Abstract
Background
Abnormalities in host mucosal immunity exist in chronic rhinosinusitis with nasal polyps (CRSwNPs), but it is unclear whether this is a cause or an effect of the eosinophilic inflammation and frequent microbial colonization that characterizes the disease. Sinonasal epithelial cells (SNECs) are critical participants in healthy antimicrobial innate immune defense. They also can promote Th2 inflammation with various mediators, including interleukin (IL)-33, which induces T helper cells to produce Th2 cytokines.
Methods
CRSwNP SNECs were obtained during sinus surgery and stored. Patients were subsequently classified as either treatment responsive or treatment recalcitrant, based on long-term outcomes of medical and surgical therapy. Epithelial cells from these patients were grown in air–liquid interface (ALI) culture and treated with IL-13, as well as the bacteria-associated molecule, CpG. Expression of IL-33 mRNA was determined by real-time polymerase chain reaction.
Results
Recalcitrant CRSwNP epithelial cells had increased baseline expression of IL-33 compared with responsive CRSwNPs, which was further increased by 24-hour exposure to CpG. Treatment-responsive epithelial cells were not induced by CpG to express IL-33. Prolonged treatment with IL-13 during differentiation at the ALI diminished the baseline expression of IL-33 and prevented the subsequent induction of IL-33 by CpG.
Conclusion
Mucosal innate immunity likely plays an important role in CRSwNP pathogenesis. A definitive link between infectious triggers and the development of Th2 inflammation has been elusive. We have found constitutive IL-33 expression by SNECs in recalcitrant CRSwNPs, which can be further induced by a bacteria-associated molecular pattern. Dysregulated epithelial cell immune interactions between host and environment may contribute to Th2 inflammation in CRSwNPs.
Keywords: Chronic, cytokines, IL-33, nasal polyps, polyps, rhinosinusitis, Th2
Chronic rhinosinusitis with nasal polyps (CRSwNPs) is an inflammatory disease that remains difficult to treat despite advances in medical and surgical therapy. Although CRSwNP patients benefit from endoscopic sinus surgery,1,2 polyp recurrence rates exceed 50% and revision surgery is required in 30% of these patients.3 Because of the significant recidivism of this disease, frequent courses or long-term usage of systemic corticosteroids remain the mainstays of treatment for CRSwNPs, despite the significant complications associated with their use. A better understanding of the pathophysiology underlying the persistent inflammatory state in CRSwNPs is necessary to ultimately develop novel pharmacotherapeutic approaches.
Sinonasal epithelial cells (SNECs) represent a frontline barrier between the host and the external environment, playing a critical role in airway mucosal defense. Resistance to potentially harmful pathogens is provided by immediate innate immune responses, as well as delayed adaptive immune responses. Although the innate immune mechanisms are classically considered constitutive and nonspecific, inducible pathogen-directed activity has recently been described. By virtue of their location, SNECs constitute an important element of the innate immunity of the sinonasal tract. Pattern recognition receptors (PRRs) allow SNECs to detect and initiate responses against pathogens present in the airway lumen. The presence of mRNA for Toll-like receptors (TLRs) 1–10 was confirmed in CRS and controlled sinonasal mucosal tissue in 2003.4 Multiple studies have established the presence of TLRs5–8 as well as innate immune effectors such as human β-defensin (HBD) 2,9 serum amyloid A,4 and surfactant proteins A and D10 in SNECs, but the specific role of epithelial cell innate immunity in CRS pathogenesis remains unclear.
The eosinophilic inflammation associated with CRSwNPs occurs in a milieu distinguished by the prevalence of Th2 cytokines such as IL-4, IL-5, and IL-13. Although the etiology of the Th2 polarization of the CRSwNP adaptive immune inflammation is not known, it is clear that these Th2-associated cytokines directly impact innate immune function. In a recent study, exposure of SNECs derived from CRSwNP patients to IL-4 and IL-13 caused a down-regulation in innate immune molecules such as TLR9, HBD-2, and surfactant protein A.11 Mechanisms through which SNECs can drive or exacerbate Th2 inflammation in CRSwNPs are suggested by studies of acid mammalian chitinase expression and the induction of eotaxin-3 expression by the molecule chitin. To date, stimulation of SNEC TLRs has been implicated in Th1 immunity only and not in the Th2 inflammatory response associated with CRSwNPs.
