Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ) is a nuclear receptor that functions as a master transcriptional regulator of adipocyte conversion. During PPARγ transactivation, multiple signaling pathways interact with one another, leading to the differentiation of both white and brown adipose tissue. Ligand activation of the PPARγ-RXR heterodimer complex also enhances insulin sensitivity, and this property has been heavily exploited to develop effective pharmacotherapies for the treatment of type 2 diabetes mellitus. PPARγ is also expressed in stem cells and plays a critical role in mesenchymal stromal cell differentiation and lineage determination events. The many facets of PPARγ activity within the bone marrow niche where adipocytes, osteoblasts, and hematopoietic cells reside make this molecule an attractive target for pharmacological investigation. Additional findings that osteoblasts can alter energy metabolism by influencing adiposity and insulin sensitivity, and observations of decreased bone turnover in diabetic subjects, underscore the contribution of the skeleton to systemic energy requirements. Studies into the role of PPARγ in skeletal acquisition and maintenance may lead to a better understanding of the molecular mechanisms governing stromal cell differentiation in the mesenchyme compartment and whether PPARγ activity can be manipulated to benefit skeletal remodeling events and energy metabolism.
Keywords: peroxisome proliferator-activated receptor-γ, bone remodeling, energy metabolism
a dramatic evolution in our understanding of adipose tissue biology has occurred over the past two decades. Our perception of adipocytes has shifted from purely inert storage vessels for triacylglyerol to central integrators of metabolic homeostasis. The discovery of leptin and the peroxisome proliferator-activated receptor (PPAR) family of nuclear receptors and transcription factors marked the beginning of a period of intense discoveries (17, 40) that ultimately led to the establishment of the PPARs (α, δ, and γ) as key regulators of fuel metabolism and energy conservation. Ligands that bind PPARγ improve glucose tolerance and insulin sensitivity in type 2 diabetic patients and in animal models of insulin resistance (66). Coincident with these findings, novel aspects of skeletal physiology emerged that challenged the sole role of calciotropic hormones as regulators of bone remodeling. Seminal studies in the early part of this century noted that leptin, an adipokine that targets the hypothalamus to control satiety, also modulates skeletal turnover through activation of the sympathetic nervous system (13). In vitro studies suggested that adipocytes and osteoblasts likely arise from the same multipotent stem cell in the bone marrow niche (5). However, the incidental finding that the PPARγ ligand rosiglitazone, an insulin sensitizer widely used for the treatment of type 2 diabetes, causes profound bone loss, fractures, and increased marrow adiposity in both C57BL/6J mice and postmenopausal women, pointed to an unexpected role for this transcription factor in marrow/bone biology (22, 35). In this report, we use these observations as a springboard to review basic and clinical studies that investigate relationships between PPARγ, the marrow microenvironment, and skeletal remodeling and define areas of potential interest for future studies.
The Biology of PPARγ
PPARγ belongs to the PPAR family of transcriptional factors and nuclear receptors that regulates lipid biosynthesis, cell differentiation, and insulin sensitivity (57). PPARγ exists in two major protein isoforms (γ1 and γ2) and two minor forms (γ3 and γ4) created by alternate promoter usage and alternative splicing at the 5′ end of the gene (57, 70). While most tissues including bone express low levels of PPARγ1, PPARγ2 is adipocyte specific and, accordingly, is expressed at high levels in both brown and white adipose tissue (8). PPARγ2 contains an additional 30 amino acids at its NH2 terminus compared with PPARγ1. All isoforms of PPARγ are found in the cytoplasm and nucleus, but it is in