Abstract
We have previously demonstrated that fetal ethanol exposure deranges the function and viability of the neonatal alveolar macrophage. Although altered differentiation of the alveolar macrophage contributes to pulmonary disease states within the adult lung, the effects of fetal ethanol exposure on the normal differentiation of interstitial to alveolar macrophage in the newborn lung are unknown. In the current study, using a mouse model of fetal ethanol exposure, we hypothesized that altered terminal differentiation of the neonatal interstitial to alveolar macrophage contributes to the observed cellular dysfunction in the ethanol-exposed newborn mouse. Control alveolar macrophage differentiation was characterized by increased expression of CD32/CD11b (P ≤ 0.05) and increased in vitro phagocytosis of Staphylococcus aureus (P ≤ 0.05) compared with interstitial macrophage. After in utero ethanol exposure, both alveolar and interstitial macrophage lacked the acquisition of CD32/CD11b (P ≤ 0.05) and displayed impaired in vitro phagocytosis (P ≤ 0.05). Ethanol significantly increased transforming growth factor-β1 (TGF-β1) in the bronchoalveolar lavage fluid (P ≤ 0.05), as well as in both interstitial and alveolar macrophages (P ≤ 0.05). Oxidant stress contributed to the ethanol-induced changes on the interstitial and alveolar cells, since maternal supplementation with the glutathione precursor S-adenosylmethionine during ethanol ingestion normalized CD32/CD11b (P ≤ 0.05), phagocytosis (P ≤ 0.05), and TGF-β1 in the bronchoalveolar lavage fluid and macrophages (P ≤ 0.05). Contrary to our hypothesis, fetal ethanol exposure did not solely impair interstitial to alveolar macrophage differentiation. Rather, fetal ethanol exposure impaired both neonatal interstitial and alveolar macrophage phagocytic function and differentiation. Increased oxidant stress and elevated TGF-β1 contributed to the impaired differentiation of both interstitial and alveolar macrophage.
Keywords: alveolar macrophage, fetal alcohol, prematurity, lung
alveolar macrophages (AM) are derived from peripheral circulating blood monocytes. The monocyte precursors within the systemic circulation constitutively move into the interstitial space of the lung, where they reside as interstitial macrophages (IM) until further migration and terminal differentiation into mature AM within the alveolar space (13, 36). As a professional phagocyte within the lung, the AM patrols the lung, defending it against foreign particles and infection by initiating an immune response, participating in phagocytosis and clearance, and regulating subsequent inflammatory processes (13, 43). The normal population of AM residing within the lung is a heterogeneous mix of immature and mature cells in both human (29) and experimental animal models (10, 24, 34). This heterogeneity results in a population of cells with variable immune function (15).
The normal terminal differentiation of the neonatal AM and its role as the modulator of the inflammatory state within the neonatal lung remain under investigation. Modulation of the innate immune responses of the fetal monocyte by systemic conditions or maternal exposures during pregnancy may affect the neonatal AM population and the inflammatory environment within the vulnerable newborn lung (26, 27). However, the normal terminal differentiation of the IM into the resident AM in the newborn lung is not well described.
Alcohol use during pregnancy remains a significant problem in our society, increasing the risk of extreme premature delivery by >34-fold (41). The occurrence of infection complicates an already tenuous clinical course for the premature neonate, causing exacerbation of primary pulmonary diseases and prolonging hospital stay (44). Limited clinical studies suggest that both term (17) and premature newborns (18) are at increased risk of sepsis after in utero alcohol exposure. Our laboratory has demonstrated in a guinea pig model of in utero ethanol (ETOH) exposure that ETOH increased oxidant stress in the developing lung and within the resident neonatal AM, deranging neonatal AM function both in vitro (19) and in vivo (35) and increasing the risk of experimental pneumonia (20). The capacity of precursors of the antioxidant glutathione (GSH) to modulate these changes in both premature and term gestations demonstrated that exaggerated oxidant stress was a central feature in the dysfunction of the ETOH-exposed developing AM (19, 20, 35).
In the adult lung, transforming growth factor-β1 (TGF-β1) plays a crucial role in lung injury and repair (2). Chronic ETOH ingestion increases TGF-β1 within the adult lung, primarily in AM and alveolar epithelial type II cells, increasing the risk of cellular dysfunction in the lung (3). In the fetal lung, TGF-β1 is essential for normal lung development and epithelial cell maturation. However, on one hand TGF-β1 positively promotes proliferation and differentiation of the macrophage, while on the other hand increased TGF-β1 decreases the expression of critical receptors on the AM (12, 22, 45).
We hypothesized that the local oxidizing environment within the ETOH-exposed lung would impair the normal differentiation of the resident AM population, thus contributing to deranged newborn AM function after fetal ETOH exposure. With the use of a mouse model of in utero ETOH exposure, the goals of this study were to 1) evaluate the normal terminal differentiation of IM and AM, and 2) determine fetal ETOH effects on TGF-β1 and macrophage differentiation in the neonatal lung.
MATERIALS AND METHODS
Mouse model of fetal ETOH exposure.
A continuous exposure of ETOH in a liquid diet (25% ETOH-derived calories; Dyets, Bethlehem, PA) especially prepared for experimentation in pregnant mice was used to establish our mouse model of fetal ETOH exposure (50, 51). Female C57BL/6 mice were shipped from the vendor and allowed to acclimate for 1 wk. The female mouse was bred and the experimental liquid diet started 1 day after the mucus plug was documented (designated E2). Dams were randomized to receive ±25% ETOH-derived calories in the liquid diet. For some pregnant dams, S-adenosylmethionine (SAM-e, 10 mM, Nature Made) was added to the experimental liquid diet beginning E2. Maternal SAM-e was chosen, as we have demonstrated that it maintains neonatal AM function despite in utero ETOH exposure at both premature and term gestations in the guinea pig model (19, 20, 35). The females were pair fed to match the ETOH-exposed dam. The only access to food was the assigned experimental liquid diet. The diet was continued until delivery of the pups at term. All animals were used with protocols reviewed and approved by the Emory University Institutional Animal Care Committee in accordance with NIH Guidelines (Guide for the Care and Use of Laboratory Animals).
Isolation of AM.