IL-33 is a recently described cytokine that was identified as a ligand for the orphan IL-1 family receptor T1/ST2.12 It is produced by airway epithelial cells, fibroblasts, and smooth muscle and its receptor is expressed in monocytes, mast cells, eosinophils, and Th2 lymphocytes.13 IL-33 drives production of Th2-associated cytokines such as IL-4, IL-5, and IL-13 and also functions as a chemoattractant for Th2 cells.13 Recent studies have shown that IL-33 may play an important role in Th2-mediated eosinophilic inflammation such as that found in asthma,14 and polymorphisms within the IL-33 receptor gene have been linked to the severity of CRS.15 Although IL-33 represents an interesting and potentially important link between SNEC function in innate immunity and the Th2 predominant inflammation in CRSwNP, its expression by SNECs has not been studied. The objective of this study was to determine whether IL-33 is expressed by SNECs and how its expression may differ in treatment-resistant forms of CRSwNPs. Using a primary epithelial cell culture model, this study also examines the modulatory effects of prolonged IL-13 exposure and acute exposure to a bacterial pathogen-associated molecule (CpG) on the expression of IL-33.
MATERIALS AND METHODS
Human Subjects
Thirty-two patients with CRSwNPs and five patients with nonpolypoid CRS (CRSsNP) were enrolled in the study. The research protocol was approved through the Johns Hopkins Institutional Review process, and all subjects gave signed informed consent. Inclusion criteria included continuous symptoms of rhinosinusitis as defined by the American Academy of Otolaryngology–Head and Neck Surgery Chronic Rhinosinusitis Task Force for >12 weeks, CT of the sinuses revealing isolated or diffuse sinus mucosal thickening or air–fluid levels, and nasal polyps visible on diagnostic endoscopy.16 Before surgery, the patients received 1 week of oral methylprednisolone. All tissue specimens were taken from the resected uncinate process or the anterior ethmoid sinus. The tissue was immediately placed in saline and processed for cell culture as described later in the SNEC Culture at the Air–Liquid Interface (ALI) section and stored frozen at −70°C. All patients received identical perioperative medications, including systemic corticosteroids.
CRSwNP patients were followed clinically for at least 6 months after surgery. Those that showed recurrence of nasal polyps despite surgery and a postoperative regimen including topical steroids and nasal saline irrigations, at minimum, were classified as recalcitrant CRSwNPs. Patients without endoscopic evidence of polyps at least 6 months after surgery were classified as responsive CRSwNPs.
SNEC Culture at the Air–Liquid Interface (ALI)
The technique of ALI cultures has been previously published by our group.17 Briefly, epithelial cells are isolated from tissue samples by enzymic digestion and grown in cell culture. Once confluent, the cells are trypsinized, suspended in bronchial epithelial growth medium, and replated onto human type IV placental collagen (type VI; Sigma, St. Louis, MO)-coated 6-well Falcon filter inserts (0.4-μm pore size; Becton Dickinson, Franklin Lakes, NJ). The P1 cells are grown to confluence with bronchial epithelial growth medium above (1 mL) and below (2 mL) the filter inserts. When confluent, medium is removed from above the cultures and the medium below the inserts is changed to ALI medium consisting of LHC Basal Medium/DMEM-H (50:50; Gibco, North Andover, MA). Each set of cultures came from a separate patient source and was maintained at the ALI with the apical surfaces remaining free of medium for at least 3 weeks before study.
Treatment of Cells with CpG or IL-13
For CpG challenge, epithelial cells at the ALI were exposed to 10 μM of CpG (Invivogen, San Diego, CA) in 250 μL of media applied to the apical surface for 24 hours. In the experiments involving IL-13 treatment, 10 ng/mL of IL-13 was added to the basal media for the first 10 days of differentiation at the ALI only, after which time the IL-13 was not present.
