Abstract
We examined intergenerational and epigenetic effects of early handling manipulations on the social behavior of the prairie vole (Microtus ochrogaster), a monogamous rodent. Laboratory-born parents and their newborn pups were assigned to either a MAN0 “zero handling” manipulation (transfer with a cup during weekly cage changes) or a MAN1 “gloved handling” manipulation (transfer with a gloved hand). Previous studies from our laboratory (Bales et al. 2007) showed that MAN0 juvenile males that received this manipulation as pups are less alloparental and that MAN0 adult females that received this manipulation as pups display impaired pair-bonding. In the present study, when MAN0 and MAN1 pups reached adulthood, they were mated in three combinations (MAN1 female × MAN1 male; MAN0 female × MAN1 male; MAN1 female and MAN0 male). Once the pairs produced offspring, we examined their parental behavior towards their own pups. The offspring of these pairings (F2 generation) also were tested as juveniles for alloparental behavior. MAN1 females paired with a MAN0 male displayed higher levels of parenting behaviors. In the F2 generation, juvenile offspring with a MAN0 parent were less alloparental than were offspring from other pairs. These results suggest that early experiences can be transmitted intergenerationally.
Keywords: development, monogamy, parental behavior, prairie vole
1. INTRODUCTION
Events occurring early in life can have significant effects on the expression of behaviors during adulthood (Denenberg and Bell, 1960; Denenberg 1962; Meaney 2001; Maestripieri et al 2005). Early experiences such as brief maternal separation can lead to individual differences in later stress reactivity and parental behaviors (e.g. Levine et al. 1967; Levine 2001, 2002; Meaney et al.1985; Meaney et al. 1989). Furthermore, natural differences in maternal behaviors (i.e. levels of licking and grooming and arched-back nursing) can affect offspring’s later behavioral and physiological responses to stress (reviewed in Meaney 2001). Rat mothers that display high levels of licking and grooming toward their pups transmit these behaviors to female offspring through experience, in a non-genomic manner. That is, female offspring of highly parental mothers are more parental toward their own offspring (Francis et al. 1999). Abusive macaque mothers also pass on this behavior to their female offspring (Maestripieri et al. 1997). In rats, gestational variation in stress levels also can also alter maternal behavior, resulting in offspring that as adults show the same tendencies in maternal behaviors as their mothers (Champagne and Meaney 2006).
Until recently, the effects of early experience had been examined primarily in species where only females care for offspring. However, in monogamous species, paternal care also may affect offspring development and later behaviors of offspring, such as the ability to form pair-bonds and to display parental care. For example, in California mice, the level of paternal pup retrieval affects subsequent aggressive behavior in male offspring (Frazier et al. 2006). Paternal care also can affect cognitive development of male offspring California mice (Bredy et al 2004). In mandarin voles (Microtus mandarinus), either short periods of social isolation during development, or rearing by the mother only instead of both parents, resulted in higher levels of anxiety and lower levels of social behavior later in life (Jia et al., 2009).
Prairie voles (Microtus ochrogaster) are socially monogamous, cooperatively breeding rodents where males and females form heterosexual pair-bonds and both parents and older offspring care for young (Getz et al.1981; Roberts et al. 1998; Solomon 1991). In laboratory experiments, pup-naïve males and females show high levels of alloparental care when exposed to an unrelated pup (approximately 80% of males and approximately 60% of females; Roberts et al. 1998). Handling studies of prairie voles by Bales et al (2007) demonstrated that early postnatal manipulation treatments affect later social behavior in the offspring. In particular, female pups that received minimal neonatal manipulation during the first days of life (transfer with a cup during weekly cage changes, or “MAN0”) were less likely to form pair-bonds as adults compared to animals that received a “gloved handling” manipulation (transfer with a gloved hand, or “MAN1”). As juveniles, MAN0 males showed lower levels of alloparental behavior than MAN1 males. It is possible that these effects are mediated by the behavior of the parent following the handling manipulation. Using the same paradigm, Tyler et al. (2005) found that, following the manipulation, MAN1 parents showed a heightened frequency of pup-directed behaviors (sniffing and retrievals), compared to MAN0 parents. This might be because a MAN1 is a more stressful manipulation, causing parents to display increased levels of stress-reducing behaviors afterward and providing extra stimulation for the offspring.
In the present study, we extend the paradigm of Bales et al (2007) to examine intergenerational effects of handling manipulations on the parenting behavior of prairie voles. We predicted that pups from different handling conditions later would vary in levels of parental care towards their own offspring. We also predicted that intergenerational transmission of such early experience effects would occur, with second generation offspring (F2) varying in their levels of alloparental care. First, we produced the MAN0 and MAN1 groups described in Bales et al. (2007); these represent the F1 generation depicted in Figure 1. We then mated the adult voles resulting from MAN0 and MAN1 manipulations in different combinations. Based on alterations in other types of social behaviors, we predicted that, compared to MAN1 animals, MAN0 animals would show lower levels of sexual behaviors (and thus possibly reduced reproduction), which has not been examined previously. We also predicted that, as parents, MAN0 animals would display lower rates of parental behaviors towards their pups. Because parental care of primiparous mothers often differs from that of multiparous females (Fleming et al 1996; Fairbanks 1996), both first and second litters were examined, particularly since deficits in parental care may be improved by experience. Finally, we predicted that juvenile F2 offspring with one MAN0 parent would display the deficits in alloparenting. Based on previous findings (Bales et al. 2007), we predicted that males in particular would be sensitive to manipulations associated with reduced levels of alloparental behavior.
Figure 1.
