Abstract
Reactive oxygen species have been shown to play a significant role in hyperoxia-induced acute lung injury, in part, by inducing apoptosis of pulmonary endothelium. However, the signaling roles of phospholipid oxidation products in pulmonary endothelial apoptosis have not been studied. Using an oxidative lipidomics approach, we identified individual molecular species of phospholipids involved in the apoptosis-associated peroxidation process in a hyperoxic lung. C57BL/6 mice were killed 72 h after exposure to hyperoxia (100% oxygen). We found that hyperoxia-induced apoptosis (documented by activation of caspase-3 and -7 and histochemical terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling staining of pulmonary endothelium) was accompanied by nonrandom oxidation of pulmonary lipids. Two anionic phospholipids, mitochondria-specific cardiolipin (CL) and extramitochondrial phosphatidylserine (PS), were the two major oxidized phospholipids in hyperoxic lung. Using electrospray ionization mass spectrometry, we identified several oxygenation products in CL and PS. Quantitative assessments revealed a significant decrease of CL and PS molecular species containing C18:2, C20:4, C22:5, and C22:6 fatty acids. Similarly, exposure of mouse pulmonary endothelial cells (MLEC) to hyperoxia (95% oxygen; 72 h) resulted in activation of caspase-3 and -7 and significantly decreased the content of CL molecular species containing C18:2 and C20:4 as well as PS molecular species containing C22:5 and C22:6. Oxygenated molecular species were found in the same two anionic phospholipids, CL and PS, in MLEC exposed to hyperoxia. Treatment of MLEC with a mitochondria-targeted radical scavenger, a conjugate of hemi-gramicidin S with nitroxide, XJB-5-131, resulted in significantly lower oxidation of both CL and PS and a decrease in hyperoxia-induced changes in caspase-3 and -7 activation. We speculate that cytochrome c driven oxidation of CL and PS is associated with the signaling role of these oxygenated species participating in the execution of apoptosis and clearance of pulmonary endothelial cells, thus contributing to hyperoxic lung injury.
Keywords: hyperoxia, endothelium, hydroperoxides, apoptosis
hyperoxia-induced acute lung injury is characterized by an influx of inflammatory cells, increased pulmonary permeability, and endothelial and epithelial cell death (41, 43, 59). Reactive oxygen species (ROS) have been postulated to have a significant role in hyperoxic acute lung injury, in part, by inducing cell death of pulmonary endothelium involving both extrinsic and intrinsic pathways (71). The mechanisms and link between two major factors contributing to genesis and maintenance of hyperoxic (acute) lung injury, an imbalance between production and elimination of partially reduced oxygen and nitrogen species and endothelial dysfunction due to endothelial cell apoptosis, are not well characterized.
Oxygenated fatty acids are well-known signaling molecules that participate in regulation and coordination of cellular and body metabolism (38, 55). Their important role in cell proliferation, i.e., modulation of apoptosis, angiogenesis, inflammation, and immune surveillance, has been well documented (44, 57). The involvement of another oxygenated product of polyunsaturated docosahexaenoic acid, resolvin E1, in the pathogenesis of lung inflammatory injury has also been suggested (2, 23, 24), even though polyunsaturated phospholipids are the major oxidation targets in sn-2 position (32). Several decades ago, an activation of lipid peroxidation in the lung under hyperoxic conditions was evidenced by the accumulation of one of the secondary products of lipid peroxidation, malonyldialdehyde (28), as well as thiobarbituric acid material in cultured endothelial cells exposed to hyperoxia (21). Moreover, an elevation of oxygenated products formed from arachidonic acid, isoprostanes and isofuranes, has been documented in hyperoxic mouse lung (17). However, the participation and signaling roles of oxidatively modified phospholipids in pulmonary endothelial apoptosis in the hyperoxic acute lung injury have not yet been established.
Recently, the accumulation of oxidation products in two anionic phospholipids, a mitochondria-specific cardiolipin (CL) and extramitochondrial phosphatidylserine (PS), has been associated with apoptosis, particularly with a release of proapoptotic factors from mitochondria into the cytosol and an externalization of PS on the cell surface of apoptotic cells, respectively (35, 36). Moreover, these two anionic phospholipids have been identified as oxidation substrates of cytochrome c (cyt c) catalyzed reactions in vitro (36, 62) and in vivo (62). Furthermore, oxidized phospholipids have been demonstrated to act as signals in monocyte activation, programmed cell death, and phagocytotic clearance of apoptotic cells (14, 22, 42, 68, 69).