the latter where heterodimerization with RXR family members occurs, and it is this complex that subsequently binds to DNA to modulate transcription. The RXR-PPARγ complex is activated by binding of endogenous (fatty acids, eicosonoids) or exogenous ligands (thiazolidinediones), which stimulate dissociation of the corepressors NCoR and SMRT (69). Histone modifications are involved in the switch of PPARγ transcriptional activity. The corepressors often form a protein complex with histone deacetylase (HDAC), and upon the ligand binding, HDACs are dissociated from the complex followed by the recruitment of coactivators and histone acetyltransferase (HAT). Histone methylation by histone methyltransferase also possesses a critical function in the transcriptional activity of PPARγ. Noncanonical Wnt signaling activated by Wnt-5a has been shown to activate histone methyltransferase, SETDB1 (SET domain bifurcated 1), through the CaMKII-TAK1/TAB2-NLK pathway (65). This exchange of coregulators facilitates the recruitment of coactivators that modify chromatin (e.g., SRC-1, -2) as well as the mediator complex (9, 18, 19, 23) to induce or suppress target gene transcription (3, 4). Recruitment to the transcriptional complex is critical for regulating PPARγ transactivational activity, and the coactivators or corepressors that bind the PPARγ-RXR transcriptional complex affect the plasticity of resident precursor cells (34, 42). DNA binding of PPARγ is another level of regulation, and there is evidence that the TAK1-TAB1-NIK signaling pathway activated by TNFα and IL-1, suppressors of adipogenesis, block DNA binding of PPARγ through the activation of NF-κB (64). While PPARγ is necessary for adipogenesis and development of white and brown adipose tissues (12, 58a), expression of PPARγ is sufficient to stimulate white, but not brown adipogenesis, of precursor cells. Brown adipogenesis requires additional factors such as PPARγ coactivators-1α or -1β (PGC-1α/β) and/or PRDM16 (31, 60, 61). Ultimately, conformational changes in the transcriptional complex due to recruitment of additional binding factors help determine cell function. Bona fide PPARγ binding sites in target gene-regulatory regions during adipogenesis have been evaluated on a genome-wide basis by using chromatin immunoprecipitation methodologies, and targets include a subset of genes involved in metabolism of lipids, carbohydrates, steroids, and amino acids (26, 39, 47) (Fig. 1).
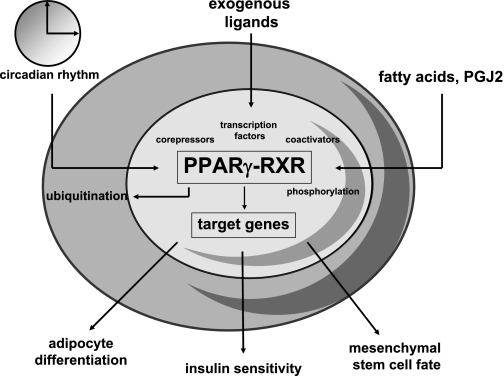
Fig. 1.
The many facets of peroxisome proliferator-activated receptor (PPAR)γ, a nuclear receptor and transcription factor. PPARγ expression and activity is dependent on heterodimerization with RXRs, binding of endogenous or exogenous ligands, other transcription factors, posttranslational modifications such as phosphorylation or ubiquitination, and cell-autonomous circadian rhythms. PPARγ has three main functions: adipocyte differentiation, insulin sensitivity, and lineage allocation of mesenchymal stem cell (MSCs). All three are important for the skeleton and are ligand dependent.
Ligands for PPARγ include the naturally occurring prostaglandin J2, 9(S)-hydroxy-octadecadienoic (HODE), compounds and HETE ligands derived from arachidonic acid as well as the thiazolidinedione (TZD) or glitazone class of synthetic compounds (15, 52). The latter are often the most recognized because rosiglitazone and pioglitazone are approved for the treatment of type 2 diabetes and are still widely prescribed despite notable side effects, including an increased risk of fractures. The TZDs were discovered by their ability to enhance insulin sensitivity. From that early work it became clear that this class of compounds bound to PPARγ. As with other activators, PPARγ first heterodimerizes with RXR to form a nuclear complex. The TZDs then bind to the ligand-binding domain of PPARγ, and this activates the heterodimeric complex. The conformational structure of PPARγ is altered, facilitating the release of corepressors and subsequent binding of nuclear coactivators resulting in the regulation of gene transcription.
