Summary
Background
The platelet α2β1 integrin functions as both an adhesion and signaling receptor upon exposure to collagen. Recent studies have indicated that α2β1 function can be activated via inside-out signaling, similar to the prototypical platelet integrin αIIbβ3. However, signaling molecules that regulate α2β1 activation in platelets are not well defined. A strong candidate molecule is the small GTPase Rap1b, the dominant platelet isoform of Rap1, which regulates αIIbβ3 activation.
Objectives
We hypothesized that Rap1b positively regulates α2β1 during agonist-induced platelet activation.
Methods
To test whether Rap1b activates α2β1 downstream of glycoprotein (GP)VI or other platelet receptors, we stimulated platelets purified from Rap1b−/− or wild-type mice with diverse agonists and measured α2β1 activation using fluorescein isothiocyanate-labeled monomeric collagen. We also examined the role of Rap1b in outside-in signaling pathways by analyzing adhesion and spreading of Rap1b−/− or wild-type platelets on monomeric, immobilized collagen. Finally, we monitored the activation status of related Rap GTPases to detect changes in signaling pathways potentially associated with Rap1b-mediated events.
Results
Rap1b−/− platelets displayed comparable ADP-induced or thrombin-induced α2β1 activation as wild-type platelets, but reduced convulxin-dependent α2β1 activation. Rap1b−/− platelets exhibited increased spreading on immobilized collagen but similar adhesion to immobilized collagen compared to wild-type platelets. Rap1b−/− platelets also showed Rap1a and Rap2 activation upon agonist stimulation, possibly revealing functional compensation among Rap family members.
Conclusions
Rap1b is required for maximal GPVI-induced but not ADP-induced activation of α2β1 in murine platelets.
Keywords: α2β1, collagen, GPVI, integrin, platelet and Rap1b
Introduction
Upon blood vessel injury, circulating platelets rapidly adhere to subendothelial collagen, spread and recruit additional platelets to initiate clot formation. Although αIIbβ3 is critical for these events and is the most abundant integrin on the platelet surface, the less abundant α2β1 provides firm adhesion to type I collagen and assists in the accumulation of platelets at sites of vascular injury [1–3]. Like αIIbβ3, α2β1 can be activated in response to physiologic agonists [4,5] and facilitates platelet signaling responses to collagen [6,7], including soluble collagen binding [8] and rapid aggregation [3,7,9,10]. Additionally, individuals with α2β1 polymorphisms that result in increased α2β1 expression have a higher risk of stroke [11], whereas individuals who lack α2β1 exhibit prolonged bleeding times [12].
Glycoprotein (GP)VI is another major collagen receptor on mouse and human platelets. Mouse platelets depleted of GPVI by intraperitoneal injection of an anti-GPVI antibody, JAQ1, show no response to fibrillar collagen [8], and in vivo depletion of GPVI protects against arterial thrombosis [13]. GPVI has been proposed to activate α2β1 as part of a redundant collagen signaling system in mouse and human platelets [7]. However, little is known about the intracellular signaling pathways leading to α2β1 activation.
Growing evidence suggests that the small GTPase Rap1b is a key regulator of integrin function in hematopoietic cells. Rap1b was recently identified as the dominant isoform involved in murine B-cell adhesion to vascular cell adhesion molecule-1 (VCAM-1) and chemotaxis [14]. In human and murine platelets, Rap1b is rapidly activated in response to diverse physiologic agonists such as collagen, convulxin (a GPVI agonist), thrombin, ADP, and epinephrine [15–18]. Rap1b−/− mice show dramatic defects in αIIbβ3-dependent processes such as hemostatic plug and arterial thrombus formation, demonstrating that Rap1b is a crucial regulator of platelet function in vivo [19]. CalDAG-GEFI, an upstream guanine nucleotide exchange factor for both Rap1a and Rap1b, is also critical for platelet integrin function [20]. CalDAG-GEFI−/− murine platelets show defective αIIbβ3-dependent aggregation induced by collagen, defective thrombus formation, defective adhesion to laminin through the α6β1 integrin, and defective adhesion to fibronectin, a process mediated by α5β1 and αIIbβ3 [20,21]. In humans, similar integrin defects were observed in leukocyte adhesion deficiency III patients, who lack normal levels of platelet CalDAG-GEFI [22].
Although it is clear that Rap1b signals to αIIbβ3 in response to fibrillar collagen and convulxin [19], it is unknown whether Rap1b signals to α2β1. We therefore tested the hypothesis that Rap1b positively regulates α2β1 by using soluble monomeric collagen to monitor the activation state of α2β1 on Rap1b−/− platelets and by analyzing platelet adherence to immobilized monomeric collagen.
Materials and methods
Reagents
See Supporting Information Doc. S1 for details.