In the present study, we used oxidative lipidomics to identify individual molecular species of phospholipids involved in the apoptosis-associated peroxidation process in hyperoxic lung and pulmonary endothelial cells. We found that hyperoxia-induced apoptosis was accompanied by nonrandom oxidation of pulmonary lipids. CL and PS were the two major oxidized phospholipids in both hyperoxic lung and mouse lung endothelial cells (MLEC). Electrospray ionization mass spectrometry (ESI-MS) analysis revealed the formation of several oxygenation products in CL and PS. Treatment of MLEC with a mitochondria-targeted scavenger of electrons and radicals, GS-nitroxide (XJB-5-131), a conjugate of hemi-gramicidin S (GS) representing a pentapeptide Leu-d-Phe-Pro-Val-Orn, with a stable nitroxide radical, 4-amino-Tempo, suppressed oxidation of both CL and PS and reduced hyperoxia-induced apoptosis. Based on previous findings and the results of this work, we speculate that cyt c driven oxidation of CL and PS is associated with the signaling roles of these oxygenated phospholipid species in the execution of apoptosis and the clearance of pulmonary endothelial cells, thus contributing to hyperoxic lung injury.
MATERIALS AND METHODS
Hyperoxia in vivo.
Wild-type C57Bl6 male mice were placed in a Plexiglass induction chamber (Vet Equip) with food and water ad libitum and exposed to 100% oxygen for up to 72 h with oxygen flowing at a rate of 15 l/min. Oxygen saturation in the chamber was measured periodically with an oxygen analyzer from Vascular Technology. All procedures were preapproved and performed according to the protocols established by the Institutional Animal Care and Use Committee of the University of Pittsburgh. The choice of 72 h of exposure to hyperoxia was based on the published data that this is immediately before the lethal endpoint of hyperoxic lung injury with concomitant increase in neutrophil recruitment to the lungs (46).
MLEC.
Mice were killed by CO2 inhalation, and the chest was opened. Lungs were flushed with HBSS containing 10 U/ml heparin, removed, finely minced, and digested in type I collagenase. The mixture was filtered, centrifuged, resuspended, and then incubated with magnetic beads coated with antibody (rat anti-mouse) to platelet endothelial cell adhesion molecule (PECAM-1; BD Pharmingen). Magnetic beads were removed gently via series of rinses (trypsin/EDTA), and cells were isolated for subculture (60). At approximately passage 2, cells were incubated with fluorescently-labeled diacetylated LDL (diI-LDL) and sorted to homogeneity by FACS. The enriched PECAM and diI-LDL population was subcultured on a collagen/gelatin matrix in 2% O2, 5% CO2, 93% nitrogen in a Coy Hypoxic Glove Box/Chamber in Opti-MEM (GIBCO), 10% FBS, 2 mM glutamine, 0.2% retinal derived growth factor (Vec Technologies), 10 U/ml heparin, 0.1 mM nonessential amino acid supplement (GIBCO), and 55 μM β-mercaptoethanol. MLEC maintain an endothelial cell morphology and phenotype (PECAM positive; uptake of dil-LDL) for longer periods (e.g., more subcultures) when grown in 2% oxygen rather than room air.
Exposure of MLEC to oxygen.
Cultured MLEC were exposed to 95% O2-5% CO2 for 72 h in a 37°C humidified BillupsRothenberg modular incubator, and their response was contrasted to MLEC cultured at 2% O2, 5% CO2, and 93% N2 in a Coy Hypoxic Glove Box/Chamber. At the end of exposure, caspase-3 and 7 activity was measured and lipids were extracted for analysis. A subgroup of MLEC was grown in room air and in early passage was placed in hyperoxia and caspase-3 and -7 activity measured after 72 h.
Caspase-3 and -7 activity.