Term pups were evaluated on day of life number one (designated P0). After anesthesia with pentobarbital sodium intraperitoneally, the pup trachea was identified under a dissecting microscope and cannulated with a 27-G catheter and the lungs serially lavaged with 60-μl sterile PBS (3×) to obtain lavage samples. The initial lavage from each pup of the litter was pooled and centrifuged (402 g for 8 min), and the supernatant [designated bronchoalveolar lavage fluid (BAL)] was saved for further analysis. The remaining cell pellet was pooled with subsequent lavage samples from each pup of the litter and similarly centrifuged, and the final cell pellet was obtained. The retrieved cells were evaluated for viability and cell type via the calcein/ethidium iodide “live-dead” stain and DiffQuik stain (Dade Behring, Newark, DE), respectively.
Apoptosis was determined by staining the cells for DNA fragmentation via terminal dUTP nick-end labeling (TUNEL) as previously described (19). Briefly, the cells were fixed with 3.7% paraformaldehyde and endogenous peroxidase was blocked with 3% H2O2 in methanol. Cells were permeabilized with 0.1% Triton X-100, stained with the in situ cell death detection kit POD (Roche), and evaluated under fluorescent microsopy. The percentage of TUNEL-positive cells was tallied from least 25 cells/litter.
Isolation of IM.
After the BAL, the pup pulmonary artery was identified under the dissecting microscope and cannulated. The fetal lung was perfused with sterile PBS (1 cc) via the pulmonary artery until white, as previously described by this laboratory (7). The perfused lungs from all the pups of one litter were pooled, minced, and serially digested with collagenase D (60 U/ml for 10 min followed by 175 U/ml for an additional 10 min). The IM were collected by sequential filtration and adherence to tissue culture plastic (37). The isolated IM were similarly evaluated for viability and cell type via the calcein/ethidium iodide live-dead stain and DiffQuik stain, respectively. Apoptosis of the cells was similarly determined by TUNEL staining.
BAL oxidant stress.
Oxidative stress was evaluated in the neonatal pup lung by determining the presence of the fatty acid oxidation product malonyldialdehyde (MDA) in the initial BAL sample. MDA was measured via ELISA (Oxis International, Foster City, CA) and normalized to sample protein as determined by the modified Bradford assay (Coomassie Plus, Thermo Scientific, Rockford, IL). Values are presented as mean MDA (μM/μg protein) ± SE.
TGF-β1 determination.
Active TGF-β1 and total TGF-β1 were measured in the initial BAL via commercially available ELISA (Promega, Madison, WI). Active TGF-β1 was measured in the sample, and then after acidification per manufacturer's instructions, the total TGF-β1 was determined. The values were similarly normalized to BAL protein. Data are presented as mean TGF-β1 (pg/μg protein) ± SE. The isolated IM and AM were also evaluated for TGF-β1 via immunostaining after fixation with 3.7% paraformaldehyde. Nonspecific binding was blocked with BSA. Cells were incubated with the primary antibody in a 1:100 dilution (Santa Cruz Biotechnology, Santa Cruz, CA), and the sample was incubated for 2 h. Cells were serially rinsed with PBS, and the secondary antibody (anti-rabbit IgG, horseradish peroxidase conjugate; Sigma-Aldrich, St. Louis, MO) was added in a 1:200 dilution for 1 h. TGF-β1 in the IM and AM was quantified with computerized analysis via ImagePro-Plus for Windows and presented as the mean density/cell ± SE as tallied from at least 25 cells/litter.
Differentiation markers of IM and AM.
To address the hypothesized effects of ETOH on the terminal differentiation of the neonatal mouse AM, we evaluated the isolated IM and the AM for cell surface markers including the alveolar macrophage marker CD32 and the β2-integrin CD11b, whose relative expression is increased on monocytes and decreased on alveolar macrophage (1, 6, 40). IM and AM were isolated and fixed with 3.7% paraformaldehyde. In some experiments, control IM and AM were incubated with TGF-β1 (5 ng/ml) for 4 h before fixation. To correct for nonspecific binding, an appropriate isotype-control antibody was used. Staining was performed in the presence of BSA to block nonspecific binding. The primary antibodies were added in a 1:100 dilution (CD32, Santa Cruz Biotechnology, Santa Cruz, CA; and CD11b, e-Bioscience, San Diego, CA), and the sample was incubated for 2 h. Cells were serially rinsed with PBS, and the secondary fluorescent antibody (FITC; Sigma, St. Louis, MO) was added in a 1:200 dilution for 1 h. Fluorescence was quantified using confocal fluorescent microscopy with quantitative digital analysis via FluoView (Olympus Corp, Melville, NY) as tallied from at least 25 cells/litter. Staining in relative fluorescent units per cell was calculated and presented as either the mean ratio of CD32 to CD11b or the mean percentage of control (relative fluorescent units/cell) for each condition ± SE.
Phagocytosis assay.
Freshly isolated AM and IM were plated at ∼106 cell/ml in DMEM-F-12 media with 2% FBS and penicillin plus streptomycin and cultured at 37°C, 5% CO2. After a 2 h incubation, the cells were washed and FITC-labeled inactivated S. aureus (Molecular Probes, Eugene, OR) added in a 1:10 ratio (cell:bacteria). The cells were incubated for an additional 2 h (19, 33). In some experiments, control IM or AM were incubated with TGF-β1 (5 ng/ml) added to the media during the phagocytosis assay.
Determination of phagocytic function.
To quantify the phagocytosis of FITC- S. aureus by the isolated AM and IM, confocal fluorescent microscopy was used as previously described by this laboratory (8, 35). After incubation, the cells were washed and then fixed with 3.7% paraformaldehyde and nonspecific binding blocked with BSA. Using three-dimensional confocal analyses (Olympus, Melville, NY), we evaluated the cell at 50% cell depth in the z plane to localize the fluorescence of phagocytosed FITC-S. aureus. Fluorescence was determined via quantitative digital analysis (FluoView; Olympus). Background fluorescence of unstained macrophages was used to account for autofluorescence (8, 35). The phagocytic index (PI) was used to determine phagocytic function as previously described by this laboratory (14) and others (5). PI was defined as the percentage of cells with internalized fluorescence × the mean relative fluorescent units internalized per cell as tallied from at least 25 cells/litter. Values for PI are presented as a percentage of control ± SE.
In vitro ETOH exposure.