Mice
Rap1b−/− mice were bred on a mixed background of 129 and C57Bl/6 as previously described [19], and used in accordance with guidelines of the IACUC of UNC-CH and the Blood Research Institute, Blood Center of Wisconsin, Milwaukee, WI, USA.
Platelet preparation
Washed murine platelets were prepared as previously described, using cardiac puncture or retro-orbital bleeding [23]. See Supporting Information Doc. S2 for more details.
Flow cytometry and data analysis
Platelets were resuspended to 5 × 107 mL−1 in modified Tyrode’s buffer. Fluorescein isothiocyanate (FITC)–collagen was added at 5–10 μg mL−1, followed by the indicated concentrations of ADP, thrombin or convulxin for 30 min in the dark at room temperature. Platelets were then diluted in Tyrode’s buffer and analyzed on a FACStar Plus, FACSCanto or LSRII flow cytometer (Becton Dickinson). Data are presented as mean fluorescence intensity from which “no agonist” control values were subtracted.
Adhesion assay
Platelet adhesion was measured as previously described, using a colorimetric phosphatase assay [24]. Briefly, platelets were adjusted to 2–5 × 107 mL−1 and preincubated with antibodies at room temperature for 30 min, where indicated. Platelets were attached to microtiter plates coated with 10 μg mL−1 soluble collagen or 10 mg mL−1 bovine serum albumin (BSA) at room temperature for 20 or 60 min, and non-adherent platelets were removed by aspiration followed by three washes with Tyrode’s buffer. Wells not subjected to washing served as a reference for total platelets. To calculate specific adhesion to collagen, raw values of BSA-coated wells were subtracted from values of corresponding collagen-coated wells and divided by values of unwashed total platelets. The values for unstimulated wild-type platelets were normalized to 100 arbitrary units, and all other data points were expressed relative to this value.
Spreading assay
Platelets were adjusted to 1 × 107 mL−1. Coverslips were coated with 10 μg mL−1 soluble collagen or 10 mg mL−1 BSA in modified Tyrode’s buffer at room temperature for 1 h. Platelets were applied to coverslips after treatment with ADP or not as indicated. Spreading was conducted at 37 °C for 1 h. Cells were washed before fixation with 1% paraformaldehyde and permeabilization with 0.1% Triton X-100. Platelets were then incubated for 30 min with Alexa Fluor 546- or Alexa Fluor 488–phalloidin and mounted with FluorSave reagent (Calbiochem, San Diego, CA, USA). Images were obtained on a Nikon TE300 inverted microscope with a 60× or 100× objective (Tokyo, Japan), and analyzed with Image Pro 5.0 software (Media Cybernetics, Inc., Bethesda, MD, USA).
Rap activation assay
Rap-GTP levels were determined essentially as described [18], except that platelets were resuspended to 6–8 × 108 mL−1 in 200 μL and stimulated with thrombin (1 U mL−1). See Supporting Information Doc. S3 for more details.
Results
Rap1b−/− platelets exhibit defective collagen binding to α2β1 upon GPVI but not ADP receptor stimulation
To determine the importance of Rap1b in α2β1 activation, we treated murine Rap1b−/− and wild-type platelets with various agonists that activate Rap1b, and used fluorescent soluble collagen (FITC–collagen) as a probe to measure α2β1 activation. Soluble FITC–collagen was used because the other major collagen receptor on platelets, GPVI, preferentially binds fibrillar collagen and because no antibody exists that specifically recognizes activated murine α2β1. ADP dose-dependently stimulated FITC–collagen binding to both Rap1b−/− and wild-type platelets to comparable levels (Fig. 1A), even at low ADP concentrations (e.g. 0.1 μM). Thrombin also stimulated FITC–collagen binding to similar levels in both Rap1b−/− and wild-type platelets. However, in agreement with a previous study, ADP-stimulated Rap1b−/− platelets displayed impaired fibrinogen binding to αIIbβ3, showing that Rap1b is required for activation of some integrins but not others downstream of ADP ([19]; data not shown).
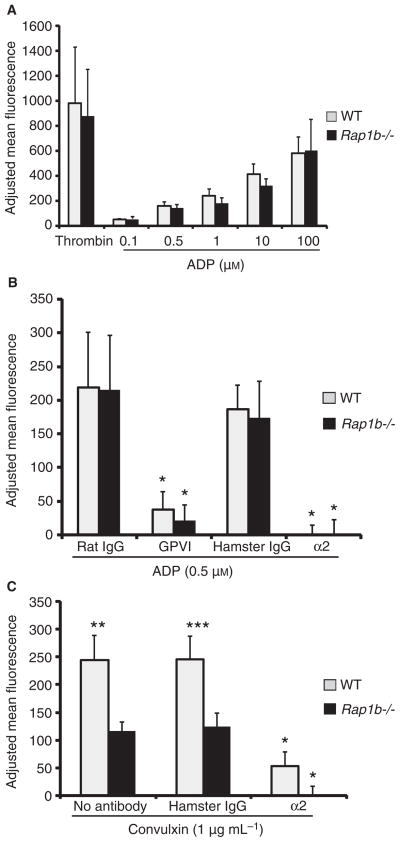
Fig. 1.
