Abstract
Antiretroviral drugs are ineffective at treating viral infection in the brain because they cannot freely diffuse across the blood-brain barrier (BBB). Therefore, HIV-1 viral replication persists in the central nervous system (CNS) and continues to augment the neuropathogenesis process. Nanotechnology can play a pivotal role in HIV-1 therapeutics as it can increase drug solubility, enhance systemic bioavailability, and at the same time offer multifunctionality. Moreover, following conjugation with transferrin (Tf), these drug-loaded nanoformulations can permeate across biological barriers such as the blood brain barrier (BBB) via a receptor mediated transport mechanism. In the current study, we have stably incorporated the antiviral drug, Saquinavir, within Tf-conjugated quantum rods (QRs), which are novel nanoparticles with unique optical properties. We have evaluated the transversing ability of the QR-Tf-Saquinavir nanoformulation across an in vitro model of BBB. In addition, we have analyzed the subsequent antiviral efficacy of this targeted nanoformulation in HIV-1 infected peripheral blood mononuclear cells (PBMCs), which are cultured on the basolateral end of the in vitro BBB model. Our results show a significant uptake of QR-Tf-Saquinavir by brain microvascular endothelial cells (BMVECs), which constitute the BBB. In addition, we observed a significant enhancement in the transversing capability of QR-Tf-Saquinavir across the BBB, along with a marked decrease in HIV-1 viral replication in the PBMCs. These observations indicate that drug-loaded nanoparticles can deliver therapeutics across the BBB. These results highlight the potential of this nanoformulation in the treatment of Neuro-AIDS and other neurological disorders.
Keywords: HIV-1, antiretroviral drugs, saquinavir, protease inhibitor, quantum rods (QR), blood brain barrier, transferrin receptor, multimodal nanoparticles and bioconjugation
INTRODUCTION
Effective treatment of neuro-AIDS requires long-term maintenance of therapeutic concentrations of an antiretroviral drug in the brain. This will lead to the sustained suppression, and eventual elimination of HIV-1 in the viral reservoirs within sequestered regions of the brain. However, the systemic delivery of antiretroviral drugs in the brain is severely hampered by the presence of the blood-brain barrier (BBB). The BBB is a complex physiological checkpoint which inhibits the free diffusion of circulating molecules from the blood into the brain. Therefore, fabrication of novel macromolecular carriers that would significantly enhance the delivery of drugs across the BBB holds the key for the treatment of neuro-AIDS and other neurological diseases.
Previously, several efforts have been made towards the transport of active molecules across the BBB via a receptor-mediated “trojan horse” approach, mainly using liposomal carriers. However, these formulations were associated with several problems such as large size, toxicity, immuno-genicity, as well as the instability of “carrier vectors” in biological media [1–3]. As a result, these efforts have met with limited success; often crossing the BBB was accompanied by severe, irreversible damage to the BBB composition. Nanoparticle-based formulations have several advantages that can enable potent drug delivery across the BBB, while avoiding any damage to the barrier. These include ultra-low size, biocompatibility, non-antigenicity, ability for targeted and controlled drug delivery, multimodality, and ability to monitor BBB permeation in real time [3–5].
Quantum rods (QRs) are semiconductor nanoparticles with unique optical properties, such as high photostability, broad absorption spectra, and narrow, size-tunable emission spectra spanning the UV-visible and near-infra-red (NIR) regions [6–8]. The use of bioconjugated QRs as luminescence probes for numerous biomedical applications has become an area of intense research over the last decade. The QR surface can be functionalized with a variety of biomolecules. These bioconjugated QRs have shown the potential to dramatically outperform conventional organic dyes in imaging of cellular and sub-cellular structures, as well as in a variety of bioassays [7–10]. In addition to their emergence as a new generation of optical bioprobes, QRs have shown promise as targeted drug carriers [9,10].
Recently, our group has shown successful transport of bioconjugated QRs across an in vitro BBB model via transferrin (Tf) receptor-mediated transport [11]. In the current study, we have integrated the potent antiretroviral drug Saquinavir into this targeted nanoplatform, in order to enable efficient drug delivery across the BBB for the treatment of neuro-AIDS. The anti retroviral drug “Saquinavir” is the first protease inhibitor approved by the Food and Drug Administration (FDA), which is a peptide-like substrate analogue that binds to the active site of the HIV protease and inhibits its activity. Saquinavir inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature noninfectious virus particles [12, 13]. Saquinavir inhibits HIV-1 activity in both acute and chronically infected cells, and its inhibitory concentrations range between 1.0 nM to 80 nM [12]. In the current study, the concentrations of Saquinavir used were 10 and 40 nM, which were well within the effective concentration range of this drug. Although it is an effective anti HIV-1 drug, Saquinavir is poorly water soluble, has low oral bioavailability, and is impermeable across the BBB. Therefore a nanoformulation of this drug may increase not only its bioavailability and solubility, but also improve its delivery to target sites. These nanoparticles are also being used as optical probes for the real-time monitoring of the BBB transcytosis process. The transcytosis efficacy of the targeted and drug-loaded nanoparticles has been investigated using the in vitro BBB model. Subsequent anti-HIV efficacy of these nanoparticles has been evaluated in HIV-1 infected peripheral blood mononuclear cells (PBMCs), which are cultured on the bottom of the lower chamber (basolateral end or CNS end) of the BBB model.
