Abstract
Severe asthma is characterized by increased airway smooth muscle (ASM) mass due, in part, to ASM cell growth and contractile protein expression associated with increased protein synthesis. Little is known regarding the combined effects of mitogens and interferons on ASM cytosolic protein synthesis. We demonstrate that human ASM mitogens including PDGF, EGF, and thrombin stimulate protein synthesis. Surprisingly, pleiotropic cytokines IFN-β and IFN-γ, which inhibit ASM proliferation, also increased cytosolic protein content in ASM cells. Thus IFN-β alone significantly increased protein synthesis by 1.62 ± 0.09-fold that was further enhanced by EGF to 2.52 ± 0.17-fold. IFN-γ alone also stimulated protein synthesis by 1.91 ± 0.15-fold; treatment of cells with PDGF, EGF, and thrombin in the presence of IFN-γ stimulated protein synthesis by 2.24 ± 0.3-, 1.25 ± 0.17-, and 2.67 ± 0.34-fold, respectively, compared with growth factors alone. The mammalian target of rapamycin (mTOR)/S6 kinase 1 (S6K1) inhibition with rapamycin inhibited IFN- and EGF-induced protein synthesis, suggesting that IFN-induced protein synthesis is modulated by mTOR/S6K1 activation. Furthermore, overexpression of tumor suppressor protein tuberous sclerosis complex 2 (TSC2), which is an upstream negative regulator of mTOR/S6K1 signaling, also inhibited mitogen-induced protein synthesis in ASM cells. IFN-β and IFN-γ stimulated miR143/145 microRNA expression and increased SM α-actin accumulation but had little effect on ASM cell size. In contrast, EGF increased ASM cell size but had little effect on miR143/145 expression. Our data demonstrate that both IFNs and mitogens stimulate protein synthesis but have differential effects on cell size and contractile protein expression and suggest that combined effects of IFNs and mitogens may contribute to ASM cell growth, contractile protein expression, and ASM remodeling in asthma.
Keywords: airway remodeling, chronic obstructive pulmonary disease, tuberous sclerosis complex 2, S6 kinase 1, miR143/145
asthma, a disease characterized by airway hyperresponsiveness, occurs in 5–8% of the U.S. population and remains an extraordinarily common illness. Despite considerable research effort, asthma mortality continues to rise, and the primary defects that underlie airway hyperresponsiveness are unknown, although an intrinsic abnormality of airway smooth muscle (ASM) has been postulated (24, 55). Increased ASM mass in the airways of patients with chronic severe or moderate asthma is a well-documented pathological finding and is due to the increased size and number of airway myocytes (4, 67). In cases of fatal asthma, a two- to fourfold increase of the ASM mass occurs compared with ASM of nonasthmatic subjects (25). Increased contractile protein expression in ASM cells is another characteristic feature of asthma that contributes to ASM remodeling (4, 67).
Considerable progress has been achieved in understanding the signaling mechanisms regulating ASM cell proliferation (5, 53, 69) demonstrating a critical role of phosphatidylinositol 3-kinase (PI3K)-mammalian target of rapamycin (mTOR)/S6 kinase 1 (S6K1) signaling cascade in mitogen-induced ASM proliferation (31). Under pathobiological conditions such as asthma, frequent stimulation of ASM by agonists, inflammatory mediators, and growth factors induces, in part, adaptive alterations in the airways that prompt an increase in protein synthesis in myocytes leading to the remodeling of ASM. Such alterations have important consequences in determining airway caliber, SM protein accumulation, and SM force generation (13, 46, 52). Thus elucidation of factors modulating ASM protein synthesis is critically important for better understanding of ASM remodeling in asthma.
Currently, “cell growth” and “cell proliferation” are used interchangeably. Cell growth and cell proliferation, however, are two distinct processes, and from yeast, Drosophila, to mammals, cell growth precedes cell proliferation (49). Importantly, inhibition of cell cycle progression has little effect on cell growth, whereas nutrient deprivation prevents cell growth and abrogates cell proliferation. Compelling evidence demonstrate that the ribosomal protein S6 kinases S6K1 and S6K2 are key regulators of protein synthesis and cell size. Our understanding of the mechanisms regulating the mTOR/S6K1 signaling pathway is fundamental to determining the mechanisms that control cell growth. Findings from our laboratory and others have identified that tumor suppressor tuberous sclerosis complex 2 (TSC2) acts as a negative regulator of mTOR/S6K1 (20). Loss-of-function mutations in Drosophila orthologs of TSC2 increases cell size (14); in contrast, overexpression of TSC2 reduces cell size (62), indicating a critical role that TSC2 plays in modulating cell size. Little is known about the role of TSC2 in human ASM cell growth.
Another characteristic feature of asthma, which requires protein synthesis and contributes to airway remodeling, is increased contractile protein expression (4, 67). Halayko et al. (21) reported that pharmacological inhibition of PI3K or mTOR blocks accumulation of 22-kDa contractile SM cell marker protein SM22 and SM myosin heavy chain (smMHC) in cultured canine ASM cells, implying that PI3K-mTOR/S6K1 signaling plays a role in contractile protein expression in ASM. However, the precise requirement of PI3K-mTOR/S6K1 for ASM remodeling has not been elucidated.
IFNs are classified as type I, which includes IFN-α and IFN-β, and type II, which includes IFN-γ. IFN-α and IFN-β are secreted by monocytes, macrophages, B cells, natural killer (NK) cells, and most virally infected cells. Conversely, IFN-γ, which is secreted by T cells, NK cells and, to lesser degree, macrophages, modestly exhibits antiviral activity but functions primarily as an immunomodulator that inhibits allergic responses by abrogating IL-4-mediated expression of low-affinity IgE receptors, isotype switching to IgE, and promoting cell-mediated immunity. Although IFN-α clearly plays a role in viral defense during asthma exacerbations (50), the role of either IFN-α or -β in modulating resident effector cell function such as airway epithelial or ASM remains unknown. Our (2, 64) published data suggest that IFN-β and IFN-γ profoundly modulate cytokine- and mitogen-induced human ASM cell proliferation. Recent evidence demonstrates that IFNs modulate PI3K-mTOR signaling cascade; the effects, however, are cell type- and context-specific (28, 30, 43, 44). TSC1/TSC2 and mTOR/S6K1 signaling are also key components in generation of IFN-dependent biological responses (27). IFN involvement in protein synthesis, ASM cell growth, and contractile protein expression, however, remains to be elucidated.
In this study, we demonstrate that human ASM mitogens PDGF, EGF, and thrombin (38, 39) stimulate protein synthesis in human ASM cells. Surprisingly, IFN-β and IFN-γ also stimulate protein synthesis in ASM cells. Using the mTOR inhibitor rapamycin, we show that mTOR/S6K1 pathway modulate growth factor- and IFN-induced protein synthesis in ASM cells. Furthermore, the overexpression of the TSC2, a negative regulator of mTOR/S6K1, abrogated mitogen-induced protein synthesis in ASM. IFN-β and IFN-γ increased SM α-actin protein levels and enhanced expression of miR143/145 microRNAs, which regulate SM-specific contractile phenotype (6). In contrast, EGF increased cell size but had little effect on miR143/145 expression. Collectively, our data demonstrate that IFNs modulate PDGF-, EGF-, and thrombin-induced protein synthesis and contractile protein expression in ASM and suggest that combined effects of IFNs and growth factors may contribute to airway remodeling and asthma pathobiology.