Rap1b−/− platelets display normal ADP signaling but abnormal convulxin signaling to α2β1. (A) Wild-type (WT) (gray bars) or Rap1b−/− (black bars) platelets were stimulated by the indicated concentrations of ADP or thrombin (1 U mL−1) followed by the addition of soluble 10 μg mL−1 fluorescein isothiocyanate (FITC)–collagen. Binding was performed for 30 min at room temperature and detected by flow cytometry. (B) Platelets were pretreated with 20 μg mL−1 control or blocking antibodies [clone JAQ1 for glycoprotein (GP)VI or HMα2 for α2β1] for 30 min at room temperature, treated with 0.5 μM ADP for 20 min, and exposed to FITC–collagen as above (*P < 0.002 for IgG vs. GPVI and IgG vs. α2). (C) Platelets were pretreated with no antibody, 20 μg mL−1 control hamster IgG or HMα2 as above before stimulation with 1 μg mL−1 convulxin (*P < 0.005 for α2 vs. IgG; **P < 0.007 and ***P < 0.02). For all graphs, the data represent means of FITC–collagen binding adjusted for no agonist background ± standard error of the mean (SEM), from three to four separate experiments.
As expected, soluble collagen binding is α2β1-dependent, as an α2 integrin blocking antibody (HMα2) completely blocked collagen binding to both wild-type and Rap1b−/− platelets in response to ADP (Fig. 1B). ADP-stimulated α2β1 activation did not require outside-in signaling from αIIbβ3, however, as the αIIbβ3 blocking antibody 1B5 had no effect on soluble collagen binding to ADP-stimulated platelets (data not shown). Interestingly, a GPVI-specific antibody (JAQ1) blocked collagen binding to wild-type and Rap1b−/− ADP-stimulated platelets (Fig. 1B), suggesting that GPVI contributes to soluble collagen binding in a Rap1b-independent manner. JAQ1 did not inhibit FITC–collagen binding to resting platelets, however, indicating that soluble collagen does not detectably bind or activate GPVI in the absence of agonist (data not shown).
As ADP and thrombin receptor signaling did not require Rap1b for α2β1 activation, we next examined whether GPVI requires Rap1b for α2β1 activation. Stimulation of Rap1b−/− platelets with the GPVI agonist convulxin resulted in 52% less FITC–collagen binding relative to wild-type platelets (Fig. 1C). Collagen binding to convulxin-stimulated platelets was primarily α2β1-dependent, as HMα2 blocked 79% of collagen binding to wild-type platelets and 100% of that to Rap1b−/− platelets. Aggregation and dense granule release of Rap1b−/− platelets in response to low concentrations of convulxin were also slower and less robust than those of wild-type platelets (data not shown). Similar to the ADP signaling mentioned above, convulxin-stimulated FITC–collagen binding did not require αIIbβ3 (data not shown). These data indicate that Rap1b is required for optimal signaling downstream of GPVI and that Rap1b may differentially activate α2β1 in response to distinct agonists.
Rap1b is not required for platelet adhesion to monomeric collagen
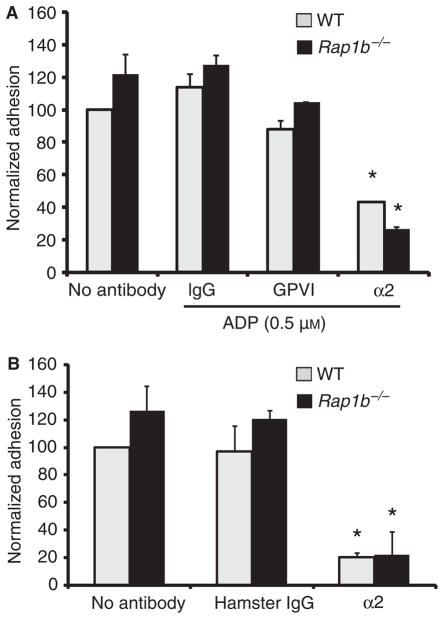
To investigate the role of Rap1b in outside-in α2β1 signaling, we evaluated platelet adhesion to immobilized monomeric collagen. Rap1b−/− platelets exhibited a trend towards increased adhesion vs. wild-type platelets at 20 and 60 min time points, but this was not statistically significant (Fig. 2A,B). ADP did not significantly enhance cell adhesion after 20 min, nor was cell adhesion blocked by the anti-GPVI antibody JAQ1 (Fig. 2A). However, cell adhesion was significantly decreased by HMα2 at 20 min (Fig. 2A) and at 60 min (Fig. 2B), indicating that Rap1b−/− and wild-type platelets adhere-normally to monomeric collagen predominantly through α2β1, independently of Rap1b.