METHODS
Study Design
Following synthesis and characterization of the nanoformulations or nanoplexes (QR-Tf-Saquinavir), we evaluated their uptake in vitro cultured monolayers of brain microvascular endothelial cells (BMVECs) using confocal imaging. We then evaluated the transversing ability of the nanoplexes, (which were added to the apical end or systemic end) across the BBB using a well validated in vitro BBB model and tested their antiviral efficacy on HIV-1 infected PBMCs, which were cultured in the bottom chamber (basolateral end or CNS end) of our in vitro BBB model. Two independent methods were used to evaluate antiviral effects and they include, 1) the measurement of p24 antigen levels using a commercially available p24 ELISA assay and 2) quantitating HIV-1 LTR-R/U5 gene expression using quantitative real time PCR.
Synthesis and Aqueous Dispersion of the QRs Terminated with Carboxyl Groups
The formation of the (Cadmium (Cd) Selelinum (Se) CdSe core nanocrystals, and thin layer of Zinc Sulfide (ZnS) shell (<1.5 nm) is grown over the nanocrystal core. CdS/ZnS graded shell on CdSe QRs in organic media was carried out as described by Yong et al. [10]. To form the nanocrystal core, 6 mmol cadmium oxide (CdO) was dissolved in 10 mL of oleic acid at 280 °C, producing a homogeneous reaction mixture of cadmium complex. Then, a solution of selenium (Se) precursor was injected into the reaction mixture. The Se:Cd molar ratio was 1:3. The length of reaction time following the injection determines the final size of the nanocrystals. The CdSe nanocrystals were then purified by precipitation from the reaction mixture with ethanol followed by centrifugation. For the growth of a ZnS shell over the CdSe cores, the CdSe cores were dispersed in 5 g of TOPO at 250°C. A mixture of diethylzinc (Et2Zn) and hexamethyldisilathiane ((TMS)2S) (Zn:S = 1:1 molar ratio) were mixed with Trioctylphosphine (TOP) and the solution precursor was added dropwise into the CdSe core nanocrystals reaction mixture at 250°C. After ~20 minutes of heating, the heating mantle was removed, and then the reaction mixture was air cooled to room temperature. Following their synthesis, the CdSe/CdS/ZnS QRs were dispersed in aqueous media by coating with mercaptosuccinic acid (MSA). Briefly, 3 mmol of MSA was dissolved in 10 mL of chloroform under vigorous stirring. After stirring for 10 to 15 minutes, 2 mL of concentrated (~40 mg/mL chloroform) CdSe/CdS/ZnS QR dispersion was added into this mixture. This solution was stirred overnight at room temperature. The QRs were separated from the surfactant solution by addition of ethanol and centrifugation. The red-brownish precipitate was re-dispersed in 10 mL HPLC water and the solution was further filtered using a syringe filter with a pore diameter of 0.45 μm. The QRs have relatively good colloidal stability and no precipitation was observed after several months of storage. This solution was kept in the refrigerator at 4°C for further use [10,11].
Formation of the QR-Tf-Saquinavir Nanoplex
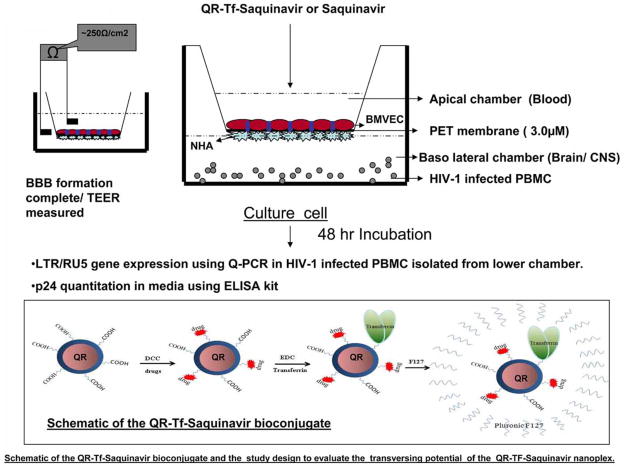
The nanoparticle synthesis process described above results in the synthesis of QRs that have terminal carboxyl groups, these carboxyl groups facilitate conjugation with Saquinavir and transferrin molecules using carbodiimide chemistry [10,11]. Briefly, 600 μL QR stock solution was mixed with 7 μL of 0.05 M EDCI solution and gently stirred for 2 to 3 minutes. Next, 7 μL of 0.25 mg/mL transferrin and 15 mL of 10 mg/mL Saquinavir were added into this mixture and gently stirred at room temperature overnight to allow the biomolecules to covalently bond to the carboxyl-terminated QRs. The bioconjugates were further purified by dialysis and centrifugation. The resulting nanoplex was then mixed with 5% (w/w) of F127 pluronic block copolymer solution, in order to enhance its colloidal stability. The prepared nanoplex was then filtered using a syringe filter with a pore diameter of 0.45 μm. The filtered nanoplex was kept at 4°C until further use. A schematic that illustrates the formation of the QR-Tf-Saquinavir nanoplex and the study design using the in vitro BBB model are shown in Scheme 1. It is worth noting that the Saquinavir is a water-insoluble drug and precipitation was observed when we try to dissolve the drug in the aqueous phase. Saquinavir was dissolved in a 100% methanol solution and a 10 and 40 nM concentration solution was prepared in the same manner as the nanoplex with the F127 pluronic block copolymer solution. However, using the nanoformulation approach, we have successfully loaded the drug within the nanoparticles formulation package and no precipitation was observed. Thus, indicating that there is a minimum lost of drug during the preparation of the nanoplex formulation. The amount of Saquinavir in nanoplex was quantitated using HPLC and was determined to be ~ 40nM.
Scheme 1.
Schematic of the QR-Tf-Saquinavir bioconjugate and the study design to evaluate the transversing potential of the QR-Tf-Saquinavir nanoformulation.