Caspase-3 and -7 activity in lung homogenates as well as in MLEC was measured using a luminescence Caspase-Glo assay kit obtained from Promega (Madison, WI). Luminescence was determined at time 0 and following 1-h incubation at 25°C using a ML1000 luminescence plate reader (Dynatech). Caspase-3 activity was expressed as the luminescence produced within 1-h incubation per microgram of protein.
Effect of the GS-nitroxide XJB-5-131 on hyperoxia exposed MLEC.
MLEC were exposed to 95% O2-5% CO2 for 72 h with or without addition of mitochondria-targeted electron scavenger, XJB-131 (20 µM), and oxidized phospholipids (see ESI-MS) and apoptosis (as determined by caspase-3 and -7 activity) were analyzed.
Immunohistochemical assessment of apoptosis.
Mice were anesthetized after 72 h of hyperoxia (or control), the chest was opened, and the vascular space was flushed with PBS. Lungs were inflated with 2% paraformaldehyde, and frozen sections were used for immunofluorescent detection of apoptosis using an Alexa 488-labeled Nick End labeling kit for assessing apoptosis from Promega. All nuclei, including apoptotic nuclei, were labeled with DAPI such that healthy nuclei appear blue and apoptotic nuclei are labeled blue and green. To define the endothelial cell population, sections were also labeled with rat anti-mouse antibody to CD31 (an endothelial cell specific marker; BD Pharmingen) and goat anti-rat Cy3 conjugated secondary antibody (Jackson ImmunoResearch). Images are confocal single plane images from an Olympus Fluoview 1000 scanning confocal microscope (Olympus America).
Lipid extraction and two-dimensional high-performance thin-layer chromatography analysis.
Total lipids were extracted from lung homogenates and MLEC by Folch procedure (18). Lipid extracts were separated and analyzed by two-dimensional high-performance thin-layer chromatography (2D-HPTLC; Ref. 53). To prevent oxidative modification of phospholipids during separation, the plates were treated with methanol containing 1 mM EDTA, 100 μM diethylenetriaminepentaacetic acid before application and separation of phospholipids by 2D-HPTLC. Total lipids (60 nmol) were applied onto plates under flow of N2, and the plates were first developed with a solvent system consisting of chloroform:methanol:28% ammonium hydroxide (65:25:5 vol/vol). After the plates were dried with a forced N2 blower to remove the solvent, they were developed in the second dimension with a solvent system consisting of chloroform:acetone:methanol:glacial acetic acid:water (50:20:10:10:5 vol/vol). The phospholipids were visualized by exposure to iodine vapors and identified by comparison with authentic phospholipid standards. For ESI-MS and analysis of phospholipid hydroperoxides (PL-OOH) by fluorescence HPLC using Amplex Red, the phospholipid spots on the silica plates were visualized by spraying the plates with deionized water. Subsequently, the spots were scraped from the silica plates and phospholipids were extracted in chloroform:methanol:water (10:5:1 vol/vol). Lipid phosphorus was determined by a micromethod (7).
Quantitation of lipid hydroperoxides.
Lipid hydroperoxides were determined by fluorescence HPLC of resorufin formed in peroxidase-catalyzed reduction of specific PL-OOH with Amplex Red after hydrolysis by porcine pancreatic phospholipase A2 (1 U/μl) in 25 mM phosphate buffer containing 1.0 mM Ca, 0.5 mM EGTA, and 0.5 mM SDS (pH 8.0 at room temperature for 30 min). For the peroxidase reaction, 50 μM Amplex Red and microperoxidase −11 (1.0 μg/μl) were added to the hydrolyzed lipids, and the samples were incubated at 4°C for 40 min. The reaction was started by addition of microperoxidase-11 and terminated by a stop reagent (100 μl of a solution of 10 mM HCl and 4 mM butylated hydroxytoluene in ethanol). The samples were centrifuged at 10,000 g for 5 min, and the supernatant was used for HPLC analysis. Aliquots (5 μl) were injected into a C-18 reverse phase column (Eclipse XDB-C18, 5 μm, 150 × 4.6 mm) and eluted using a mobile phase composed of 25 mM KH2PO4 (pH 7.0)/methanol (60:40 vol/vol) at a flow rate of 1 ml/min. The resorufin fluorescence was measured at 590 nm after excitation at 560 nm. A Shimadzu LC-100AT vp HPLC system equipped with a fluorescence detector (model RF-10Axl) and an autosampler (model SIL-10AD vp) was used (64, 67).
