Abstract
Alveolar surfactant protein A (SP-A) is endocytosed by type II epithelial cells through clathrin-dependent uptake and targeted to lamellar bodies for resecretion. However, the mechanism for secretion of newly synthesized SP-A, whether regulated exocytosis of lamellar bodies or constitutive secretion, is unresolved. If it is the latter, lamellar body SP-A would represent endocytosed protein. Amantadine, an inhibitor of clathrin-coated vesicle budding, was used to evaluate the role of endocytosis in accumulation of SP-A in lamellar bodies. In isolated rat lungs, amantadine (10 mM) inhibited uptake of endotracheally instilled 35S-labeled biosynthesized surfactant proteins by >80%. To study trafficking of newly synthesized SP-A, lungs were perfused for up to 6 h with [35S]methionine, and surfactant was isolated from lung lavage fluid and lamellar bodies were isolated from lung homogenate. With control lungs, the mean specific activity of [35S]SP-A (disintegrations per minute per microgram of SP-A) increased linearly with time of perfusion: it was significantly higher in isolated lamellar bodies than in surfactant and was increased in both compartments by 50–60% in the presence of 0.1 mM 8-bromo-cAMP. These results suggest a precursor-product relationship between lamellar body and extracellular [35S]SP-A. Specific activities in both compartments were unaffected by addition of amantadine (10 mM) to the lung perfusate, indicating that uptake from the alveolar space was not responsible for the increase in lamellar body [35S]SP-A. Thus the pathway for secretion of newly synthesized SP-A is by transfer from the site of synthesis to the storage/secretory organelle prior to lamellar body exocytosis.
Keywords: protein trafficking, surfactant secretion, alveolar epithelial type II cell, protein synthesis, clathrin-dependent endocytosis
there is general agreement that lung surfactant phospholipids and surfactant proteins B and C (SP-B and SP-C) are synthesized in the endoplasmic reticulum of lung alveolar type II epithelial cells and transported to the lamellar bodies for processing and storage prior to secretion into the alveolar space. However, there is less agreement concerning the intracellular trafficking of SP-A. Like SP-B and SP-C, SP-A is synthesized by type II cells and is secreted into the alveolar space, where it associates with the lung surfactant phospholipids (19, 22, 37). Extracellular SP-A appears to play crucial roles in the formation of tubular myelin, in the regulation of lamellar body exocytosis, and in the uptake of phospholipids by endocytosis and their subsequent remodeling (20, 23, 30, 40). The uptake process is dependent on the association of phospholipids with SP-A, followed by binding of the SP-A-phospholipid complex to a specific cell membrane-localized SP-A receptor (4, 5, 16), and then internalization of the complex by a clathrin-dependent pathway (21, 32, 35). Although the pathway for uptake of SP-A seems to be fairly well understood, there is uncertainty regarding the pathway for secretion of newly synthesized SP-A by type II cells.
Initial autoradiographic studies of protein synthesis and trafficking in type II cells indicated transport from endoplasmic reticulum to Golgi and then to multivesicular bodies that subsequently fused with lamellar bodies prior to secretion (10). However, these studies were carried out prior to the molecular identification of SP-A. Subsequent studies indicated that the kinetics for appearance of SP-A and phospholipids in the surfactant were similar (22) and that surfactant secretagogues had a similar effect on exocytosis of SP-A and phospholipid (13, 37). These studies were interpreted to indicate transfer of newly synthesized SP-A from the endoplasmic reticulum to storage organelles (lamellar bodies) prior to exocytosis, similar to the pathways for processing of lung surfactant phospholipid. However, more recent studies using the intact lung (11, 19) or isolated type II cells (28, 31) have indicated possible differences in kinetics for the appearance of SP-A in surfactant and lamellar bodies. These latter publications have suggested an alternative pathway in which newly synthesized SP-A is secreted constitutively, i.e., by a nonregulated secretory pathway. In this scenario, the presence of SP-A in lamellar bodies is accounted for entirely by recycling following endocytosis of extracellular SP-A (11, 19, 28, 29, 36). Because of its cellular complexity, it has been difficult to evaluate the relative roles for these two potential pathways (regulated vs. constitutive secretion) in the intact lung, and the majority of recent studies related to intracellular SP-A trafficking have been carried out with isolated type II cells (28, 29, 31). Unfortunately, these cells in primary culture differentiate relatively rapidly following their isolation and appear to transform into type I cells, which lack the machinery for SP-A synthesis and trafficking. Thus the suitability of these cells for study of the intracellular SP-A trafficking pathways is uncertain.
Our prior studies have utilized the isolated perfused lung to evaluate the endocytic uptake (recycling) of radiolabeled SP-A following endotracheal instillation (15, 21). These studies have demonstrated disappearance of radiolabeled SP-A from the alveolar space and its appearance in lamellar bodies. Treatment of isolated lungs with amantadine, an inhibitor of clathrin-mediated endocytosis, markedly inhibited the uptake of radiolabeled SP-A (as well as associated phospholipids) from the alveolar space (21, 32). If the secretory pathway for newly synthesized SP-A is through nonregulated (constitutive) secretion, the inhibition of uptake from the alveolar space should prevent the appearance of radiolabeled protein in the lamellar bodies. On the other hand, if newly synthesized SP-A is transferred to lamellar bodies prior to secretion, amantadine should have no effect. In the present study, we have evaluated the appearance of newly synthesized SP-A in lamellar bodies and lung surfactant in the presence and absence of an inhibitor of SP-A endocytosis (amantadine) to gain insight into the SP-A trafficking pathway.