In addition to activation of PPARγ by ligand binding to the heterodimeric complex, other transcription factors can also modulate PPARγ expression. For example, during adipogenesis, CCAAT/enhancer-binding protein (C/EBP)β and C/EBPδ have been shown to induce the expression of the master adipogenic transcription factors PPARγ and C/EBPα (10). Additional transcription factors upstream of PPARγ include SREBP-1c, KLF5, KLF15, Zfp423, and early B-cell factor (Ebf1) (24, 29, 58). Ebf1 is a helix-loop-helix DNA-binding protein instrumental for B-cell development. Expression of this nuclear factor has been found in a variety of regions, including bone marrow and white adipose tissue (27, 58). Recent studies have shown that Ebf1 binds directly to the PPARγ promoter and may act between C/EBPβ and C/EBPα/PPARγ in the adipocyte differentiation cascade (16, 27, 29). Finally, gene expression studies in mice have revealed that PPARγ has an inherent circadian rhythm that appears to be responsive to extracellular nutrients. However, the regulatory determinants of these changes have not been defined (21).
PPARγ transcripts rise within 36 hours of 3T3-L1 adipocyte differentiation and generally are increased more than tenfold over baseline. During late differentiation, PPARγ continues to play a crucial role in the function of many, and perhaps most, fat cell-specific genes. In fact, PPARγ binding is absolutely required for the function of the fat-selective enhancers for the FABP4/aP2 and PEPCK genes in cultured fat cells (66a). The process of PPARγ inhibition is less clear, although several mechanisms, including targeting to proteosome by β-catenin, SUMOylation, phosphorylation, and deadenylation of PPARγ 3′-UTRs have been implicated (41, 48, 57). As noted, PPARγ is a circadian-regulated gene and its expression is likely cued by dietary intake (68). This observation is supported by additional studies showing that PPARγ ligands themselves can serve as entrainment factors for endogenous clock genes. Srebp-1c and PGC-1a are also circadian-regulated genes, and these determinants coupled with the downstream effects of PPARγ are critical for insulin sensitivity. Although the downstream transcriptional targets of PPARγ have been well described, separating the adipogenic and insulin-sensitizing properties of PPARγ represents a major therapeutic hurdle for the development of more efficacious agonists in the treatment of type 2 diabetes mellitus.
PPARγ and the Skeleton
Bone remodeling is a dynamic process of skeletal dissolution followed by new bone formation that occurs in discrete units composed of osteoblasts, osteoclasts, and osteocytes. Bone turnover is essential for maintaining skeletal integrity as well as providing a ready source of calcium for homeostatic processes. Remodeling occurs in both cortical and trabecular compartments, although in the latter, turnover is more frequent. During adult life, bone resorption and formation are coupled by numerous cytokines and growth factors secreted by mesenchymal progenitors and mature osteoblasts. Imbalances in the remodeling unit, either by excessive resorption or impaired bone formation, lead to bone loss and eventual skeletal failure.
Emerging evidence demonstrates the critical roles of the skeleton as a metabolic integrator. Mice that lack osteocalcin produce less insulin and become obese and diabetic (38). In addition to the effects of adipose tissue on energy regulation, these newer findings place healthy bone and pathological bone conditions as major determinants of energy balance. In the context of PPARγ activity, treatment of rodents or humans with rosiglitazone causes bone loss and structural fragility, in part due to impaired bone formation (22). This phenotype resembles the process of aging in mammalian bone, which is also characterized by significant marrow adiposity, reduced trabecular bone volume fraction, and increased bone resorption. Consistent with these findings, rosiglitazone-induced bone loss is much more pronounced in older female C57BL/6J mice and is associated with significant infiltration of the marrow with large adipocytes. Histomorphometric indexes in these mice reveal a marked suppression in bone formation and increases in bone resorption consistent with a profound imbalance in bone remodeling. Not surprisingly, this effect is strain specific, such that some mice show only increased marrow adipogenesis but no bone loss (e.g., C3H/HeJ), while others display no skeletal or marrow effects from rosiglitazone (2). Interestingly, both cortical and trabecular bone compartments are negatively affected by TZD exposure in mice.
