staining kit (Sigma). Briefly, EPCs after transfection were first fixed for 6–7 min at room temperature in fixation buffer. After washing with PBS, cells were incubated with β-gal staining solution for 12 h at 37°C without CO2. The reaction was stopped by the addition of PBS. Statistical analysis was performed by counting ≥600 cells for each sample (25).
Statistical analysis.
Data were expressed as means ± SE. Differences between two treatment groups were compared using Student's t-test. Results were considered statistically significant when P < 0.05. All analyses were performed with GraphPad Prism 5.0 (GraphPad, San Diego, CA).
RESULTS
Characterization of bone marrow-derived EPCs.
To characterize bone marrow-derived EPCs, the stem cell markers (CD34, CD133), endothelial cell markers (VEGFR-2, VE-cadherin), and a hematopoietic cell marker (CD45) were examined by flow cytometry according to recent publications (10, 18). Compared with freshly isolated mononuclear cells, the percentages of cell population-bearing adult stem cell markers (CD34+ cells, CD133+ cells, CD34+CD133+ cells, and CD34+VEGFR-2+ cells) in 7-day cultured cells were increased from 19.44 ± 2.16 to 48.09 ± 3.18%, 16.45 ± 0.41 to 42.86 ± 2.36%, 12.67 ± 0.85 to 34.16 ± 4.58%, and 6.28 ± 1.41 to 15.26 ± 4.74%, respectively (Table 1). The percentages of endothelial lineage cell population (VEGFR-2+ cells and VE-cadherin+ cells) were elevated from 9.97 ± 3.49 to 15.44 ± 1.23% and 7.29 ± 3.35 to 16.28 ± 4.25%, respectively. However, the percentage of hematopoietic CD45+ cells decreased from 64.10 ± 10.49 to 9.71 ± 1.02%. Additionally, the expression of endothelial cell markers in human umbilical vein endothelial cells (HUVECs) was also detected by flow cytometry. Results showed that HUVECs expressed these two markers at significantly higher levels (VEGFR-2: 84.1 ± 6.65%; VE-cadherin: 86.43 ± 8.71%), which is consistent with their fully differentiated status.
Table 1.
Characterization of bone marrow-derived MNCs and cultured EPCs
| MNCs (day 0), % | EPCs (day 7), % | |
|---|---|---|
| CD34 | 19.44 ± 2.16 | 48.09 ± 3.18 |
| CD133 | 16.45 ± 0.41 | 42.86 ± 2.36 |
| VEGFR-2 | 9.97 ± 3.49 | 15.44 ± 1.23 |
| VE-cadherin | 7.29 ± 3.35 | 16.28 ± 4.25 |
| CD34/CD133 | 12.67 ± 0.85 | 34.16 ± 4.58 |
| CD34/VEGFR2 | 6.28 ± 1.41 | 15.26 ± 4.74 |
| CD45 | 64.10 ± 10.49 | 9.71 ± 1.02 |
Data are expressed as means ± SE; n = 3 for each group. MNCs, mononuclear cells (freshly isolated); EPCs, endothelial progenitor cells (cultured for 7 days); VEGFR-2, VEGF receptor-2.
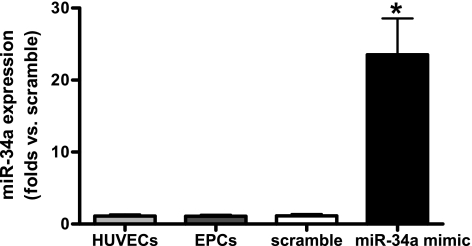
miR-34a was expressed in normal EPCs and increased significantly after transfection of miR-34a mimic.
Real-time PCR results showed that miR-34a was expressed in normal EPCs (Fig. 1). To upregulate its expression in EPCs, cells were transfected with miR-34a mimic. After 72 h of miR-34a transfection, miR-34a level was increased ∼23-fold in EPCs compared with those transfected with scramble miRNA mimic (Fig. 1).