Physical Characterization of QRs
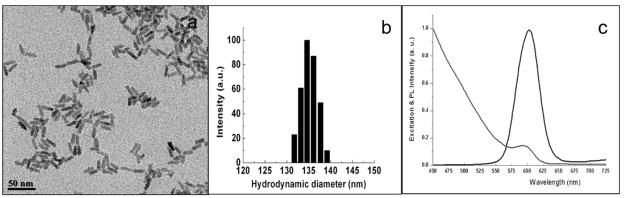
The physical properties of the nanoplex were established using transmission electron microscopy (TEM), dynamic light scattering (DLS), spectrophotometry and spectrofluorimetry. TEM was used to determine the particle size and size distribution. The aqueous dispersion of the nanoparticles was drop cast on a TEM copper grid (Electron Microscopy Sciences, Inc.) and visualized using a JEOL JEM 2020 electron microscope. DLS measurements were carried out using a Brookhaven 90Plus particle size analyzer, with a scattering angle of 90°. UV-vis absorption spectra were recorded using a Shimadzu UV-3101 PC spectrophotometer, using a quartz cuvette with 1 cm path length. Fluorescence spectra were recorded on a Fluorolog-3 (Jobin Yvon, Longjumeau, France) spectrofluorimeter (Fig. 1a–c).
Fig. 1.
Physical characterization of the QR-Tf-Saquanavir nanobioconjugates. Their size and size-distribution are shown using (a) Transmission electron microscopy (TEM) and (b) dynamic light scattering (DLS). Their absorption (grey line) and emission (black line) properties are shown in (c).
In Vitro BBB Model
The in vitro BBB model [14–18] uses primary cultures of both BMVECs (Cat# ACBRI-376) and normal human astrocytes (NHAs, Cat# ACBRI-371), which were obtained from Applied Cell Biology Research Institute (ACBRI) Kirkland, WA. Characterization of BMVECs demonstrated that >95% cells were positive for cytoplasmic von Willibrand’s factor/Factor VIII. BMVECs were cultured in CS-C complete serum-free medium (ABCRI, Cat # SF-4Z0-500) with attachment factors (ABCRI, Cat # 4Z0-210) and Passage Reagent Group™ (ABCRI, Cat # 4Z0-800). NHAs were cultured in the CS-C medium, supplemented with 10 μg/ml human epidermal growth factor, 10 mg/ml insulin, 25 μg/ml progesterone, 50 mg/ml transferrin, 50 mg/ml gentamicin, 50 μg/ml amphotericin-B, and 10% FBS. NHAs were characterized on the basis of >99% of these cells being positive for glial fribrillary acidic protein (GFAP). Both BMVECs and NHAs were obtained at passage 2 for each experiment and were used for all experiments between 2–8 passages; within the 6 to 27 cumulative population doublings.
The BBB model used consists of 2-compartment wells in a 6 well culture plate, with the upper compartment separated from the lower by a 3μM polyethylene terephthalate (PET) insert (surface area = 4.67 cm2). The BMVECs were grown to confluency on the upper side of the insert, while a confluent layer of NHAs were grown on the underside. The formation of a functional and intact BBB takes a minimum of 5 days, which can be confirmed by determining the transendothelial electrical resistance (TEER) value, as described below [14–19]. Using the in vitro BBB model we examined BBB permeability, transendothelial migration, and the efficacy of drug delivery of the QR-Tf-Saquinavir nanoplex, along with the various control formulations.
Measurement of TEER
TEER across the in vitro BBB was measured using an ohm meter Millicell ERS system (Millipore, Bedford, MA Cat # MERS 000 01). Electrodes were sterilized using 95% alcohol and rinsed in distilled water prior to measurement. A constant distance of 0.6 cm was maintained between the electrodes at all times during TEER measurement.
Imaging of Nanoparticles
The cellular uptake of the QR-Tf-Saquinavir was studied using confocal microscopy (Fig. 3). A Nikon Eclipse TE2000 microscope equipped with the Nuance GNIR imaging system (Cambridge Research & Instrumentation Inc., Cambridge, MA) was employed, which is capable of multispectral (wavelength-resolved) imaging in the range of 500–950 nm. Custom-designed filter cubes, with corresponding dichroic and emission filters acquired from Omega Optical, were used to cut off the excitation light and obtain high contrast fluorescence images.
Fig. 3.
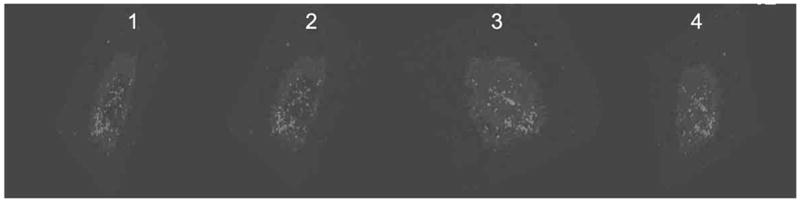
Frames 1–4: Sequential 3-D confocal images of BMVEC cells showing uptake of QR-Tf-Saquinavir showing internalization (light grey dots) of the nanoplex.