ESI-MS.
ESI-MS analysis was performed by direct infusion into linear ion-trap mass spectrometer LXQ with the Xcalibur operating system (ThermoFisher Scientific, San Jose, CA) as previously described (64, 67). Samples collected after 2D-HPTLC separation were evaporated under N2, resuspended in chloroform:methanol 1:1 vol/vol (20 pmol/μl) and directly utilized for acquisition of negative-ion or positive-ion ESI mass spectra at a flow rate of 5 μl/min. The ESI probe was operated at a voltage differential of 3.5–5.0 kV in the negative or positive ion mode. Capillary temperature was maintained at 70 or 150°C. With the use of full range zoom [mass-to-charge ratio (m/z) of 200–2,000] in positive and negative ion mode, the profile spectra were acquired. Tandem mass spectrometry (MS/MS analysis) of individual phospholipid species was employed to determine the fatty acid composition. The MS/MS spectra were acquired using an isolation width of m/z 1.0. Singly charged ions were used for structural identification of CL as described by Hsu et al (25). The scan time setting of ion trap for full MS (range of m/z 1,400–1,600) was set at 50 microscans with maximum injection time 1,000 msec. MSn analysis was performed using isolation width of m/z 1 and five microscans with a maximum injection time of 1,000 ms. Two ion activation techniques were used for MS analysis: collision-induced dissociation (with Q = 0.25 and low mass cut off at 28% of the precursor m/z) and pulsed-Q dissociation technique (with Q = 0.7 and no low mass cut off for analysis of low molecular weight fragment ions). Based on the MS fragmentation data, the chemical structures of lipid molecular species were drawn using ChemDraw and confirmed by comparing with the fragmentation patterns presented in the Lipid Map Data Base (www.lipidmaps.org). Additionally, to quantitatively assess different molecular species of CL and PS, liquid chromatography (LC)/ESI-MS was performed using a Dionex Ultimate 3000 HPLC coupled online to a linear ion trap mass spectrometer (LXQ ThermoFisher). The lipids were separated on a normal phase column (Luna 3 μm Silica 100A, 150 × 2 mm; Phenomenex, Torrance, CA) with a flow rate of 0.2 ml/min using gradient solvents containing 5 mM CH3COONH4 [A: n-hexane:2-propanol:water, 43:57:1 (vol/vol/vol); and B: n-hexane:2-propanol:water, 43:57:10 (vol/vol/vol)]. Analysis of phospholipid oxidized molecular species (hydroperoxy and hydroxy) was performed as described (67). To account for isotopic interferences, we performed isotopic corrections by entering the chemical composition of each species into the Qual browser of Xcalibur (operating system) and using the simulation of the isotopic distribution to make adjustments for the major peaks. To minimize isotopic interferences between isolated masses M+2, the MS/MS spectra were acquired using an isolation width of m/z 1.0. For identification and characterization of phospholipid molecular species, the spectra of CL and PS were acquired in negative mode and the scan data type was set to profile. For quantitative assessments of CL and PS molecular species in control and hypoxic lung, we used LC/MS. Because of the large size of LC/MS files obtained in the profile mode, the scan data type was set to centroid.
Statistics.
The results are means ± SD from at least three experiments, and statistical analyses were performed by either paired/unpaired Student's t-test or one-way ANOVA. The statistical significance of differences was set at P < 0.05.
RESULTS
Effect of hyperoxia on phospholipid composition in mouse lung.