Fig. 1.
miR-34a is expressed in normal endothelial progenitor cells (EPCs) and increases significantly following transfection with miR-34a mimic. miR-34a expression was determined by real-time PCR. The relative gene expression was normalized to U6 small nucleolar RNA, and analysis was performed by the 2−ΔΔCT method. Data were expressed as means ± SE; n = 3. *P < 0.05 vs. scramble. HUVECs, human umbilical vein endothelial cells.
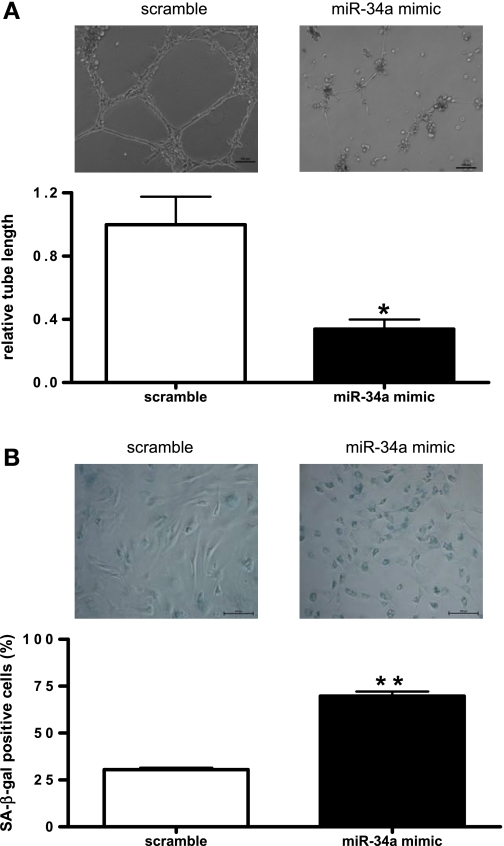
Overexpression of miR-34a impaired in vitro angiogenesis and induced senescence in normal EPCs.
To investigate the role of miR-34a on EPC-mediated angiogenesis, in vitro Matrigel tube formation assay was performed (25). Fifteen hours after seeding on Matrigel, EPCs transfected with miR-34a mimic showed a significant impairment of tube-forming activity, suggesting that miR-34a overexpression decreased EPC angiogenic function (Fig. 2A). Considering that EPC regenerative capacity contributes greatly to EPC function, we further examined cellular senescence in miR-34a mimic-transfected EPCs. SA-β-gal activity, which is a characteristic feature of senescence-related growth arrest (8), was tested in EPCs with miR-34a overexpression. Results revealed that overexpression of miR-34a significantly increased the percentage of SA-β-gal-positive cells by ∼40% compared with that of scramble miRNA mimic (Fig. 2B).
Fig. 2.
Overexpression of miR-34a impairs angiogenesis in vitro and induces senescence in normal EPCs. A, top: representative micrographs of Matrigel angiogenesis assays in EPCs transfected with miR-34a mimic or scramble microRNA (miRNA) mimic. Tube formation was defined as a tube-like structure exhibiting a length 4 times its width (magnification ×100). Bar = 100 μm. A, bottom: statistical analysis of cumulative tube lengths. Image Pro plus 5.1 was used to determine the cumulative length of the tube-like structures in images. Data were normalized to EPCs transfected with scramble miRNA mimic and expressed as means ± SE; n = 3. *P < 0.05 vs. scramble. B, top: representative micrographs of senescence-associated β-galactosidase (SA-β-gal) staining in EPCs transfected with miR-34a mimic or scramble miRNA mimic (magnification ×200). Bar = 100 μm. B, bottom: statistical analysis of SA-β-gal-positive cells. Data are expressed as means ± SE; n = 3. **P < 0.01 vs. scramble.
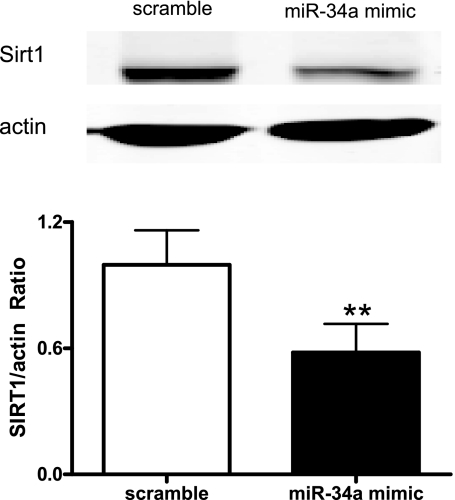
miR-34a downregulated endogenous Sirt1 expression in EPCs.