MATERIALS AND METHODS
Human ASM cell culture.
Human tracheas were obtained from lung transplant donors in accordance with procedures approved by the University of Pennsylvania Committee on Studies Involving Human Beings. Details regarding the isolation and characterization of human ASM cells by immunostaining for SM-specific α-actin and agonist-induced changes in cytosolic calcium have been reported by our laboratory (54). All studies were performed on confluent, growth-arrested for 48 h cells when they morphologically most resemble the in vivo state.
[3H]leucine incorporation protein synthesis assay.
Serum-deprived ASM cells were treated with agonists as indicated in figure legends for each specific experiment for 16 h; then, cells were labeled with 2.0 μCi/ml [methyl-3H]leucine (Amersham) for 6 h and treated with trypsin, and the proteins were precipitated with 20% TCA; the precipitate was aspirated onto glass filters, extensively washed, dried, and counted (3, 11, 15, 68). To ensure changes in protein synthesis were not due to changes in cell number, cell counts were performed in parallel.
Transient infection with replication-deficient adenovirus.
Recombinant adenovirus expressing green fluorescent protein (GFP)-tagged TSC2 and control GFP constructs were created using the AdEasy vector system and were generously provided to us by Dr. Noonan (12). Infection with replication-deficient adenovirus was performed as previously described (40). Briefly, preconfluent cells were serum-deprived for 24 h, rinsed with PBS, and then incubated with 5.8 × 106 plaque-forming units (pfu) of GFP or GFP-TSC2 in Ham's F-12 media without serum, penicillin, and streptomycin for 1 h; then, cells were rinsed twice with 10% FBS in Ham's F-12 and incubated with complete media for 24 h before [3H]leucine incorporation protein synthesis assay. Mock-infected cells were used as negative control. No significant differences in protein synthesis were detected in GFP-infected cells vs. mock-infected cells.
Immunohistochemistry and immunocytochemistry.
Cells or tissue sections were fixed with 3.7% paraformaldehyde (Polysciences, Warrington, PA), permeabilized with 0.1% Triton X-100 (Sigma Chemical, St. Louis, MO), and then blocked with blocking solution as we previously described (16, 18, 19). Anti-phospho-ribosomal protein S6 (Ser235) antibody (Upstate Biotechnology, Lake Placid, NY) was used at a 1:50 dilution; secondary antibody Alexa Fluor 488 goat anti-rabbit IgG conjugate (Molecular Probes, Eugene, OR) was used at a 1:400 dilution; anti-SM α-actin clone 1A4 FITC-conjugated antibody (Sigma Chemical) was used at a 1:200 dilution; anti-human-IFN-β goat antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:50 dilution; and secondary antibody Alexa Fluor 594 anti-goat chicken IgG conjugate (Molecular Probes) was used at a 1:400 dilution. Cells and tissue sections were visualized using a Nikon Eclipse TE2000-E or a Nikon Eclipse E400 microscope under appropriate filters.
Immunoblot analysis.
Serum-deprived cells were lysed in S6K1 lysis buffer (36, 38), and equal amounts of lysate, adjusted by protein content, were subjected to immunoblot analysis with anti-ribosomal protein S6, anti-phospho-ribosomal protein S6 (Ser235/236), anti-S6K1, anti-phospho-Thr389 S6K1, anti-SM α-actin, and anti-total actin antibodies (Cell Signaling Technology, Beverly, MA) as we previously described (16, 18, 19). Image analysis was performed using the Gel-Pro Analyzer program (Media Cybernetics, Silver Spring, MD).
Cell size analysis.
Serum-deprived cells were treated with diluent, 100 U/ml IFN-β, or 100 U/ml IFN-γ in the presence or absence of 10 ng/ml EGF for 22 and 72 h, and then cell size was examined by volumetric analysis using Multisizer 3 Coulter Counter (Beckman Coulter, Brea, CA) according to manufacturer's protocol. Cell volume analysis was performed using Multisizer 3 3.51 software (Beckman Coulter). For each measurement, 1 × 106 cells per 20 ml of electrolyte were used; three measurements per each experimental condition were performed in each experiment.
Detection of miR143 and miR145 expression.
miR143 and miR145 expression was examined as described previously (6). Serum-deprived cells were incubated with 100 U/ml IFN-β, 100 U/ml IFN-γ, or diluent alone or in combination with 10 ng/ml EGF for 22 h followed by total RNA extraction using miRNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Then, cDNA synthesis was performed using Universal cDNA synthesis kit (Exiqon, Woburn, MA) according to the manufacturer's instructions. Briefly, total RNA containing microRNA was polyadenylated, and cDNA was synthesized using a poly(T) primer with a 3′ degenerate anchor and a 5′ universal tag. Then, cDNA was served as a template for microRNA quantitative real-time PCR (qPCR) using miRCURY LNA Universal RT microRNA PCR kit (Exiqon). Primers were included Exiqon-validated miR143- and miR145-specific primer sets (hsa-miR-143 primer: 5′-UGAGAUGAAGCACUGUAGCUC-3′; hsa-miR-145 primer: 5′-GGAUUCCUGGAAAUACUGUUCU-3′) and 5S rRNA control primers (Exiqon). qPCR assays were performed using Realplex 2 Mastercycler (Eppendorf). The amplification profile was denatured at 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C for 60 s. At the end of the PCR cycles, melting curve analyses were performed.
Data analysis.
Data points from individual assays represent the mean values ± SE. Statistically significant differences among groups were assessed with ANOVA (Bonferroni-Dunn), with values of P < 0.05 sufficient to reject the null hypothesis for all analyses. All experiments were designed with matched control conditions within each experiment to enable statistical comparison as paired samples.
RESULTS
Human ASM expresses IFN-β in vivo.
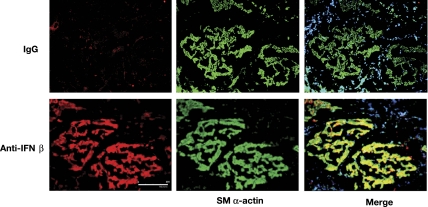
Because IFNs play a role in modulating airway inflammation in asthma (48, 50, 60), we examined whether IFNs are secreted in vivo. Dual immunohistochemical analysis of freshly harvested human trachealis was performed with anti-SM α-actin antibody to localize ASM and with either anti-IFN-β or anti-IFN-γ antibodies to determine whether human ASM expresses IFN-β or IFN-γ in vivo. As seen in Fig. 1, ASM markedly expressed IFN-β in histological sections obtained from the otherwise healthy lung transplant donors. In comparison, sections stained with anti-IFN-γ antibody or an isotype-matched IgG had little specific staining. These data demonstrate that ASM constitutively expresses IFN-β but not IFN-γ, which may modulate ASM functions.