To mimic chronic in utero ETOH exposure to the neonatal IM and AM, control cells were similarly isolated at P0 and placed in modular chambers (Billups-Rothenberg, Del Mar, CA). The cells were incubated with or without ETOH in vitro (0.1%, 25 mM,) added to the culture media for a duration of 4 days with daily media changes. We chose this in vitro ETOH concentration based on studies of alcohol's effect on other immune cells with 25 mM ETOH described to correlate with moderate alcohol consumption (30, 46). The use of modular chambers (Billups-Rothenberg) allowed for the maintenance of ETOH concentrations with daily media changes during the incubation period. In some experiments the cells were incubated with or without a monoclonal anti-TGF-β1 antibody (mAbTGF-β1; 10 ng/ml; R&D Systems, Minneapolis, MN) or a nonspecific mouse IgG added to the media at the same concentration. After 4 days of in vitro ETOH exposure, the phagocytic ability of the AM and IM to ingest FITC-S. aureus was similarly investigated.
Statistical analyses.
Sigma Stat for Windows (Systat Sotware, San Jose, CA) was used for all statistical analyses. ANOVA or ANOVA on Ranks was used as appropriate. Student Newmans Keul's or Dunn's test was used for multiple comparisons, respectively. A P ≤ 0.05 was considered significant. Each n represents a separate mouse litter.
RESULTS
Pup characteristics.
The liquid diet was well tolerated by the pregnant mouse without significant loss of pregnancy or distress. Tail vein blood ETOH levels were measured in the dams ∼4 days before the expected term delivery. Blood ETOH levels were significantly elevated in both the ETOH dams and the ETOH + SAM-e dams compared with control (control: 17.4 ± 5 μg/dl vs. ETOH: 76.7 ± 9.5 μg/dl vs. ETOH + SAM-e: 75.1 ± 6.9 μg/dl; P < 0.05 vs. control). There was no difference in blood ETOH levels between the dams fed ETOH vs. those on ETOH + SAM-e (P = NS). There was no difference in the average number of pups delivered/litter (n = 6–8; P = NS), and pup weight was similar among the experimental groups (control: 1.35 ± 0.05 g, control + SAM-e: 1.16 ± 0.06 g, ETOH: 1.25 ± 0.04 g, and ETOH + SAM-e: 1.28 ± 0.06 g; P = NS; n = at least 5 separate litters per group).
Isolation of IM and AM from P0 pups.
At isolation, the IM and AM cell yields/pup were similar among the experimental groups (P = NS). However, significantly more IM were isolated from any experimental group than AM (IM: range 75–210 × 103 cells/pup vs. AM: range 8–18 × 103 cells/pup; P < 0.05). The majority of both isolated IM and AM were macrophages (IM: range 91–96% macrophage; AM: range 93–97% macrophage) from all groups as determined by DiffQuik staining.
Viability and apoptosis of the IM and AM.
For the IM, the addition of maternal SAM-e to control litters did not significantly change IM viability (control + SAM-e: 87.9 ± 1.8% viable). There was a significant reduction in the viability of the ETOH-exposed IM at the time of isolation compared with control (control: 89.8 ± 0.9% vs. ETOH: 81.8 ± 1.1% viable; P < 0.05). The addition of maternal SAM-e during ETOH ingestion resulted in increased viability of the P0 IM (ETOH + SAM-e: 87.0 ± 1.3%; P < 0.05 vs. ETOH alone), which reached control levels. For the AM, maternal SAM-e did not alter AM viability compared with control (control: 95.1 ± 0.5% vs. control + SAM-e: 95.9 ± 1.1%; P = NS). The viability of the ETOH-exposed AM was also significantly decreased compared with control (control: 95.1 ± 0.5% vs. ETOH: 89.1 ± 0.9%; P < 0.05), while viability in ETOH + SAM-e AM was maintained and significantly higher than ETOH-exposed AM (ETOH: 89.1 ± 0.9% vs. ETOH + SAM-e: 93.3 ± 1.2%; P < 0.05 vs. ETOH).
Apoptosis of the IM and AM was determined by TUNEL staining (n = at least 4 separate litters/group). There was no difference in apoptosis between control and control + SAM-e IM (control: 5.4 ± 1% vs. control + SAM-e: 4.9 ± 0.6%; P = NS) or AM (control: 1.99 ± 1.2% vs. control + SAM-e: 2.45 ± 1.5%; P = NS). However, the addition of ETOH significantly increased both IM apoptosis (ETOH: 33.4 ± 2.7%; P < 0.05 vs. control) and AM apoptosis (ETOH: 21.8 ± 2.3%; P < 0.05 vs. control). The addition of maternal SAM-e during ETOH ingestion significantly protected both the IM (ETOH + SAM-e: 7.5 ± 1.1%; P = NS vs. ETOH) and the AM (ETOH + SAM-e: 7.3 ± 1.2%: P = NS vs. ETOH) from ETOH-induced apoptosis.
Fetal ETOH exposure increased lipid peroxidation within the pup airway.
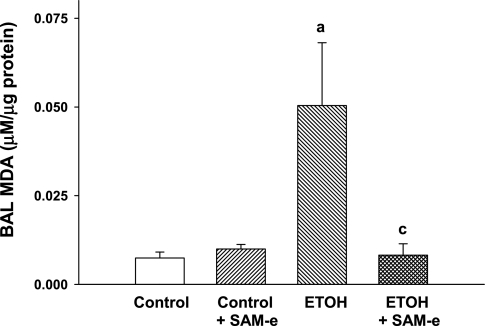
In a guinea pig model of fetal ETOH exposure, we demonstrated that fetal ETOH exposure increased oxidant stress in the fetal lung, as evidenced by increased lipid peroxidation products in the BAL (19). In the current mouse model, fetal BAL oxidant stress was demonstrated in the ETOH P0 lung with an ∼10-fold increase in MDA compared with control (Fig. 1; P < 0.05 vs. control). The addition of SAM-e to control litters did not alter BAL MDA levels compared with control (P = NS). ETOH + SAM-e diminished BAL MDA to control levels. BAL protein did not significantly differ among the experimental groups (P = NS). Thus similar to the guinea pig model of fetal ETOH exposure (19, 35), the ETOH-exposed mouse pup lung demonstrated evidence of increased macromolecular damage from oxidant stress.