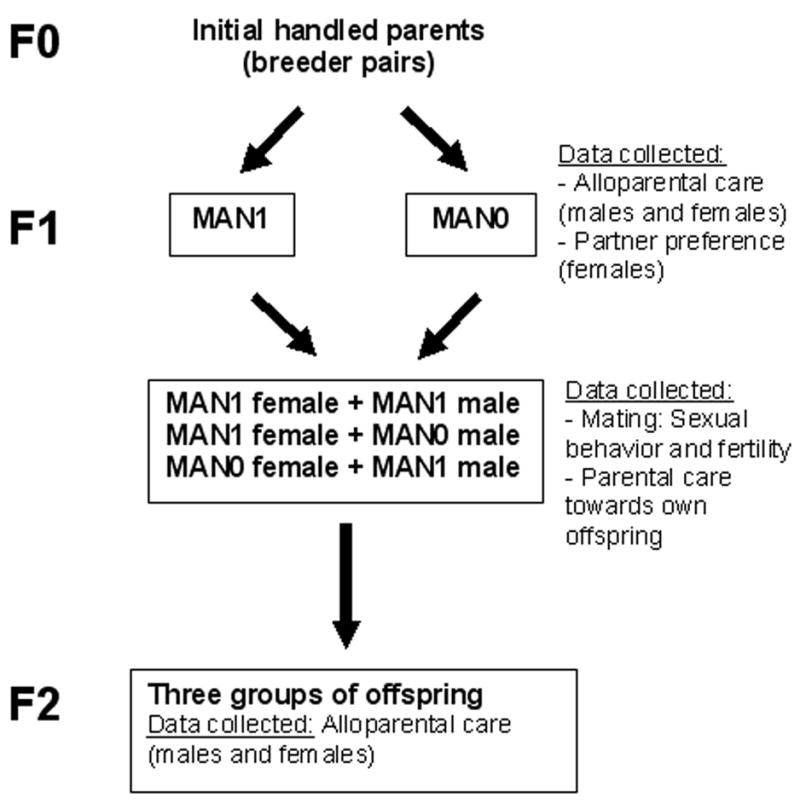
Study design, detailing handling manipulation treatments and behavioral tests administered to animals in the first generation and their offspring.
2. METHODS
2.1. Subjects
Figure 1 summarizes the paradigm used in the study. Thirty pairs of laboratory-bred male and female prairie voles were used as breeders. Voles were descendants of a wild stock originally caught near Champaign, Illinois, and it had been approximately three-four years since the introduction of wild-born voles to the colony. Stock was systematically outbred. Animals were maintained on a 14 h light: 10 h dark cycle and allowed food (high-fiber Purina rabbit chow) and water ad libitum. Breeding pairs were maintained in large polycarbonate cages (44 × 22 × 16 cm) and provided with cotton for nesting material.
2.2. Original Manipulation
Two early manipulation treatments were used. All breeder pairs had previously raised at least one litter. Each breeder pair received each treatment one time, in random order. No order effects were found. This study design has allowed us to rule out many genetic effects (Bales et al., 2007), since litters from the same breeder pair differed significantly in behavior based on treatment group. In the “zero handling” treatment (MAN0), litters received a cage change within the first two days of life, which involved moving parents between cages via a plastic cup. Animals were kept in the plastic cup for 10 seconds. Pups were attached to the mother by milk-teeth and therefore not directly manipulated. In the “one handling” treatment (MAN1), animals were lifted by the scruff of the neck with a gloved hand for 10 seconds and placed in a new cage. In both treatments, the cotton nesting material was not transferred between cages; rather, new nesting material was provided in the clean cage. Only litter sizes containing a minimum of four pups and a maximum of six pups were used, and there were always at least two pups of each sex. Litters received weekly cage changes and manipulations during the next two weeks, and were separated from parents at 20 days. Pups and parents thus received a total of three manipulations. When pups were too large to be attached to the mother’s nipples (during the third cage change), they were transferred in the same manner as their parents.
2.3. Behavioral Testing
After weaning, juveniles were housed in same-sex sibling pairs, in polycarbonate cages (27 × 16 × 13 cm). One male and one female from each litter were used for behavioral testing of alloparental care, yielding group sizes of 12–20 for behavioral measures. Behavioral testing of the F1 generation (alloparental care testing and partner preference testing) was performed in order to document that the early manipulation had the same effects seen by Bales et al (2007), with similar results. Details of both procedures are available in Bales et al. (2007), and details of the alloparental care test are also included below. Results of those tests are not presented here for the sake of brevity but confirmed those results found in the original study. All behavioral tests and observations were carried out with lights on except for the overnight sex tests (below).
2.3.1. Sex Tests
Immediately after the partner preference test, all pairs were tested for sexual behavior. Three experimental types of matings were produced: MAN1 female × MAN1 male; MAN1 female × MAN0 male; and MAN0 female × MAN1 male. We considered MAN1 × MAN1 the control group, and therefore were changing one member of the pair at a time against the background of the control group. In addition, we aimed to minimize the number of animals utilized in the study, according to one of the priorities of the U.S. Government Principles for the Utilization and Care of Vertebrate Animals used in Research, Testing and Training (Guide for the Use and Care of Laboratory Animals, National Research Council, National Academy Press, Washington. D.C., 1996).
Animals were placed in a cage (27 × 16 × 13 cm) and remained together for 48 hours. Behavior of the pairs was recorded in time-lapsed video. After lights out, recording was carried out under red light. At the end of 48 hours, the male and female were placed in a normal breeder cage (44 × 22 × 16 cm). Behaviors scored in males included number of mounts, number of intromissions and ejaculations (Bales et al. 2004). These behaviors were analyzed with a Kruskal-Wallis test due to non-normality of the data, with type of pair as the treatment variable.