Fig. 2.
Rap1b−/− and wild-type (WT) platelets adhere to immobilized collagen via α2β1 with similar avidity. (A) Wild-type (gray) and Rap1b−/− (black) platelets were pretreated with no antibody, or 20 μg mL−1 hamster IgG, JAQ1 (GPVI) or HMα2 (α2) as in Fig. 1B. All samples except no-antibody controls were stimulated with 0.5 μM ADP and allowed to adhere to microtiter wells coated with 10 μg mL−1 collagen or bovine serum albumin for 20 min at 37 °C before total phosphatase activity was measured using a colorimetric assay (*P < 0.03 for α2 vs. IgG). (B) Platelets were treated with antibodies as in Fig. 1C without agonist and allowed to adhere as above for 60 min at 37 °C for 1 h. Data represent normalized specific adhesion ± standard error of the mean (see Materials and methods) from four separate experiments (*P < 0.0005 for α2 vs. IgG). GP, glycoprotein.
Rap1a and Rap2 are expressed and can be activated in Rap1b−/− platelets
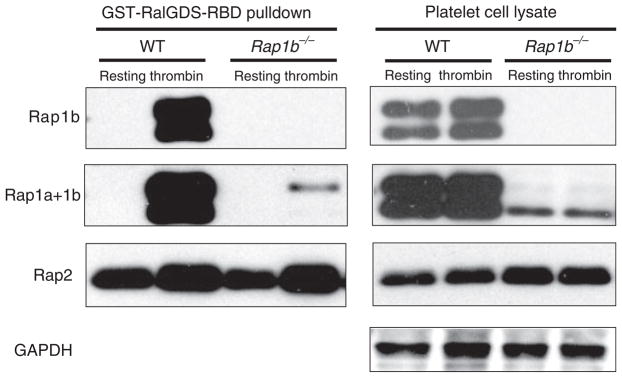
To investigate whether loss of Rap1b might influence the activity or expression of related GTPases in Rap1b−/− platelets, we evaluated Rap1a and Rap2 activation in the presence or absence of thrombin, using a pulldown assay and specific antibodies. As expected, we detected robust Rap1b activation in response to thrombin in wild-type platelets (Fig. 3, top left panels). Using an antibody that detects both Rap1a and Rap1b, we also detected Rap1a activation in thrombin-stimulated but not resting Rap1b−/− platelets (Fig. 3, left ‘Rap1a+1b’ panel). Rap1 proteins ran as doublets, possibly due to protein phosphorylation [25] or oxidation status [26]. Rap2 is also expressed in both wild-type and Rap1b−/− platelets, and can be activated by thrombin (Fig. 3). Rap2 displayed relatively high levels of activation in resting platelets, but even higher levels upon thrombin treatment. These data reveal that Rap1b deficiency does not affect Rap2 activity, and that both Rap1a and Rap2 could potentially compensate for loss of Rap1b in knockout platelets.
Fig. 3.
Thrombin stimulates Rap1a and Rap2 activation in Rap1b−/− platelets. Thrombin (1 U mL−1) or buffer was added to 6–8 × 108 platelets mL−1 in modified Tyrode’s buffer for 3 min. Platelets were lysed, and equal amounts of lysates were used in a pulldown assay (left panels) followed by non-reduced sodium dodecylsulfate polyacrylamide gel electrophoresis and western blotting with Rap1b, Rap1a+1b (which recognizes both Rap1a and Rap1b) or Rap2 antibodies. Note that the Rap1b antibody does not crossreact with Rap1a in lysates from Rap1b−/− mice (Rap1b panels). Total cell lysates were used as loading controls (right panels). Data are representative of three independent experiments. GST, glutathione-S-transferase; RalGDS, Ral guanine nucleotide dissociation stimulator; RBD, Ras binding domain; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; WT, wild-type.