METHODS
Male Sprague-Dawley rats (180–220 g body wt) were obtained from Charles River Breeding Laboratories (Kingston, NY). All protocols for animal use were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Rats were anesthetized with pentobarbital sodium (30 mg/kg ip), the trachea was cannulated, and lungs were continuously ventilated and cleared of blood by perfusion through the pulmonary artery. The cleared lungs were used to isolate alveolar type II epithelial cells for histological analysis of SP-A or were placed in the isolated lung perfusion chamber for study of SP-A uptake.
Alveolar type II epithelial cells were isolated by digestion of lungs with elastase (Worthington, Lakewood, NJ) followed by panning on IgG-coated dishes and overnight culture on tissue culture plastic (Costar, Sigma-Aldrich, St. Louis, MO), as described previously (3). For immunocytochemistry, cells were fixed for 5 min in ice-cold methanol-acetone (1:1), treated with 5% BSA-10% goat serum in PBS, incubated with anti-SP-A polyclonal antibody (PAb) (13) and anti-ABCA3 monoclonal antibody (MAb 3C9) (44) for 2 h, incubated for 1 h with FITC-labeled goat anti-mouse and Texas red-labeled goat anti-rabbit antibodies (Invitrogen, Carlsbad, CA), and then washed with PBS. Staining for ABCA3 was used as a marker for lamellar body membranes (26). In some experiments, 0.3% Triton X-100 (Roche, Mannheim, Germany) was added to the blocking and wash solutions. In other experiments, cells were fixed with ice-cold 4% paraformaldehyde (EMS, Hatfield, PA) for 10 min. After the final wash, coverslips were air-dried and mounted with Mowiol (Vector Labs, Burlingame, CA) on glass slides. Details concerning the immunofluorescence methods are given in Table 1.
Table 1.
Immunofluorescence methods
| Method 1 | Method 2 | Method 3 | |
|---|---|---|---|
| Fixative (time) | 4% Paraformaldehyde-PBS, ice-cold (10 min) | Methanol-acetone (1:1), ice-cold (5 min) | Methanol-acetone (1:1), ice-cold (5 min) |
| Permeabilization | PBS-0.3% Triton X-100 | (Methanol-acetone) | (Methanol-acetone) |
| Blocking | Solution A: PBS-5% BSA-10% NGS-0.3% Triton X-100 | Solution A | Solution B: PBS-5% BSA-10% NGS (no Triton X-100) |
| Primary Abs (time) | MAb 3C9-anti SP-A in solution A (2 h) | MAb 3C9-anti SP-A in solution A (2 h) | MAb 3C9-anti SP-A in solution B (2 h) |
| Secondary Abs (time) | FITC-GAM/TXRD-GAR in solution A (1 h) | FITC-GAM/TXRD-GAR in solution A (1 h) | FITC-GAM/TXRD-GAR in solution B (1 h) |
| Washes | PBS + Triton X-100 | PBS + Triton X-100 | PBS |
Abs, antibodies; SP-A, surfactant protein A; NGS, normal goat serum; GAM, goat anti-mouse antibody; GAR, goat anti-rabbit antibody; TXRD, Texas red.
For intact-lung studies, isolated lungs in the perfusion chamber were continuously perfused for 1–6 h with 40 ml of recirculating Krebs-Ringer bicarbonate medium containing 5% fatty acid-free BSA and 10 mM glucose at pH 7.4 and 37°C, as described previously (13, 21, 32). 35S-translabel (200 μCi; MP Biomedicals, Santa Ana, CA) was added to the perfusate, which also contained, depending on the experiment, 8-bromo-cAMP (8-BrcAMP, 100 μM) and amantadine (generally 10 mM; both from Sigma-Aldrich, St. Louis, MO). 35S-translabel contains ∼85% [35S]methionine and ∼15% [35S]cysteine; for simplicity, we will refer to the substrate as [35S]methionine. To measure SP-A trafficking, the appearance of [35S]SP-A was measured in the lamellar body and surfactant compartments. At the end of perfusion, lungs were lavaged through the trachea five times, each with 7 ml of PBS. The aliquots of lung lavage fluid were combined and used to isolate a lung surfactant fraction by NaCl-NaBr density gradient centrifugation (37). After the lungs were lavaged, they were homogenized using a Polytron apparatus, and a lamellar body-enriched fraction was isolated by upward flotation on a sucrose density gradient (8). To subfractionate the organelles, isolated lamellar bodies were frozen overnight in a hypotonic solution of 50 mM sucrose in 10 mM Tris·HCl (pH 7.2); after they were thawed, the disrupted lamellar body samples were loaded on a cushion of 0.5 M sucrose (3 ml) and centrifuged for 1 h at 100,000 g using a swinging-bucket rotor (44).