PBMC Isolation
Peripheral Blood leukocytes were isolated from leukocyte depletion filters obtained from the blood bank at Upstate New York Transplant Services, Buffalo, NY. Leukocytes are eluted aseptically from these filters using standard protocols to yield the so called “Filter Buffy coats” [20]. Briefly, filters were flushed at room temperature with sterile filter elution medium (Dulbecco’s PBS without MgCl2 and CaCl2, containing 5mM Na2-EDTA and 2.5% w/v sucrose) using a sterile 60 ml syringe. A total volume of 200 ml elution medium was collected using gentle pressure to avoid cell disruption or filter leakage. These filter buffy coats were then overlaid on 15ml Ficoll-Paque® Plus (Amersham-Pharmacia, Piscataway, NJ Cat # 17-1440-03), in a 50 ml culture tube. Samples were centrifuged for 20 min at 700 × g and 20°C. The filter-PBMC interface was carefully removed by pipetting and washed twice with PBS/EDTA and resuspended in complete RPMI media. Then, the total number of cells was counted using a hemocytometer.
Cell Viability Measurement Using an MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-DiphenylTetrazolium Bromide, a Tetrazole) Assay
MTT cell proliferation assay measures the reduction of a tetrazolium component (MTT) into an insoluble formazan product by the mitochondria of viable cells. The MTT assay is a quantitative, sensitive detection of cell proliferation since it measures the growth rate of cells by virtue of a linear relationship between cell activity and absorbance. The MTT assay was done to evaluate the cell viability of all cells in culture, namely the PBMC’s, BMVEC and NHA. Cell viability was tested in the PBMC prior to being infected by HIV-1 as well as after 7 days post infection. PBMC viability was also measured before and after treatment with the nanoplexes. Viability of the BMVEC and the NHA were measured before growing them on the cell culture inserts for formation of the in vitro BBB model. Viability of the BMVEC was also measured prior to and post treatment with the nanoplexes. Typically 10,000 cells suspended in 100 μl of media were incubated with 10 μl of MTT reagent (Cat # 30-1010K; ATCC) for approximately 3 hours, followed by addition of a detergent solution to lyse the cells and solubilize the colored crystals. Colorimetric detection was done at a wavelength of 570 nm. The amount of color produced is directly proportional to the number of viable cells.
HIV-1 Infection of PBMCs
PBMCs (1×106 cells/ml) were infected for 3 hours with HIV-1 IIIB (NIH AIDS Research and Reference Reagent Program) at a concentration of 103.0TCID 50/ml cells, equivalent to 10ng viral isolate/ml of culture media. Following that, the infected cells were washed with Hanks buffered saline, reconstituted in RPMI media (fortified with 10 % FBS) and incubated at 37°C/5% CO2 for 7 days. Levels of p24 in the culture supernatants were measured using a commercially available p24 ELISA kit (Zeptometrix, Buffalo, NY) 7 days post-infection. These infected PBMCs were then washed and reconstituted in fresh culture medium and used for evaluating the anti HIV-1 efficacy of the nanoformulations.
Evaluation of the Ability of the Nanoplex to Transverse the BBB and Antiviral Efficacy of the QR-Tf-Saquinavir Nanoplex
QR-Tf-Saquinavir nanoplex, free Saquinavir, QR alone, reconstituted in 300μl of the media, were added to the upper chamber of the in vitro BBB. 200,000 HIV-1 infected PBMC’s that were suspended in 1ml of RPMI complete media were plated in the lower chamber (basolateral end) of the in vitro BBB model. The in vitro BBB cell culture chambers were incubated at 37°C and 5% CO2 for a period of 24–48 hrs after addition of the QR-Tf-Saquinavir nanoplexes, free Saquinavir, QR to the upper chambers (apical end). At the end of this incubation, the PBMCs were harvested from the lower chamber, washed, and then analyzed for antiviral efficacy of the nanoplex by measurement of (a) p24 antigen levels via p24 ELISA assay, and (b) LTR-R/U5 amplification via quantitative real time PCR, as described below. Triplicate wells were used for each condition tested.
Real Time Quantitative PCR (Q-PCR)
Cytoplasmic RNA was extracted by an acid guanidinium-thiocyanate-phenol-chloroform method using Trizol reagent (Invitrogen, Carlsbad, CA) [21]. The amount of RNA was quantified using a Nano-Drop ND-1000 spectrophotometer (Nano-Drop™, Wilmington, DE) and the isolated RNA was stored at −80°C until used. The LTR-R/U5 region represents early stages of reverse transcription of HIV-1. Following conversion of RNA to cDNA using reverse transcription, relative abundance of mRNA species was quantified by real time quantitative PCR using the LTR/RU5 specific primers and the Brilliant® SYBR® green QPCR master mix (Stratagene Inc, La Jolla, CA; Cat# 600548-51). The followings are the primer sequences used for LTR/RU5 (Forward primer 5′-TCTCTCTGGTTAGACCAGATCTG-3′ and Reverse primer 5′-ACTGCTAGAGATTTTCCAC ACTG-3′). Relative expression of mRNA species was calculated using the comparative CT method [22,23]. All data were controlled for quantity of RNA input by performing measurements on an endogenous reference gene, β-actin. In addition, results obtained on RNA from treated samples were normalized to results obtained on RNA from the control, untreated sample. Results were expressed as transcript accumulation index (TAI) as described earlier [23]. This calculation assumes that all PCR reactions are taking place with 100% efficiency.
RESULTS
Characterization of the Nanoplexes
The physical properties of the nanoparticle formulations, before and after bioconjugation, are usually established using the following techniques: a) Dynamic light scattering to estimate the hydrodynamic diameter and surface charge of the aqueous dispersed nanoparticles, b) Transmission Electron Microscopy (TEM) to determine particle size and size distribution, and c) evaluation of the spectral properties of the fluorescent nanomaterials using simple spectrophotometry and spectrofluorimetry. Fig. (1) shows the size-distribution of the QR-Tf-Saquinavir bioconjugates, via TEM and DLS measurements. TEM image (Fig. 1a) shows the rods with average length and diameter of ~25 nm and ~5 nm, respectively. On the other hand, the DLS data (Fig. 1b) shows their hydrodynamic diameter around 130 to 140 nm. The discrepancy between these two data can be explained by the fact that while TEM only measures the size of the inorganic semiconductors, it does not consider the thick surface coating with Mercapto succinic acid (MSA), along with the bioconjugated drug and transferrin molecules. On the other hand, DLS assumes the particles to be spherical, and measures their hydrodynamic diameter, which includes the overall size of the hydrated bioconjugated lysine-coated QRs, thus showing a much bigger particle size. Fig. (1c) shows the absorption and emission spectra of the bioconjugates, with the emission wavelength around 605 nm.