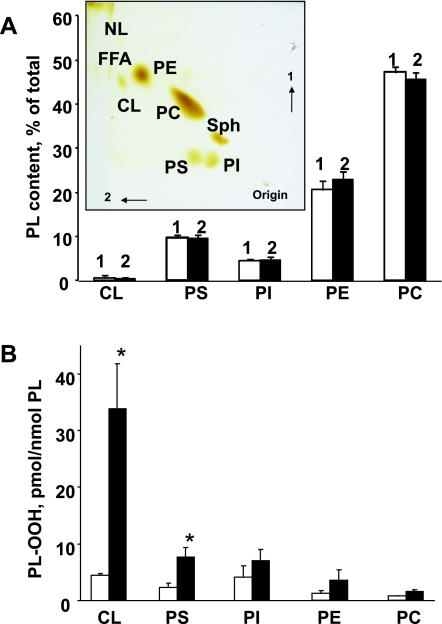
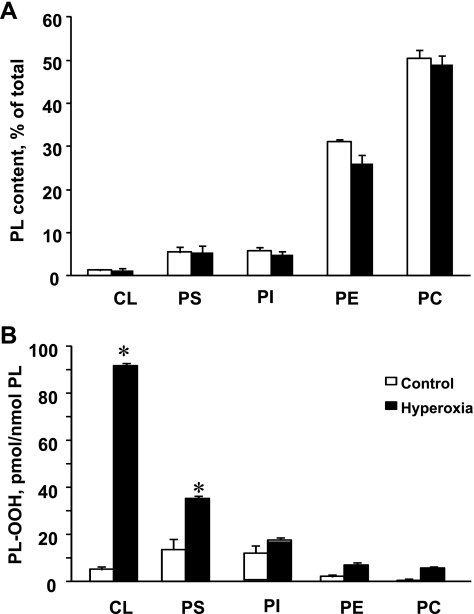
First, we studied the effects of hyperoxia on the composition of the major phospholipids in the mouse lung. Lipids from the lungs of normal mice and mice exposed to hyperoxia (100% oxygen, 72 h) were isolated and separated by 2D-HPTLC (Fig. 1A, inset). To quantitate phospholipids, the silica spots were scraped off the plates and lipid phosphorus was determined. The major classes of mouse lung phospholipids, in the order of their abundance, were as follows: phosphatidylcholine (PC) > phosphatidylethanolamine (PE) > PS = phosphatidylinositol (PI) > CL > sphingomyelin (Fig. 1A). No significant changes in the composition of the phospholipids were detected after exposure to hyperoxia (Fig. 1A).
Fig. 1.
Phospholipid (PL) composition and accumulation of phospholipid hydroperoxides in lung of mouse exposed to hyperoxia. A: phospholipid composition of control and hyperoxic lung. Mice were exposed to hyperoxia (100% of oxygen) for 72 h and killed thereafter. Lipids were extracted and separated by 2-dimensional high-performance thin-layer chromatography (2D-HPTLC). Spots of phospholipids were scraped, and lipid phosphorus was determined. Inset: typical 2D-HPTLC chromatogram of total lipids extracted from control mouse lung. NL, neutral lipids; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; CL, cardiolipin; Sph, sphingomyelin. Open bars or number 1 are control; closed bars or number 2 are hyperoxia. Data are means ± SD; n = 3 (for control); n = 4 (for hyperoxia). B: accumulation of phospholipid hydroperoxides (PL-OOH) in lung of mice exposed to hyperoxia (100% of oxygen for 72 h). Lipids were extracted and separated by 2D-HPTLC. PL-OOH were detected using Amplex Red protocol. Open bars are control; closed bars are hyperoxia. Data are means ± SD. *P < 0.05 vs. control; n = 3 (for control); n = 4 (for hyperoxia).
Effect of hyperoxia on the accumulation of hydroperoxy phospholipids in the mouse lung.
The amounts of PL-OOH in major phospholipids were detected after their separation by 2D-HPTLC using the HPLC Amplex Red protocol (64, 67). Only two anionic phospholipids, CL and PS, underwent robust oxidation in the hyperoxic mouse lung (Fig. 1B). The CL hydroperoxide (CL-OOH) content was as high as 33.8 ± 8.0 vs. 4.5 ± 0.3 pmol/nmol of CL in the lung of hyperoxia and control mice, respectively. The amount of accumulated PS hydroperoxide (PS-OOH) in the hyperoxic lung was 7.6 ± 1.7 of vs. 2.6 ± 0.7 pmol/nmol in the control lung, respectively. Hyperoxia-induced increments of CL-OOH and PS-OOH constituted 29.3 and 5.0 pmol/nmol for CL and PS, respectively. No significant oxidation in the major phospholipid classes such as PC, PE, and PI was determined in the hyperoxic lung.