RNA Extraction/Reverse Transcription
Total RNA was isolated with the RNeasy Mini kit (Qiagen, Valencia, CA) using the manufacturer’s protocol. RNA was quantified spectrophotometrically and absorbance ratios at 260/280 nm were >1.80 for all samples studied. Five hundred nanograms of total RNA was reverse transcribed in a 20-μL volume with random hexamer primers (Invitrogen, Carlsbad, CA), 20 U of RNase inhibitor (Applied Biosystems, Foster City, CA), and the Omniscript RT kit (Qiagen) under conditions provided by the manufacturer.
Real-Time Polymerase Chain Reaction (PCR)
Real-time PCR was performed in a Light-Cycler 1.2 (Roche, Indianapolis, IN) using the SYBR Green PCR Kit (Qiagen). Primers were commercially synthesized by Invitrogen: 18S (sense, 5′-GTAACCCGTTGAACCCCATT-3′; antisense, 5′-CCATCCAATCGGTAGTAGCG-3′) and IL-33 (sense, 5′-CATGCCAACAACAAGGAACA-3′; antisense, 5′-AGGACAAAGAAGGCCTGGTC-3′). The cycle parameters used were 95°C for 15 minutes, and then 45 cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Amplicon expression in each sample was normalized to its 18S RNA content. The level of expression of target mRNA was determined as the ΔCT, as previously described.17 Negative controls, consisting of reaction mixtures containing all components except target RNA, were included with each PCR run. Finally, amplified products were sequenced to verify authenticity.
Immunochemistry
Adherent cells at the ALI were washed and fixed with ice-cold methanol for 10 minutes at 4°C. After blocking nonspecific binding sites (1% bovine serum albumin), inserts were incubated (room temperature, 2 hours) with Alexa 488–conjugated monoclonal anti–β-IV-tubulin antibody conjugated (Millipore, Billerica, MA) and rabbit anti-ZO-1(Invitrogen). Inserts were then incubated for 45 minutes with Alexa 594–conjugated donkey anti-rabbit IgG. The cell layers were cut away from the inserts and mounted on glass slides with Vectashield mounting medium (Vector Labs, Burlingame, CA). A negative control was performed by omitting primary antibody. The cells were observed under a Zeiss 510 meta confocal microscope equipped with epifluorescence illumination (Carl Zeiss, Thornwood, NY). Images were displayed as Z stacks with a 0.5-μm slice thickness.
Statistical Analysis
Raw data from real-time PCR were entered into a spreadsheet (Excel; Microsoft Corp., Redmond, WA). Statistical analysis was performed using a software program (StatView; SAS Institute, Inc., Cary, NC). Data are expressed as mean ± SEM. Statistical significances of differences in IL-33 expression were determined using either paired or unpaired t-tests assuming unequal variances. Differences were considered statistically significant at p < 0.05. Regression analysis was performed using Excel software.
RESULTS
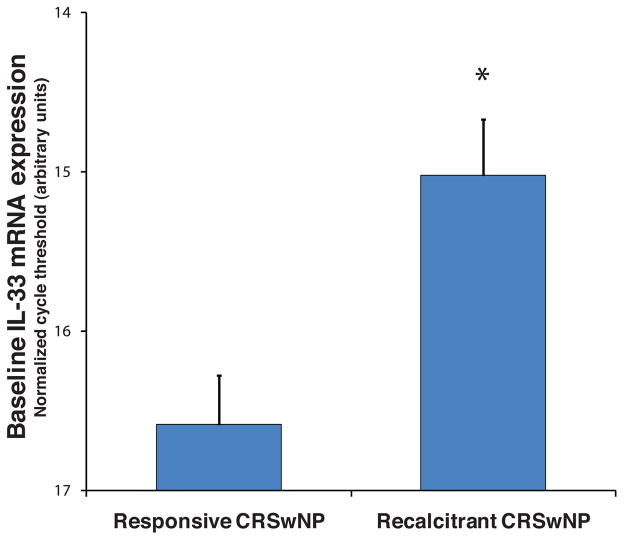
SNECs were obtained from 32 patients with CRSwNPs at the time of endoscopic sinus surgery. The patients underwent identical medical management in the perioperative period and were followed for at least 6 months thereafter. Eleven of the patients had persistent nasal polyposis, despite continued aggressive medical therapy with systemic and topical corticosteroids. The remaining 21 patients either did not have recurrence of polyps or were controllable with medical therapy. Immediately after surgery, the epithelial cells were grown to confluence in submerged cell culture, and mRNA was harvested. Real-time PCR revealed that the level of expression of IL-33 was threefold greater in the recalcitrant CRSwNP group (n = 5) than it was in cells from responsive CRSwNP patients (n = 5; p = 0.001; Fig. 1).