Fig. 1.
In utero ethanol (ETOH) increased lipid peroxidation in the bronchoalveolar lavage (BAL). Timed pregnant mice were randomized to receive ±25% ETOH-derived calories in a liquid diet beginning on E2. For some pregnant dams, S-adenosylmethionine (SAM-e; 10 mM) was added to the experimental liquid diet. Pups were allowed to deliver at term, and lung BAL was obtained under a dissecting microscope. The lipid peroxidation product malonyldialdehyde (MDA) was determined in the BAL via ELISA. Bar heights represent means ± SE of MDA (μM) as normalized to BAL protein (μg). aP < 0.05 vs. control; cP < 0.05 vs. ETOH; n = at least 4 separate litters.
In utero ETOH increased TGF-β1 in the neonatal mouse lung and the macrophage.
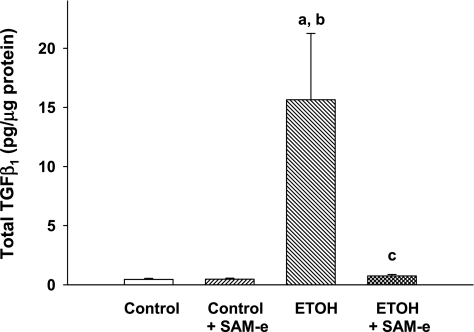
There was no statistical difference in active TGF-β1 in the pup BAL across experimental groups (P = NS). However, ETOH-exposed pups demonstrated dramatically higher total TGF-β1 levels in the BAL compared with either control or control + SAM-e pups (Fig. 2; P < 0.05 vs. control; P < 0.05 vs. control + SAM-e). GSH availability modulated ETOH-induced increases in BAL TGF-β1, since maternal SAM-e during ETOH exposure significantly attenuated BAL total TGF-β1 in the neonatal mouse BAL to minimally detected levels (Fig. 2; P < 0.05 vs. ETOH). For control BAL, active TGF-β1 constituted the majority (100%) of the total TGF-β1 measurable. Similar findings were evident in control + SAM-e BAL (100% of total) and ETOH + SAM-e (78% of total). However, in the ETOH-exposed BAL, active TGF-β1 constituted 9.5% of the total TGF-β1 pool.
Fig. 2.
Elevated total transforming growth factor-β1 (TGF-β1) in the ETOH-exposed BAL. After randomization to ±25% ETOH-derived calories in a liquid diet beginning on E2, timed pregnant mice were allowed to deliver at term gestation. For some pregnant dams, SAM-e was added to the ETOH diet. Total TGF-β1 was determined in the BAL of day of life number one (P0) pups via ELISA with values normalized to BAL protein. Bar heights are means ± SE of TGF-β1 (pg/μg protein). aP < 0.05 vs. control; bP < 0.05 vs. control + SAM-e; cP < 0.05 vs. ETOH; n = at least 7 separate litters.
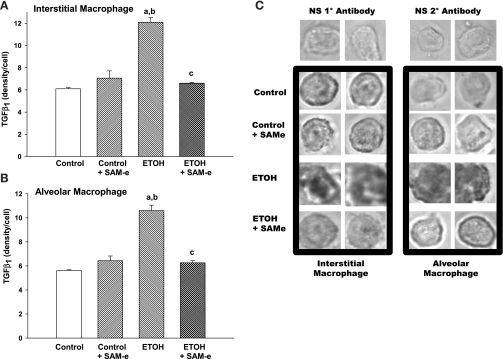
To evaluate the in utero ETOH effect on macrophage TGF-β1 in the neonatal mouse lung, we evaluated isolated IM and AM for TGF-β1 by immunostaining after fetal ETOH exposure. As demonstrated in Fig. 3, A–C, maternal SAM-e did not alter TGF-β1 staining compared with control in either IM (A) or AM (B). However, ETOH IM demonstrated a 50% increase in TGF-β1 and in utero ETOH exposure increased TGF-β1 staining for the AM by >40% (Fig. 3, A and B; P < 0.05 vs. respective control; P < 0.05 vs. respective control + SAM-e). Maternal SAM-e during ETOH ingestion significantly blunted the increased TGF-β1 in both the IM and the AM compared with ETOH alone (Fig. 3, A and B; P < 0.05 vs. ETOH, respectively). Representative photomicrographs of the IM and AM (Fig. 3C) demonstrate the ETOH effect on TGF-β1 density on both the IM and the AM. Therefore, the significant increase in total TGF-β1 found in the neonatal BAL was mirrored in both the ETOH-exposed IM and AM, suggesting that these cells were an important source of the increased TGF-β1 evident in the ETOH-exposed mouse lung.
Fig. 3.
The effect ETOH on TGF-β1 density for isolated interstitial macrophage (IM) and alveolar macrophage (AM). After term delivery, P0 AM were isolated via BAL. IM were isolated from the P0 lungs via collagenase digestion and adherence. The density of TGF-β1 was determined on isolated IM (A) and AM (B) cells via immunostaining (C) with analysis via ImagePro Plus. Bar heights represent mean density per cell of TGF-β1 ± SE as tallied from at least 25 cells/litter. aP < 0.05 vs. respective control; bP < 0.05 vs. respective control + SAM-e; cP < 0.05 vs. respective ETOH; n = at least 4 separate litters. C: representative photomicrographs of IM and AM stained for TGF-β1 (NS = nonspecific).