To determine specific activity of [35S]SP-A, the SP-A protein content and the disintegrations per minute (dpm) associated with SP-A were measured in the surfactant and lamellar body fractions. The content of SP-A in these fractions was calculated on the basis of gel electrophoresis, as described previously (6) and shown in Fig. 1. Total protein in the subcellular fractions was measured by the Coomassie blue reagent assay (Bio-Rad), with bovine γ-globulin used as standard. The fraction of total surfactant protein represented by SP-A was estimated from Coomassie blue-stained gels obtained by SDS-PAGE (10% Bis-Tris) under reducing conditions (50 mM dithiothreitol). A second gel run in parallel was transferred to a nitrocellulose membrane and subjected to Western blotting to confirm the identity of the SP-A bands. After the membrane was blocked with 3% nonfat milk in Tris-buffered saline (TBS), it was incubated with the SP-A pAb in a solution of 1.5% milk and TBS containing 0.1% Tween 20 (TTBS) for 2 h, washed in TTBS, and incubated with goat anti-rabbit secondary antibody. For visualization of the protein bands, the Odyssey infrared scanner (Li-Cor Bioscience, Lincoln, NE) was used according to the manufacturer's instructions. Density of the Coomassie blue-stained bands was quantitated by scanning using computer-assisted densitometric software (Image J software), and the percentage of the total protein corresponding to the SP-A bands was calculated. This method gave values for percent SP-A content that are similar to our previous report, where SP-A was quantitated by ELISA (37). The individual SP-A bands were cut from the gels and combined; dpm were measured by scintillation counting, and the specific activity was calculated as dpm per microgram of SP-A.
Fig. 1.
Representative SDS-polyacrylamide gel with Coomassie blue staining and Western blot analysis using anti-surfactant protein A (SP-A) antibody for lamellar bodies and lung surfactant. Samples were isolated following a 6-h perfusion period under basal conditions without (lane 1) or with (lane 2) addition of 10 mM amantadine to the perfusate. Images were scanned using the Odyssey system, and results are shown as “Quantitation.” MW lane shows molecular mass standards, with size shown in kDa.
The effect of amantadine on uptake of radiolabeled SP-A from the air spaces was determined using a previously described method (15, 21). Biosynthesized radiolabeled natural surfactant was prepared by perfusion of lungs for 6 h in the presence of 8-BrcAMP with 35S-translabel added to the perfusate. The lung surfactant fraction was isolated, and analysis by SDS-PAGE indicated that ∼50% of the recovered dpm was present in SP-A. To measure uptake, the biosynthesized radiolabeled surfactant (1.4 × 105 dpm in 0.1 ml of saline) was instilled into the trachea of anesthetized rats; then the lungs were immediately isolated and perfused for 2 h in the presence of 8-BrcAMP (100 μM) and varying concentrations of amantadine. At the end of perfusion, the lungs were lavaged to remove unincorporated dpm, homogenized, and analyzed for dpm. Uptake of 35S-labeled protein was calculated from the dpm in lung homogenate as a percentage of the total dpm instilled.
RESULTS
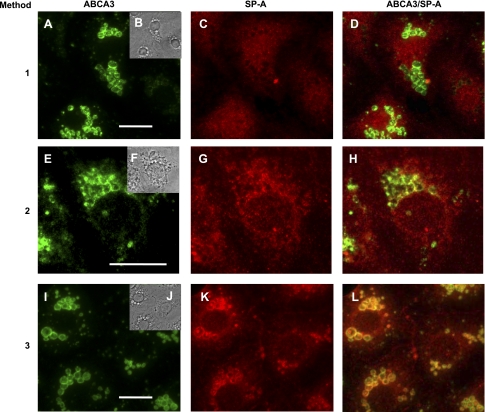
Our initial investigation addressed whether lamellar bodies contain SP-A, since other reports noted difficulties in the immunohistochemical evaluation of lamellar bodies for this protein (2, 24, 42). An mAb to ABCA3, a 180-kDa protein that is present in the limiting membrane of lamellar bodies, was used to identify and localize lamellar bodies (Fig. 2) (26, 44). SP-A was identified using a pAb and was readily visualized in the cytoplasm (presumably in vesicles) of isolated type II cells at 24 h in culture (Fig. 2). However, there was no reaction to the anti-SP-A antibody within lamellar bodies and only weak colocalization of SP-A with the outer lamellar body membrane marker protein (ABCA3) when the cell samples were fixed/permeabilized with paraformaldehyde-Triton X-100 (method 1, Fig. 2, A–D). When a modified fixation technique with ice-cold methanol-acetone (50:50) in the presence of Triton X-100 was employed, there was distinct colocalization of SP-A and ABCA3 on the membrane region of the type II lamellar body-like structures but no visible intralamellar body SP-A (method 2, Fig. 2, E–H). However, when methanol-acetone without Triton X-100 was used (method 3, Fig. 2, I–L), colocalization of SP-A and ABCA3 in the lamellar body periphery was obvious, and bright fluorescence was visible within the organelles, indicating immunoreaction with SP-A.
Fig. 2.
Immunofluorescent localization of SP-A in lamellar bodies of isolated type II cells. Type II cells were fixed and permeabilized using methods 1, 2, and 3 (see Table 1). Cells were labeled with the primary antibodies to ABCA3 [monoclonal antibody 3C9 (A, E, and I)] or to SP-A [rabbit polyclonal anti-rat SP-A (C, G, and K)] and secondary antibodies FITC-goat anti-mouse antibody (green) or Texas red-goat anti-rabbit antibody (red). Insets (B, F, and J): phase images of matching cell samples. ABCA3/SP-A, merged images of ABCA3 and SP-A (D, H, and L). A–D correspond to method 1, E–H to method 2, and I–L to method 3. Scale bar, 10 μm.