Fig. 1.
Human airway smooth muscle (ASM) expresses IFN-β. Freshly harvested human trachealis was fixed, sectioned, and coimmunostained with anti-SM α-actin and an anti-IFN-β antibody or an isotype-matched IgG. The images are representative of 3 separate experiments. Scale bar is 200 μm.
IFN-β, IFN-γ, and growth factors stimulate protein synthesis in the human ASM.
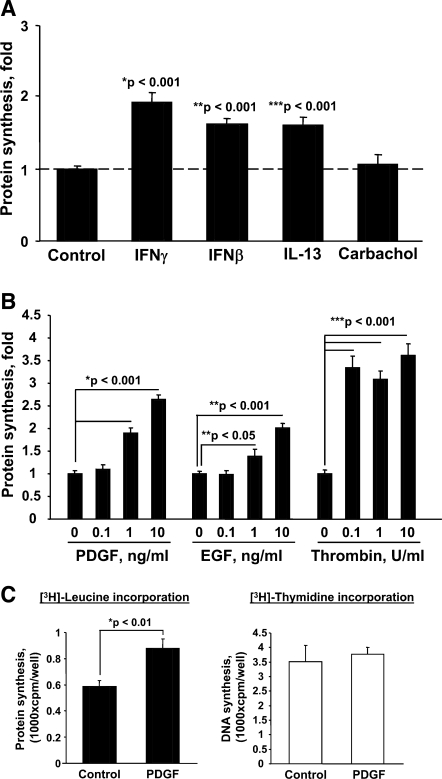
Because ASM growth is associated with increases in cell mass, we examined whether contractile agonists and or mitogens promote protein synthesis in human ASM cells. Serum-deprived ASM cells were stimulated with IFN-β, IFN-γ, IL-13, and carbachol followed by measurements of protein synthesis as assessed by [3H]leucine incorporation (3, 11) (Fig. 2A). In parallel experiments, protein synthesis was examined in ASM treated with PDGF, EGF, or thrombin at concentrations (Fig. 2B) that are known to promote ASM cell growth (17, 33, 35, 37–39). Cells were stimulated with agonists for 18 h, and [3H]leucine was added in the last 6 h of stimulation. After examining time-dependent [3H]leucine incorporation, the 22-h time point was chosen because, by this time, synchronized ASM cells progress into late G1 phase of the cell cycle without entering S phase, initiating DNA synthesis and cell doubling, which makes it difficult to interpret the relevance of increases in protein synthesis. As seen in Fig. 2A, IFN-β and IFN-γ stimulated protein synthesis compared with diluent-treated cells. Interestingly, the proinflammatory mediator IL-13 also stimulated protein synthesis (Fig. 2A), in contrast to the contractile agonists carbachol and bradykinin (data not shown). Treatment with PDGF, EGF, and thrombin also increased protein synthesis in ASM cells (Fig. 2B). Importantly, IFN-, IL-13-, and growth factor-induced increase in protein synthesis was not accompanied by increased DNA synthesis, as confirmed by [3H]thymidine incorporation assay performed in parallel (Fig. 2C; data not shown), suggesting that increased protein synthesis is not due to ASM cell proliferation. These data demonstrate that not only growth factors, but also IFN-β, IFN-γ, and some (thrombin) but not all contractile agonists and cytokines promote protein synthesis in human ASM cells, which may contribute to ASM remodeling in asthma.
Fig. 2.
A: the effect of IFN-β, IFN-γ, IL-13, and carbachol on protein synthesis in human ASM cells. Serum-deprived ASM were treated with 100 U/ml IFN-β, 100 U/ml IFN-γ, 10 ng/ml IL-13, and 10 μM carbachol for 22 h; [3H]leucine was added in the last 6 h of stimulation followed by the [3H]leucine incorporation assay. B: PDGF, EGF, and thrombin stimulate protein synthesis. Serum-deprived cells were stimulated with agonists for 22 h followed by the [3H]leucine incorporation assay; [3H]leucine was added in the last 6 h of stimulation. C: stimulation of protein synthesis is not associated with cell proliferation. [3H]leucine and [3H]thymidine incorporation was performed in parallel using cells stimulated with 10 ng/ml PDGF for 22 h. [3H]leucine was added in the last 6 h of stimulation. The results represent mean values ± SE from 6 replicates per condition performed in 2 separate experiments. cpm, Counts per minute.
IFN-β and IFN-γ augment agonist-induced protein synthesis.
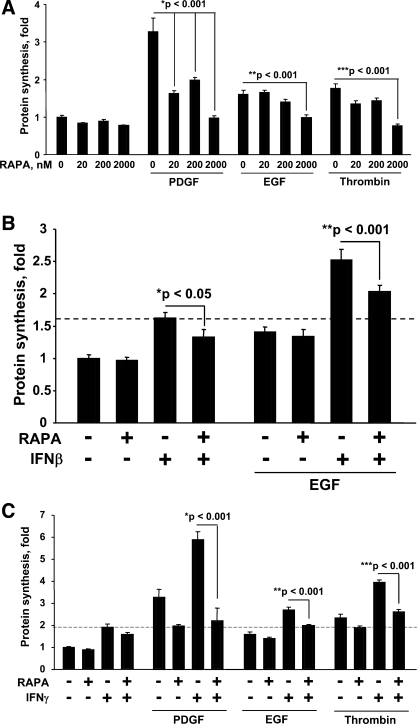
To address whether IFNs modulate ASM protein synthesis, serum-deprived human ASM cells were treated with IFN-β and IFN-γ, with or without agonists, and then protein synthesis was measured. IFN-β and IFN-γ alone significantly stimulated protein synthesis in a concentration-dependent manner (Fig. 3, A and B); furthermore, the simultaneous treatment of ASM cells with IFN-β and IFN-γ and with PDGF, EGF, or thrombin markedly augmented, in a concentration-dependent manner, ASM cell protein synthesis compared with the effect of PDGF, EGF, or thrombin alone (Fig. 3, A and B). Importantly, at these concentrations, IFN-β and IFN-γ inhibit ASM DNA synthesis, as we (2, 64) demonstrated in our published studies.
Fig. 3.
IFN-β and IFN-γ increase PDGF-, EGF-, and thrombin-induced protein synthesis in human ASM cells. Serum-deprived cells were treated with 100 U/ml IFN-β (A) and 100 U/ml IFN-γ (B) in the presence of 10 ng/ml EGF (A and B), 10 ng/ml PDGF (B), and 1 U/ml thrombin (B) for 22 h followed by the [3H]leucine incorporation assay. [3H]leucine was added in the last 6 h of stimulation. The results represent mean values ± SE from 6 replicates per condition performed in 4 separate experiments. P was determined by ANOVA (Bonferroni-Dunn).
mTOR/S6K1 activation is critical for protein synthesis in human ASM cells.