We recorded reproductive variables including latency to produce first and second litters and litter size surviving to weaning. A total of 42 pairs were produced. Although we aimed for 30 pairs for the purposes of statistical analyses (10 pairs × 3 intergenerational treatments), additional pairs were generated because several pairs did not produce offspring or suffered mortality for reasons unrelated to this experiment (see Results).
2.4. Intergenerational Pairings and Behavioral Testing
The male and female pairs that participated in the sex test then entered the second part of the study in which intergenerational effects of the early manipulation were examined (Fig 1). Once pairs produced their first litter, parental behaviors of the male and the female toward their own pups were examined. During twenty-minute focal observation sessions on each parent, we continuously recorded the duration of huddling behavior, pseudohuddling, non-huddling contact, retrieving and licking/grooming, and nursing postures such as lateral nursing and nursing while locomoting (definitions in Table 1). We also recorded how much time the animal spent inside or outside the nest. Observations were conducted on two days during the pups’ first week of life (ranging from post-natal days 1–3), on two days during the second week (days 8–10) and on two days during their third week (days 15–17). Observations were recorded during morning hours (0800 and 1200 hours) and afternoons (0100 and 0500 hours), on each day observations were conducted. Individual parental behaviors were analyzed with a repeated-measures ANOVA, with early handling treatment and parity as independent variables, as well as a handling treatment by parity interaction.
Table 1.
Ethogram of behaviors for scoring parent-young interactions and for alloparental care tests.
| Postures | |
| Huddling | All four paws on ground. Legs holding self up over pups. Arched back, head tucked. |
| Pseudohuddling | A more relaxed version of huddling. Standing over pups in a crouched position. For parental behaviors toward own pups, can be lying on pups. |
| Lateral Nursing | Lying on side with pups. Also coded when father is lying with female and pups. |
| Nursing while locomoting | Female has pup(s) attached behind her. Can be walking or be stationary. |
| Non-huddling contact | Vole in contact with pups and quiescent. |
| Other Behaviors | |
| In nest | Inside nest area and/or is in contact with pups and other parent. |
| Out of nest | Does not meet above criteria |
| Licking and sniffing | Licking and sniffing pups, including anogenital licking. |
| Retrieving | Lifting pup in mouth and carrying at least one inch. |
| Non-pup directed | Self-grooming, grooming partner, locomoting, drinking, eating. |
| Autogroom | Grooming self |
Following standard husbandry procedures, during weekly cage changes the intergenerational pairs were handled in a similar way to the MAN1 manipulation. We followed this cage-changing procedure in order to minimize the chance that any deficiencies in social behavior seen in the F2 generation were due to the way the F1 generation was handled, thus ensuring that any effects were due to the handling treatment of the initial breeder pairs.
Offspring of these pairs were weaned at 20 days of age and one male and one female of each litter given an alloparental care test, as described below. Identical handling, weaning and testing procedures were followed for the second litter.
2.4.1. Alloparental Care Testing
When animals were between 20–25 days of age (average: 22 days), we tested their response toward an unrelated vole pup (Bales et al 2007; Bales et al. 2004; Roberts et al. 1999). Animals were always weaned before the birth of the next litter to their parents, thus ensuring no previous exposure to pups. Test animals were introduced into an apparatus that consisted of two cages (27 × 16 × 13 cm) connected by a 5 cm clear plastic tube, and given 45 minutes to acclimate. One pup (1–4 days old) was then introduced into the front cage. The test animal was exposed to the pup for 10 minutes (methods based on Bales et al., 2004). If the test animal showed pup-directed aggression (biting), the test was stopped immediately and the pup removed and treated as necessary. The tests also were video-recorded and later scored from the videotapes by an experimentally-blind observer, using the software Behavior Tracker 1.5 (www.behaviortracker.com). Behaviors scored included: huddling, pseudohuddling, non-huddling contact (contact with pup not covered by another category), retrieval frequency and licking/grooming (see Table 1 for full behavioral definitions). Animals performing huddling, pseudohuddling or retrievals were classified as “parental”. Proportional data (i.e. proportion of animals that were “alloparental”) were examined via a chi-squared test, while tests of individual, continuous behaviors were analyzed via Kruskal-Wallis due to non-normality. All alloparental care behaviors were analyzed via one-tailed tests due to a strong a priori prediction that offspring of MAN0 parents would show deficits in alloparental behavior (Bales et al 2007). Statistical significance was set at p < 0.05.
Spearman’s correlations between parental behavior of the F1 generation, and alloparental behavior of the F2 generation, were calculated for four main behaviors (huddling, non-huddling contact, licking, and retrievals). The p-values were then adjusted for multiple comparison by the Benjamini-Hochberg false discovery rate method (Benjamini and Hochberg 1995).
3. RESULTS
3.1. First Generation (Mating)
3.1.1. Sexual Behavior
Mounts, intromissions, ejaculations, and ejaculations per intromissions were scored. Males in MAN1 × MAN1 pairs (the first term always indicates the treatment of the female of the pair, the second the treatment of the male) showed an average of 3.7 mounts and 7.6 intromissions during sex tests, while males in other groups showed higher levels of mounts and intromissions (Table 2). However, differences between treatment pairs were not significant (Kruskal-Wallis test: mounts: H=0.05, df=2, p= 0.90; intromissions: H=0.37, p=0.80). No variables were significantly different at p<0.05 when analyzed by Kruskal-Wallis tests.
Table 2.