To substantiate the immunolocalization of SP-A, a lamellar body-enriched fraction was isolated from rat lungs, and the amount of associated SP-A was compared with that found in lysates of isolated rat type II cells. Western blotting showed that lamellar bodies were greatly enriched in SP-A on a protein basis compared with type II cells, indicating accumulation within the storage organelles (Fig. 3A). To determine localization within the organelles, lamellar bodies were subfractionated in a sucrose gradient. Four distinct layers formed: the supernatant with soluble proteins, the lipid layer with lipid-associated proteins, the 0.5 M sucrose cushion, and the pellet with membrane-associated proteins (Fig. 3B). Western blot analysis of samples loaded on an equal-protein basis indicated that the bulk of the SP-A was associated with the lipid fraction, with some SP-A recovered with the membrane pellet and even less in the soluble protein fraction (Fig. 3B).
Fig. 3.
SP-A protein in rat isolated type II (TII) cells and lung lamellar bodies (LBs). A: freshly isolated type II cells and lamellar bodies isolated from whole rat lung were run on 10% gels using SDS-PAGE techniques under reducing conditions and transferred to nitrocellulose membranes. Amount of protein loaded per lane is indicated. Membranes were probed with an antibody against SP-A using Western blot procedures. B: lamellar bodies were lysed in hypertonic sucrose by freeze-thawing and subfractionated by centrifugation over a sucrose cushion. Soluble fraction (sol), lipid band, and pellet were run on an SDS-polyacrylamide gel and probed with an antibody against SP-A. Type II whole cell lysate was used as a standard (TII std).
The isolated perfused lung preparation was used to study SP-A trafficking. As an initial experiment, we evaluated whether amantadine would inhibit the cellular uptake of intratracheally instilled 35S-labeled protein in biosynthesized natural rat lung surfactant, as we demonstrated previously for iodinated human SP-A (21). Amantadine was added to the perfusate at concentrations of 2.5–15 mM. Uptake of 35S-labeled protein at 2 h of isolated lung perfusion under control conditions was ∼45% of the instilled dpm (Table 2), similar to our previously reported values (15). Uptake was decreased significantly by amantadine, with maximal inhibition (82%) at 10 mM (Fig. 4, Table 2). All further studies were carried out with this concentration. Thus, amantadine essentially prevents SP-A trafficking from the alveolar space to intracellular organelles.
Table 2.
Effect of amantadine on uptake of 35S-labeled protein from alveolar space into tissue of isolated perfused rat lung
| Uptake in 2 h | |
|---|---|
| Control | 44.6 ± 0.7 |
| Amantadine (10 mM) | 8.1 ± 0.5* |
Values are means ± SE (n = 3), expressed as percentage of instilled disintegrations per minute.
P < 0.05 vs. control.
Fig. 4.
Effect of varying concentrations of amantadine in lung perfusate on uptake of 35S-labeled surfactant during 2 h of isolated rat lung perfusion. Uptake was calculated from disintegrations per minute (dpm) present in lung tissue following lavage as a percentage of initial dpm instilled into the lungs. Each point represents a separate isolated perfused lung preparation.
The next step was to measure the incorporation of [35S]methionine into the lung surfactant and lamellar body fractions. Measurement of 35S specific activity in lung fractions required measurement of SP-A content. Under basal and 8-BrcAMP-stimulated conditions, SP-A comprised ∼35% of the total protein in lung surfactant and ∼10% of the total protein in lung lamellar bodies (Table 3). Amantadine treatment did not alter the level of SP-A in the lung compartments (Table 3). Incorporation of [35S]methionine into SP-A in the lung lamellar body and the lung surfactant fractions increased progressively during 1–6 h of perfusion of lungs in the presence of 8-BrcAMP (Fig. 5, control). The addition of amantadine to the perfusate had no effect on the rate of increase of 35S incorporation with time. An expanded study (i.e., more lungs) of the 6-h perfusion time point confirmed the similar results for the presence and absence of amantadine (Fig. 6). Lung perfusion in the absence of secretagogue gave lower rates for 35S incorporation and, like the stimulated rate, showed no effect of amantadine (Fig. 6). For basal (Fig. 6) and 8-BrcAMP-stimulated (Figs. 5 and 6) conditions, the calculated specific activity for [35S]methionine in SP-A was ∼50% greater in lamellar bodies than surfactant.
Table 3.
SP-A content of rat lung surfactant and lamellar bodies
| Lung Surfactant | Lung Lamellar Bodies | |
|---|---|---|
| Control | 34.3 ± 0.6 | 10.9 ± 0.6 |
| +8-BrcAMP | 34.6 ± 0.8 | 10.5 ± 0.5 |
| Amantadine | 32.8 ± 0.7 | 10.6 ± 0.3 |
| +8-BrcAMP | 33.6 ± 0.5 | 10.1 ± 0.6 |
Values are means ± SE, expressed as percentage of total protein. SP-A content was measured after perfusion of isolated lungs for 6 h and was determined by densitometry of polyacrylamide gels. 8-BrcAMP, 8-bromo-cAMP.