Sirt1 has been reported to have a miR-34a binding site within its 3′-untranslated region (38). To test the hypothesis that miR-34a negatively regulates Sirt1 expression in EPCs, miR-34a mimic was transfected into EPCs to upregulate miR-34a expression, and Sirt1 protein level was examined afterward. After 72 h of miR-34a mimic transfection, Western blot analysis demonstrated that Sirt1 level was diminished by ∼40% in EPCs compared with that of scramble miRNA mimic (Fig. 3).
Fig. 3.
miR-34a suppresses silent information regulator 1 (Sirt1) expression in EPCs. Sirt1 expression in EPCs transfected with miR-34a mimic or scramble miRNA mimic was determined by Western blot analysis. Top: representative blots of 3 individual experiments. Bottom: quantitative analysis of band density. Data were expressed as means ± SE normalized to EPCs transfected with scramble miRNA mimic; n = 3. **P < 0.01 vs. scramble.
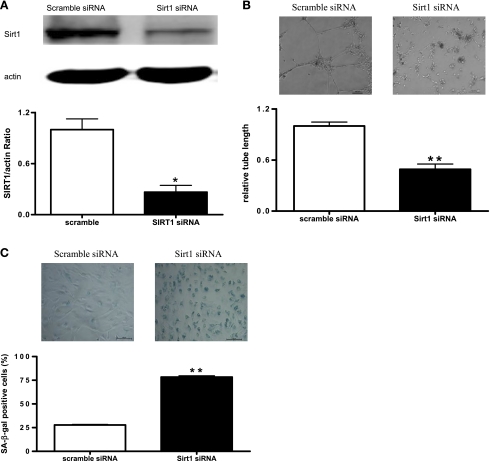
Silencing of Sirt1 impeded angiogenesis and increased senescence in EPCs.
Sirt1 inhibition has been reported to be associated with premature senescence and impaired angiogenic activity in HUVECs (28). To test whether Sirt1 plays key roles in EPC angiogenesis and senescence, Sirt1 level in normal EPCs was knocked down by Sirt1 siRNA transfection. Sirt1 expression was decreased by 70% in EPCs transfected with Sirt1 siRNA compared with those transfected with scramble siRNA (Fig. 4A). The effect of Sirt1 knockdown on in vitro angiogenesis was then examined using Matrigel assay. Results showed that EPCs transfected with Sirt1 siRNA formed significantly more incomplete and poorly connected tube networks than those of scramble siRNA-treated EPCs (Fig. 4B). Consistently, the percentage of SA-β-gal-positive senescence cells was significantly increased following Sirt1 knockdown (Fig. 4C).
Fig. 4.
Silencing of Sirt1 results in decreased angiogenesis and increased senescence in EPCs. A: Sirt1 expression in EPCs transfected with Sirt1 small interfering RNA (siRNA) or scramble siRNA was determined by Western blot analysis. A, top: representative blots of 4 individual experiments. A, bottom: quantitative analysis of band density. Data were normalized to EPCs transfected with scramble siRNA and expressed as means ± SE; n = 4. *P < 0.05 vs. scramble. B, top: representative micrographs of Matrigel angiogenesis assay in EPCs transfected with Sirt1 siRNA or scramble siRNA. Bar = 100 μm. B, bottom: statistical analysis of cumulative tube lengths. Data were normalized to EPCs transfected with scramble siRNA and expressed as means ± SE; n = 5. **P < 0.01 vs. scramble. C, top: representative micrographs of SA-β-gal staining in EPCs transfected with Sirt1 siRNA or scramble siRNA. Bar = 100 μm. C, bottom: statistical analysis of SA-β-gal-positive cells. Data were expressed as means ± SE; n = 3. **P < 0.01 vs. scramble.