Male mating behaviors recorded during 48-hr sex tests. Means ± SE. No variables were significantly different at p<0.05 when analyzed by Kruskal-Wallis test
| Variable | MAN1 × MAN1 (n=14) | MAN1 × MAN0 (n=13) | MAN0 × MAN1 (n=16) |
|---|---|---|---|
| Mounts | 3.7 ± 1.7 | 7.3 ± 3.2 | 8.6 ± 1.7 |
| Intromissions | 7.6 ± 4.6 | 25.7 ± 13.1 | 29.9 ± 18.1 |
| Ejaculations | 0.14 ± 0.1 | 0.37 ± 0.2 | 0.54 ± 0.2 |
| Ejaculations/intromissions | 0.01 ± 0.01 | 0.03 ± 0.02 | 0.03 ± 0.01 |
3.1.2. Reproductive Success
Several pairs did not produce offspring, even after 60 days of pairing. 31% of MAN1 × MAN0 pairs did not breed (4 out of 13 pairs) and 20% of MAN0 × MAN1 pairs did not breed (3 out of 15 pairs), compared to only 7% of MAN1 × MAN1 pairs. This comparison between proportion of pairs not producing offspring was not significant (χ2= 2.45, df= 2, p=0.29). No differences were found in latency to produce first litter when all pairs, including those that did not produce litters were included (H=0.89, df=2, p= 0.64; Table 3). When only pairs that did reproduce were included, there were also no treatment effects on latency to produce first litter (H = 0.30, df=2, p = 0.86). Surviving litter sizes and days to second litter were not statistically significant.
Table 3.
Reproductive parameters for intergenerational pairs. Means SE. No variables were significantly different at p<0.05 when analyzed by Kruskal-Wallis test
| Variable | MAN1 × MAN1 (n=14) a | MAN1 × MAN0 (n=13) b | MAN0 × MAN1 (n=15) c |
|---|---|---|---|
| Days to first litter | 29.9 ± 2.5 | 38.9 ± 4.8 | 33.0 ± 3.7 |
| Surviving litter size at weaning (1st litter) | 4.4 ± 0.3 | 3.7 ± 0.5 | 4.0 ± 0.5 |
| Days to second litter | 24.1 ± 1.3 | 27.9 ± 4.4 | 22.4 ± 0.2 |
| Surviving litter size at weaning (2nd litter) | 4.2 ± 0.5 | 5.0 ± 0.4 | 4.5 ± 0.4 |
= this group had 1 pair that never gave birth. Data for days to 1st litter for that pair are included but estimated at 60 days.
= this group had 4 pairs that never gave birth. Data for days to 1st litter for that pair are included but estimated at 60 days.
= this group had 3 pairs that never gave birth. Data for days to 1st litter for that pair are included but estimated at 60 days.
3.1.3. Patterns of Parental Behavior
3.1.3.1. Females
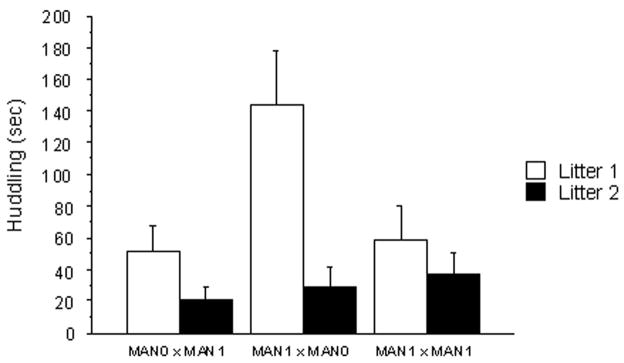
In females, there was an interaction between early treatment and parity for huddling (F 2, 188=3.17, p=0.04), with females in MAN1 × MAN0 pairs huddling more in their first litters than other groups but all groups reducing huddling with their second litters. The main effect of early treatment on later huddling behavior was also significant (repeated measures ANOVA; F 2, 118= 2.87, p=0.05; Fig 2a), primarily because of the females in MAN1 × MAN0 pairs showing higher levels of huddling than MAN0× MAN1 females (Fisher’s PLSD, p= 0.01) and MAN1 × MAN1 females (p= 0.04). The main effect of parity on female huddling was also significant, with mothers huddling more in their first litters compared to their second litters (F 1, 118= 11.42, p=0.008; Fig 2a).
Figure 2.
Duration of parental behaviors in seconds (measured in a 20 min observation period) for males and females, according to handling treatment and parity. A) Female huddling: MAN1 × MAN0 females huddled more over pups compared to other treatments; across treatments, females huddled more in their first litters compared to second litters. B) Female retrievals: MAN1 × MAN0 females retrieved pups more often than females in other treatments; across treatments, females retrieved more in their second litters compared to second litters.
Female retrievals also displayed an interaction between early treatment and parity (F 2, 188= 6.15, p=0.002), with females in MAN1 × MAN0 pairs retrieving more in their second litters than other groups. There was a significant main effect of early treatment on female retrievals (F2, 118= 3.55; Fig 2b), primarily because of the MAN1 × MAN0 females retrieving their pups more than MAN0× MAN1 females (p= 0.01) and MAN1 × MAN1 females (p= 0.03). The main effect of parity on female retrievals of their pups was also significant, with mothers retrieving pups more in their second litters (F1, 118= 14.12, p=0.0002; Fig 2b).
Finally, there was a main effect of early treatment on lateral nursing (F 2, 118= 3.38, p=0.03) and nursing while locomoting (F 2, 118= 5.82, p=0.003). MAN1 × MAN0 females engaged in less lateral nursing compared to MAN1 × MAN1 females (p= 0.009) and in more nursing while locomoting compared to MAN1 × MAN1 females (p= 0.002).