Postmenopausal females tend to be the most sensitive to bone loss by the TZDs (22). In one report, older women lost more than 2% of their bone density after only 14 weeks of rosiglitazone treatment (22). In several large randomized trials of rosiglitazone and pioglitazone, fractures were reported to be twice as likely in diabetic women treated with a TZD compared with those administered metformin, insulin, or sulfonylureas (25). To date, no human studies examining fatty infiltration in the marrow of individuals receiving the aforementioned drugs have been performed. Thus, it remains unclear whether marrow adiposity occurs in humans as it does in mice after exposure to TZDs. However, biochemical markers of bone turnover reveal uncoupling with enhanced bone resorption and suppressed bone formation that are consistent with experimental findings in mice (35). Furthermore, the predisposition of postmenopausal women to the skeletal effects of TZDs suggests that an altered bone remodeling state due to greater rates of resorption than formation enhances the susceptibility to TZD-induced bone loss. Interestingly, the skeletal sites most frequently reported to fracture in diabetics are those that are predominantly cortical in nature rather than trabecular (25). This would imply that drug-induced changes in metabolic factors such as adipokines may play a systemic role in TZD-induced bone loss, independent of changes in the trabecular compartment that can be related to enhanced marrow adiposity.
Osteoblasts and adipocytes are thought to originate from a common marrow mesenchymal stem cell (MSC), which also serves as a source of progenitors for marrow fibroblasts, chondrocytes, and supporting stroma for hematopoietic cells (5, 28) (Fig. 2). Indeed, this triad of osteoblasts, adipocytes, and blood cells, along with stromal precursors and true “stem cells,” comprise the bone marrow niche essential for differentiation, mobilization, and exit of hematopoietic stem cells from the marrow compartment (45). Commitment of marrow MSCs toward the adipocyte or osteoblast fate occurs through a highly regulated mechanism in which lineage-specific transcription factors (such as Runx2 for osteoblasts and PPARγ2 for adipocytes) are ultimately activated. Notably, embryonic stem cells with a null mutation in PPARγ spontaneously differentiate into osteoblasts and are unable to enter the adipogenic program due to alterations in their “stemness” profile (63). Suppression of PPARγ by Wnt signaling or shRNAs is both necessary and sufficient to stimulate osteoblastogenesis, and represses adipogenesis of bipotential ST2 cells (32). As such, PPARγ2 acts as a dominant negative regulator of osteoblast differentiation. These observations are recapitulated in older mice, where PPARγ2 and FABP4/aP2 transcripts are markedly increased in marrow-derived MSCs, marrow adiposity is pronounced, and bone mass is reduced (44). Expression studies in bone marrow-derived MSCs from C57BL/6J mice at 20 months also reveals suppression of Runx2, Dlx5, Col1A1, and osteocalcin, a gene expression profile consistent with reduced osteoblastogenic potential (44). Similarly, in mice heterozygous for the Pparg gene (homozygosity for the null allele of Pparg is embryonic lethal), bone mass is high, and osteoblast differentiation markers are enhanced (33). Wan et al. (67) reported that conditional inactivation of Pparg in hematopoietic precursors using the Tie2-Cre system, resulted in very high bone mass as a result of impaired osteoclastogenesis. Additionally, we recently observed that conditional deletion of Pparg in differentiated adipocytes resulted in lipodystrophy by 26 weeks accompanied by high bone mass (M. Kawai, personal observation). Interestingly, these mice exhibit increased osteoclast activity, suggesting that the phenotypic effects of PPARγ are highly context specific, with temporal effects from Pparg deletion varying with the timing of inactivation.
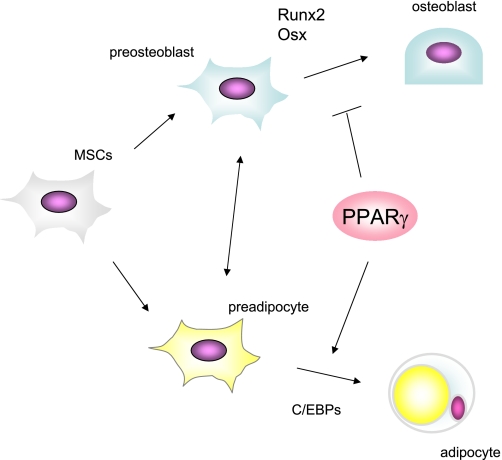
Fig. 2.