The mTOR/S6K1 signaling pathway is a key regulator of protein synthesis across species (65). To determine the mechanism of ASM cell growth, we examined whether mTOR/S6K1 activation modulates protein synthesis in ASM cells. Serum-deprived ASM cells were pretreated with rapamycin, a specific mTOR/S6K1 inhibitor, followed by the stimulation of cells with PDGF, EGF, and thrombin in the presence or absence of IFN-β and IFN-γ. Inhibition of mTOR/S6K1 attenuated not only PDGF-, EGF-, and thrombin-induced protein synthesis (Fig. 4A), but also the IFN-β and IFN-γ effects on protein synthesis (Fig. 4, B and C, respectively). Importantly, pharmacologically relevant concentrations of rapamycin (20 and 200 nM) attenuated but did not completely inhibit ASM protein synthesis, suggesting that, in parallel to mTOR/S6K1, other signaling pathway(s) might be involved in the regulation of protein synthesis in ASM cells. Our data show that the mTOR/S6K1 pathway is critical for regulating protein synthesis in ASM cells and suggest that mTOR/S6K1 play a role in regulating ASM remodeling.
Fig. 4.
Rapamycin (RAPA) inhibits mitogen-induced protein synthesis in human ASM cells. Serum-deprived cells were pretreated with RAPA for 30 min at the indicated concentrations followed by stimulation with 10 ng/ml PDGF, 10 ng/ml EGF, and 10 U/ml thrombin in the absence (A) or the presence of IFN-β (B) and IFN-γ (C); 200 nM RAPA was used in B and C; then, the [3H]leucine incorporation assay was performed. [3H]leucine was added in the last 6 h of stimulation. The results represent mean values ± SE from 6 replicates per condition performed in 2 separate experiments. P was determined by ANOVA (Bonferroni-Dunn).
IFN-β, IFN-γ, and EGF activate ribosomal protein S6.
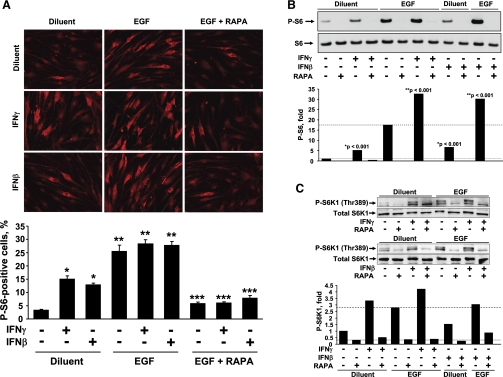
We next examined the activation of ribosomal protein S6, as a molecular signature of mTOR/S6K1 activation (18), to determine whether mTOR/S6K1 signaling pathway is activated by IFN-β and IFN-γ and is involved in the control of ASM protein synthesis. The activation of ribosomal protein S6 was determined by immunostaining with anti-phospho-specific antibody. As seen in Fig. 5A, IFN-β and IFN-γ alone significantly stimulated S6 phosphorylation; EGF alone also markedly increased S6 activation; and simultaneous treatment of cells with IFNs and EGF augmented S6 phosphorylation. Similar results were obtained using immunoblot analysis (Fig. 5B). Additionally, as seen in Fig. 5C, IFNs and EGF also induced activatory phosphorylation of S6K1, an upstream regulator of ribosomal protein S6 activity. Importantly, activation of S6 was abrogated by rapamycin (Fig. 5, A–C), which indicates that mTOR activation is critical for IFN- and EGF-induced ribosomal protein S6 activation.
Fig. 5.
IFN-β, IFN-γ, and EGF stimulate ribosomal protein S6 phosphorylation (P-S6) and S6 kinase 1 (S6K1) activation. Serum-deprived cells were pretreated with 200 nM RAPA for 30 min followed by stimulation with 10 ng/ml EGF, 100 U/ml IFN-β, 100 U/ml IFN-γ, or diluent for 22 h. A, top: representative images of immunostaining with P-S6 antibody of ASM treated with IFNs, RAPA, and EGF were taken on Nikon Eclipse E400 microscope. A, bottom: statistical analysis. Data represent a percentage of P-S6-positive cells per total number of cells. Data are mean values ± SE by ANOVA (Bonferroni-Dunn). *P < 0.01 for IFN-γ vs. diluent and for IFN-β vs. diluent; **P < 0.001 for EGF vs. diluent, EGF + IFN-γ vs. IFN-γ, and EGF + IFN-β vs. IFN-β; ***P < 0.001 for EGF + RAPA vs. EGF, EGF + IFN-γ + RAPA vs. EGF + IFN-γ, and EGF + IFN-β + RAPA vs. EGF + IFN-β by ANOVA (Bonferroni-Dunn). B and C: immunoblot analysis of cell lysates equalized in protein content was performed to detect activation levels of S6 (B) and S6K1 (C). Top: images are representative of 2 independent experiments. Bottom: statistical analysis of immunostaining was performed using Gel-Pro Analyzer software. The results represent mean values ± SE from 2 separate experiments. Phosphorylation levels of S6 or S6K1 in diluent-treated cells were taken as 100%. P was determined by ANOVA (Bonferroni-Dunn).
TSC2 inhibits agonist-induced ASM protein synthesis.
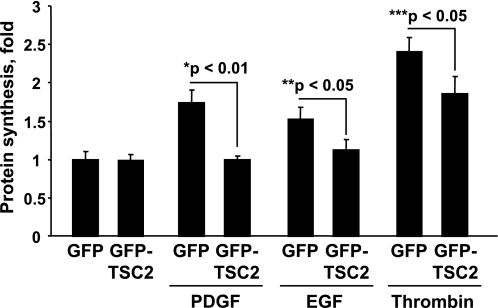
Tumor suppressor TSC2 is an upstream negative regulator of mTOR/S6K1 activation; growth factors or nutrients induce phosphorylation of TSC2, release mTOR/S6K1 from negative regulation by TSC2, activate mTOR/S6K1, phosphorylate ribosomal protein S6, and stimulate protein synthesis (16, 18, 34). We next examined whether TSC2 modulates agonist-induced protein synthesis in ASM cells. Transient transfection was performed as we (19) described previously with 40–60% efficiency. As seen in Fig. 6, protein synthesis in control cells infected with GFP construct was significantly stimulated by PDGF, EGF, and thrombin. In contrast, expression of GFP-tagged TSC2 significantly inhibited PDGF-, EGF-, and thrombin-induced protein synthesis in human ASM cells. These data suggest that TSC2 regulates mitogen-induced protein synthesis in ASM and that TSC2 is critical for modulating ASM cell growth.
Fig. 6.
Tumor suppressor protein tuberous sclerosis complex 2 (TSC2) inhibits agonist-induced ASM protein synthesis. Serum-deprived cells expressing green fluorescent protein (GFP)-TSC2 or control GFP were stimulated with 10 ng/ml PDGF, 10 ng/ml EGF, and 1 U/ml thrombin for 22 h followed by the [3H]leucine incorporation assay. [3H]leucine was added in the last 6 h of stimulation. The results represent mean values ± SE from 6 replicates per condition performed in 2 separate experiments. P was determined by ANOVA (Bonferroni-Dunn).