ESI-MS analysis of CL and PS molecular species of lung phospholipids.
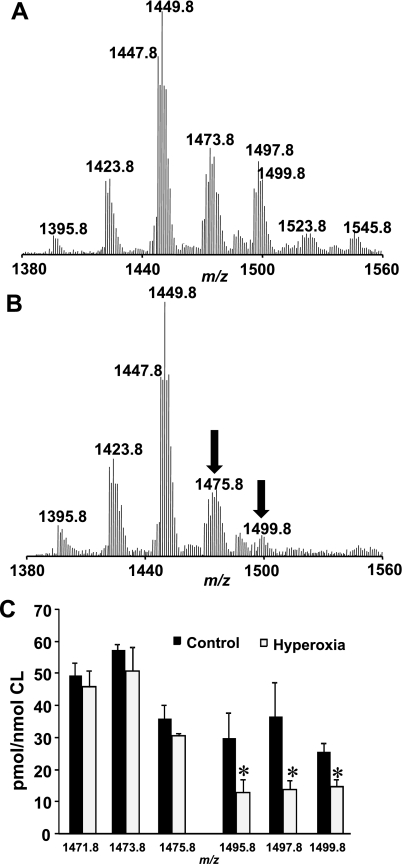
Molecular species of CL and PS were characterized by ESI-MS using the negative mode. Direct infusion experiments as well as LC/ESI-MS were employed in MS and MSn analysis for identification of the molecular species containing polyunsaturated fatty acid residues that are highly susceptible to oxidation (64). Typical full LC/ESI-MS spectra of lung CL and PS are presented in Figs. 2A and 3A, respectively. CL molecular species were represented by four clusters (Fig. 2A). The major cluster contained molecular ions with m/z 1,447.8 and 1,449.8. Molecular clusters at m/z 1,421.8, 1,473.8, and 1,497.9 were also detectable in the full MS spectrum but at relatively lower abundances. Analysis of PS revealed three major molecular clusters with m/z 788.5, 810.5, and 834.5. To identify molecular species of phospholipids, we performed MS2 experiments using the pulsed-Q dissociation technique. Fragmentation (MS2) analysis showed that both CL and PS contained linoleic (C18:2), arachidonic (C20:4), docosapentaenoic (C22:5), and docosahexaenoic acid (C22:6; Tables 1 and 2). A quantitative assessment of these phospholipids revealed that CL was enriched with molecular species containing C18:2 (Table 1). PS was mainly represented by molecular species containing C20:4 and C22:6 (Table 2).
Fig. 2.
Oxidation of CL in lung of mice exposed to hyperoxia. A: typical full negative liquid chromatography (LC)/electrospray ionization mass spectrometry (ESI-MS) spectrum of CL isolated from control mouse lung. Major cluster contains molecular ions with a mass-to-charge ratio (m/z) of 1,447.8 and 1,449.8 along with clusters at m/z 1,421.8, 1,473.8, and 1,497.9 were detectable in the spectrum. B: typical full negative LC/ESI-MS spectrum of CL isolated from hyperoxic mouse lung. MS analysis revealed significant reduction of CL molecular ions with m/z 1,495.8, 1,497.8, and 1,499.8. C: quantitative assessment of CL molecular species in control lung and lung isolated from mice exposed to hyperoxia. Open bars are control; closed bars are hyperoxia. Data are means ± SD. *P < 0.05 vs. control; n = 3 (for control); n = 4 (for hyperoxia).