3.1.3.2. Males
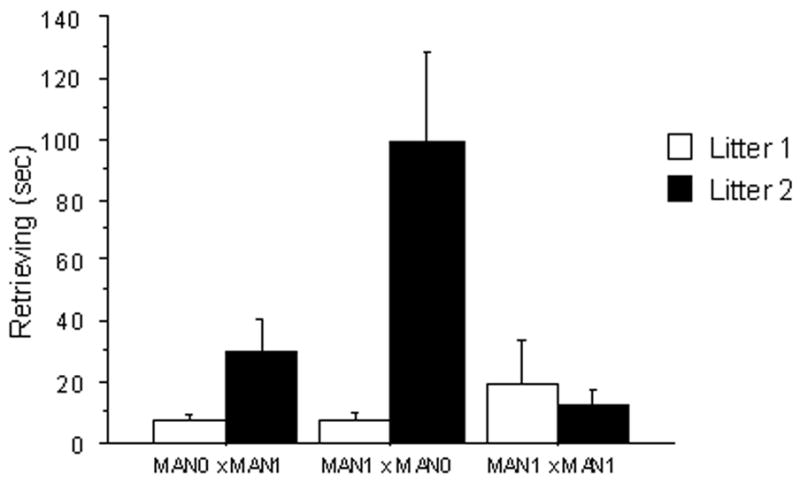
In males, there was also an interaction between treatment and experience for retrievals (F 2, 116=3.12, p=0.04), with MAN1 × MAN0 males retrieving more in their second litters than other groups. There was also a significant effect of early treatment on male retrievals (F 2, 116= 2.97, p =0.05), with males in MAN1 × MAN0 pairs retrieving pups more compared to MAN1 × MAN1 males (p= 0.04) and to MAN0 × MAN1 males (p=0.05; Fig 2d). There was a main effect of parity on male retrievals, with fathers retrieving pups more in their second litter (F 1, 116 = 23.43, p < 0.0001; Fig 3a).
Figure 3.
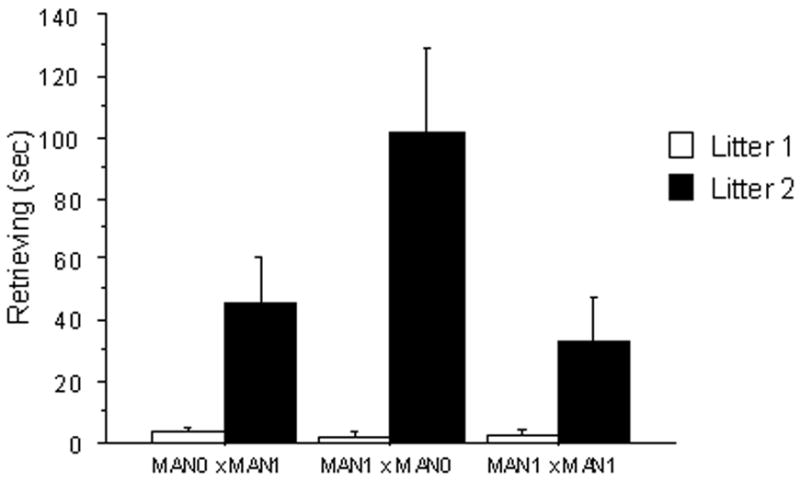
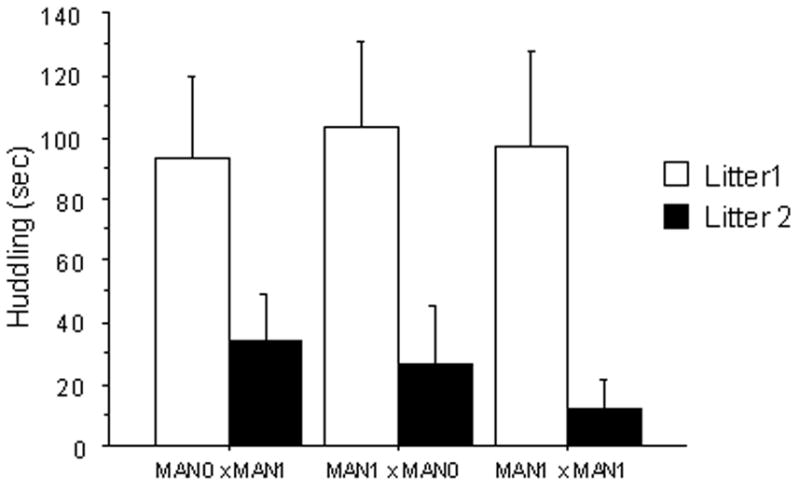
Duration of parental behaviors in seconds (measured in a 20 min observation period). A) Male huddling: across groups, males huddled more in their first litters compared to second litters. B) Male retrievals: MAN1 × MAN0 males retrieved pups more often than males in other groups; across treatments, males retrieved more in their second litter compared to second litters.
Male huddling and licking pups was also affected by parity, with males performing more of these behaviors in their first litters (huddle: F 1, 116= 17.48, p < 0.0001, Fig 3b; lick: F 1,116= 11.39, p=0.008).
3.2. Intergenerational Effects
3.2.1. Alloparental Care by F2 Voles
Due to the fact that several intergenerational pairs did not breed (see above) and often litters were small or sex-biased, sample sizes are reduced for certain treatments (Table 4). For the first litter, a comparison of the overall proportion of animals that were classified as parental by intergenerational treatment was not statistically significant (χ2= 0.87, df= 2, p=0.33, one-tailed). However, examinations of individual behaviors showed that male offspring of MAN1 × MAN0 pairs took longer to approach pups (Kruskal-Wallis test: H=8.75, df=2, p=0.006; one-tailed) and spent less time engaging in non-huddling contact (H=7.76, df=2, p=0.015; one-tailed; Table 4).
Table 4.