Determination of the Integrity of the In Vitro BBB During Transversing of the Nanoplex Across the BBB
Structurally, the BBB is composed of BMVEC connected by tight junctions (TJ) and TJ proteins which together ensure the BBB’s structural integrity. Additional components of the BBB are the surrounding capillary basement membrane and astrocytes. The Astrocytic end-feet are in close apposition to the abluminal surface of the brain endothelium and assist in the barrier function by coordinating the functional activities of BMVEC. Our in vitro BBB model is a well validated co-culture model and reflects several characteristics of the in vivo BBB. We have validated the BBB model of Persidsky et al. [12–16], which is used for all experiments in this study. Formation of an intact BBB was measured by determining TEER using Millicell-ERS microelectrodes (Millipore, Bedford, MA). A mean TEER value of ~250 ohms/cm2 is consistent with the formation of the BBB. No significant change was observed in the TEER values of the BBB, before (261±7.91 ohm/cm2) and after (267.983±5.43 ohm/cm2) treatment with the nanoformulations for 48hrs. Since TEER values indicate the integrity of the BBB, the above data shows that the QR-Tf-Saquinavir nanoplex, free Saquinavir, or the QR alone did not cause any damage to the BBB while traversing through this barrier.
Evaluation of Toxicity and Uptake of QR-Tf-Saquinavir Nanoplexes by BMVEC Monolayers
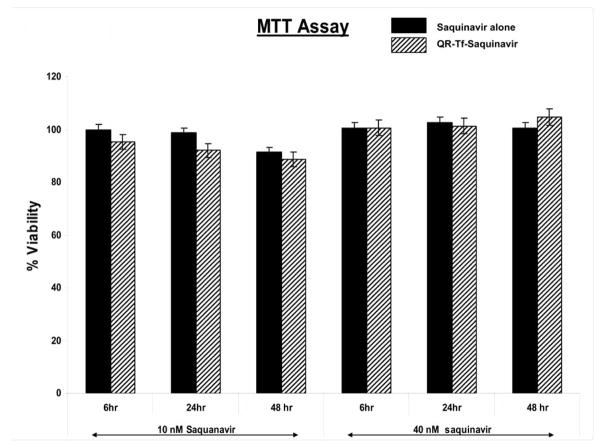
Evaluation of the toxicity of the nanoplexes on cells in culture is crucial to their eventually use for biomedical application. We used a MTT cell viability assay (Promega, Madision, WI) to evaluate if the prepared nanoplexes were toxic to the BMVECs. A dose and time-dependent study was carried out to evaluate any change in cell viability. Additionally, trypan blue staining of the cells was done to determine their percentage viability. Results from our MTT assay show that the treatment of the BMVECs with free Saquinavir and the QR-Tf-Saquinavir nanoplex containing 10nM and 40nM Saquinavir concentrations over a 6–48 hr time period did not result in any toxicity (Fig. 2). The cell viability was greater than 90% for all three time points tested. Additionally, Trypan blue staining was also done using the same samples and indicated >93% viability over the 6–48 hr time period (data not shown). Note: We used the QR-Tf-Saquinavir nanoplexes and free Saquinavir formulations, that contained 40nM Saquinavir concentration for all subsequent experiments in the study.
Fig. 2.
The dose and time-dependent cytotoxicity of Saquinavir and QR-Tf-Saquinavir nanoformulations on BMVECs using MTT assay. Our results show absence of any toxicity upon treatment of the BMVECs with the Saquinavir and QR-Tf-Saquinavir nanoformulations over a 6–48 hr time period, at two concentrations of Saquinavir (10 and 40nM). The results shown are mean ± SD of 3 separate experiments.
Additionally, uptake of the nanoplexes by BMVEC cells was examined using confocal imaging and a 3-D confocal images of BMVEC cells (Fig. 3) shows internalization of the QR-Tf-Saquinavir nanoplex confirmed by local spectral analysis.