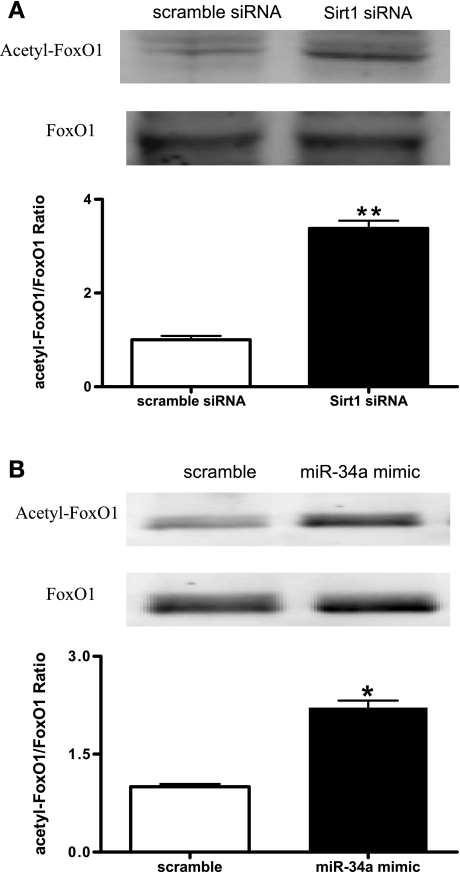
miR-34a increased the expression of acetylated FoxO1 in EPCs.
FoxO1 has been reported to be a key Sirt1 downstream target that regulates other target proteins in a deacetylation-dependent manner. Western blot analysis showed that Sirtl knockdown led to augmented FoxO1 acetylation in EPCs (Fig. 5A). To test whether the processes controlled by miR-34a could be mediated by a deacetylation-dependent regulation of Sirt1, the expression of FoxO1 acetylation was analyzed following miR-34a upregulation in EPCs. Results showed that overexpression of miR-34a caused significantly increased FoxO1 acetylation in EPCs (Fig. 5B).
Fig. 5.
miR-34a augments acetylated forkhead box O transcription factor (FoxO1) expression in EPCs. A: expressions of FoxO1 and acetylated FoxO1 in EPCs transfected with Sirt1 siRNA or scramble siRNA were determined by Western blot analysis. A, top: representative blots of 3 individual experiments. A, bottom: quantitative analysis of band density. Data were normalized to EPCs transfected with scramble siRNA and expressed as means ± SE; n = 3. **P < 0.01 vs. scramble. B: expressions of FoxO1 and acetylated FoxO1 in EPCs transfected with miR-34a mimic or scramble miRNA mimic were determined by Western blot analysis. B, top: representative blots of 3 individual experiments. B, bottom: quantitative analysis of band density. Data were expressed as means ± SE normalized to EPCs transfected with scramble miRNA mimic; n = 3. *P < 0.05 vs. scramble.
DISCUSSION
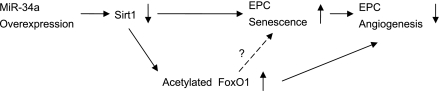
The major new findings of the present study were that miR-34a significantly 1) inhibited EPC-mediated angiogenesis in vitro, 2) augmented EPC senescence, with a concomitant Sirt1 reduction, and 3) enhanced the expression of Sirt1 effector protein-acetylated FoxO1 in EPCs, an effect mimicked by Sirt1 knockdown (see Fig. 6).
Fig. 6.
Schematic illustration of the possible signaling cascade through which miR-34a regulates EPC angiogenesis. Overexpression of miR-34a in EPCs induces cellular senescence and impedes in vitro angiogenesis via suppressing Sirt1. Expression of acetylated FoxO1 was augmented with miR-34a upregulation, which may mediate the effect of miR-34a on EPC angiogenesis.
Angiogenesis plays an essential role during the whole lifespan, and an imbalance of angiogenesis leads to many disorders (7). Accordingly, precise control of angiogenesis has been identified as a potential therapeutic strategy by, for instance, either promoting angiogenesis of ischemic tissues or inhibiting angiogenesis in tumor cells (11). Among the types of cells involved in the process of angiogenesis, EPCs are prominent (1). EPCs are defined as endothelial cell precursors that are derived from bone marrow, can home to sites of neovascularization, differentiate into endothelial cells, and participate in angiogenesis and maintenance of endothelial integrity (19).