Rap1b−/− platelets exhibit increased spreading on monomeric collagen
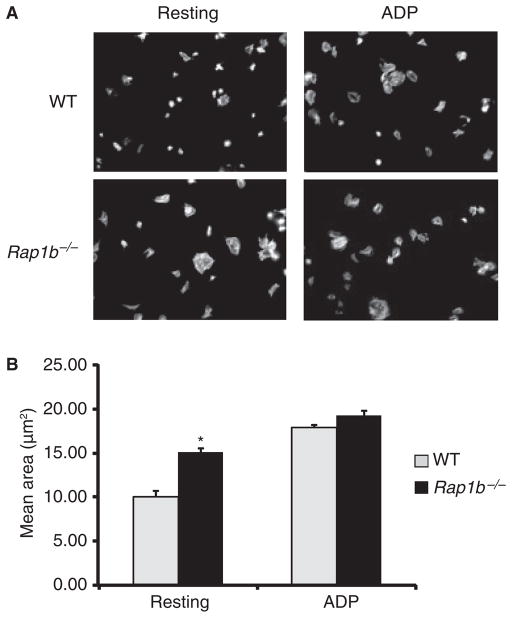
Platelet spreading on immobilized monomeric collagen was assessed by immunofluorescence microscopy (Fig. 4). Surprisingly, unstimulated Rap1b−/− murine platelets showed increased filopodial and lamellipodial extensions on immobilized collagen as compared with wild-type platelets, resulting in increased surface area and a more circular, flattened morphology. Digital image analysis confirmed that the mean polygonal area of the Rap1b−/− platelets was significantly greater than that of wild-type platelets (Fig. 4B). However, upon ADP stimulation, wild-type and Rap1b−/− platelets exhibited a similar spreading response. JAQ1 did not inhibit spreading of wild-type platelets on immobilized soluble collagen (data not shown), demonstrating that GPVI does not play a critical role in cell attachment or spreading on immobilized monomeric collagen.
Fig. 4.
Rap1b−/− platelets show increased spreading on immobilized collagen. (A) Coverslips were coated with 10 μg mL−1 soluble, monomeric collagen. Washed platelets (1 × 107 mL−1) were pretreated with ADP (300 μM) where indicated, and plated for 45–60 min at 37 °C to allow spreading; this was followed by fixation, permeabilizaton and staining of F-actin with Alexa Fluor 488–phalloidin. Data represent two to six independent experiments. (B) The mean area of resting and ADP-stimulated platelets from two experiments [*P < 0.0007 for resting wild-type (WT) vs. Rap1b−/−]. The area was calculated for 130–291 cells per condition using ImagePro software.
Rap1b−/− platelets express more α2β1 and are larger than wild-type platelets
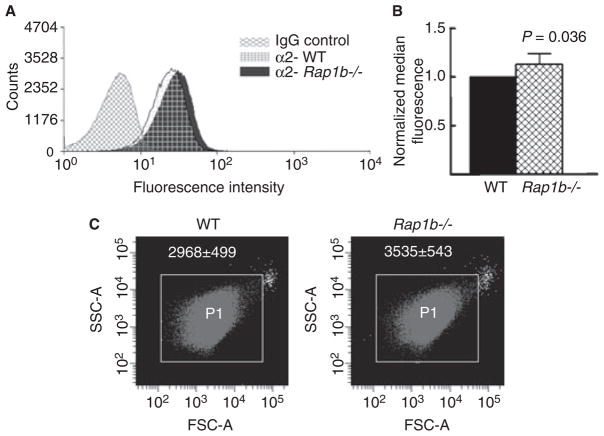
To investigate the mechanism of increased α2β1-dependent spreading in Rap1b−/− platelets, we evaluated α2β1 surface expression. Rap1b−/− platelets showed a slight increase in α2β1 expression as compared with wild-type platelets, as indicated by a rightward shift of the flow cytometry histogram (Fig. 5A,B). No difference in surface expression of GPVI was observed between wild-type and Rap1b−/− platelets (data not shown). We also found that Rap1b−/− platelets display a larger mean forward scatter than wild-type platelets, which may indicate a larger size (Fig. 5C). These data are consistent with a model in which larger platelet size accounts for increased α2β1 expression in Rap1b−/− platelets, resulting in enhanced α2β1-dependent spreading on immobilized monomeric collagen.
Fig. 5.
Rap1b−/− platelets display increased α2β1 expression and size. (A) Wild-type (WT) or Rap1b−/− platelets were treated with fluorescein isothiocyanate- conjugated IgG control or α2 antibody (grid for wild type, solid for Rap1b−/−), incubated for 30 min on ice in the dark, and analyzed via flow cytometry. IgG control readings for wild-type and Rap1b−/− platelets were the same, and are represented as one histogram (IgG control). The histograms shown are representative of three separate experiments. (B) Quantification of data shown in (A) ± standard deviation. (C) The size of wild-type and Rap1b−/− platelets was measured by flow cytometry. Shown is a forward scatter (FSC-A) (x-axis) vs. side scatter (SSC-A) (y-axis) dot plot from one representative experiment gated on P1. Rap1b−/− platelets have an increased mean forward scatter (white numbers, P < 0.05), but not side scatter, as compared with wild-type platelets.