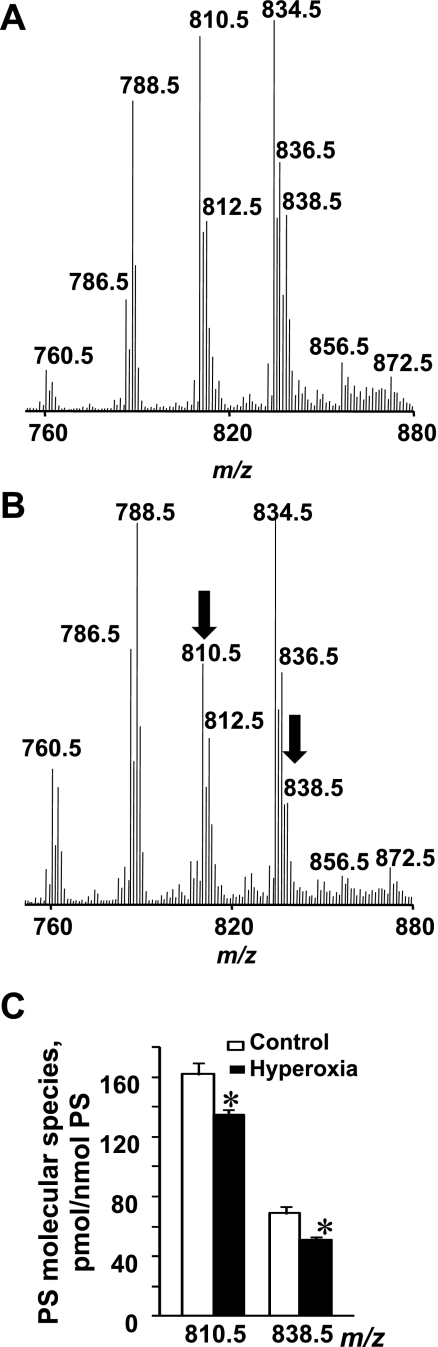
Fig. 3.
Oxidation of PS in lung of mice exposed to hyperoxia. A: typical full negative LC/ESI-MS spectrum of PS isolated from control mouse lung. Major molecular ions of PS with m/z 788.5, 810.5, and 834.5 were detectable in spectrum. B: typical full negative LC/ESI-MS spectrum of PS isolated from hyperoxic mouse lung. A decrease of intensity of 2 molecular ions with m/z 810.5 (C18:0/C20:4) and 838.5 (C18:0/C22:4) in hyperoxic lung was detected. C: quantitative assessment of PS molecular species in normal and hyperoxic lung. Open bars are control; closed bars are hyperoxia. Data are means ± SD. *P < 0.05 vs. control; n = 3 (for control); n = 4 (for hyperoxia).
Table 1.
Identification and content of major CL molecular species in mouse lung and mouse lung endothelial cells
| m/z | CN:DB | Molecular Species | Whole Mouse Lung, pmol/nmol total CL | MLEC, pmol/nmol total CL |
|---|---|---|---|---|
| 1,421.8 | 70:7 | C16:1/C18:2/C18:2/C18:2 | 68.8 ± 7.8 | 25.1 ± 3.8* |
| 1,423.8 | 70:6 | C16:0/C18:2/C18:2/C18:2 | 56.4 ± 9.5 | 87.7 ± 4.3 |
| 1,425.8 | 70:5 | C16:1/C18:2/C18:1/C18:1 | 16.6 ± 5.7 | 111.4 ± 6.5* |
| C16:0/C18:2/C18:2/C18:1 | ||||
| 1,447.8 | 72:8 | C18:2/C18:2/C18:2/C18:2 | 155.7 ± 2.3 | 43.9 ± 2.4* |
| 1,449.8 | 72:7 | C18:2/C18:2/C18:2/C18:1 | 140.6 ± 4.1 | 99.4 ± 7.0* |
| 1,451.8 | 72:6 | C18:2/C18:2/C18:1/C18:1 | 75.5 ± 7.9 | 41.3 ± 5.8* |
| 1,453.8 | 72:5 | C18:2/C18:1/C18:1/C18:1 | ND | 137.1 ± 7.2 |
| 1,455.8 | 72:4 | C18:1/C18:1/C18:1/C18:1 | ND | 17.9 ± 37 |
| 1,471.8 | 74:10 | C18:2/C18:2/C18:2/C20:4 | 49.2 ± 4.3 | 6.9 ± 0.7* |
| 1,473.8 | 74:9 | C18:1/C18:2/C18:2/C20:4 | 57.2 ± 1.8 | 64.8 ± 4.8 |
| 1,475.8 | 74:8 | C18:1/C18:1/C18:2/C20:4 | 35.8 ± 4.4 | 59.0 ± 6.1* |
| 1,477.8 | 74:7 | C18:1/C18:1/C18:1/C20:4 | 19.1 ± 1.2 | 60.5 ± 4.7* |
| 1,495.8 | 76:12 | C18:2/C18:2/C20:4/C20:4 | 29.6 ± 8.3 | 10.7 ± 0.2 |
| 1,497.9 | 76:11 | C18:1/C18:2/C20:4/C20:4 | 36.3 ± 11.0 | 16.8 ± 3.0 |
| C18:1/C18:2/C18:2/C22:6 | ||||
| 1,499.8 | 76:10 | C18:1/C20:4/C20:4/C18:1 | 25.6 ± 2.7 | 35.8 ± 4.7 |
| C18:0/C18:2/C18:2/C22:6 | ||||
| C18:1/C18:2/C18:2/C22:5 |
Data are means ± SE; n = 3 for whole lung; n = 4 for mouse pulmonary endothelial cells (MLEC). CN, carbon number; DB, double bonds; m/z, mass-to-charge ratio; CL, cardiolipin; ND, not determined.