PPARγ regulates MSC allocation. Determination of lineage allocation of MSCs is regulated by several transcription factors including PPARγ and CCAAT/enhancer binding proteins (C/EBPs), which govern adipogenesis, Runx2 (runt-related transcription factor), and Osx (osterix), which are necessary for osteoblastogenesis. PPARγ favors adipogenesis and suppresses osteoblastogenesis partly through inhibiting Runx2 function, resulting in the reduction of osteoblast pool in bone marrow.
In sum, these data provide strong evidence that PPARγ is a gate-keeper for MSC differentiation into one of two lineages and that these two programs (i.e., adipogenesis and osteoblastogenesis) are mutually exclusive. However, several lines of evidence contradict mutual exclusivity for lineage allocation. For example, netoglitazone, another ligand of PPARγ, although only a partial agonist, does not cause trabecular bone loss despite a more than sixfold increase in the number of marrow adipocytes (35). Similarly, C3H/HeJ, an inbred mouse strain, has very high bone mass but more marrow adiposity than C57BL/6J throughout development. In addition, Ebf1−/− mice are lipodystrophic but have high bone mass and large marrow adipocytes with enhanced PPARγ expression (27). These experimental models do not completely contradict the presumption of a linear specification scheme that diverges early between fat and bone but instead support temporal and ligand specificity for PPARγ activity.
PPARγ and Bone Marrow Mesenchymal Cells
PPARγ is expressed in pluripotent stem cells and in more restricted progenitor cells. To more fully understand the molecular signature of PPARγ in MSCs, we utilized a clonal, marrow-derived cell model and performed timed gene array studies. Marrow stromal cells (U-33) were transfected with PPARγ2 (U-33/γ2cells) and then exposed to rosiglitazone for 2, 24, or 72 hours (36, 55, 62, 63). These studies demonstrated that activation of the PPARγ2 isoform by rosiglitazone converted cells from the osteoblast lineage to terminally differentiated adipocytes that express adipokines, genes required for lipid metabolism, and other adipocyte markers (20, 37, 62). Not surprisingly, gene expression profiles at all three time points revealed that several adipogenic genes, including for CideC, CD36, adipoq, Cebpα and FABP4, were markedly upregulated. Genes encoding enzymes that function principally in lipid storage, but not oxidation, were also markedly increased. In contrast, virtually all osteogenic genes, including for Runx2, BMP2, Sox9, Dlx5, Osterix, VDR, and Col1a1, were suppressed more than 75% by rosiglitazone exposure (63). This pattern was consistent with previous in vivo data demonstrating that rosiglitazone preferentially suppresses bone formation at the expense of adipogenesis. It should be noted that synthetic ligands modulate cell processes in ways that may vary from those by endogenous ligands. To address this issue, we examined the differences between MSCs expressing PPARγ2 treated with or without rosiglitazone. Strikingly, hundreds of genes were differentially expressed between the two cell groups; i.e., the gene expression profile of U-33 cells transfected with PPARγ2 (U-33/γ2) compared with U-33/γ2 cells activated with rosiglitazone. Notably, PPARγ-expressing MSCs induced expression of genes important for the early differentiation of hematopoietic and neurotropic cells, including CXCL-12, CXL-16, CXCL-4, Ntf3, and Kitl, whereas activation of PPARγ2-expressing MSCs with rosiglitazone markedly suppressed these same genes (62). These data suggest that rosiglitazone may enhance stemness, particularly for cells destined to undergo hematopoiesis. Interestingly, PPARγ alone also suppressed expression of LIF and its receptor, two determinants required for the maintenance of the undifferentiated stem cell phenotype, whereas rosiglitazone had no effect (62). Taken together, these lines of evidence suggest that endogenous PPARγ2 ligands may differ in their biological activity from synthetic ligands (37). If true, these results have profound implications for our understanding of how PPARγ regulates marrow stromal cell lineage. Importantly, they raise the possibility that, at least during the early differentiation steps of MSCs, multiple fates are not only possible, but necessary.