Fig. 5.
Time course for the effect of amantadine on incorporation of [35S]methionine into the SP-A fraction of lamellar bodies (A) and surfactant (B). Lungs were perfused for 1–6 h following the addition of 35S-translabel to the lung perfusate in the absence (control) or presence (+Amantadine) of 10 mM amantadine. Lung perfusate contained 100 μM 8-bromo-cAMP. At the end of perfusion, lung surfactant was isolated from lung lavage fluid and lamellar bodies from postlavage lung homogenate. Fractions were analyzed for SP-A content and dpm for calculation of specific activity. Each point is a separate lung perfusion experiment. Separate regression lines for values obtained in the absence (solid line) and presence of amantadine (dashed line) are drawn by the least-mean-squares method.
Fig. 6.
Effect of amantadine (10 mM) on incorporation of [35S]SP-A into lamellar bodies or surfactant. Lungs were perfused for 6 h in the absence (A) or presence (B) of a secretagogue, 8-bromo-AMP (100 μM), and in the absence (control) or presence of amantadine (10 mM). SP-A specific activity was calculated as described in Fig. 5 legend. Values are means ± SE (n = 6 for basal and n = 3 for cAMP). For cAMP values, results include 6-h perfusion experiments shown in Fig. 5. *P < 0.05 vs. corresponding value for surfactant.
DISCUSSION
This study was designed specifically to determine the trafficking of newly synthesized SP-A in the type II cell. One possible pathway involved SP-A synthesis in the endoplasmic reticulum followed by transport directly to lamellar bodies for storage and subsequent secretion. An alternative is that SP-A after synthesis enters a vesicular pathway and is secreted constitutively. If the latter pathway is the only route for secretion, the presence of SP-A in lamellar bodies would result from its reuptake from the alveolar space by endocytosis. It has been shown that inhibitors of oligosaccharide processing in the Golgi apparatus inhibit the appearance of SP-A in lamellar bodies (1), although these results would not differentiate between protein that is sorted into a regulated vs. a constitutive secretory pathway. This present study used amantadine, an inhibitor of endocytotic uptake of SP-A (21), to examine the relative role of these two pathways.
Several contentious issues in the literature require examination before focusing on the pathway for SP-A trafficking. The first is whether lamellar bodies contain SP-A, since difficulties in visualizing SP-A in lamellar bodies by microscopy have raised questions as to whether SP-A is actually located within these organelles. Immunohistochemistry of lung tissue (25, 42) and of isolated cells by light microscopy (24, 41) has demonstrated SP-A in type II cells, but the lamellar bodies generally appear as empty vesicles that fail to label with anti-SP-A antibody. Studies at the electron-microscopic level using immunogold techniques have produced similar results: gold-labeled anti-SP-A antibody could be localized to the lamellar body membrane, but with few exceptions, very little gold labeling was observed within the lamellar body contents (12, 27, 33, 34, 38). It has been recognized that this relatively poor detection of SP-A may be due to various technical issues (17, 38). Access of antibodies to antigenic sites could be limited by the presence of compact phospholipid lamellae within the lamellar bodies. Treatment of tissue or cells with Triton X-100, as generally used to permeabilize cells, can extract phospholipids and its associated SP-A. A brief (5- to 10-min) treatment with Triton X-100 did increase gold-labeled SP-A antibody particles over lamellar bodies, but longer treatments decreased particle numbers (38). In view of these observations, we developed a procedure to fix and permeabilize isolated type II cells in a single step that did not utilize Triton X-100. With this method, SP-A colocalized with ABCA3 on the membrane of the lamellar body-like vesicles and was present within the ABCA3-positive organelles. Western blot analysis of an isolated lamellar body-enriched fraction demonstrated the presence of SP-A and showed significant enrichment over whole lung content of the protein. Localization of SP-A within the lipid-rich environment of the internal compartment of the lamellar body was confirmed by identification of SP-A in the lipid fraction of the disrupted organelles. Thus the present studies indicate the presence of SP-A within the lamellar body contents.
A second question germane to the discussion is whether SP-A that is internalized by type II cells via endocytosis is targeted to lamellar bodies or is directed to other organelles. A relatively recent study demonstrated internalization of SP-A by isolated type II cells but failed to detect localization of the endocytosed protein in lamellar bodies (39). This latter study postulated the rapid recycling (resecretion) of endocytosed SP-A through an early endosome. On the other hand, studies of the intact lung following endotracheal instillation of labeled SP-A have demonstrated its uptake from the alveolar space and subsequent appearance in lamellar bodies (15, 18, 19), as confirmed in the present study. One possibility for these different results is that the isolated type II cells had lost the mechanism for normal SP-A trafficking to lamellar bodies, consistent with their differentiation in culture and loss of other phenotypic characteristics. Another possibility is that uptake of SP-A into submembrane vesicles and its resecretion occurs on a rapid time scale with a slower progress to lamellar bodies. Indeed, a longer-duration (6-h) study of isolated type II cells did show uptake of extracellular SP-A into lamellar bodies (28). Thus, endocytosis leading to lamellar body accumulation of SP-A would appear to be physiological, although these results by themselves do not exclude internalization into an intermediate compartment such as early endosomes (39) or multivesicular bodies (43) and do not reflect the pathway for secretion of newly synthesized protein.