Effects of IFN-β, IFN-γ, and EGF on ASM cell size and contractile protein expression.
Because increased protein synthesis in ASM may contribute to increased cell size and/or increased contractile protein expression, we next examined effects of IFNs and EGF on ASM cell size and contractile protein expression. As seen in Fig. 7, bottom, incubation with EGF for 22 and 72 h increased ASM cell volume compared with diluent-treated cells. IFN-β or IFN-γ had little effect on ASM cell volume at 22 or 72 h of incubation compared with diluent-treated cells (Fig. 7, top), and IFNs had little effect on EGF-induced cell size increase (Fig. 7B, bottom). These data demonstrate that IFN-dependent elevation of protein synthesis is not associated with increase in ASM cell size.
Fig. 7.
Effects of EGF, IFN-β, and IFN-γ on ASM cell size. Serum-deprived cells were treated with diluent, 100 U/ml IFN-β, or 100 U/ml IFN-γ in presence or absence of 10 ng/ml EGF for 22 (A) and 72 (B) h followed by cell size analysis using Multisizer 3 Coulter Counter. For each experimental condition, 1 × 106 cells were analyzed in each replication; 3 replications had been performed for each experimental condition in each experiment.
Because, as shown in Figs. 2A and 3, IFNs increase protein synthesis in ASM cells, we next examined whether IFNs modulate SM α-actin protein levels. ASM cells were treated with IFN-β and IFN-γ for 22 h followed by immunoblot analysis with SM α-actin-specific antibody. We found that both IFN-β and IFN-γ increase SM α-actin protein levels in ASM cells (Fig. 8A). These data demonstrate that IFNs, although having little effect on cell volume, increase contractile protein expression. Because our data demonstrate that IFNs promote ribosomal protein S6 activation (Fig. 5), we next determined whether increase in SM α-actin protein levels correlates with S6 activation. ASM cells, incubated with IFN-β and IFN-γ for 22 h, were subjected to dual immunostaining with anti-SM α-actin and anti-phospho-S6 antibodies. As seen in Fig. 8B, top and middle, ASM cells treated with IFNs had increased SM α-actin and phospho-S6 levels compared with diluent-treated cells. Importantly, the majority of SM α-actin-positive cells showed increased S6 phosphorylation (Fig. 8B, bottom), demonstrating that correlation exists between IFN-dependent increase of SM α-actin protein levels and S6 activation.
Fig. 8.
IFN-β and IFN-γ modulate SM α-actin protein levels. A: serum-deprived cells were incubated with 100 U/ml IFN-β, 100 U/ml IFN-γ, or diluent for 22 h, and then immunoblot analysis with anti-SM α-actin antibody was performed. Immunoblot for total actin was used as a control for equal loading. Images are representative of 3 independent experiments. B: serum-deprived cells were treated with 100 U/ml IFN-β, 100 U/ml IFN-γ, or diluent for 22 h, and then SM α-actin (red) and P-S6 (green) were detected using coimmunostaining with specific antibodies. 4′,6′-Diamidino-2-phenylindole (DAPI) staining (blue) was performed to detect cell nuclei. Images are representative of 3 human ASM cell cultures obtained from 3 different donors. Images were taken using Nikon Eclipse E400 microscope under ×200 magnification. Arrows indicate cells with SM α-actin and P-S6 colocalization.
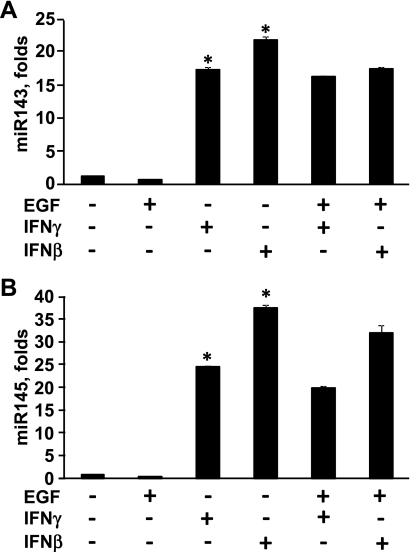
Studies suggest that expression of contractile proteins in ASM regulated at a posttranscriptional levels (4, 67), and recent data demonstrate that microRNAs miR143 and miR145 modulate posttranscriptional regulation of contractile protein expression in SM cells (6, 10). We next performed analysis of miR143/145 expression in ASM cells incubated for 22 h with IFN-β, IFN-γ, or EGF separately or in combination. Our data demonstrate that either IFN-β or IFN-γ markedly increased miR143 and miR145 expression compared with diluent-treated cells (Fig. 9, A and B, respectively). Interestingly, EGF alone or in combination with IFNs had little effect on miR143/145 expression (Fig. 9). Collectively, these data show that IFN-β and IFN-γ promote SM α-actin accumulation and miR143/145 expression without increase of ASM cell volume. In contrast, EGF increases size but had little effect on miR143/145 expression in ASM cells. Thus the combination of IFNs and EGF increases ASM cell size and promotes contractile protein expression, which can have a combined effect on ASM remodeling.
Fig. 9.
IFN-β and IFN-γ stimulate miR143 and miR145 microRNA expression. miR143 (A) and miR145 (B) expression was examined in serum-deprived ASM cells treated with IFN-β, IFN-γ, or EGF separately or in combination. miR143 or miR145 expression in diluent-treated cells was taken as 1-fold. Data are mean values ± SE from 2 independent experiments; *P < 0.001 for IFN-β and IFN-γ vs. control by ANOVA (Bonferroni-Dunn).
DISCUSSION
An understanding of the molecular signaling mechanisms involved in modulating ASM mass is critically important to identify a potential therapeutic target(s) to treat asthma and chronic obstructive pulmonary disease (COPD). Our study shows that whereas IFNs inhibit human ASM cell proliferation (2, 64), IFN-β and IFN-γ alone or in combination with mitogens stimulate protein synthesis in ASM cells. Importantly, the inhibition of mTOR/S6K1 signaling pathway by specific pharmacological inhibitor rapamycin or overexpression of TSC2, an obligated upstream negative regulator of mTOR/S6K1, inhibits mitogen- and IFN-induced protein synthesis in ASM cells (Fig. 8). Our data also show that although both EGF and IFNs stimulate protein synthesis, they have differential effects on cell size and SM α-actin expression. Thus IFNs stimulated miR143/145 microRNA and SM α-actin expression but had little effect on cell size. In contrast, EGF markedly increased cell size but had little effect on miR143/145 and SM α-actin expression. Whether the specific mechanism of IFN-dependent SM α-actin expression involves IFN-dependent gene expression remains to be elucidated (Fig. 8). Our study, however, shows that both effects, growth factor-dependent cell growth, e.g., increase in cell size, and IFN-dependent miR143/145 and SM α-actin expression, are modulated by TSC2-mTOR/S6K1 signaling, suggesting that targeting of this pathway may have therapeutic benefits in preventing ASM remodeling.