Individual behaviors in the alloparental care test for intergenerational animals. Numbers in bold are significantly different at p<0.05 when analyzed by Kruskal-Wallis test
| Litter 1 | Litter 2 | |||||
|---|---|---|---|---|---|---|
| Female Behaviors (sec) | MAN1 × MAN1 (n=10) | MAN 1 × MAN0 (n=6) | MAN0 × MAN1 (n=10) | MAN1 × MAN1 (n=7) | MAN 1 × MAN0 (n=5) | MAN0 × MAN1 (n=10) |
| Total pup-directed behavior | 123 ± 59 | 117 ± 66 | 252 ± 54 | 363 ± 40 | 233 ± 84 | 186 ± 34 |
| Huddling and pseudohuddling | 0.3 ± 0.3 | 20 ± 19 | 31 ± 17 | 28 ± 20 | 18 ± 18 | 15 ± 12 |
| Non-huddling contact | 28 ± 22 | 29 ± 28 | 71 ± 44 | 58 ± 29 | 64 ± 43 | 57 ± 21 |
| Licking/grooming | 77 ± 36 | 38 ± 36 | 92 ± 30 | 251 ± 33 | 115 ± 54 | 88 ± 24 |
| Retrievals (frequency) | 2 ± 1.3 | 0.8 ± 0.7 | 0.8 ± 0.3 | 2.5 ± 1.1 | 0.2 ± 0.1 | 2 ± 1.1 |
| Latency to approach pup | 72 ± 37 | 54 ± 29 | 97 ± 54 | 19 ± 6 | 21 ± 11 | 77 ± 52 |
| Male Behaviors (sec) | MAN1 × MAN1 (n=10) | MAN 1 × MAN0 (n=6) | MAN0 × MAN1 (n=12) | MAN1 × MAN1 (n=7) | MAN 1 × MAN0 (n=5) | MAN0 × MAN1 (n=10) |
| Total pup-directed behavior | 130 ± 58 | 122 ± 85 | 216 ± 44 | 198 ± 60 | 264 ± 90 | 201 ± 53 |
| Huddling and pseudohuddling | 1 ± 0.7 | 0 ± 0 | 0 ± 0 | 1.2 ± 1 | 7 ± 7 | 8 ± 7 |
| Non-huddling contact | 60 ± 28 | 5 ± 5 | 46 ± 24 | 30 ± 22 | 120 ± 67 | 49 ± 19 |
| Licking/grooming | 90 ± 26 | 70 ± 55 | 116 ± 31 | 135 ± 42 | 94 ± 31 | 106 ± 37 |
| Retrievals (frequency) | 1.2 ± 0.7 | 0.2 ± 0.2 | 2 ± 1 | 1.6 ± 0.8 | 0 ± 0 | 4 ± 2 |
| Latency to approach pup | 75 ± 53 | 179 ± 106 | 76 ± 23 | 224 ± 98 | 12 ± 4 | 39 ± 8 |
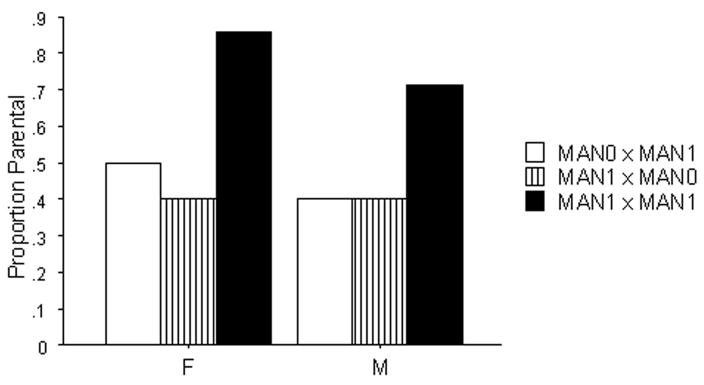
For the second litter, a greater proportion of offspring of MAN1 × MAN1 pairs were alloparental than offspring of the two other groups (χ2= 4.84, df= 2, p=0.04, one-tailed; Fig 4). Female offspring from MAN1× MAN1 pairs spent more time in pup-directed behavior (H=6.65, df= 2, p=0.015; one-tailed) and more time licking the pup (K-W test: H=9.92, df= 2, p=0.005, one-tailed; Table 4) than females from other intergenerational treatments. Male offspring from MAN1 × MAN1 pairs took longer to approach pups (H=6.43, df=2, p=0.04; two-tailed as direction was opposite to that predicted).
Figure 4.
Proportion of intergenerational animals that behaved parentally as a function of handling manipulations, Litter 2. Sample sizes are as given in Table 4. Asterisks indicate statistically significant difference when analyzed with a Chi-square test.
3.2.2. Relationships between Parental Care Received and Performance in Alloparental Care Test
Several components of maternal behavior received as pups and later performance of F2 offspring in alloparental care tests were correlated. We found trends for mothers that huddled and retrieved more to have daughters that huddled and retrieved more during alloparental care tests (huddle: r32= 0.69, adjusted p-value=0.06; retrieve: r32= 0.50, adjusted p-value=0.06). Mothers that huddled and retrieved more had sons that huddled and retrieved significantly more during parental care tests (r32= 0.68, adjusted p=0.034; retrieval, r32= 0.49, adjusted p=0.034).
Similarly, paternal behavior and offspring performance on alloparental care tests were significantly correlated. Fathers that showed more non-huddling contact with their pups had sons that showed more non-huddling contact during alloparental care tests (NHC: r30= 0.65, adjusted p=0.0267). Fathers that huddled more and retrieved more had sons that huddled and retrieved more during parental care tests (huddle: r30= 0.66, adjusted p=0.0267; retrieve: 0.80, adjusted p=0.02). Fathers that huddled and retrieved more tended to have daughters that huddled and retrieved more (r30= 0.71, adjusted p=0.06; retrievals: r30= 0.71, adjusted p=0.06) during alloparental care tests.