Discussion
The small GTPase Rap1b regulates multiple integrins and is strongly activated by agonists that also activate α2β1 in murine platelets. Therefore, we tested the hypothesis that Rap1b regulates platelet α2β1 activation using the recently described Rap1b−/− mice [19]. These mice have a severe bleeding phenotype resulting from defective platelets that do not aggregate normally in response to fibrillar collagen or other agonists such as thrombin. Because of this phenotype, we originally predicted that agonist-induced inside-out signals leading to α2β1 activation would also be attenuated in Rap1b−/− platelets. Indeed, we found that convulxin-induced but not ADP-induced α2β1 activation is impaired in Rap1b−/− platelets. Soluble collagen binding is reduced by more than 50% in Rap1b-deficient platelets in response to convulxin, which mimics the defective convulxin-dependent αIIbβ3 activation originally observed in Rap1b−/− platelets [19]. However, collagen binding to α2β1 is not completely inhibited in convulxin-stimulated Rap1b−/− platelets, indicating the existence of Rap1b-independent pathways from GPVI to α2β1. Convulxin-induced collagen binding was completely blocked by an α2 antibody, revealing the essential role of α2β1 in this process. Collectively, these data demonstrate that Rap1b is critical for GPVI-dependent α2β1 activation, which highlights the sequential nature of α2β1 activation downstream of GPVI and supports the reciprocal signaling model previously proposed for these two collagen receptors [7,8].
Like convulxin, ADP requires GPVI for α2β1 activation. Soluble collagen binding in response to a low concentration of ADP is significantly blocked by the GPVI antibody JAQ1. JAQ1 may interfere with α2β1 activation by preventing subtle interactions between soluble collagen and GPVI in the presence of ADP, for the following reasons. First, the soluble collagen used here does not activate platelets in the absence of agonist, and therefore does not generate strong GPVI signals. Second, soluble collagen may trigger weak Rap1b-independent signals through GPVI that cooperate with ADP-dependent signals, leading to α2β1 activation. Third, inhibition of α2β1 completely abrogates ADP-induced soluble collagen binding, whereas blockade of GPVI does not. Taken together, these data suggest that weak GPVI signaling and ADP receptor stimulation collaboratively regulate α2β1 via an unknown mechanism that does not involve Rap1b. Another interpretation that is formally possible concerns ADP regulation of GPVI. Although GPVI has not been described as a receptor that undergoes agonist-dependent ‘activation’, these data may be the first to show that ADP enhances the ability of GPVI to bind certain ligands such as soluble collagen.
In contrast to soluble collagen binding, neither GPVI nor Rap1b signaling is required for platelet adhesion to immobilized monomeric collagen. Rap1b-deficient platelets adhered to immobilized monomeric collagen to the same extent as wild-type platelets in an α2β1-dependent but GPVI-independent manner, suggesting that Rap1b may not be necessary for adhesion in murine platelets or for solution-phase collagen binding initiated by certain G-protein-coupled receptors.
Previous studies also support our finding that Rap1 is not universally required for integrin activation. For example, α4β1 on human T cells does not require Rap1 activity for ligation to its counter-receptor VCAM-1 [27]. Likewise, sickle red blood cells do not require Rap1 for adhesion to VCAM-1 via α4β1 [28], and adhesion to VCAM-1 is not completely abolished in Rap1b-knockout B cells [14]. These data, combined with our own showing that lack of Rap1b only partially blocks GPVI signaling, strongly suggest the existence of alternative pathways to integrin activation that do not require Rap1.
What are these alternative pathways? We reasoned that ADP-stimulated or thrombin-stimulated collagen binding to Rap1b−/− platelets may be catalyzed by other Rap family proteins such as Rap1a and/or Rap2, which are 95% and 65% identical to Rap1b respectively [29]. In a Rap1 pulldown assay, we detected Rap1a activation upon thrombin stimulation in Rap1b−/− platelets. We also observed a considerable amount of Rap2 activation in both Rap1b−/− and wild-type platelets, indicating that Rap1a and/or Rap2 signaling to α2β1 may occur in the absence of Rap1b. Previous studies have shown that Rap2b is activated by some of the same agonists that activate Rap1b [30] and that Rap1b-independent signaling pathways are operative in response to ADP [19]. For example, Rap1b-deficient platelets show only a partial aggregation response to ADP, but Rap1b-deficient platelets treated with ADP receptor antagonists show a complete loss of aggregation [19], suggesting that Rap1b cannot account for the entire signaling output of ADP receptors and that other GTPases, such as like Rap1a and/or Rap2, may be involved.
Unexpectedly, we found that resting Rap1b−/− platelets demonstrated enhanced spreading on collagen relative to wild-type platelets, which may be due to altered cytoskeletal dynamics and organization [31]. To explore whether this increased spreading may be due to enhanced ADP release, we looked for increased dense granule release in knockout platelets. Dense granule secretion was decreased in Rap1b−/− platelets as compared with wild-type platelets in response to convulxin. Although dense granule release does not appear to contribute to the enhanced spreading of Rap1b−/− platelets, these data imply that Rap1b may negatively regulate some aspects of α2β1-dependent signaling, such as cell spreading.