Figure 1.
Expression of interleukin (IL)-33 mRNA was assessed by real-time polymerase chain reaction (PCR) in primary cultures of sinonasal epithelial cells (SNECs) derived from chronic rhinosinusitis with nasal polyps (CRSwNP) patients (n = 32). The baseline level of IL-33 mRNA was threefold greater in epithelial cells from patients with recalcitrant CRSwNP (n = 11) who continue to have nasal polyps despite sinus surgery and continuous medical therapy. Error bars denote standard error of the mean (*p = 0.001). Increased expression of IL-33 mRNA in cultured SNECs obtained from patients with treatment-recalcitrant CRSwNPs.
Epithelial cells from five recalcitrant and five responsive patients were grown at the ALI until fully differentiated, and then challenged with 10 μM of CpG for 24 hours. RNA was extracted from the cells, and IL-33 mRNA expression was assessed by real-time PCR. The baseline level of IL-33 expression was not significantly different between the two groups. However, CpG induced a threefold increase in expression of IL-33 by recalcitrant CRSwNP epithelial cells (p = 0.002), but no increase was observed in responsive CRSwNP cells (Fig. 2).
Figure 2.
In differentiated sinonasal epithelial cells (SNECs), exposure to the bacterial pathogen-associated molecule CpG (10 μM) for 24 hours induces increased expression of interleukin (IL)-33 mRNA only in cells derived from patients with recalcitrant chronic rhinosinusitis with nasal polyps (CRswNPs). Each symbol denotes an individual subject’s SNECs (n = 5 for each group). There was a threefold induction for the recalcitrant group as a whole. CpG induces increased expression of IL-33 mRNA in differentiated SNECs derived from patients with recalcitrant CRswNP.
To study the effect of the Th2 cytokine environment on IL-33 expression, we generated epithelial cell cultures from five CRSsNP patients. Unlike cells derived from CRSwNP mucosa, cells from CRSsNP subjects have not been chronically exposed in vivo to Th2 cytokines before isolation. CRSsNP patients were otherwise similar to CRSwNP patients in terms of medical therapy and the presence of sinonasal mucosal inflammation. The CRSsNP epithelial cells were grown in media containing10 ng/mL of IL-13 from day 3 through day 13 of differentiation at the ALI to model the Th2 milieu present in CRSwNP sinonasal mucosa. After exposure to IL-13 for 10 days, the cells were grown in standard media without IL-13 for at least 7 more days, until full differentiation had occurred. Immunochemical staining for β-IV-tubulin in our cultures revealed a decreased number of ciliated cells (Fig. 3 A) shown by a decreased expression of β-IV-tubulin mRNA (Fig. 3 B). Baseline expression of IL-33 mRNA was reduced 3.65-fold in cells differentiated in the presence of IL-13, when compared with cells from the same patients grown in the absence of IL13 (p = 0.03; Fig. 3 C). Subsequent exposure to CpG did not stimulate an increase in IL-33 mRNA expression (data not shown).
Figure 3.