4. DISCUSSION
In our laboratory as in many other vole laboratories, transfer of animals between cages with a gloved hand (a MAN1 manipulation) is the norm during routine husbandry. Bales et al (2007) demonstrated that key traits that have been used to define monogamy, such as paternal care and the capacity to form pair bonds, can be altered by small changes in early experience such as the MAN1 vs. MAN0 manipulation. Furthermore, the behavioral effects of these manipulations vary in a sexually dimorphic manner. The present study further demonstrates that such changes can be long-lasting, can affect parenting behavior as adults and can be transmitted intergenerationally to offspring. These findings are consistent with previous studies on rats, showing that variations in maternal care can change the behavior and neurobiology of adult offspring (Francis et al 2002) and that these changes translate into changes in maternal behavior during adulthood (Francis et al 1999; Pedersen and Boccia 2002). However, in many cases these findings did not support the predictions that we made prior to beginning the study, with the early experience paradigm used in this experiment causing long-lasting changes but not in predictable ways.
We first predicted that pairs containing MAN0 animals would display lower levels of reproduction, possibly due to altered socio-sexual behavior. This prediction was not upheld, suggesting that regardless of social deficits in other areas, sexual behavior is sufficient to support normal reproduction.
We then predicted that MAN0 parents would display lower rates of parental behaviors towards their pups because MAN0 animals showed impairment in other social behaviors in previous studies (Bales et al 2007). We did not see changes in parental behavior in MAN0 females, although we did see what appeared to be compensatory changes in mates of MAN0 males; most of the observed changes were in female MAN1 (control) animals that were paired with a MAN0 male. These females spent more time engaging in parental behaviors toward their own pups (e.g. huddling and retrievals) than MAN0 females or MAN1 females paired with MAN1 males. This was true for both first and second litters, and suggests that these females could be compensating for lower levels of parental behaviors displayed by their pair-mates. However, MAN0 males paired with MAN1 females did not have detectably lower levels of huddling or other parenting behaviors examined in this study. It is possible that other behaviors, not recorded here, were altered in MAN0 males. For example, nest-building, an important activity in nature for male prairie voles (Gruder-Adams and Getz 1985), was not recorded and it is possible that MAN0 males nest-build less than MAN1 voles.
While we found no changes in parenting behavior in MAN0 females, MAN0 males paired with MAN1 females showed increased retrievals compared to males in other pairs. This was not consistent with our original hypothesis of reduced parenting behavior by MAN0 males. However, it is still possible that the quality of the parental behavior differed between MAN0 and MAN1 males. While retrievals are a parental behavior, in this species they are sometimes associated with arousal and anxiety and thus not always a “positive” experience for the offspring; there is some evidence that increased retrievals by fathers can result in higher anxiety in offspring (Delaney-Busch and Bales, unpublished data).
We also predicted that juvenile F2 offspring with one MAN0 parent would display deficits in alloparenting, and that males would be particularly affected. These predictions were partially upheld, with offspring of both sexes displaying less alloparental behavior. In particular, consistent with the lower alloparental care displayed by the MAN0 males before pairing, the male offspring of MAN0 fathers took longer to approach pups and spent less time in non-huddling contact with pups. In California mice, paternal care also had a sex-specific effect in which males but not females had changes in learning and memory depending on whether they were reared with their fathers, with those whose fathers were present having higher discrimination ratios in an object recognition task (Bredy et al 2004). In our study, second litter offspring of either a MAN0 mother or a MAN0 father behaved less alloparentally, and this was true for both males and females. The fact that behavioral effects of having a MAN0 mother only appeared in the second litter could be due to the fact that animals generally behaved less parentally in their second litter, or due to the fact that MAN0 females started retrieving more in their second litter (see Figure 2b). This reduction in maternal care was true for all treatments, including females in MAN0 × MAN1 pairs, which licked and huddled less over pups in their second litter compared to levels for their first litters. Therefore, it is possible that the decrease in parental behaviors of MAN0 mothers did not reach some critical threshold until the second litter; it is also possible that high levels of retrievals from mothers also generate anxiety in offspring as discussed above.
We also found significant positive correlations between certain parental behaviors of parents and those of offspring. In particular, sons’ behaviors seemed particularly correlated with those displayed by their parents. In rats, intergenerational studies have shown that daughters display parental behaviors shown by their mothers (Gonzalez et al. 2001; Meaney 2001). Here we demonstrate an intergenerational effect for males as well.
One puzzling result is that second litter male offspring of MAN1 × MAN1 pairs had longer latencies to approach infants than male offspring of other pairings. These males did eventually show alloparental behavior (Figure 3), unlike the other group that displayed long latencies (the first litter male offspring of MAN1 × MAN0 pairs), which also showed lower pup contact (Table 3). This longer latency to approach pups could be due to a competing activity, such as exploration of the novel testing area. This is not a simple question to answer, but the key is to distinguish between a long latency to approach driven by fear of the pup, vs. one driven by exploration of the arena. One way to get at whether this group was more afraid is to examine whether or not the MAN1 × MAN1 males autogroomed more than other groups, and they did not (F=0.12, p=0.88, df=2). Therefore exploration remains the most plausible alternative explanation.
The expression of parental behaviors in prairie voles is linked to the neuropeptide oxytocin (Williams et al. 1994; Cho et al. 1999), providing a physiological mechanism by which parental stimulation might affect later behavior of offspring. Neonatal injections of oxytocin can increase the later expression of social behaviors (Bales and Carter 2003), while neonatal exposure to an oxytocin antagonist reduced alloparental behaviors in males, similar to the effect found in MAN0 males (Bales et al. 2004). Female alloparenting and pair-bonding was affected by neonatal exposure to oxytocin, with direction dependent on the dosage (Bales et al.2007). Oxytocin is released by warmth and touch (Uvnas-Moberg 1998), and thus oxytocin release in the pup may be induced by differential parenting following a handling episode. Oxytocin may also mediate the intergenerational transmission of these behaviors. In female rats, oxytocin can increase expression of maternal behaviors such as licking pups (Pedersen and Prange 1979), which then increases oxytocin receptor expression in female pups (Francis et al. 2002). This has been associated with an increase in maternal behaviors when the pups reach adulthood themselves (Pedersen and Boccia 2002).