PPARγ and the Bone-Fat Relationship: Experimental Models
Infiltration of bone marrow with adipocytes is not uncommon and is observed in patients with multiple myeloma, advanced age, anorexia nervosa, or following exposure to drugs such as glucocorticoids and TZDs (1, 14, 30, 43, 53, 55). All of these conditions are associated with low bone mass and, in particular, with impaired bone formation. Experimental rodent models and some human studies have confirmed that PPARγ expression is increased in marrow stromal cells from aging individuals as well as those exposed to glucocorticoids (54, 55). These findings are consistent with the tenet that MSCs shift their lineage in response to PPARγ activation. However, the functional contribution of marrow adiposity and the role of PPARγ in these pathophysiological states remain largely unknown. Experimental models, however, provide some interesting insights into states of impaired bone formation and marrow “stress”. During bone marrow transplantation studies in mice, lethal irradiation is followed by hematopoietic reconstitution from a transfusion of donor cells. However, prior to donor repopulation of host marrow, at 7–10 days following radiation, the marrow that initially is characterized by cell death begins to reappear in the form of adipocytes. These cells are transient in nature, and by 16–20 days hematopoietic repopulation occurs. The presence of marrow fat at this critical time is also observed in humans with acute lymphocytic leukemia following chemotherapy (59). The adipogenesis appears to be timed with the earliest appearance of donor “stem cells” prior to differentiation, and Naveiras et. al. (46) hypothesize that adipogenesis impairs hematopoiesis. On the basis of the array studies discussed earlier, PPARγ activation is likely a critical part of this early event and may act to either maintain stemness or establish a gene expression program required later during differentiation. This hypothesis is supported by potent inhibition of marrow adipogenesis in several studies of irradiated or streptozoticin-induced diabetic mice treated with the PPARγ inhibitor bisphenol A diglycidyl ether (BADGE) (6, 46).
Another scenario where adipogenesis makes an unlikely appearance is in heterotopic ossification, a condition characterized by bone formation in nonosseous tissues. Olmsted-Davis et al. (49) hypothesized that brown adipogenesis was critical for heterotopic bone formation. To test this, MC3T3-E1 cells overexpressing BMP-2 were ectopically placed on muscle tissue and cell tracing studies were performed. Within 24 hours, adipocytes appeared at the site of cell placement. This was followed by both vascular invasion and chondrocytic infiltration, ultimately leading to new bone formation and marrow development (49). The presence of adipocytes was transient, but these cells stained positively for UCP1, and the authors postulated that the hypoxia induced by mitochondrial consumption enhanced vascular invasion and stimulated endochondral bone formation. A similar process of transient adipogenesis followed by vascular invasion may also occur during distraction osteogenesis, a surgical intervention aimed at reconstructing skeletal deformities, and an experimental model of bone formation (7, 50; B. Lecka-Czernik, personal communication). Taken together with the irradiation model, it is apparent that adipogenesis and PPARγ activation are critical components of several dynamic and physiological processes related to bone acquisition. Whether the transient appearance of adipocytes is a function of brown adipogenesis in these models is unclear. However, it does reinforce the reoccurring theme that PPARγ and its temporal expression may serve several purposes within the skeletal milieu.
Future Directions and Controversies
Understanding the multiple facets of PPARγ will undoubtedly have implications for our understanding of normal skeletal remodeling and maintenance. It is conceivable that these insights can be parlayed into new therapeutic modalities for individuals with low bone mass. Several questions remain unanswered and will likely require significantly more effort to answer. In particular, the function of marrow adiposity needs to be understood, especially in pathological states where stress in bone-forming osteoblasts is a consistent feature. The current dogma suggests that marrow adiposity results as a default pathway for MSCs to differentiate into in the absence of a signal driving osteoblastogenesis or chondrogenesis. In agreement with this, studies in young adults and elderly subjects describe an inverse relationship between the amount of cortical bone and marrow adiposity (11). However, as discussed, marrow fat may accompany high bone mass and may also be an early feature of endochondral bone formation. Better animal models and novel tracing studies are necessary to define when, where, and how PPARγ is activated and whether the end result, particularly in marrow, is brown or white adipose tissue. Importantly, determining the transcriptional components that define the function of brown vs. white fat is a major priority. Another critical area of study is defining how PPARγ is regulated in a circadian fashion and what downstream targets are affected by meal-induced PPARγ activation (21, 56). Finally, the interface between the PPARγ system and the Wnt/β-catenin signaling pathway in the bone marrow compartment requires further delineation (51). These and other questions will dictate an area of investigation that a decade ago was unheard of and will likely provide key insights into physiological and pathological skeletal conditions.