Effect of 10-day exposure to interleukin (IL)-13 on differentiating sinonasal epithelial cells (SNECs). (A) Confocal microscopy of differentiated epithelial cell cultures after 21 days at the air–liquid interface (ALI). The green label shows β-IV-tubulin expression denoting cilia, and the red label shows the cell boundaries with the marker Zo-1, which labels intercellular tight junctions. The top left panel shows the distribution of ciliated cells without IL-13 treatment and the bottom panel shows the effect of IL-13 exposure during days 3–13 of differentiation. (B) Real-time polymerase chain reaction (PCR) shows decreased β-IV-tubulin mRNA expression in cells exposed to IL-13 for 10 days during differentiation. (C) Real-time PCR reveals a 3.65-fold decreased expression of IL-33 by cells exposed to IL-13 during differentiation when compared with unexposed cells derived from the same patients. Error bars denote standard error of the mean (*p = 0.03). Effect of IL-13 exposure during epithelial cell differentiation at the ALI on ciliation (panels A and B) and level of IL-33 mRNA expression (panel C).
DISCUSSION
CRSwNPs is a disease marked by persistent mucosal inflammation despite medical and surgical therapy designed to relieve ostial obstruction. Although CRSwNP inflammation is clearly associated with exaggerated production of Th2 cytokines and proeosinophilic mediators, the underlying cause remains unknown. Multiple groups have proposed that abnormal immune responses may accompany exposure to microorganisms and/or their products including fungi, S. aureus, or bacterial biofilms.18–23 However, these agents do not typically generate Th2 response in healthy hosts, suggesting that an additional pathogenic mechanism is present in affected CRSwNP patients.
Adaptive and innate immunity work synergistically to protect the host from pathogens entering the nasal cavity. Adaptive host defenses are responsible for the maintenance of lymphocytic memory responses and effective pathogen-specific clearance of infections on reexposure. Sinonasal mucosal innate immunity is a first line of defense that includes the physical barrier created by epithelial cells, mucociliary clearance, and specific PRRs, as well as immunologic effectors such as cathelicidins and antimicrobial peptides. Innate immune responses mediated by known PRRs generate predominately antimicrobial responses and activation of Th1 pathways. Although a limited number of exogenous and endogenous agents are capable of eliciting pro-Th2 gene expression by epithelial cells, the relationship between bacterial infection and exacerbation of eosinophilic inflammation of CRSwNPs has not been defined.
IL-33 is a cytokine produced by airway epithelial cells and fibroblasts, in which its receptor (T1/ST2) is found in mast cells, eosinophils, and Th2 lymphocytes.13 IL-33 activation of T1/ST2 stimulates the production of pro-Th2 inflammatory mediators and cytokines.13 In this study, we show the expression of IL-33 in primary cultures of human SNECs derived from patients with CRSwNPs. Although previous studies have confirmed the presence of IL-33 in the epithelial cells of the bronchus and small airways,12 this is the first study to establish evidence that IL-33 is produced by SNECs. We observed that baseline IL-33 mRNA expression was threefold higher in SNECs derived from recalcitrant CRSwNP patients than those with responsive disease. This analysis was performed within 1 week of epithelial cell isolation from the patient tissue and presumably is reflective of in vivo levels of expression. Although our study population was limited to 11 recalictrant CRSwNP and 21 responsive CRSwNP patients, these results suggest that IL-33 levels may correlate with nonresponsiveness to medical and surgical therapy. Although levels of Th2 cytokine have not been shown to directly correlate with CRSwNP disease severity, it is conceivable that IL-33–driven increases in Th2 cytokine expression after surgery may hasten the return of nasal polyps in those patients with recalcitrant CRSwNPs, when compared with those with responsive disease. Interestingly, a recent report has suggested that polymorphisms in the IL-33 receptor gene may be associated with a protective effect against development of severe CRS disease.15
Unmethylated CpG oligonucleotides are molecular patterns associated with bacterial infection that are recognized by the innate immune receptor TLR9. TLR9 activation stimulates antimicrobial effector expression and elaboration of Th1 cytokines in multiple cell types and may simultaneously down-regulate Th2 inflammation.24–26 Previous studies have supported a role of SNECs in the innate immune response by showing expression of TLR9-related effectors such as HBD-2 and IL-8 after exposure to CpG.27 TLR9 expression was also found to be decreased in human SNECs from patients with recalcitrant CRSwNPs compared with those with responsive disease, suggesting potential deficiency in Th1 immune responses and predisposition toward Th2-skewed inflammation.5 In the present study, exposure to CpG of differentiated SNECs from patients with recalcitrant CRSwNPs caused a threefold increase in their expression of IL-33. Given the ability of IL-33 to generate Th2 inflammation and tissue eosinophilia, CpG-induced IL-33 expression may contribute to recurrent or persistent eosinophilic inflammation in CRSwNPs after surgery. Although the exact mechanism by which CpG induces increased expression of IL-33 remains unclear, this pathway represents a potential link between the innate immune activity of SNECs and Th2-skewed adaptive immune responses in CRS. It is interesting that TLR9 normally induces a Th1-like pattern of gene expression, and that TLR9 levels are decreased in recalcitrant CRSwNP SNECs. Our findings raise the possibility of a TLR9-independent CpG signaling pathway that can result in IL-33 expression. Additional work is required to explore this mechanism.