We also found that Rap1b−/− platelets have increased cell surface expression of α2β1 and are larger than wild-type platelets. The larger size of Rap1b-deficient platelets may account for their increased α2β1 expression, and other studies have shown that collagen receptor density plays an important role in a cell’s ability to bind collagen [32,33]. Increased α2β1 expression did not correlate with a younger, more reticulated population, as Rap1b−/− platelets contain slightly less RNA than wild-type platelets (data not shown). Therefore, the larger size and elevated α2β1 surface expression of Rap1b−/− platelets may represent a compensatory developmental mechanism that explains the observed adhesion and spreading phenotypes.
In conclusion, we provide evidence for both Rap1b-dependent and Rap1b-independent pathways leading to α2β1 activation. Rap1b is required for efficient α2β1 activation downstream of GPVI, but is not required for ADP-induced or thrombin-induced α2β1 activation or platelet adhesion to immobilized monomeric collagen. Even though these data are the first to reveal Rap1b involvement in GPVI-mediated α2β1 activation, GPVI probably initiates additional Rap1b-independent pathways as well, which impinge upon α2β1. Other small GTPases such as Rap1a and/or Rap2 may function redundantly with Rap1b, especially during ADP signaling or cell adhesion in murine platelets. We have raised important questions about signaling specificity to integrins, and although our study addresses some of these issues, more research is needed to precisely define how signaling pathways are productively coupled to Rap1b activation and what molecular events subsequent to Rap1b activation are critical for α2β1 function.
Supplementary Material
Acknowledgments
We thank J. Dargatz, H. Cheeseman, H. Berlin, E. Hilliard and S. Smyth for excellent technical help with the Rap1b−/− mice. We also thank T. Leisner and J. Brittain for critical reading of the manuscript. We acknowledge support from NIH1T32DE017245-01 (Z. Wang), a Howard Hughes Medical Institute Predoctoral Fellowship (M. K. Larson), NIH 2-P01- HL06350 (L. V. Parise) and 2-P01-HL45100 (L. V. Parise and G. C. W.), and AHA Scientist Development Grant 0235127N (M. Chrzanowska-Wodnicka).
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no confiict of interest.
Additional Supporting Information may be found in the online version of this article:
Doc. S1. Reagents.
Doc. S2. Platelet preparation.
Doc. S3. Rap activation assay.
Doc. S4. Statistics.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Moroi M, Onitsuka I, Imaizumi T, Jung SM. Involvement of activated integrin alpha2beta1 in the firm adhesion of platelets onto a surface of immobilized collagen under flow conditions. Thromb Haemost. 2000;83:769–76. [PubMed] [Google Scholar]
- 2.Gralnick HR, Kramer WS, McKeown LP, Garfinkel L, Pinot A, Williams SB, Krutzsch H. Platelet adhesion at high shear rates: the roles of von Willebrand factor/GPIb and the beta 1 integrin alpha 2 beta 1. Thromb Res. 1996;81:113–19. doi: 10.1016/0049-3848(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 3.Li TT, Larrucea S, Souza S, Leal SM, Lopez JA, Rubin EM, Nieswandt B, Bray PF. Genetic variation responsible for mouse strain differences in integrin alpha 2 expression is associated with altered platelet responses to collagen. Blood. 2004;103:3396–402. doi: 10.1182/blood-2003-10-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung SM, Moroi M. Platelets interact with soluble and insoluble collagens through characteristically different reactions. J Biol Chem. 1998;273:14827–37. doi: 10.1074/jbc.273.24.14827. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Leisner TM, Parise LV. Platelet alpha2beta1 integrin activation: contribution of ligand internalization and the alpha2-cytoplasmic domain. Blood. 2003;102:1307–15. doi: 10.1182/blood-2002-09-2753. [DOI] [PubMed] [Google Scholar]
- 6.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160:769–80. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Kahn ML. Reciprocal signaling by integrin and nonintegrin receptors during collagen activation of platelets. Mol Cell Biol. 2003;23:4764–77. doi: 10.1128/MCB.23.14.4764-4777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieswandt B, Brakebusch C, Bergmeier W, Schulte V, Bouvard D, Mokhtari-Nejad R, Lindhout T, Heemskerk JW, Zirngibl H, Fässler R. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–30. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–44. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, Eckes B. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–94. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 11.Furihata K, Nugent DJ, Kunicki TJ. Influence of platelet collagen receptor polymorphisms on risk for arterial thrombosis. Arch Pathol Lab Med. 2002;126:305–9. doi: 10.5858/2002-126-0305-IOPCRP. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwenhuis HK, Akkerman JW, Houdijk WP, Sixma JJ. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985;318:470–2. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- 13.Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad R, Rackebrandt K, Cazenave JP, Ohlmann P, Gachet C, Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193:459–69. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Yu M, Podd A, Wen R, Chrzanowska-Wodnicka M, White GC, Wang D. A critical role of Rap1b in B-cell trafficking and marginal zone B-cell development. Blood. 2008;111:4627–36. doi: 10.1182/blood-2007-12-128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke B, Akkerman JW, Bos JL. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 1997;16:252–9. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke B, van Triest M, de Bruijn KM, van Willigen G, Nieuwenhuis HK, Negrier C, Akkerman JW, Bos JL. Sequential regulation of the small GTPase Rap1 in human platelets. Mol Cell Biol. 2000;20:779–85. doi: 10.1128/mcb.20.3.779-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by G(i) family members in platelets. J Biol Chem. 2002;277:23382–90. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 18.Larson MK, Chen H, Kahn ML, Taylor AM, Fabre JE, Mortensen RM, Conley PB, Parise LV. Identification of P2Y12-dependent and - independent mechanisms of glycoprotein VI-mediated Rap1 activation in platelets. Blood. 2003;101:1409–15. doi: 10.1182/blood-2002-05-1533. [DOI] [PubMed] [Google Scholar]
- 19.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC. Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–7. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–6. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 21.Bergmeier W, Goerge T, Wang HW, Crittenden JR, Baldwin AC, Cifuni SM, Housman DE, Graybiel AM, Wagner DD. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, Crittenden JR, Amariglio N, Safran M, Graybiel AM, Rechavi G, Ben Dor S, Etzioni A, Ronen A. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDA-GGEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–82. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jantzen HM, Milstone DS, Gousset L, Conley PB, Mortensen RM. Impaired activation of murine platelets lacking G alpha(i2) J Clin Invest. 2001;108:477–83. doi: 10.1172/JCI12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellavite P, Andrioli G, Guzzo P, Arigliano P, Chirumbolo S, Manzato F, Santonastaso C. A colorimetric method for the measurement of platelet adhesion in microtiter plates. Anal Biochem. 1994;216:444–50. doi: 10.1006/abio.1994.1066. [DOI] [PubMed] [Google Scholar]
- 25.Lapetina EG, Lacal JC, Reep BR, Vedia LM. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci USA. 1989;86:3131–4. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Silva NJ, Jacobson KL, Ott SM, Watson EL. Beta-adrenergic-induced cytosolic redistribution of Rap1 in rat parotid acini: role in secretion. Am J Physiol Cell Physiol. 1998;274:C1667–73. doi: 10.1152/ajpcell.1998.274.6.C1667. [DOI] [PubMed] [Google Scholar]
- 27.Ghandour H, Cullere X, Alvarez A, Luscinskas FW, Mayadas TN. Essential role for Rap1 GTPase and its guanine exchange factor Cal-DAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood. 2007;110:3682–90. doi: 10.1182/blood-2007-03-077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MM, Zayed MA, Evans A, Parker CE, Ataga KI, Telen MJ, Parise LV. Role of Rap1 in promoting sickle red blood cell adhesion to laminin via BCAM/LU. Blood. 2005;105:3322–9. doi: 10.1182/blood-2004-07-2881. [DOI] [PubMed] [Google Scholar]
- 29.Klinz FJ, Seifert R, Schwaner I, Gausepohl H, Frank R, Schultz G. Generation of specific antibodies against the rap1A, rap1B and rap2 small GTP-binding proteins. Analysis of rap and ras proteins in membranes from mammalian cells. Eur J Biochem. 1992;207:207–13. doi: 10.1111/j.1432-1033.1992.tb17039.x. [DOI] [PubMed] [Google Scholar]
- 30.Greco F, Sinigaglia F, Balduini C, Torti M. Activation of the small GTPase Rap2B in agonist-stimulated human platelets. J Thromb Haemost. 2004;2:2223–30. doi: 10.1111/j.1538-7836.2004.01018.x. [DOI] [PubMed] [Google Scholar]
- 31.Bertoni A, Tadokoro S, Eto K, Pampori N, Parise LV, White GC, Shattil SJ. Relationships between Rap1b, affinity modulation of integrin alpha IIbbeta 3, and the actin cytoskeleton. J Biol Chem. 2002;277:25715–21. doi: 10.1074/jbc.M202791200. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Locke D, Liu Y, Liu C, Kahn ML. The platelet receptor GPVI mediates both adhesion and signaling responses to collagen in a receptor density-dependent fashion. J Biol Chem. 2002;277:3011–19. doi: 10.1074/jbc.M109714200. [DOI] [PubMed] [Google Scholar]
- 33.Furihata K, Clemetson KJ, Deguchi H, Kunicki TJ. Variation in human platelet glycoprotein VI content modulates glycoprotein VI-specific prothrombinase activity. Arterioscler Thromb Vasc Biol. 2001;21:1857–63. doi: 10.1161/hq1001.096643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.