Because there is no single definitive marker for EPCs (34), the combination of adult stem cell and endothelial cell markers is commonly used for their identification. According to the latest publications (10, 18), 7-day cultured EPCs in rodents exhibit typical phenotypes with the expression of Sca-1, CD34, Flk-1 (VEGFR-2), VE-cadherin, CD105, and CD11b. Consistent with these studies, our data showed that the expression of both stem cell and endothelial cell markers in 7-day cultured EPCs was significantly increased, whereas the expression of hematopoietic cell marker CD45 was decreased compared with freshly isolated mononuclear cells, suggesting that 7-day cultured EPCs are a heterogeneous cell population possessing stem cell characteristics while undergoing differentiation into endothelial cells. These results also confirmed the successful isolation of the bone marrow-derived EPCs, as described previously (10, 18).
Accumulating evidence suggests that miRNAs are involved in the process of angiogenesis, modulating new vessel formation through their upregulation or downregulation (20, 32, 37). Among these miRNAs, let7-f, miR-27b, and miR-130a have been identified as proangiogenic miRNAs, whereas miR-221 and miR-222 exert antiangiogenic effects. Some miRNAs, such as the miR-17–92 cluster and miR-378, are also involved in tumor angiogenesis (37). miR-34a, known as a tumor suppressor, induces cell cycle arrest and apoptosis by downregulating cell cycle and apoptosis-related proteins (13). Recent studies have shown that transient expression of miR-34a induces growth arrest and cellular senescence, whereas loss or reduced expression of miR-34a has been detected in a variety of tumor tissues and cell lines (13, 21, 22, 26, 33, 35, 41). Although miR-34a has been reported to express ubiquitously (17), neither its expression nor a role in endothelial lineage cells has been defined to date. In the present study, we demonstrated the expression of miR-34a in normal EPCs for the first time.
To investigate the role of miR-34a in EPC angiogenesis, we transfected miR-34a mimic into EPCs to upregulate its expression and then examined their phenotypic changes. Our results clearly showed that miR-34a inhibited Matrigel tube formation, a hallmark of in vitro angiogenesis. Regenerative capacity of EPCs is closely related to their functions. In agreement with this principle, aged EPCs exhibit decreased cell proliferation, survival rate, and migration capacity (16). To better understand how miR-34a impairs EPC angiogenesis, we investigated EPC senescence under the same condition under which in vitro angiogenesis was studied. Our data showed that overexpression of miR-34a resulted in an increased percentage of SA-β-gal-positive senescence cells, which is consistent with the findings in fibroblasts and cancer cells (13, 23, 33). Collectively, the above observations suggest that miR-34a overexpression induces senescence in normal EPCs and may thereby contribute to their impaired angiogenic function.
Sirt1, well known as a longevity gene, has been shown to protect cells against stress-induced senescence and extends organismal lifespan in response to calorie restriction (6). Considering the opposite role of miR-34a and Sirt1 in senescence and that Sirt1 has been reported to be one of the potential targets of miR-34a (38), we further examined the effect of miR-34a on Sirt1 expression in EPCs. MiR-34a was overexpressed in EPCs, and Sirt1 protein level was found to be diminished by 40%, demonstrating that miR-34a negatively regulates Sirt1 expression in EPCs. However, similar to a recent report (38), overexpression of miR-34a did not completely abolish Sirt1 expression. We suspect that either miR-34a does not exactly match its Sirt1 binding site or every Sirt1 binding site does not interact with miR-34a, which warrants further comprehensive studies in the future.
Besides its role against aging, Sirt1 is also involved in the maintenance of vascular endothelial homeostasis (29). Indeed, Sirt1 protects endothelial cells against vascular dysfunction by preventing stress-induced senescence, promotes endothelium-dependent vascular relaxation, and plays a critical role in angiogenesis (24, 27, 28). Our observations that silencing of Sirt1 in EPCs led to their impaired angiogenesis and increased senescence are highly consistent with the above findings. Taken together, our results suggest that miR-34a-induced Sirt1 decrease may represent an important mechanism underlying EPC senescence and subsequent angiogenesis reduction.