To Evaluate the Specificity of the Uptake of Targeted and Non Targeted Nanoplexes by PBMCs
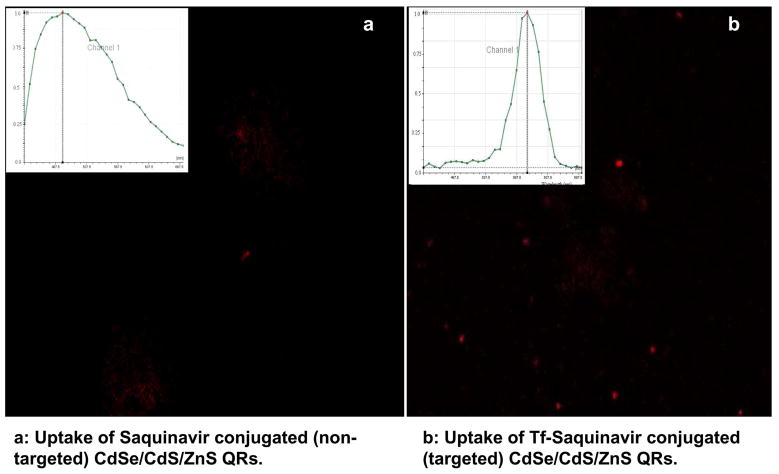
In addition to uptake of the nanoplexes by BMVEC cells we had to evaluate the specificity of the uptake of the targeted (QR-Tf-Saquinavir) and non-targeted (QR-Saquinavir) nanoplex by the PBMCs for which approximately, 200,000 PBMC/ml media were incubated with 300 μl of the targeted (QR-Tf-Saquinavir) and non-targeted (QR-Saquinavir) nanoplexes for 24 hrs, followed by imaging using confocal microscopy. Our results show a significant uptake of the targeted QR-Tf-Saquinavir by the PBMC (Fig. 4b), as compared to the non-targeted QR-Saquinavir which showed relatively poor staining (Fig. 4a). Furthermore, local spectral analysis confirmed the origin of fluorescence in the targeted cells from the QRs (Figure 4b, inset), as seen from the sharp emission spectra peaking around 605 nm (please refer to Fig. 1c). In comparison, the local spectra generated from the non-targeted cells show broad emission spectra peaking around 500 nm, which is a characteristic of cellular autofluorescence (Figure 4a, inset). Local spectra analysis is used to determine whether the nanoparticles are successfully migrate into the cells. If the cells are not labeled with the QRs, the emission spectra from the cells will be solely coming from the auto-fluorescence of the cells. When the cells are labeled with functionalized QRs, one can easily use laser scanning confocal microscope to quantitatively determine the uptake of the QRs because the emission spectra QRs can be well separately under same excitation wavelength. Our results indicate enhanced cellular uptake of targeted (QR-Tf-Saquinavir) over the non-targeted (QR-Saquinavir) nanoplexes.
Fig. 4.
Confocal images of PBMCs showing differential uptake of (a) non-Tf conjugated, and (b) Tf-conjugated Saquinavir-QRs. Confocal microscopy images were obtained with laser excitation at 405 nm (Insets) Localized spectral analyses from the treated cells.
Effect of the QR-Tf-Saquinavir Nanoplex on HIV-1 Replication
The ultimate goal of our studies was to evaluate not only if the QR-Tf-Saquinavir nanoplexes can transverse the in vitro BBB, and to investigate if there is significant uptake of the nanoplex by the PBMC cultured at the basolateral end of the BBB model, but also to confirm if the nanoplex uptake by the PBMC has resulted in a decrease in antiviral activity. The following experiments were carried out to determine if nanoplex uptake by the PBMC resulted in a decrease in HIV-1 replication. On the day of the experiment, the infected PBMCs (200,000 cells/ml media) were plated at the bottom of the lower chamber of our in vitro BBB model, the p24 levels were measured prior to and at the end of the experiment. 300ul of the QR-Tf-Saquinavir nanoplexes, as well as Saquinavir alone, were added to the upper chamber and incubated for a 48 hr time period. At the end of the incubation period, the PBMCs from the lower chamber were harvested and the antiviral activity was measured using the p24 ELISA assay, as well as gene expression of HIV-1 LTR was quantitated using real-time PCR. Our results (Fig. 5) showed a 40% and 62% decrease in p24 production in PBMC harvested from cell culture chambers which were treated with free Saquinavir and QR-Tf-Saquinavir nanoplexes, respectively. Statistical comparisons were made with the control well, wherein media alone was added. Real-time PCR data indicated that PBMC harvested from cell culture chambers which were treated with free Saquinavir and QR-Tf-Saquinavir nanoplexes, showed a significant decrease of 67% and 91%, respectively in HIV-1 LTR gene expression when compared to the control (media alone) (Fig. 6).
Fig. 5.
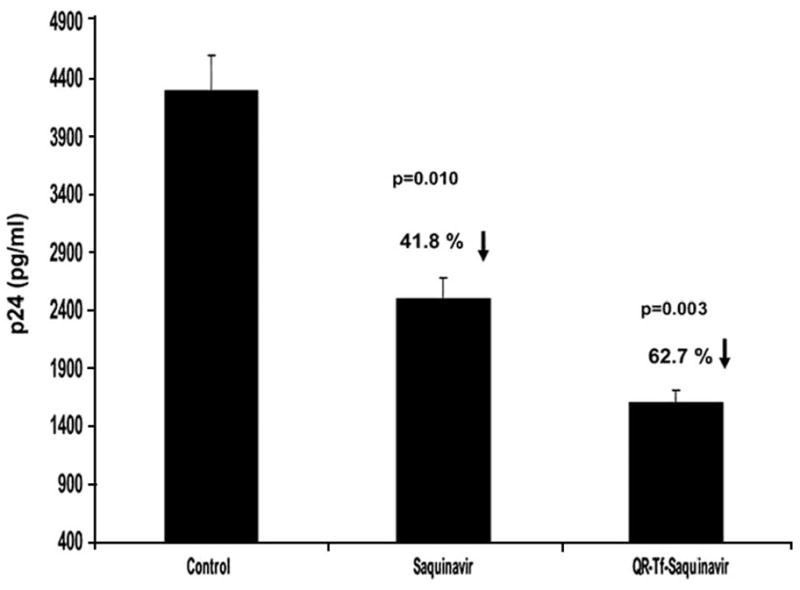
Effect of QR bioconjugates on p24 production in HIV-1 infected PBMC. Culture media from the lower chamber (basolateral end) was harvested 48 h after the QR-Tf-Saquinavir (40nM) nanoplex/Saquinavir (40nM) alone was added to the apical chamber of the in vitro BBB model. p24 levels in these supernatants were measured using a commercially available ELISA kit. Our results showed a significant decrease in p24 production in the culture supernatants of HIV-1 infected PBMC harvested from the basolateral end of the culture wells of the in vitro BBB model in which the QR-Tf-Saquinavir bioconjugate or Saquinavir alone were added at the apical end and compared to control culture wells in which media alone was added. The results shown are mean ± SD of 3 separate experiments done in duplicate.