GRANTS
K. M. Sousa has received support from a Regenerative Sciences Training Grant (DK-070071), a TEAM Postdoctoral Fellowship in Regenerative Sciences (DE-007057), and an Eli Lilly Postdoctoral Fellowship. C. J. Rosen and O. A. MacDougald are jointly supported by Grant R24 DK-084970 from National Institute of Diabetes and Digestive and Kidney Diseases. Additional support came from National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-45433 and AR-54604 to C. J. Rosen and DK-51563 and DK-62876 to O. A. MacDougald.
DISCLOSURES
No conflicts of interest are reported by the authors.
REFERENCES
- 1.Abella E, Feliu E, Granada I, Millá F, Oriol A, Ribera JM, Sánchez-Planell L, Berga LI, Reverter JC, Rozman C. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and notwith other clinical findings. Am J Clin Pathol 118: 582–588, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology 150: 1330–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O'Rahilly S. Activators of peroxisome proliferator-activated receptor γ have depot-specific effects on human preadipocyte differentiation. J Clin Invest 100: 3149–3153, 1997a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272: 5128–5132, 1997b [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19: 180–192, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Botolin S, McCabe L. Inhibition of PPARγ prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol 209: 967–976, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bouletreau PJ, Warren SM, Longaker MT. The molecular biology of distraction osteogenesis. J Craniomaxillofac Surg 31: 173–178, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology 137: 354–66, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Bugge A, Grontved L, Aagaard MM, Borup R, Mandrup S. The PPARγ2 A/B-domain plays a gene-specific role in transactivation and cofactor recruitment. Mol Endocrinol 23: 794–808, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273: 30057–30060, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Di Lorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 93: 2281–2286, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan SZ, Ivashchenko CY, Whitesall SE, D'Alecy LG, Duquaine DC, Brosius FC, Gonzalez FJ, Vinson C, Pierre MA, Milstone DS, Mortensen RM. Hypotension, lipodystrophy, and insulin resistance in generalized PPARy-deficient mice rescued from embryonic lethality. J Clin Invest 117: 812–822, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducy P, Amling M, Takeda S, Priemel FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibitsbone formation through a hypothalimic relay: a central control of bone mass. Cell 100: 197–207, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab 95: 407–413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 83: 803–812, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Fretz JA, Nelson T, Xi Y, Adams DJ, Rosen CJ, Horowitz MC. Altered metabolism and lipodystrophy in the early B-cell factor 1-deficient mouse. Endocrinology 151: 1–11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–70, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Ge K, Guermah M, Yuan CX, Ito M, Wallberg AE, Spiegelman BM, Roeder RG. Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis. Nature 417(6888): 563–567, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Ge K, Cho YW, Guo H, Hong TB, Guermah M, Ito M, Yu H, Kalkum M, Roeder RG. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferators-activated receptor gamma-stimulated adipogenesis and target gene expression. Mol Cell Biol 28: 1081–1091, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimble JM, Robinson CE, Wu X, Kelly K, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol 50: 1087–1094, 1996 [PubMed] [Google Scholar]
- 21.Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci USA 104: 9888–9893, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92: 1305–1310, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Grøntved L, Madsen MS, Boergsen M, Roeder RG, Mandrup S. MED14 tethers Mediator to the N-terminal domain of PPARgamma and is required for full transcriptional activity and adipogenesis. Mol Cell Biol 30: 2155–2169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature 464(7288): 619–623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95: 592–600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamza MS, Pott S, Vega VB, Thomsen JS, Kandhadayar GS, Ng PW, Chiu KP, Pettersson S, Wei CL, Ruan Y, Liu ET. De-novo identification of PPARγ/RXR binding sites and direct targets during adipogenesis. PLoS ONE 4: e4907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hesslein DG, Fretz JA, Xi Y, Nelson T, Zhou S, Lorenzo JA, Schatz DG, Horowitz MC. Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone 44: 537–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Jahagirdar BN, Reinhardt RL. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418(6893): 41–49, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Jiminez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol 27: 743–757, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2: 165–171, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA, Spiegelman BM. Regulation of the brown and white fat gene program though a PRDM16/CtBP transcriptional complex. Genes Dev 22: 1397–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, MacDougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferators-activated receptor gamma. J Biol Chem 282: 14515–14524, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi H, Akune T, Yamaguchi M, Ohba S, Ogata N, Chung UI, Kubota N, Terauchi Y, Kadowaki T, Nakamura K. Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J Bone Miner Metab 23: 275–279, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev 10: 1096–1107, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 148: 2669–2680, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem 74: 357–371, 1999 [PubMed] [Google Scholar]