While handling paradigms may not seem to relate intuitively to the natural habitat or lifestyle of most animal species, they offer us a controllable window into the early experience of a pup. By handling the parents and thus changing parental behaviors (Tyler et al. 2005) and possibly some aspect of novelty, such as being exposed to the plastic cup (Tang et al. 2006), we can cause long-term changes in behavior that will then allow us to identify underlying mechanisms. In conclusion, in this study we showed that individual differences in vole parental behavior, caused by early experience, can be transmitted across generations through behavioral transmission, although not always in predictable ways. These findings suggest that in species with biparental care, early postnatal experience of both males and females can significantly change their parenting behavior later in life, and affect the behavior of the next generation of offspring. In addition, we have found that MAN0 voles show reductions in oxytocin peptide immunoreactivity compared to MAN1 voles and widespread changes in oxytocin receptors (increased or decreased depending on brain area) (Bales et al., unpublished data). We are currently examining whether similar neurobiological correlates are present in the offspring of MAN0 animals, which would further elucidate the mechanisms through which behaviors are being transmitted from one generation to the next.
Acknowledgments
We thank Luana Griffin, Caroline Hostetler, Denise Mathieu, Ashley Montross and Julie van Westerhuyzen for help with animal care and data collection. Funding for this project was provided by NIH 073022 to C. Sue Carter and KLB, and by NSF 0437523 to KLB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2003;117:854–859. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol. 2004;44:122–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- Bales KL, Abdelnabi M, Cushing BS, Ottinger MA, Carter CS. Effects of neonatal oxytocin manipulations on male reproductive potential in prairie voles. Physiol Behav. 2004;81:519–526. doi: 10.1016/j.physbeh.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS. Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev Psychobiol. 2007;49:335–342. doi: 10.1002/dev.20216. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc. 1995;57:289–300. [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and development of the offspring in a rodent model. Biol Psych. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Bell RW. Critical periods for the effects of infantile experience on adult learning. Science. 1960;131:227–228. doi: 10.1126/science.131.3395.227. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Ottinger DR, Stephans MW. Effects of maternal factors upon growth and behavior of the rat. Child Dev. 1962;33:65–71. doi: 10.1111/j.1467-8624.1962.tb05988.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Individual differences in maternal style: causes and consequences for mothers and offspring. In: Rosenblatt JS, Snowdon CT, editors. Parental Care: Evolution, Mechanisms, and Adaptive Significance. Academic Press; San Diego: 1996. pp. 579–611. [Google Scholar]
- Fleming AS, Morgan HD, Walsh C. Experiential factors in postpartum regulation of maternal care. In: Rosenblatt JS, Snowdon CT, editors. Parental Care: Evolution, Mechanisms, and Adaptive Significance. Academic Press; San Diego: 1996. pp. 295–332. [Google Scholar]
- Francis DD, Dorio J, Liu D, Meaney MJ. Nongenomic transmission across generations in maternal behavior and stress responses in the rat. Science. 1999;286:1155–58. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendoc. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Frazier CR, Trainor BC, Cravens CJ, Whitney TK, Marler CA. Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm Behav. 2006;50:669–707. doi: 10.1016/j.yhbeh.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Devel Psychobiol. 2001;8:11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gruder-Adams S, Getz LLK. Comparison of the mating system and paternal care in Microtus ochrogaster and M. pennsylvanicus. J Mammal. 1985;66:165–167. [Google Scholar]
- Jia R, Tai F, An S, Zhang X, Broders H. Effects of neonatal paternal deprivation or early deprivation on anxiety and social behaviors of the adults in mandarin voles. Behav Proc. 2009 doi: 10.1016/j.beproc.2009.07.006. in press. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Enduring effects of early experience on adult behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. Academic Press; New York: 2002. pp. 535–542. [Google Scholar]
- Levine S, Haltmeyer GC, Karas GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiol Behav. 1967;2:55–63. [Google Scholar]
- Maestripieri D, Wallen K, Carroll KA. Infant abuse runs in families of group-living pigtail macaques. Child Abuse and Neglect. 1997;21:465–71. doi: 10.1016/s0145-2134(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci Behav Rev. 2005;29:51–57. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. Early, postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav Neurosci. 1985;99:760–765. doi: 10.1037//0735-7044.99.4.765. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Sharma S, Viau V, Sarrieu A. Postnatal handling increased hippocampal type II, glucocorticoid receptors and enhances adrenocortical negative-feedback efficacy in the rat. Neuroendocrinol. 1989;51:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann Rev Neurosci. 2001;24:1161–192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress. 2002;5:259–267. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ. Induction of maternal behavior in virgin female rats after introcerebroventricular administration of oxytocin. PNAS. 1979;7:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Miller AK, Taymans SE, Carter CS. Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster) Can J Zool. 1998;76:1862–1868. [Google Scholar]
- Solomon NC. Current indirect fitness benefits associated with philopatry in juvenile prairie voles. Behav Ecol Sociobiol. 1991;29:277–282. [Google Scholar]
- Tang AC, Ayers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. PNAS. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler AN, Michel GF, Bales KL, Carter CS. Do brief early disturbances of parents affect parental care in the bi-parental prairie vole (Microtus ochrogaster)? Dev Psychobiol. 2005;47:451. [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm Behav. 1994;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]