Chronic airway inflammation and airway remodeling due to abnormal proliferation, hypertrophy, and elevated expression of contractile proteins in ASM cells are pathological hallmarks of asthma (22). Whereas increases in ASM mass due to ASM cell proliferation were reported for patients with mild-to-moderate asthma (67), in cases of severe asthma, increase in ASM cell diameter is not associated with cell proliferation and is, in part, due to ASM cell hypertrophy (4), suggesting that both ASM cell hypertrophy and proliferation may contribute to asthma pathobiology at different stages of disease development. Increase in SM protein levels have been described in patients with both mild-to-moderate and severe asthma (4, 67). However, the mechanisms of ASM cell growth and contractile protein expression are not fully established.
Our studies show that IFNs, although inhibiting ASM cell proliferation (2, 64), stimulate protein synthesis in ASM cells. IFN-β and IFN-γ levels in airways of subjects with asthma, especially after acute exacerbations, are induced by viral infections (48, 50, 66). IFN-γ also modulates resident cell function by increasing adhesion molecules and integrin expression on epithelial (7) and ASM cells (41) and by inducing leukotriene receptor expression and class II, but not class I, MHC expression in ASM (1, 42, 63). Although IFN-α clearly plays a role in viral defense during asthma exacerbations (48, 50, 66), the role of either IFN-α or -β in modulating resident effector cell function, such as airway epithelial or SM, remains unknown. Our data show that IFN-β but neither -α nor -γ is constitutively expressed in ASM and profoundly modulates cytokine- and mitogen-induced cell proliferation, as demonstrated by our (2, 64) published studies. To date, however, little information is available about a role of IFN-β and IFN-γ in regulating protein synthesis, cell growth, and contractile protein expression in ASM cells. In a pathological condition such as asthma, in situ, growth factors, contractile agonists, proinflammatory mediators, and cytokines have a differential effect on ASM protein synthesis, which may deregulate ASM growth and contractile protein expression and promote ASM remodeling.
Whereas the antiproliferative, antiviral, and immunomodulatory effects of IFNs are well-established, little is known about their role in regulating protein synthesis, cell hypertrophy, and SM protein expression. In Drosophila, the orthologs of Janus kinases (JAK)/signal transducers and activators of transcription (STAT) have profound effects on cell size. Thus loss-of-function mutations in genes encoding Drosophila STAT and JAK reduce cell size, suggesting that both JAK and STAT functions are required for cell hypertrophy (8). In contrast, stabilization of STAT by cyclin D/Cdk4 increases its protein activity and promotes increase in cell size of the eye in Drosophila (9). Controversy, however, exists regarding the role of IFNs in protein synthesis and cell size in vertebrates. Depending on cell type and experimental conditions, IFNs inhibit (59, 61) or have little effect on transforming growth factor-β (TGF-β)-induced SM α-actin expression (23). Evidence also suggests that IFN-γ may induce an increase in cell size (26); the mechanism of this effect and its relevance to protein synthesis and cell growth of ASM cells, however, remain to be determined. Our data show that IFN-β and IFN-γ alone are sufficient to promote protein synthesis in human ASM cells; furthermore, the presence of growth factors such as PDGF and EGF or contractile promitogenic agonist thrombin augments protein synthesis induced by IFN-β and IFN-γ.
The biological effects of IFNs require the activation of both STAT-dependent and STAT-independent signaling pathways (57). In human ASM cells, IFN-β and IFN-γ activate the classical STAT-dependent signaling cascade (2, 64). The STAT-independent mechanism involves the coordination and cooperation of the IFN-activated p38 MAPK cascade and the PI3K cascade (29, 58). Importantly, the activation of the PI3K signaling cascade regulates IFN-inducible activation of the mTOR/S6K1 pathway, which mediates the control of protein synthesis and cell growth (44). Importantly, PI3K/mTOR/S6K1 activation also contributes to SM22 and smMHC protein expression in primary canine ASM cells (21).
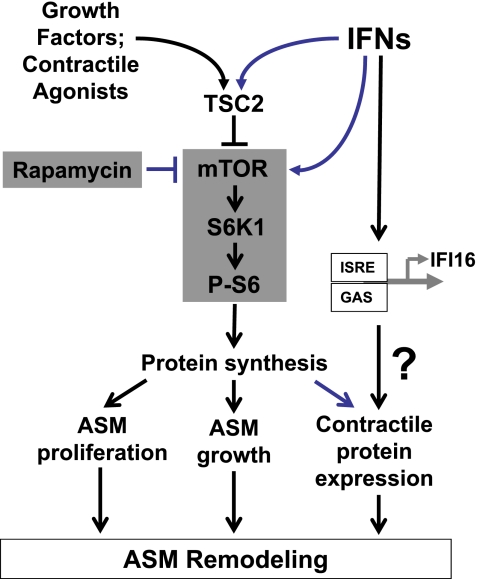
We found that IFN-β and IFN-γ alone stimulate phosphorylation of ribosomal protein S6 in ASM cells, which is further enhanced by the combined treatment of IFN-β and IFN-γ with EGF. Furthermore, inhibition of mTOR with its specific inhibitor, rapamycin, attenuates IFN-β- and IFN-γ-induced protein synthesis, demonstrating that mTOR/S6K1 signaling cascade modulates IFN-induced protein synthesis. The critical role of S6 phosphorylation, which is a molecular signature of mTOR/S6K1 activation, in the regulating of protein synthesis and cell growth is well-established (34). Gene deletion studies in mice and Drosophila determined that the ribosomal protein S6 kinases, dS6K in Drosophila and S6K1 and S6K2 in mice, are key regulators of protein synthesis and cell size. Thus loss-of-function mutations in the dS6K gene reduce cell and organ size without affecting cell numbers (47). The deletion of both the S6K1 and S6K2 genes in mice results in reduced muscle mass (56); however, the total number of muscle fibers and nuclei does not change, indicating that the S6K deletion induces an atrophic phenotype (51). Our data demonstrate that EGF, which stimulates mTOR/S6K1 activation and protein synthesis, also increases ASM cell size. IFN-β and IFN-γ also promote protein synthesis but have little effect on the size of ASM cells and do not modulate EGF-induced ASM cell size increase. Importantly, IFN-β and IFN-γ markedly increased SM α-actin protein levels in ASM cells. Published data demonstrate that contractile protein accumulation in asthmatic ASM is not associated with mRNA expression of contractile protein genes, suggesting that contractile protein expression may be regulated at a posttranscriptional levels (4, 67). Recent studies show that posttranscriptional regulation of SM gene expression is through microRNAs miR143 and miR145 that regulate the maintenance and/or acquisition of contractile proteins (6). Our data demonstrate that IFN-β and IFN-γ markedly upregulated miR143 and miR145 expression in ASM cells. Interestingly, EGF had little effect on miR143/145 expression and did not augment IFN-dependent elevation of miR143/135. Collectively, our data demonstrate that IFN-β- and IFN-γ-induced protein synthesis in ASM cells is associated with increased SM α-actin accumulation and miR143/145 expression; EGF stimulated protein synthesis and ASM cell growth but had little effect on miR143/145 expression. These data suggest that combination of IFNs and growth factors promote ASM hypertrophy and SM protein accumulation that may contribute to ASM remodeling in asthmatic airways. Schematic representation of potential IFN-dependent modulation of mitogen-induced protein synthesis in ASM is shown in Fig. 10.