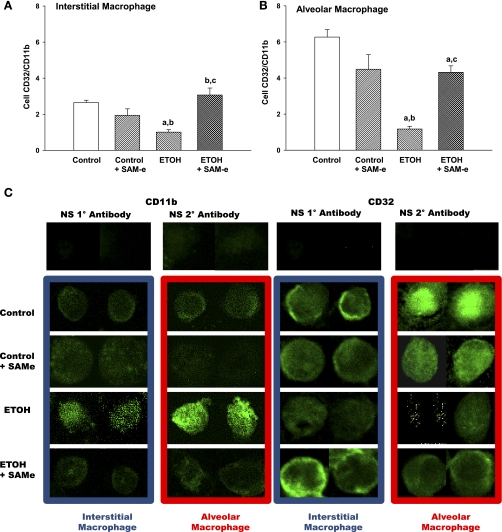
In utero ETOH delayed terminal differentiation and function of the neonatal AM. Both IM and AM were evaluated for the expression of CD32, a marker of a terminally differentiated AM, relative to the expression of the monocytic β2-integrin CD11b (Fig. 4). Maternal SAM-e did not alter CD32/CD11b in either IM (Fig. 4A) or AM (Fig. 4B) compared with control. Both control and control + SAM-e demonstrated a significant increase in CD32/CD11b on the AM compared with the resident IM, a characteristic of terminal differentiation of the neonatal AM in the mouse lung (P < 0.05, respective AM vs. IM). However, within the ETOH-exposed lung, the AM demonstrated CD32/CD11b staining equivalent to that seen for the ETOH-exposed IM (P = NS ETOH IM vs. ETOH AM). Furthermore, compared with control or control + SAM-e, both ETOH-exposed IM and AM demonstrated blunted CD32/CD11b expression (Fig. 4, A and B; P < 0.05 vs. respective control; P < 0.05 vs. respective control + SAM-e). This suggested a lack of normal terminal differentiation for the ETOH-exposed cells in the neonatal lung. While there was no difference in CD32/CD11b between ETOH + SAM-e IM vs. control (P = NS), maternal SAM-e during ETOH exposure significantly increased CD32/CD11b on IM and AM compared with ETOH alone (Fig. 4, A and B; P < 0.05 vs. respective ETOH), although staining did not reach control AM levels (Fig. 4; P < 0.05 vs. control AM). Representative confocal photomicrographs of CD11b and CD32 staining on the IM and AM are presented in Fig. 4C.
Fig. 4.
Markers of terminal differentiation on the IM and AM. To determine the phenotype of the isolated IM and AM, CD32 and the β2-integrin CD11b were evaluated on fixed cells using incubation with primary antibodies and secondary labeling via FITC. Cells were evaluated via confocal fluorescent microscopy, and fluorescence was quantified using digital analysis via FluoView as tallied from at least 25 cells/litter. IM (A) and AM (B) staining is presented as the mean ratio of CD32 to CD11b ± SE. aP < 0.05 vs. respective control; bP < 0.05 vs. respective control + SAM-e; cP < 0.05 vs. respective ETOH; n = at least 5 separate litters. C: Representative confocal photomicrographs of IM and AM stained for CD11b or CD32 with FITC.
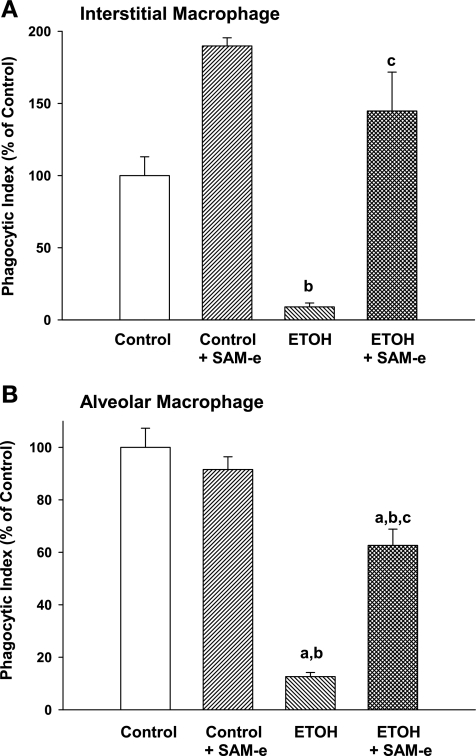
The expression of CD32/CD11b on the IM and AM paralleled phagocytic function. In both control and control + SAM-e pups, the observed gain in CD32/CD11b in the AM compared with IM was accompanied by a significant increase in the phagocytic function of the cell by 1.6-fold (control) and 1.2-fold (control + SAM-e) (P < 0.05, AM vs. IM; data not shown). SAM-e did not significantly alter IM or AM phagocytosis compared with control (Fig. 5, A and B; P = NS). However, ETOH-exposed cells demonstrated significant dysfunction in phagocytosis. Although phagocytosis by the ETOH AM was greater than the ETOH IM (P < 0.05, ETOH AM vs. ETOH IM; data not shown), phagocytosis remained severely depressed in both ETOH IM and ETOH AM compared with control IM and AM (Fig. 5, A and B; P < 0.05 vs. respective control; P < 0.05 vs. respective control + SAM-e). Maternal SAM-e during ETOH exposure significantly improved both IM and AM phagocytic function compared with ETOH alone (Fig. 5; P < 0.05 vs. respective ETOH), although AM function failed to reach either control or control + SAM-e levels (Fig. 5B; P < 0.05 vs. control or control + SAM-e AM, respectively).
Fig. 5.
In utero ETOH impaired phagocytosis of both IM and AM. Freshly isolated IM and AM from the 3 experimental groups were incubated with inactivated FITC-labeled S. aureus in a 1:10 ratio (macrophage:bacteria) for 4 h. Phagocytosis was determined using 3-dimensional confocal fluorescent analyses. The cell was evaluated at 50% cell depth and 50% diameter in the z plane and internalization of the FITC-labeled S. aureus was determined. Phagocytic index (PI) was calculated as the percentage of cells with internalized fluorescence × the mean relative fluorescent units internalized per cell (RFU/cell). Bar heights represent mean PI (presented as %control) ± SE as tallied from at least 25 cells/litter from IM (A) and AM (B). aP < 0.05 vs. respective control; bP < 0.05 vs. respective control + SAM-e; cP < 0.05 vs. respective ETOH; n = at least 4 separate litters.
Effect of in vitro TGF-β1 on CD32/CD11b and phagocytic function.
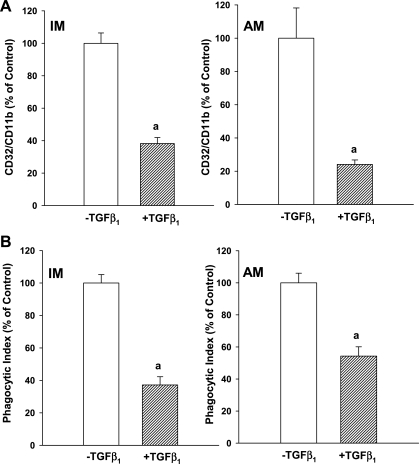
Because of the observed increase in TGF-β1 in the ETOH-exposed fetal lung and cells, we sought to determine the direct effect of TGF-β1 on the function and expression of the differentiation markers in the P0 IM and AM in the neonatal mouse. Direct incubation with TGF-β1 significantly decreased CD32/CD11b immunostaining in the control IM by >60% and the control AM by >70% (Fig. 6A; P < 0.05, vs. without TGF-β1). Furthermore, the TGF-β1-induced decrease in CD32/CD11b expression was paralleled by significantly decreased phagocytosis of FITC-labeled S. aureus by IM (∼60%) and AM (∼45%; Fig. 6B; P < 0.05 vs. without TGF-β1).