- 37.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143: 2376–2384, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endrocrine regulation of energy metabolism by the skeleton. Cell 130: 456–469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, Lazar MA. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev 22: 2941–2952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J Biol Chem 270: 12953–12956, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol 26: 5827–5837, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem 64: 345–73, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthoped Relat Res 80: 147–154, 1971 [DOI] [PubMed] [Google Scholar]
- 44.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPARγ2 transcription factor and TGF-β/BMP signalling pathways. Aging Cell 3: 379–389, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med 226: 507–520, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460 (7252): 259–263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Borgesen M, Francoijs KJ, Mandrup S, Stunnenberg HG. Genome-wide profiling of PPARgamma: RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimmer composition during adipogenesis. Genes Dev 22: 2953–2967, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oberfield JL, Collins JL, Holmes CP, Goreham DM, Cooper JP, Cobb JE, Lenhard JM, Hull-Ryde EA, Mohr CP, Blanchard SG, Parks DJ, Moore LB, Lehmann JM, Plunket K, Miller AB, Milburn MV, Kliewer SA, Willson TM. A peroxisome proliferator-activated receptor gamma ligand inhibits adipocyte differentiation. Proc Natl Acad Sci USA 96: 6102–6106, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol 170: 620–632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA. Expression of angiogenic factors during distraction osteogenesis. Bone 33: 889–898, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19: 612–617, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor γ. J Biol Chem 273: 1855–1858, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients isreflected by altered mesenchymal stem cells dynamics. J Cell Biochem 75: 414–423, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Rodríguez JP, Montecinos L, Ríos S, Reyes P, Martínez J. Mesenchymal stem cells from osteoporotic patientsvproduce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem 79: 557–565, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Express 19: 109–124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen CJ. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab 7: 7–10, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 276: 37731–37734, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896, 2006 [DOI] [PubMed] [Google Scholar]
- 58a.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4: 611–617, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Schick F, Einsele H, Lutz O, Claussen CD. Lipid selective MR imaging and localized 1H spectroscopy of bone marrow during therapy of leukemia. Anticancer Res 16: 1545–1551, 1996 [PubMed] [Google Scholar]
- 60.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PDRM16. Cell Metab 6: 38–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454 (7207): 961–967, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shockley KR, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 regulates a molecular signature of marrow mesenchymal stem cells. PPAR Res 81219, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 106: 232–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, Kadowaki T, Takeuchi Y, Shibuya H, Gotoh Y, Matsumoto K, Kato S. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol 5: 224–230, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 9: 1273–1285, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77: 289–312, 2008 [DOI] [PubMed] [Google Scholar]
- 66a.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol 15: 351–357, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med 13: 1496–1503, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Yamashita D, Yamaguchi T, Shimizu M, Nakata N, Hirose F, Osumi T. The transactivating function of peroxisome proliferator-activated receptor γ is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes to Cells 9: 1017–1029, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Yu C, Markan K, Temple KA, Deplewski D, Brady MJ, Cohen RN. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J Biol Chem 280: 13600–13605, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor γ (mPPARγ) gene: alternative promoter use and different splicing yield two mPPARγ isoforms. Proc Natl Acad Sci USA 92: 7921–7925, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]