Fig. 10.
Schematic representation of a potential mechanism of IFN-dependent modulation of mitogen-induced protein synthesis in ASM. See discussion for details. mTOR, mammalian target of rapamycin; ISRE, IFN-stimulated response element; IFI16, IFN-γ-inducible protein 16; GAS, IFN-γ-activated sequence.
Our understanding of the mechanisms regulating the mTOR/S6K1 signaling pathway is fundamental to determining the mechanisms that control protein synthesis. Recent findings from our laboratory (32) and others have identified that tumor suppressor TSC2 acts as a negative regulator of mTOR/S6K1 signaling pathway and protein synthesis. Loss-of-function mutations in Drosophila orthologs of TSC2 increase cell size (14); in contrast, overexpression of TSC2 reduces cell size (62), indicating a critical role that TSC2 plays in modulating cell size. We show that expression of TSC2 in ASM cells inhibits PDGF-, EGF-, and thrombin-induced protein synthesis, demonstrating that ASM cell protein synthesis is regulated by tumor suppressor TSC2, which acts upstream of mTOR/S6K1. Importantly, activity of TSC2 is regulated by p38 MAPK (45), suggesting that IFN-dependent modulation of mTOR/S6K1 signaling may act through p38 MAPK.
ASM hypertrophy and increased contractile protein expression are characteristic features of chronic severe asthma. This study presents evidence that the TSC2-mTOR/S6K1 signaling pathway is critical for mitogen-induced protein synthesis in human ASM cells. Furthermore, we show that antiproliferative cytokines IFN-β and IFN-γ, while inhibiting ASM cell proliferation (2, 64), are sufficient for stimulation of protein synthesis, which requires mTOR/S6K1 activation and that presence of PDGF, EGF, or thrombin augments IFN-β- and IFN-γ-induced protein synthesis. Treatment with IFN-β and IFN-γ promoted SM α-actin accumulation and miR143/145 expression without affecting ASM cell size. Conversely, EGF increased ASM cell volume but had little effect on miR143/145 expression. These data suggest that combination of growth factors, contractile agonists, and proinflammatory mediators may deregulate ASM cell growth and SM protein expression promoting airway remodeling. Further studies determining the precise molecular signaling mechanism of the IFN-dependent mTOR/S6K1 activation and regulating protein synthesis of human ASM cells may provide new insights into the therapeutic targets to prevent or abrogate ASM remodeling in asthma.
GRANTS
This work was supported by National Institutes of Health Grants 2RO1-HL-71106 (V. P. Krymskaya), RO1-HL-090829 (V. P. Krymskaya), HL-067663 (R. A. Panettieri, Jr.), ES-03508 (R. A. Panettieri, Jr.), AI-068871 (R. A. Panettieri, Jr.), and HL-097796 (R. A. Panettieri, Jr.) and the American Thoracic Society/LAM Foundation Research Grant LAM-07-001 (E. A. Goncharova). E. A. Goncharova is a Parker B. Francis Fellow in Pulmonary Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the National Disease Research Interchange for providing whole human lungs for ASM cell culture.
P. N. Lim is a PhD candidate at Institute of Molecular Plant Sciences, University of Edinburgh, King's Buildings, Mayfield Road, Edinburgh EH9 3JH, Scotland, UK.
Present address of A. Chisolm: Drexel University College of Medicine, Philadelphia, PA.
REFERENCES
- 1.Amrani Y, Moore PE, Hoffman R, Shore SA, Panettieri RA., Jr Interferon-gamma modulates cysteinyl leukotriene receptor-1 expression and function in human airway myocytes. Am J Respir Crit Care Med 164: 2098–2101, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Amrani Y, Tliba O, Choubey D, Huang CD, Krymskaya VP, Eszterhas A, Lazaar AL, Panettieri RA., Jr IFN-γ inhibits human airway smooth muscle cell proliferation by modulating the E2F-1/Rb pathway. Am J Physiol Lung Cell Mol Physiol 284: L1063–L1071, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Andrawis NS, Wang E, Abernethy DR. Endothelin-1 induces an increase in total protein synthesis and expression of the smooth muscle α-actin gene in vascular smooth muscle cells. Life Sci 59: 523–528, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 167: 1360–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 4: 2, 2003 [PMC free article] [PubMed] [Google Scholar]
- 6.Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest 119: 2634–2647, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang YJ, Holtzman MJ, Chen CC. Differential role of Janus family kinases (JAKs) in interferon-gamma-induced lung epithelial ICAM-1 expression: involving protein interactions between JAKs, phospholipase Cgamma, c-Src, and STAT1. Mol Pharmacol 65: 589–598, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chen HW, Chen X, Oh SW, Marinissen MJ, Gutkind JS, Hou SX. mom Identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Gene Dev 16: 388–398, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Oh SW, Zheng Z, Chen HW, Shin Hh, Hou SX. Cyclin D-Cdk4 and cyclin E-Cdk2 regulate the JAK/STAT signal transduction pathway in Drosophila. Dev Cell 4: 179–190, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee T, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460: 705–710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension 33: 177–182, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Finlay GA, York B, Karas RH, Fanburg BL, Zhang H, Kwiatkowski DJ, Noonan DJ. Estrogen-induced smooth muscle cell growth is regulated by tuberin and associated with altered activation of platelet-derived growth factor receptor-β and ERK-1/2. J Biol Chem 279: 23114–23122, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gabella G. Hypertrophic smooth muscle. I. Size and shape of cells, occurence of mitosis. Cell Tissue Res 201: 63–78, 1979 [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Gene Dev 15: 1383–1392, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 34: 247–254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. J Cell Biol 167: 1171–1182, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L354–L363, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA, Krymskaya VP. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. J Biol Chem 277: 30958–30967, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Goncharova EA, Goncharov DA, Spaits M, Noonan D, Talovskaya E, Eszterhas A, Krymskaya VP. Abnormal smooth muscle cell growth in lymphangioleiomyomatosis (LAM): role for tumor suppressor TSC2. Am J Respir Cell Mol Biol 34: 561–572, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncharova EA, Krymskaya VP. Pulmonary lymphangioleiomyomatosis (LAM): progress and current challenges. J Cell Biochem 103: 369–382, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, Forsythe SM, Hershenson MB, Solway J. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol 31: 266–275, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Hershenson MB, Brown M, Camoretti-Mercado B, Solway J. Airway smooth muscle in asthma. Annu Rev Pathol 3: 523–555, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Hindman HB, Marty-Roix R, Tang JB, Jupiter JB, Simmons BP, Spector M. Regulation of expression of α-smooth muscle actin in cells of Dupuytren's contracture. J Bone Joint Surg Br 85: 448–455, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology 20: 28–35, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 1: 176–183, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kang W, Rathinavelu S, Samuelson LC, Merchant JL. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest 85: 702–715, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kaur S, Lal L, Sassano A, Majchrzak-Kita B, Srikanth M, Baker DP, Petroulakis E, Hay N, Sonenberg N, Fish EN, Platanias LC. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. J Biol Chem 282: 1757–1768, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kaur S, Sassano A, Joseph AM, Majchrzak-Kita B, Eklund EA, Verma A, Brachmann SM, Fish EN, Platanias LC. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J Immunol 181: 7316–7323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaur S, Uddin S, Platanias LC. The PI3' kinase pathway in interferon signaling. J Interferon Cytokine Res 25: 780–787, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kroczynska B, Kaur S, Katsoulidis E, Majchrzak-Kita B, Sassano A, Kozma SC, Fish EN, Platanias LC. Interferon-dependent engagement of eukaryotic initiation factor 4B via S6 kinase (S6K)- and ribosomal protein S6K-mediated signals. Mol Cell Biol 29: 2865–2875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krymskaya VP. Targeting phosphatidylinositol 3-kinase pathway in airway smooth muscle: rationale and promise. BioDrugs 21: 85–95, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Krymskaya VP. Tumor suppressors hamartin and tuberin: intracellular signaling. Cell Signal 15: 729–739, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Krymskaya VP, Ammit AJ, Hoffman RK, Eszterhas A, Panettieri RA. Activation of class IA phosphatidylinositol 3-kinase stimulates DNA synthesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 280: L1009–L1018, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle 8: 403–413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krymskaya VP, Goncharova EA, Ammit AJ, Lim PN, Goncharov DA, Eszterhas A, Panettieri RA. Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. FASEB J 19: 428–430, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Krymskaya VP, Hoffman R, Eszterhas A, Ciocca V, Panettieri RA. TGF-β1 modulates EGF-stimulated phosphatidylinositol 3-kinase activity in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 273: L1220–L1227, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Krymskaya VP, Hoffman R, Eszterhas A, Kane S, Ciocca V, Panettieri RA. EGF activates ErbB2 and stimulates phosphatidylinositol 3-kinase in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 276: L246–L255, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Krymskaya VP, Orsini MJ, Eszterhas AJ, Brodbeck KC, Benovic JL, Panettieri RA, Jr, Penn RB. Mechanisms of proliferation synergy by receptor tyrosine kinase and G protein-coupled receptor activation in human airway smooth muscle. Am J Respir Cell Mol Biol 23: 546–554, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Krymskaya VP, Penn RB, Orsini MJ, Scott PH, Plevin RJ, Walker TR, Eszterhas AJ, Amrani Y, Chilvers ER, Panettieri RA. Phosphatidylinositol 3-kinase mediates mitogen-induced human airway smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 277: L65–L78, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Lanuti M, Kouri CE, Force SD, Chang MY, Amin K, Xu K, Blair IA, Kaiser LR, Albelda SM. Use of protamine to augment adenovirus-mediated cancer gene therapy. Gene Ther 6: 1600–1610, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Lazaar AL, Albelda SM, Pilewski JM, Brennan B, Pure E, Panettieri RA. T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J Exp Med 180: 807–816, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazaar AL, Reitz HE, Panettieri RA, Jr, Peters SP, Pure E. Antigen receptor-stimulated peripheral blood and bronchoalveolar lavage-derived T cells induce MHC class II and ICAM-1 expression on human airway smooth muscl. Am J Respir Cell Mol Biol 16: 38–45, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Lekmine F, Sassano A, Uddin S, Smith J, Majchrzak B, Brachmann SM, Hay N, Fish EN, Platanias LC. Interferon-γ engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp Cell Res 295: 173–182, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Lekmine F, Uddin S, Sassano A, Parmar S, Brachmann SM, Majchrzak B, Sonenberg N, Hay N, Fish EN, Platanias LC. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J Biol Chem 278: 27772–27780, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Inoki K, Vacratsis P, Guan KL. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J Biol Chem 278: 13663–13671, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Malqvist U, Arner A. Contractile properties during development of hypertrophy of the smooth muscle in the rat portal vein. Acta Physiol Scand 133: 49–61, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science 285: 2126–2129, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Mouthon L, Guillevin L. Interferon-alpha in corticosteroid-resistant asthma and Churg-Strauss syndrome. Allergy 58: 1244–1246, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Nurse P. Cyclin dependent kinases and cell cycle control. Biosci Rep 22: 487–499, 2002 [DOI] [PubMed] [Google Scholar]
- 50.O'Sullivan S, Cormican L, Burke CM, Poulter LW. Fluticasone induces T cell apoptosis in the bronchial wall of mild to moderate asthmatics. Thorax 59: 657–661, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1−/− skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol 7: 286–294, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Owens GK, Geisterfer AA, Yang YW, Komoriya A. Transforming growth factor-β-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol 107: 771–780, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panettieri RA., Jr Airway smooth muscle: an immunomodulatory cell. J Allergy Clin Immunol 110: S269–S274, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Panettieri RA, DePalo LR, Murray RK, Yadvich PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol Cell Physiol 256: C329–C335, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Panettieri RA, Grunstein MM. Airway smooth muscle hyperplasia and hypertrophy. In: Asthma, edited by Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ. Philadelphia: Lippincott-Raven, 1997, p. 823–842 [Google Scholar]
- 56.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1−/−/S6K2−/− mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol 24: 3112–3124, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5: 375–386, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Platanias LC. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol Ther 98: 129–142, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Shen H, Zhang M, Minuk G, Gong Y. Different effects of rat interferon alpha, beta and gamma on rat hepatic stellate cell proliferation and activation. BMC Cell Biol 3: 1–8, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Moleculae mechanisms regulating Th1 immune responses. Annu Rev Immunol 21: 713–758, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Tanaka K, Sano K, Tanaka K, Kobayashi M, Katsumura K, Ikeda T, Abe M. Demonstration of downregulation of α-smooth muscle actin in interferon-γ-treated myofibroblast by a novel cell-capture enzyme immunoassay. Int Immunopharmacol 1: 769–775, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell 105: 345–355, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Tliba O, Amrani Y. Airway smooth muscle cell as an inflammatory cell: lessons learned from interferon signaling pathways. Proc Am Thorac Soc 5: 106–112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tliba O, Tliba S, Da Huang C, Hoffman RK, DeLong P, Panettieri RA, Jr, Amrani Y. Tumor necrosis factor α modulates airway smooth muscle function via the autocrine action of interferon β. J Biol Chem 278: 50615–50623, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 3: 393–402, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Wong CK, Ho CY, Ko FW, Chan CH, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol 125: 177–183, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med 169: 1001–1006, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Zhou L, Goldsmith AM, Bentley JK, Jia Y, Rodriguez ML, Abe MK, Fingar DC, Hershenson MB. 4E-binding protein phosphorylation and eukaryotic initiation factor-4E release are required for airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 33: 195–202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou L, Hershenson MB. Mitogenic signaling pathways in airway smooth muscle. Respir Physiol Neurobiol 137: 295–308, 2003 [DOI] [PubMed] [Google Scholar]