Fig. 6.
Direct effect of TGF-β1 on markers of differentiation and phagocytosis. To determine the direct effects of TGF-β1 on the IM or AM phenotype and phagocytic function, control P0 IM and AM were isolated from the pups via BAL. The cells were incubated with ±TGF-β1 added to the culture media (5 ng/ml, 4 h). After culture, the cells were incubated with primary antibodies to CD32 and CD11b with secondary labeling via FITC. In parallel, control P0 cells were incubated ±TGF-β1 ±FITC-labeled S. aureus and phagocytosis was similarly determined. Confocal fluorescent microscopy was used with the fluorescence quantified using digital analysis via FluoView as tallied from at least 25 cells/litter. A: bar heights represent mean RFU/cell ± SE as expressed as a percentage of the control (−TGF-β1) for CD32/CD11b staining for interstitial (left) and alveolar (right) macrophage. aP < 0.05 vs. −TGF-β1; n = 3 separate litters. B: bar heights represent mean PI ± SE for interstitial (left) and alveolar (right) macrophage as expressed as a percentage of the control (−TGF-β1). aP < 0.05 vs. −TGF-β1; n = 3 separate litters.
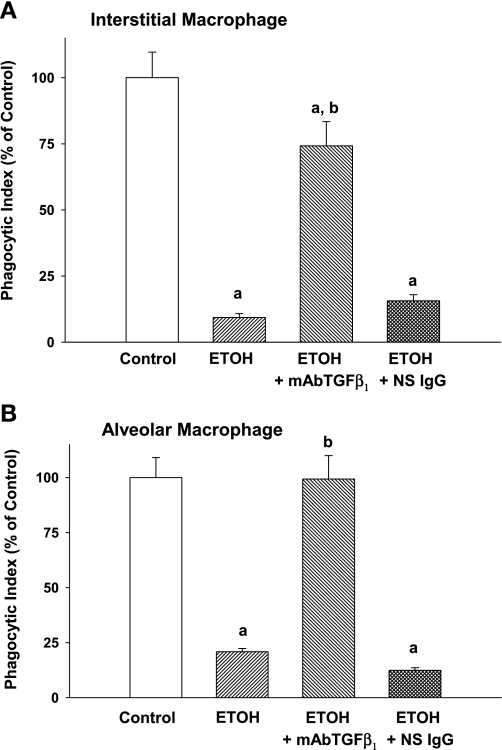
In vitro ETOH impaired phagocytosis via TGF-β1. To further provide a mechanistic link between excessive TGF-β1 demonstrated with in utero ETOH exposure and the observed dysfunction of the exposed macrophage, we exposed IM and AM to in vitro ETOH and evaluated phagocytic function. In vitro ETOH significantly impaired IM phagocytosis by 90% and AM phagocytosis by 79% compared with control (Fig. 7, A and B, respectively; P < 0.05 vs. control). However, the addition of mAbTGF-β1 during the ETOH exposure protected both the IM and the AM from ETOH-induced dysfunction, increasing phagocytosis by the IM to near 75% of control and maintaining AM phagocytosis at control levels despite ETOH exposure (Fig. 7, A and B, respectively; P < 0.05 vs. ETOH). The nonspecific mouse IgG demonstrated no protection to either IM or AM in the presence of ETOH (Fig. 7, A and B, respectively; P < 0.05 vs. control; P = NS vs. ETOH).
Fig. 7.
In vitro ETOH impaired phagocytosis via TGF-β1. Isolated IM and AM from control P0 pups were incubated with or without ETOH (0.1%, 25 mM) added to the culture media in vitro for 4 days with daily media changes. In some experiments, the cells were incubated with or without a monoclonal anti-TGF-β1 antibody (mAbTGF-β1) or a nonspecific IgG. After 4 days, FITC-labeled S. aureus was added and phagocytosis was determined. PI was calculated as the percentage of cells with internalized fluorescence × the mean RFU/cell. Bar heights represent mean PI (presented as %control) ± SE for interstitial (A) and alveolar (B) macrophage. aP < 0.05 vs. control; bP < 0.05 vs. ETOH alone; n = at least 4 separate litters.
DISCUSSION
The goals of this study were to describe the normal terminal differentiation of IM and AM in the neonatal lung and evaluate whether in utero ETOH exposure impaired AM differentiation. In the current study, we characterized the IM and the AM in the P0 mouse pup. A significant gain in the alveolar macrophage marker CD32 compared with the relative monocytic marker CD11b was demonstrated in the AM vs. the IM. This was accompanied by a gain of in vitro phagocytic function by the AM for the control groups. With in utero ETOH exposure, the fetal lung was under increased oxidant stress as evidenced by elevated MDA in the BAL. This oxidant stress was accompanied by ETOH-exposed AM with a phenotype more characteristic of IM, with increased apoptosis, reduced CD32/CD11b expression, and impaired phagocytic function. Furthermore, ETOH-exposed IM also demonstrated increased apoptosis, diminished CD32/CD11b expression, and impaired phagocytosis, suggesting that in utero ETOH adversely affected both the resident AM and their precursor the IM.
With inflammatory states, chronic disease, infection, and lung injury, the heterogeneity of the AM population shifts to a more immature, monocytic phenotype and these changes in AM function can contribute to the severity of the local disease state (9, 23, 32, 39). The current study suggested that in utero alcohol exposure was another condition that altered the resident AM population in the neonatal lung by increasing oxidant stress within the lung and delaying the differentiation of both the IM and the AM. Furthermore, the current results suggested that ETOH-induced oxidant stress mediated these changes, since SAM-e maintained the differentiation of both the IM and the AM in terms of phenotype and function.