The expression of IL-33 was greatly reduced in cells differentiated in the presence of the Th2 cytokine, IL-13. Our findings confirm reports in the literature that IL-13 exposure during differentiation leads to a relative decrease in the proportion of ciliated epithelial cells in ALI cell cultures.18 Given this relationship, we hypothesize that IL-33 may be produced principally by ciliated SNECs rather than mucus-producing epithelial cells. Future studies to analyze and compare the expression of IL-33 in SNECs exposed to IL-13 from CRSwNP and non-CRS patients may help to better elucidate the clinical relevance of these findings.
As with all in vitro studies, it is important to recognize that this model does not perfectly replicate the SNEC’s environment in CRSwNPs. These cells lack their normal interactions with other resident cell populations, and growth in cell culture may lead to phenotypic changes that in and of themselves affect genetic transcription and translation. Also, the method of cell culture (submerged versus ALI) must be taken into account when interpreting in vitro experimental results. For example, the baseline expression of IL-33 was threefold greater in submerged cultures from recalcitrant CRSwNP when compared with responsive CRSwNP patients, but were similar between these same groups when an ALI culture technique was used (Fig. 2). We believe that the difference in experimental conditions is sufficient to explain the variance in baseline IL-33 expression. Differentiation that occurs when cells are grown in an ALI induces alteration in the expression of many epithelial cell genes. In addition, the prolonged period of time required to grow ALI cultures likely accounts for shifts in baseline mRNA expression. There are strengths and weaknesses to studying submerged versus ALI cultures. Although studying differentiated cells likely better simulates the physiology of the innate immune response in vivo, baseline in vivo gene expression for a particular patient may be better represented in submerged cultures closer to the time of collection. Despite limitations of sample size and restrictions inherent to cell culture techniques, the present study clearly shows that SNECs produce IL-33 and that expression may be modulated by host and environmental factors. Importantly, IL-33 represents a new link between innate and adaptive immune responses in the sinonasal tract, and its dysregulation in severe forms of CRSwNPs may suggest a novel target for future therapy.
Acknowledgments
The authors would like to thank Ms. Leda, Pawliuk and Ms. Linsdey May for their excellent technical assistance with polymerase chain reaction and confocal microscopy, respectively.