As a first step to evaluate SP-A trafficking in this study, we utilized radiolabeled biosynthesized rat surfactant to confirm that amantadine treatment inhibits uptake of surfactant proteins from the alveolar space. Biosynthesized rat 35S-labeled surfactant protein was used for these studies and should represent a more physiological preparation (for the study of rat lungs) than the iodinated human SP-A from alveolar proteinosis patients, which was used in our previous experiments (21). The results obtained with these two different preparations were similar, confirming that amantadine inhibits alveolar uptake of SP-A by >80%. The previous study showed similar inhibition of SP-A uptake by amantadine and phenylarsine oxide, another inhibitor of clathrin-mediated endocytosis (21). We chose amantadine for this study, because toxic effects within the inhibitory dose range have not been described for this agent, whereas phenylarsine oxide can also inhibit metabolism and ATP generation.
For the next phase of the study, isolated lungs were perfused with [35S]methionine for up to 6 h and the incorporation of [35S]SP-A into surfactant and lamellar body fractions was analyzed. Several characteristics of the SP-A labeling are noteworthy. First, consistent with our previous study (6), a greater lag was noted in the appearance of 35S in surfactant SP-A than in lamellar body SP-A; the inverse might be expected if reuptake from the alveolar space were the only mechanism for SP-A incorporation into these organelles. Another study from our laboratory evaluating [3H]phenylalanine incorporation into surfactant SP-A showed an even greater lag than was shown by our 32S results, but a time course for lamellar bodies was not determined (13). The greater lag noted for 3H incorporation is perhaps related to the differential handling of the two labeled amino acids (phenylalanine vs. methionine).
As a second observation of labeling, the estimated lamellar body specific activity was higher than surfactant specific activity under basal and cAMP-stimulated conditions, compatible with lamellar body SP-A as the source of the extracellular protein. Of course, calculation of specific activity does depend on accurate measurement of SP-A content; the present results for SP-A show good agreement with past results using a different (ELISA) method (37). As a third observation, SP-A specific activity in lamellar bodies and surfactant was increased by the presence of 8-BrcAMP. This observation is compatible with, although not specific for, regulated secretion, since cAMP also regulates SP-A synthesis and, possibly, endocytosis (9, 13, 15, 20, 37).
Finally and most importantly, treatment with amantadine markedly inhibited uptake of [35S]SP-A from the alveolar space but had no effect on specific activity of newly synthesized SP-A in the lamellar body fraction. On the basis of the results with amantadine, uptake of radiolabeled SP-A from the alveolar space could not be solely responsible for the appearance of radiolabeled SP-A in lamellar bodies. However, while amantadine is a clinically approved drug and a relatively nontoxic inhibitor, its precise mechanism related to endocytosis and its possible effects on other cellular functions are not fully understood. Thus the usual caveats related to the use of inhibitors apply, namely, basing conclusions solely on the use of one inhibitor (amantadine) poses a potential limitation in data interpretation. On the other hand, it is difficult to envision possible side effects of amantadine as a mechanism for the lack of effect on SP-A appearance in lamellar bodies.
The interpretation of results also depends on the nature of the isolated subcellular fractions, i.e., surfactant and lamellar bodies. Clearly, it is not possible to isolate absolutely pure fractions; in any event, the organelles, and even the extracellular material, may represent heterogeneous populations. If so, it is possible that only some lamellar bodies are destined to contain newly synthesized SP-A; there is insufficient evidence at present to support that possibility. Our prior measurements of the isolated lamellar body fraction showed a phospholipid-to-protein ratio >9 and minimal contamination on analysis by electron microscopy and by measurement of the content of marker enzymes for other subcellular organelles (6–8, 15). Furthermore, disaturated phosphatidylcholine represented 66% of total phosphatidylcholine in surfactant and 62–69% in lamellar bodies (7, 14). These results suggest sufficient purity of the preparations to preclude contamination as a major influence on the results.
The cumulative evidence presented here indicates that newly synthesized SP-A is transported to lamellar bodies, where it is available for secretion into the extracellular space. These results conflict with the labeling kinetics observed in lungs of intact rabbits (19) and lambs (18), where labeling of surfactant preceded labeling of lamellar bodies. We, like the authors of the previous study, are unable to fully interpret the previously observed kinetics, which might have been hampered by the lower labeling efficiency in the intact animal. It would seem unlikely that the diametrically opposed conclusions between the previous and present results are based on species (rats vs. rabbits/lambs) or preparation (intact lung vs. intact animal). In any event, the present results using amantadine to block endocytosis of SP-A indicate that alveolar uptake is not the major source of lamellar body SP-A. Thus the primary pathway for secretion of newly synthesized SP-A must be through lamellar bodies.