Results from these studies strongly implicated a role for elevated TGF-β1 in the observed ETOH-induced changes. ETOH exposure in utero elevated TGF-β1 within the IM as well as the AM in our mouse model. Furthermore, elevated TGF-β1 was apparent in the BAL of the ETOH pup lung at baseline. Exogenous in vitro TGF-β1 suppressed CD32/CD11b in control AM and IM and diminished phagocytic function. Furthermore, a mAbTGF-β1 protected both IM and AM from the impaired phagocytosis demonstrated with in vitro ETOH exposure, suggesting an autocrine role for TGF-β1-induced injury in the ETOH-exposed macrophage. TGF-β1 is well described as an anti-inflammatory mediator, downregulating CD32 in monocytic cells (38), inhibiting phagocytosis (47), and diminishing inflammatory cytokine release (11, 31). Results from the current study suggested that in utero ETOH impaired both IM and AM differentiation and phagocytosis, in part via increased TGF-β1 in the developing cells. Furthermore, attenuating oxidant stress with maternal SAM-e despite ETOH exposure blunted the increased TGF-β1 and resultant alterations in the cells. Although an anti-inflammatory response from ETOH exposure may be perceived as beneficial in the developing lung, such an atmosphere may prove detrimental to the premature lung already at risk for infection (4, 21) and the alcohol-exposed newborn at increased risk for systemic sepsis (17, 18).
We chose to evaluate TGF-β1 as a possible mediator in the ETOH-exposed neonatal lung, since chronic ETOH ingestion increased TGF-β1 primarily in AM and alveolar epithelial type II cells, increasing the risk of cellular dysfunction in the adult lung (3). TGF-β1 plays a crucial role in lung injury and repair in the adult (2), and TGF-β1 has both positive and inhibitory effects on AM differentiation and proliferation (12, 45). However, an inappropriate increase in TGF-β1 in the developing lung, particularly localized to the AM, is a hallmark for the development of bronchopulmonary dysplasia in the premature newborn (2, 25, 28, 42, 48, 49). Conditional overexpression of TGF-β1 postnatally during alveolar development in mice (48) or genetic overexpression in rats (16) resulted in lung injury characteristic of this condition. Thus relative amounts of TGF-β1 are essential for AM differentiation, but excess amounts are detrimental within the neonatal lung. In the in utero ETOH model, there is no overt injury to the neonatal lung at baseline. However, these results suggested that given the excessive total TGF-β1 in the BAL, the ETOH-exposed neonatal lung would be at risk for amplified injury in the face of a second insult. Results from the current study support further investigations to ascertain the risk of neonatal lung injury after in utero alcohol exposure.
The current study expands on the evidence of our previous studies that demonstrated similar AM dysfunction with in utero ETOH exposure, but extended the investigation into the IM precursors. In the neonatal guinea pig exposed to ETOH in utero, AM were dysfunctional at both premature (19) and term gestations (20, 35). These results in the mouse model suggested that in utero ETOH effects were not species specific but a universal finding in the developing animal lung.
Since AM are derived from peripheral circulating blood monocytes that constitutively move into the interstitial space of the lung and differentiate into mature AM in the alveolar space (13, 36), it was important to begin the evaluation of the IM pool of cells as well as the AM in the ETOH-exposed mouse lung. The mouse provides an important model for continued investigation into the detriments of exaggerated oxidant stress, such as in utero ETOH exposure, on the mechanisms that modulate the differentiation and function of the neonatal AM. Given the ETOH-induced changes in the IM pool demonstrated in the current study, we cannot exclude the possibility that in utero ETOH also adversely affected the monocyte and/or the bone marrow of the developing mouse.
In summary, the neonatal mouse model allows for the mechanistic evaluation of terminal differentiation of the neonatal AM. Fetal ETOH exposure impaired neonatal mouse IM and AM phagocytic function, in part due to impaired differentiation of both IM and AM in the lung. Increased oxidant stress and elevated TGF-β1 in the ETOH-exposed fetal lung contributed to the impaired differentiation of the dysfunctional macrophage. In this scenario, altered IM and AM differentiation and dysfunction may contribute to an increased risk of infection and/or lung injury in the developing newborn.
GRANTS
This study was funded by National Institute on Alcohol Abuse and Alcoholism Grants R01-AA-139879 and AA-016348 (to T. W. Gauthier) and P50-AA-135757 (to L. A. S. Brown and T. W. Gauthier) and grants from the Children's Research Center of Children's Healthcare of Atlanta at Egleston (to T. W. Gauthier and L. A. S. Brown).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75: 1037–1050, 1990 [PubMed] [Google Scholar]
- 2.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest 125: 754–765, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med 170: 188–194, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bellanti JA, Zeligs BJ. Developmental aspects of pulmonary defenses in children. Pediatr Pulmonol Suppl 11: 79–80, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Berclaz PY, Zsengeller Z, Shibata Y, Otake K, Strasbaugh S, Whitsett JA, Trapnell BC. Endocytic internalization of adenovirus, nonspecific phagocytosis, and cytoskeletal organization are coordinately regulated in alveolar macrophages by GM-CSF and PU1. J Immunol 169: 6332–6342, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS, Thomassen MJ. PU1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol 285: L1132–L1136, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brown LA, Harris FL, Guidot DM. Chronic ethanol ingestion potentiates TNF-α-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol 281: L377–L386, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol 292: L824–L832, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Calhoun WJ, Salisbury SM. Heterogeneity in cell recovery and superoxide production in buoyant, density-defined subpopulations of human alveolar macrophages from healthy volunteers and sarcoidosis patients. J Lab Clin Med 114: 682–690, 1989 [PubMed] [Google Scholar]
- 10.Chandler DB, Fuller WC, Jackson RM, Fulmer JD. Fractionation of rat alveolar macrophages by isopycnic centrifugation: morphological, cytochemical, biochemical, and functional properties. J Leukoc Biol 39: 371–383, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan K, Ruan Q, Sensenbrenner L, Chen B. Transforming growth factor-beta 1 bifunctionally regulates murine macrophage proliferation. Blood 79: 1679–1685, 1992 [PubMed] [Google Scholar]