Footnotes
Presented at the American Rhinologic Society meeting, Rhinology World, Philadelphia, Pennsylvania, April 17, 2009
References
- 1.Poetker DM, Mendolia-Loffredo S, Smith TL. Outcomes of endoscopic sinus surgery for chronic rhinosinusitis associated with sinonasal polyposis. Am J Rhinol. 2007;21:84–88. doi: 10.2500/ajr.2007.21.2978. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. Influence of polyps on outcomes after endoscopic sinus surgery. Laryngoscope. 2007;117:1834–1838. doi: 10.1097/MLG.0b013e3180caa19d. [DOI] [PubMed] [Google Scholar]
- 3.Wynn R, Har-El G. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 2007;114:811–813. doi: 10.1097/00005537-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Vandermeer J, Sha Q, Lane AP, et al. Innate immunity of the sinonasal cavity: Expression of messenger RNA for complement for complement cascade components and toll-like receptors. Arch of Otolaryngol Head Neck Surg. 2004;130:1374–1380. doi: 10.1001/archotol.130.12.1374. [DOI] [PubMed] [Google Scholar]
- 5.Schleimer RP, Lane AP, Kim J. Innate and acquired immunity and epithelial cell function in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:51–78. [PubMed] [Google Scholar]
- 6.Lane AP, Truong-Tran Q, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol. 2006;20:138–144. [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Z, Yang Z, Wang C. Expression of TLR2 and TLR4 messenger RNA in the epithelial cells of the nasal airway. Am J Rhinol. 2005;19:236–239. [PubMed] [Google Scholar]
- 8.Fransson M, Adner M, Erjafalt J, et al. Up-regulation of Toll-like receptors 2, 3, and 4 in allergic rhinitis. Respir Res. 2005;6:100. doi: 10.1186/1465-9921-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SH, Kim JE, Lim HH, et al. Antimicrobial defensin peptides of the human nasal mucosa. Ann Otol Rhiol Laryngol. 2002;111:135–141. doi: 10.1177/000348940211100205. [DOI] [PubMed] [Google Scholar]
- 10.Woodworth BA, Smythe N, Spicer SS, et al. Presence of surfactant lamellar bodies in normal and diseased sinus mucosa. ORL J Otorhinolaryngol Relat Spec. 2005;67:199–202. doi: 10.1159/000087093. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan M, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008;22:115–121. doi: 10.2500/ajr.2008.22.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz J, Owyang A, Oldham E, et al. IL-33 an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type-2 associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Arend WP, Palmer G, Gabay C. IL-1, IL-18, IL-33 families of cytokines. Immunological Reviews. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 14.Cherry WB, Yoon J, Bartemes KR, et al. A novel IL-1 family cytokine, IL-33, potentially activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castano R, Bosse Y, Endam LM, Desrosiers M. Evidence of association of interleukin-1 receptor-like 1 gene polymorphisms with chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23:377–384. doi: 10.2500/ajra.2009.23.3303. [DOI] [PubMed] [Google Scholar]
- 16.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan M, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol. 2007;21:373–377. doi: 10.2500/ajr.2007.21.3034. [DOI] [PubMed] [Google Scholar]
- 18.Laoukili J, Perret E, Willems T, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108:1817–1824. doi: 10.1172/JCI13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponikau JU, Sherris DA, Kephart GM, et al. The role of ubiquitous airborne fungi in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2005;5:472–476. doi: 10.1007/s11882-005-0028-6. [DOI] [PubMed] [Google Scholar]
- 20.Bachert C, Gevaert P, Zhang N, et al. Superantigens and nasal polyps. Curr Allergy Asthma Rep. 2003;3:523–531. doi: 10.1007/s11882-003-0065-y. [DOI] [PubMed] [Google Scholar]
- 21.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2006;134:991–996. doi: 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Palmer JN. Bacterial biofilms: Do they play a role in chronic rhinosinusitis? Otolaryngol Clin North Am. 2005;38:1193–1201. doi: 10.1016/j.otc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein JM, Ballow M, Schlievert PM, et al. A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol. 2003;17:321–326. [PubMed] [Google Scholar]
- 24.Serebrisky D, Teper AA, Huang CK, et al. CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J Immunol. 2000;165:5906–5912. doi: 10.4049/jimmunol.165.10.5906. [DOI] [PubMed] [Google Scholar]
- 25.Broide DH, Stachnick G, Castaneda D, et al. Immunostimulatory DNA mediates inhibition of eosinophilic inflammation and airway hyperreactivity independent of natural killer cells in vivo. J Allergy Clin Immunol. 2001;108:759–763. doi: 10.1067/mai.2001.118795. [DOI] [PubMed] [Google Scholar]
- 26.Farkas L, Kvale EO, Johansen FE, et al. Plasmacytoid dendritic cells activate allergen-specific TH2 memory cells: Modulation by CpG oligodeoxynucleotides. J Allergy Clin Immunol. 2004;114:436–443. doi: 10.1016/j.jaci.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan M, Lee WK, Dubin MG, et al. Sinonasal epithelial cell expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am J Rhinol. 2007;21:110–116. doi: 10.2500/ajr.2007.21.2997. [DOI] [PubMed] [Google Scholar]