FoxO transcription factors are critical regulators of stress responses, longevity, and oncogenesis and directly control the expressions of genes involved in apoptosis, cell cycle progression, and stress responses (40). FoxO1 has also been reported to be involved in angiogenesis, and its acetylation was shown to negatively regulate vascular growth (30). Sirt1 was found to bind to and deacetylate FoxO1 and then suppress its transcriptional activity (40). In the present study, we showed that overexpression of miR-34a augments FoxO1 acetylation, which is known to negatively affect cell survival and angiogenesis. Importantly, Sirt1 knockdown produced a similar effect in EPCs. Collectively, these data suggest that the effect of miR-34a on EPC angiogenesis could be mediated by increased FoxO1 acetylation following Sirt1 reduction. Although miR-34a has been implicated in tumor-suppressive function (38), the findings reported here imply that the angiogenesis inhibition function exhibited by miR-34a may also contribute to impaired angiogenesis in ischemia, a common phenomenon underlying a number of chronic cardiovascular diseases.
In conclusion, we have determined that miR-34a acts as an endogenous suppressor of Sirt1 to impair EPC-mediated angiogenesis by senescence induction, which may provide a potential miRNA-based mechanism in regulating angiogenesis in health and disease.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant R01-GM-077352, American Diabetes Association Research Award 7-08-RA-23 (to Dr. A. F. Chen), and grants from National Basic Research Program of China (2006CB503910) and National High-Tech Research and Development Program of China (2006AA 02A 408; to Dr. J. Li).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Ahn GO, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis 12: 159–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem 73: 417–435, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bordone L, Guarente L. Caloric restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6: 298–305, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campini J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117: 1045–1054, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, van Eck M, Van Craeyveld E, Jacobs F, Carlier V, Van Linthout S, Erdel M, Tjwa M, De Geest B. Critical role of scavenger receptor-BI-expressing bone marrow-derived endothelial progenitor cells in the attenuation of allograft vasculopathy after human apo A-I transfer. Blood 113: 755–764, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 438: 967–974, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fish JE, Srivastava D. MicroRNAs: opening a new vein in angiogenesis research. Sci Signal 2: pe1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun 377: 114–119, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Haigis MC, Guarente LP. Mammalian sirtuins: emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors α and β mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation 114: 2261–2270, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 45: 1441–1448, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ 17: 193–199, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: 1584–1595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krenning G, van Luyn MJ, Harmsen MC. Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol Med 15: 180–189, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 101: 59–68, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y, Zheng X. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett 275: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Korner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 7: 2591–2600, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Maes OC, Sarojini H, Wang E. Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J Cell Physiol 221: 109–119, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 120: 1524–1532, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Mraz M, Malinova K, Kotaskova J, Pavlova S, Tichy B, Malcikova J, Kozubik KS, Smardova J, Brychtova Y, Doubek M, Mayer J, Pospisilova S. miR-34a, miR-29c and miR-17–5p are downregulated in CLL patients with TP53 abnormalities. Leukemia 23: 1159–1163, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J, Komuro I. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol 29: 889–894, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol 43: 571–579, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Potente M, Dimmeler S. Emerging roles of SIRT1 in vascular endothelial homeostasis. Cell Cycle 7: 2117–2122, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 100: 11618–11623, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res 104: 442–454, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA 104: 15472–15477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med 13: 87–102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tryndyak VP, Ross SA, Beland FA, Pogribny IP. Down-regulation of the microRNAs miR-34a, miR-127, and miR-200b in rat liver during hepatocarcinogenesis induced by a methyl-deficient diet. Mol Carcinog 48: 479–487, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95: 343–353, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res 79: 581–588, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105: 13421–13426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle 8: 712–715, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J 24: 1021–1032, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zenz T, Mohr J, Eldering E, Kater AP, Bühler A, Kienle D, Winkler D, Dürig J, van Oers MH, Mertens D, Döhner H, Stilgenbauer S. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood 113: 3801–3808, 2009 [DOI] [PubMed] [Google Scholar]