- 13.Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol 60: 353–369, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol 121: 1372–1378, 1378.e1–1378.e3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke-Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes ML, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol 157: 3097–3104, 1996 [PubMed] [Google Scholar]
- 16.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauthier TW, Drews-Botsch C, Falek A, Coles C, Brown LA. Maternal alcohol abuse and neonatal infection. Alcohol Clin Exp Res 29: 1035–1043, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Gauthier TW, Manar MH, Brown LA. Is maternal alcohol use a risk factor for early-onset sepsis in the premature newborn? Alcohol 33: 139–145, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Gauthier TW, Ping XD, Harris FL, Wong M, Elbahesh H, Brown LA. Fetal alcohol exposure impairs fetal alveolar macrophage function via decreased glutathione availability. Pediatr Res 57: 76–81, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gauthier TW, Young PA, Gabelaia L, Tang SM, Ping XD, Harris FL, Brown LA. In utero ethanol exposure impairs defenses against experimental group b streptococcus in the term guinea pig lung. Alcohol Clin Exp Res 33: 300–306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall SL, Sherman MP. Intrapulmonary bacterial clearance of type III group B streptococcus is reduced in preterm compared with term rabbits and occurs independent of antibody. Am Rev Respir Dis 145: 1172–1177, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Jr, Nicholson AC. Transforming growth factor-beta1 (TGF-beta1) and TGF-beta2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-gamma. J Biol Chem 275: 1241–1246, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hance AJ, Douches S, Winchester RJ, Ferrans VJ, Crystal RG. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol 134: 284–292, 1985 [PubMed] [Google Scholar]
- 24.Holian A, Dauber JH, Diamond MS, Daniele RP. Separation of bronchoalveolar cells from the guinea pig on continuous gradients of Percoll: functional properties of fractionated lung macrophages. J Reticuloendothel Soc 33: 157–164, 1983 [PubMed] [Google Scholar]
- 25.Kotecha S. Cytokines in chronic lung disease of prematurity. Eur J Pediatr 155, Suppl 2: S14–17, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res 55: 764–768, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med 171: 73–77, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kwong KY, Niang S, Literat A, Zhu NL, Ramanathan R, Jones CA, Minoo P. Expression of transforming growth factor beta (TGF-b1) by human preterm lung inflammatory cells. Life Sci 79: 2349–2356, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol 70: 163–170, 2001 [PubMed] [Google Scholar]
- 30.Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol 173: 3398–3407, 2004 [DOI] [PubMed] [Google Scholar]
- 31.McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol 163: 6164–6172, 1999 [PubMed] [Google Scholar]
- 32.Meyer KC, Powers C, Rosenthal N, Auerbach R. Alveolar macrophage surface carbohydrate expression is altered in interstitial lung disease as determined by lectin-binding profiles. Am Rev Respir Dis 148: 1325–1334, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Moffat FL, Jr, Han T, Li ZM, Peck MD, Jy W, Ahn YS, Chu AJ, Bourguignon LY. Supplemental l-arginine HCl augments bacterial phagocytosis in human polymorphonuclear leukocytes PG-26–33. J Cell Physiol 168: 26–33, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Oghiso Y. Morphologic and functional heterogeneity among rat alveolar macrophage fractions isolated by centrifugation on density gradients. J Leukoc Biol 42: 188–196, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Ping XD, Harris FL, Brown LA, Gauthier TW. In vivo dysfunction of the term alveolar macrophage after in utero ethanol exposure. Alcohol Clin Exp Res 31: 308–316, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol 156: 191–211, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Prokhorova S, Lavnikova N, Laskin DL. Functional characterization of interstitial macrophages and subpopulations of alveolar macrophages from rat lung. J Leukoc Biol 55: 141–146, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Reterink TJ, Klar-Mohamad N, Nibbering PH, van Es LA, Daha MR. CD32 expression and signaling is down-regulated by transforming growth factor-beta 1 on human monocytes. Eur J Immunol 26: 1970–1973, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Rosseau S, Hammerl P, Maus U, Walmrath HD, Schutte H, Grimminger F, Seeger W, Lohmeyer J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 279: L25–L35, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Senft AP, Korfhagen TR, Whitsett JA, LeVine AM. Surfactant protein D regulates the cell surface expression of alveolar macrophage beta(2)-integrins. Am J Physiol Lung Cell Mol Physiol 292: L469–L475, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sokol RJ, Janisse JJ, Louis JM, Bailey BN, Ager J, Jacobson SW, Jacobson JL. Extreme prematurity: an alcohol-related birth effect. Alcohol Clin Exp Res 31: 1031–1037, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol 8: 29–38, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Standiford TJ, Kunkel SL, Lukacs NW, Greenberger MJ, Danforth JM, Kunkel RG, Strieter RM. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol 155: 1515–1524, 1995 [PubMed] [Google Scholar]
- 44.Stoll BJ, Holman RC, Schuchat A. Decline in sepsis-associated neonatal and infant deaths in the United States, 1979 through 1994. Pediatrics 102: e18, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Strassmann G, Cole MD, Newman W. Regulation of colony-stimulating factor 1-dependent macrophage precursor proliferation by type beta transforming growth factor. J Immunol 140: 2645–2651, 1988 [PubMed] [Google Scholar]
- 46.Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcohol Clin Exp Res 25: 1766–1772, 2001 [PubMed] [Google Scholar]
- 47.Tridandapani S, Wardrop R, Baran CP, Wang Y, Opalek JM, Caligiuri MA, Marsh CB. TGF-beta 1 suppresses [correction of supresses] myeloid Fc gamma receptor function by regulating the expression and function of the common gamma-subunit. J Immunol 170: 4572–4577, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 31: 650–656, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Viscardi R, Manimtim W, He JR, Hasday JD, Sun CC, Joyce B, Pierce RA. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol 9: 143–151, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Zhou H, Moscatello KM, Dixon C, Brunson LE, Chervenak R, Chervenak DC, Zhao X, Wolcott RM. In utero exposure to alcohol alters cell fate decisions by hematopoietic progenitors in the bone marrow of offspring mice during neonatal development. Cell Immunol 239: 75–85, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Wolcott RM, Jennings SR, Chervenak R. In utero exposure to ethanol affects postnatal development of T- and B-lymphocytes, but not natural killer cells. Alcohol Clin Exp Res 19: 170–176, 1995 [DOI] [PubMed] [Google Scholar]