Fig. 6.

Effect of QR bioconjugates on LTR/RU5 gene expression in HIV-1 infected PBMC. HIV-1 infected PBMC were harvested from the lower chamber, 48 hr after the QR-Tf-Saquinavir (40nM) nanoplex/Saquinavir (40nM) alone was added to the apical chamber of the in vitro BBB model. RNA was extracted, reverse transcribed and the LTR/RU5 gene expression quantitated using Q-PCR. Our results show a significant decrease in LTR/RU5 gene expression in HIV-1 infected PBMC harvested from the basolateral end of the culture wells of the in vitro BBB model in which the QR-Tf-Saquinavir bioconjugate or Saquinavir alone were added at the apical end and compared to PBMC harvested from control culture wells in which media alone was added. The results shown are mean ± SD of 3 separate experiments done in duplicate.
DISCUSSION
Involvement of the CNS in HIV-1 infection occurs early in infection and the CNS serves as a sanctuary for HIV-1. Microglia, macrophages and neurons may all harbor the virus and contribute to viral persistence in the CNS [24,25]. Antiretroviral drugs are ineffective at lowering viral levels in the brain because they cannot permeate across the BBB. As a consequence, viral replication could persist in the brain and can continue to augment the neuropathogenesis process, even in the absence of detectable levels of HIV-1 in the peripheral blood. Further, this ongoing viral replication in the CNS could lead to drug resistance, that may then be disseminated into peripheral circulation [26]. Therefore, it is imperative to develop novel, nanotechnology-based drug delivery platforms, which can permeate across the BBB and release therapeutic concentrations of desired drugs in the brain. Nanoparticle-based formulations have several advantages that can enable potent drug delivery across the BBB, while avoiding any damage to the barrier.
QR are a new generation of optical bioprobes, that have great potential as targeted drug carriers due to the fact that they are multimodal. Multimodal (i.e. the ability for multiple functions for the same agent) nature of a nanoparticle allows it to have multiple properties that are easily integrated to form a nanoparticle complex or a nanoplex. Such a nanoplex can simultaneously: a) carry a payload of the contrast-generating material, which greatly improves its detectability; b) permit the modification of its surface properties to improve circulation half-lives or to attach targeting groups; c) allow its detection with several imaging techniques and d) include therapeutic modalities. Based on our previous studies [11], we have shown that the QRs must be conjugated with specific biorecognition molecules, such as transferrin (Tf), to allow targeted delivery across the BBB. Since the transferrin receptor (TfR) is highly localized on the endothelial surface of the brain, it is ideally suited to trigger receptor-mediated transport across the BBB.
The nanoplexes specifically targets the transferrin receptor on endothelial cells and facilitates their passage across the BBB. The transferrin-receptor (TfR), is an iron-transporting protein-receptor which is present on the BBB in elevated amounts. It is known that the transferrin receptor (TfR) is a kind of specific BBB transporter that allows selected biomolecules to move across the BBB. In our study Transferrin (Tf)-conjugated QRs were used for transport across the in vitro BBB model via a receptor-mediated transport mechanism. We have previously shown that the migration rate of Tf-conjugated QRs crossing the in vitro BBB is concentration and time-dependent [11]. The specific nature of the targeting is confirmed by pre-saturating the BMVECs with free antibodies prior to treatment with the targeted nanoparticles, where we anticipate reduced uptake of the nanoparticles. Tf will generally trigger the receptor mediated transport across the BBB because it undergoes transcytosis through the BBB. Based on these properties transferrin (Tf), was chosen as one of the specific ligands for facilitating transport across the BBB. We hypothesize that integrating the antiretroviral drug Saquinavir within this QR-Tf nanoplatform will markedly enhance its transversing ability across the BBB.
To ensure the use of nanoparticles for applications in drug delivery, evaluation of their safety is critical and will determine how significant a role nanotechnology will play in biomedical applications. Therefore, following the characterization of these QR-Tf-Saquinavir nanoplexes, these nanoformulation were evaluated for potential cytotoxicity in BMVEC and PBMC. Our results indicated absence of any toxicity over different time and concentration conditions tested (Fig. 2).
We observed significant transversing of QR-Tf-Saquinavir nanoplexes, across the BBB, 3-D confocal imaging of BMVEC cells treated with the QR-Tf-Saquinavir nanoplexes shows internalization of the QR-Tf-Saquinavir nanoplex (Fig. 3). Additionally, local spectra analysis is obtained from laser scanning confocal microscopy of the BMVEC cells. Generally, local spectra analysis is used to determine whether the nanoparticles are internalized into the cells. We have shown that the QR-Tf-Saquinavir nanoplexes are taken up by the BMVEC cells, this is confirmed by measuring the emission spectra from the cells which matches with the QR solution spectra emission.
We observed no appreciable change in the TEER measurements of the in vitro BBB following treatment with the QR-Tf-Saquinavir nanoplexes, free Saquinavir, and QR’s alone indicating the absence of any functional damage to the BBB and therefore indicates that these targeted nanoplexes may be extremely effective in carrying therapeutic payload across the BBB.