While the present results indicate SP-A secretion through lamellar bodies, they do not exclude the possibility of additional secretion through a constitutive pathway. Measurements of SP-A as a percentage of total protein in extracellular surfactant compared with lamellar bodies suggests an excess of SP-A in the former. That is, SP-A is ∼35% of surfactant protein but only ∼10% of lamellar body protein. There are several possible reasons for this difference of SP-A content. First, the lamellar bodies contain membrane and other (nonsurfactant) proteins that are not secreted with the lamellar body contents, so the secreted material (i.e., surfactant) would have a higher percentage due to SP-A. In addition to intrinsic lamellar body proteins, extrinsic proteins that co-isolate with the lamellar body fraction would artificially lower the percentage of SP-A. As discussed above, the purity of the preparation is probably not a major factor. Other possibilities for the presence of excess SP-A in the extracellular material include SP-A secretion by Clara cells, which could co-isolate with alveolar surfactant obtained by lung lavage, or differential kinetics for clearance of SP-A from the alveolar space relative to other proteins (15, 36). Finally, the higher levels of SP-A in alveolar surfactant could reflect an alternative source for the protein, such as constitutive secretion by type II cells, in addition to regulated secretion. The potential for constitutive secretion of SP-A is suggested by some studies with isolated type II cells (28, 29), but definitive evidence for a possible constitutive pathway in the intact lung has not been presented.
In summary, the present study indicates that newly synthesized SP-A is targeted to lamellar bodies for storage and subsequent secretion by regulated exocytosis as a component of the lung surfactant.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-19737.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. Altaf Kazi and Bruno Schremmer for assistance with gel electrophoresis and Victoria Brown for typing the manuscript.
This work was presented in part at the FASEB Conference on Lung Surfactant: Cell and Molecular Biology, Snowmass, CO, July 2006; and the Experimental Biology Meeting, Washington, DC, April 2007.
Present address of P. Ruckert: Praxis Rolke/Ruckert, Aschaffenburg, Germany.
REFERENCES
- 1.Alcorn JL, Mendelson CR. Trafficking of surfactant protein A in fetal rabbit lung in organ culture. Am J Physiol Lung Cell Mol Physiol 264: L27–L35, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Ballard PL, Ertsey R, Gonzales LK, Liley HG, Williams MC. Isolation and characterization of differentiated alveolar type II cells from fetal human lung. Biochim Biophys Acta 883: 335–344, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Bates SR, Dodia C, Fisher AB. Surfactant protein A regulates uptake of pulmonary surfactant by lung type II cells on microporous membranes. Am J Physiol Lung Cell Mol Physiol 267: L753–L760, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bates SR, Dodia C, Tao JQ, Fisher AB. Surfactant protein-A plays an important role in lung surfactant clearance: evidence using the surfactant protein-A gene-targeted mouse. Am J Physiol Lung Cell Mol Physiol 294: L325–L333, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bates SR, Kazi AS, Tao JQ, Yu KJ, Gonder DS, Feinstein SI, Fisher AB. Role of P63 (CKAP4) in binding of surfactant protein-A to type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 295: L658–L669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beers MF, Kim CY, Dodia C, Fisher AB. Synthesis of type II cell lamellar body lysozyme-15 kD protein (lbl-15) by perfused rat lung. Am J Respir Cell Mol Biol 11: 240–248, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Chander A, Dodia CR, Gil J, Fisher AB. Isolation of lamellar bodies from rat granular pneumocytes in primary culture. Biochim Biophys Acta 753: 119–129, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Chander A, Johnson RG, Reicherter J, Fisher AB. Lung lamellar bodies maintain an acidic internal pH. J Biol Chem 261: 6126–6131, 1986 [PubMed] [Google Scholar]
- 9.Chen Q, Bates SR, Fisher AB. Secretagogues increase the expression of surfactant protein A receptors on lung type II cells. J Biol Chem 271: 25277–25283, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Chevalier G, Collet AJ. In vivo incorporation of choline-3H, leucine-3H and galactose-3H in alveolar type II pneumocytes in relation to surfactant synthesis. A quantitative radioautographic study in mouse by electron microscopy. Anat Rec 174: 289–310, 1972 [DOI] [PubMed] [Google Scholar]
- 11.Doyle IR, Barr HA, Davidson KG, Nicholas TE. Differential changes in SP-A and disaturated phospholipids in the isolated perfused rat lung and in vivo. Am J Physiol Lung Cell Mol Physiol 271: L374–L382, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Fehrenbach H, Tews S, Fehrenbach A, Ochs M, Wittwer T, Wahlers T, Richter J. Improved lung preservation relates to an increase in tubular myelin-associated surfactant protein A. Respir Res 6: 60, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher AB, Arad I, Dodia C, Chander A, Feinstein SI. cAMP increases synthesis of surfactant-associated protein A by perfused rat lung. Am J Physiol Lung Cell Mol Physiol 260: L226–L233, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Fisher AB, Dodia C. Lysosomal-type PLA2 and turnover of alveolar DPPC. Am J Physiol Lung Cell Mol Physiol 280: L748–L754, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Fisher AB, Dodia C, Chander A. Alveolar uptake of lipid and protein components of surfactant. Am J Physiol Lung Cell Mol Physiol 261: L334–L340, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Gupta N, Manevich Y, Kazi AS, Tao JQ, Fisher AB, Bates SR. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 291: L436–L446, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Haller EM, Shelley SA, Montgomery MR, Balis JU. Immunocytochemical localization of lysozyme and surfactant protein A in rat type II cells and extracellular surfactant forms. J Histochem Cytochem 40: 1491–1500, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Ikegami M, Lewis JF, Tabor B, Rider ED, Jobe AH. Surfactant protein A metabolism in preterm ventilated lambs. Am J Physiol Lung Cell Mol Physiol 262: L765–L772, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Ikegami M, Ueda T, Purtell J, Woods E, Jobe A. Surfactant protein A labeling kinetics in newborn and adult rabbits. Am J Respir Cell Mol Biol 10: 413–418, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Jain D, Dodia C, Bates SR, Hawgood S, Poulain FR, Fisher AB. SP-A is necessary for increased clearance of alveolar DPPC with hyperventilation or secretagogues. Am J Physiol Lung Cell Mol Physiol 284: L759–L765, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Jain D, Dodia C, Fisher AB, Bates SR. Pathways for clearance of surfactant protein A from the lung. Am J Physiol Lung Cell Mol Physiol 289: L1011–L1018, 2005 [DOI] [PubMed] [Google Scholar]
- 22.King RJ, Martin H. Intracellular metabolism of the apoproteins of pulmonary surfactant in rat lung. J Appl Physiol 48: 812–820, 1980 [DOI] [PubMed] [Google Scholar]
- 23.Kuroki Y, Mason RJ, Voelker DR. Alveolar type II cells express a high-affinity receptor for pulmonary surfactant protein A. Proc Natl Acad Sci USA 85: 5566–5570, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liley HG, Ertsey R, Gonzales LW, Odom MW, Hawgood S, Dobbs LG, Ballard PL. Synthesis of surfactant components by cultured type II cells from human lung. Biochim Biophys Acta 961: 86–95, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Madsen J, Tornoe I, Nielsen O, Koch C, Steinhilber W, Holmskov U. Expression and localization of lung surfactant protein A in human tissues. Am J Respir Cell Mol Biol 29: 591–597, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem 277: 22147–22155, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Ochs M, Johnen G, Muller KM, Wahlers T, Hawgood S, Richter J, Brasch F. Intracellular and intraalveolar localization of surfactant protein A (SP-A) in the parenchymal region of the human lung. Am J Respir Cell Mol Biol 26: 91–98, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Osanai K, Mason RJ, Voelker DR. Trafficking of newly synthesized surfactant protein A in isolated rat alveolar type II cells. Am J Respir Cell Mol Biol 19: 929–935, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Osanai K, Tsuchihara C, Hatta R, Oikawa T, Tsuchihara K, Iguchi M, Seki T, Takahashi M, Huang J, Toga H. Pulmonary surfactant transport in alveolar type II cells. Respirology 11Suppl: S70–S73, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Palaniyar N, Zhang L, Kuzmenko A, Ikegami M, Wan S, Wu H, Korfhagen TR, Whitsett JA, McCormack FX. The role of pulmonary collectin N-terminal domains in surfactant structure, function, and homeostasis in vivo. J Biol Chem 277: 26971–26979, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Rooney SA, Gobran LI, Umstead TM, Phelps DS. Secretion of surfactant protein A from rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 265: L586–L590, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Ruckert P, Bates SR, Fisher AB. Role of clathrin- and actin-dependent endocytotic pathways in lung phospholipid uptake. Am J Physiol Lung Cell Mol Physiol 284: L981–L989, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Schmiedl A, Ochs M, Muhlfeld C, Johnen G, Brasch F. Distribution of surfactant proteins in type II pneumocytes of newborn, 14-day old, and adult rats: an immunoelectron microscopic and stereological study. Histochem Cell Biol 124: 465–476, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Schmiedl A, Vieten G, Muhlfeld C, Bernhard W. Distribution of intracellular and secreted surfactant during postnatal rat lung development. Pediatr Pulmonol 42: 548–562, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Stevens PA, Wissel H, Zastrow S, Sieger D, Zimmer KP. Surfactant protein A and lipid are internalized via the coated-pit pathway by type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 280: L141–L151, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Ueda T, Ikegami M, Jobe AH. Clearance of surfactant protein A from rabbit lungs. Am J Respir Cell Mol Biol 12: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Wali A, Beers MF, Dodia C, Feinstein SI, Fisher AB. ATP and adenosine 3′,5′-cyclic monophosphate stimulate the synthesis of surfactant protein A in rat lung. Am J Physiol Lung Cell Mol Physiol 264: L431–L437, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Walker SR, Williams MC, Benson B. Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem 34: 1137–1148, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Wissel H, Zastrow S, Richter E, Stevens PA. Internalized SP-A and lipid are differentially resecreted by type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 278: L580–L590, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Wu YZ, Manevich Y, Baldwin JL, Dodia C, Yu K, Feinstein SI, Fisher AB. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J Biol Chem 281: 7515–7525, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Xu X, McCormick-Shannon K, Voelker DR, Mason RJ. KGF increases SP-A and SP-D mRNA levels and secretion in cultured rat alveolar type II cells. Am J Respir Cell Mol Biol 18: 168–178, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Yogalingam G, Doyle IR, Power JH. Expression and distribution of surfactant proteins and lysozyme after prolonged hyperpnea. Am J Physiol Lung Cell Mol Physiol 270: L320–L330, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Young SL, Fram EK, Larson E, Wright JR. Recycling of surfactant lipid and apoprotein-A studied by electron microscopic autoradiography. Am J Physiol Lung Cell Mol Physiol 265: L19–L26, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Zen K, Notarfrancesco K, Oorschot V, Slot JW, Fisher AB, Shuman H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. Am J Physiol Lung Cell Mol Physiol 275: L172–L183, 1998. [DOI] [PubMed] [Google Scholar]