In addition to investigating if there was significant uptake of the nanoplex by the PBMC cultured at the basolateral end of the BBB model, we also wanted to evaluate if the nanoplex uptake by the PBMC had resulted in a significant decrease in HIV-1 replication. We observed a significant decrease in p24 production and LTR/RU5 gene expression in the HIV-1 infected PBMCs, harvested from the lower chamber of the in vitro BBB model (Figs. 5, 6). The decrease in HIV-1 p24 is a consequence of the decrease in the HIV-1 LTR transcript. We do not anticipate the percentage decrease in viral replication measured by both methods to be identical due to the fact although there is a significant decrease in viral transcription, it may not result in the same degree of decrease in HIV-1 p24 protein production. These two experiments independently verify that the functional, antiviral efficacy of Saquinavir is significantly enhanced when delivered via a targeted nanocarrier across the BBB. Anti HIV-1 efficacy of Saquinavir is significantly enhanced upon incorporation within targeted nanoplex such as QR-Tf-Saquinavir.
In conclusion, we have demonstrated the ability of our QR-Tf-Saquinavir nanoformulation to transverse the BBB and significantly inhibit HIV-1 replication in the infected PBMCs, demonstrating their anti-HIV-1 efficacy in the brain. These results will provide the basis for the development of novel QR based nanoformulations with conjugated anti-retrovirals, which could not only effectively cross the BBB, but also facilitate significant uptake of the nanoplex by HIV-1 infected cells in the CNS, thereby permitting increased bioavailability of the antiretroviral drug resulting in significantly reducing viral infection in the brain. This will provide an effective therapeutic strategy in the treatment of Neuro-AIDS and other neurological disorders.
Acknowledgments
Saquinavir was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. This study was supported by grants from the National Institute of Health ARRA Grant # 1RO1AI08556901A, RO1CA119397, K01DA024577, Pfizer Inc (Grant # GA 400IN3) the John R. Oishei and Kaleida Health Foundations.
References
- 1.Gulyaev AE, Gelperina SE, Skidan IN, et al. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:1564–9. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 2.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674:171–4. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder U, Sommerfeld P, Ulrich S, Sabel BA. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J Pharm Sci. 1998;87:1305–7. doi: 10.1021/js980084y. [DOI] [PubMed] [Google Scholar]
- 4.Pathak S, Cao E, Davidson MC, Jin S, Silva GA. Quantum dot applications to neuroscience: new tools for probing neurons and glia. J Neurosci. 2006;26:1893–5. doi: 10.1523/JNEUROSCI.3847-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva GA. Nanotechnology approaches for drug and small molecule delivery across the blood brain barrier. Surg Neurol. 2007;67:113–6. doi: 10.1016/j.surneu.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Michalet X, Pinaud FF, Bentolila LA, et al. Quantum Rods for Live Cells, in Vivo Imaging, and Diagnostics. Science. 2005;307:538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad PN. Introduction in Biophotonics. Wiley; New York: 2003. [Google Scholar]
- 8.Prasad PN. Nanophotonics. Wiley; New York: 2004. [Google Scholar]
- 9.Qian J, Yong KT, Roy I, et al. Imaging Pancreatic Cancer Using Surface-Functionalized Quantum Rods. J Phys Chem. 2007;111:6969–72. doi: 10.1021/jp070620n. [DOI] [PubMed] [Google Scholar]
- 10.Yong KT, Qian J, Roy I, et al. Quantum rod bioconjugates as targeted probes for confocal and two-photon fluorescence imaging of cancer cells. Nano Lett. 2007;7:761–5. doi: 10.1021/nl063031m. [DOI] [PubMed] [Google Scholar]
- 11.Xu G, Yong KT, Roy I, et al. Bioconjugated quantum rods as targeted probes for efficient transmigration across an in vitro blood-brain barrier. Bioconjug Chem. 2008;19:1179–85. doi: 10.1021/bc700477u. [DOI] [PubMed] [Google Scholar]
- 12.Kravcik S, Gallicano K, Roth V, et al. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and Saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–5. [PubMed] [Google Scholar]
- 13.Dragsted UB, Gerstoft J, Pedersen C, et al. Randomized trial to evaluate indinavir/ritonavir versus Saquinavir/ritonavir in human immunodeficiency virus type 1-infected patients: the MaxCmin 1 Trial. J Infect Dis. 2003;188:635–42. doi: 10.1086/377288. [DOI] [PubMed] [Google Scholar]
- 14.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Ann Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–8. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 16.Persidsky Y, Gendelman HE. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol. 1997;62:100–6. doi: 10.1002/jlb.62.1.100. [DOI] [PubMed] [Google Scholar]
- 17.Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukocyte Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- 18.Persidsky Y, Stins M, Way D, et al. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997;158:3499–510. [PubMed] [Google Scholar]
- 19.Mahajan SD, Aalinkeel R, Sykes DE, et al. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–41. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 20.Meyer TP, Zehnter I, Hofmann B, et al. Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods. 2005;307:150–66. doi: 10.1016/j.jim.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Saachi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 23.Radonić A, Thulke S, Mackay IM, et al. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–62. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 24.Letendre SL, Ellis RJ, Everall I, et al. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2009;17:46–56. [PMC free article] [PubMed] [Google Scholar]
- 25.Enting RH, Hoetelmans RM, Lange JM, et al. Antiretroviral drugs and the central nervous system. AIDS. 1998;12:1941–55. doi: 10.1097/00002030-199815000-00005. [DOI] [PubMed] [Google Scholar]
- 26.DeLuca A, Ciancio B, Larussa D, et al. Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology. 2002;59:342–7. doi: 10.1212/wnl.59.3.342. [DOI] [PubMed] [Google Scholar]