P < 0.01 vs. whole lung.
Table 2.
Identification and content of major PS molecular species in mouse lung and mouse lung endothelial cells
| m/z | CN:DB | Molecular Species | Whole Mouse Lung, pmol/nmol total PS | MLEC, pmol/nmol total PS |
|---|---|---|---|---|
| 786.5 | 36:3 | C18:0/C18:2; C18:1/C18:1 | 49.9 ± 2.2 | 40.8 ± 4.7 |
| 788.5 | 36:1 | C18:0/C18:1 | 128.4 ± 8.3 | 175.9 ± 6.8* |
| 810.5 | 38:4 | C18:0/C20:4 | 158.7 ± 5.8 | 111.7 ± 10.0* |
| 812.5 | 38:3 | C18:0/C20:3 | 60.0 ± 2.1 | 77.4 ± 7.9 |
| 832.5 | 40:7 | C18:1/C22:6 | 13.9 ± 2.0 | 3.6 ± 2.0* |
| 834.5 | 40:6 | C18:0/C22:6 | 158.4 ± 5.4 | 131.8 ± 11.0 |
| 836.5 | 40:5 | C18:0/C22:5 | 79.3 ± 3.1 | 166.7 ± 8.9* |
| 838.5 | 40:4 | C18:0/C22:4 | 65.8 ± 3.8 | 97.7 ± 5.1* |
Data are means ± SE; n = 3 for whole lung; n = 4 for MLEC. PS, phosphatidylserine.
P < 0.01 vs. whole lung.
Identification and quantitative analysis of oxidized molecular species of CL in hyperoxic mouse lung.
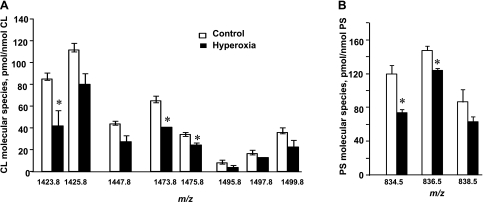
Mouse lung CL was enriched with molecular species containing C18:2 and less unsaturated acyl groups (Table 1). For comparison, the sum of these species was 444.8 vs. 227.2 pmol/nmol CL for molecular species containing at least one acyl group with four, five, and six double bonds, a twofold difference. Oxidation should cause disappearance of “oxidizable” species and appearance of their oxygenated products. Quantitative assessments revealed a significant reduction of CL molecular ions with m/z 1,495.8, 1,497.8, and 1,499.8, i.e., less abundant but more unsaturated species (Fig. 2, B and C). The amount of these molecular species was decreased 2.3-, 2.7-, and 1.8-fold, respectively, after hyperoxia vs. the control mice. Essentially no quantitative changes were found in the clusters of less polyunsaturated CL molecules with m/z 1,471.8–1,475.8. While the relative intensity of the most abundant molecular ion with m/z 1,447.8, containing four C18:2 acyl residues, was slightly decreased in the hyperoxic lung compared with the control lung, quantitative assessments revealed no significant differences between the two types of samples. In line with this, we found that CL oxidation involved less abundant molecular species containing C18:2, C20:4, C22:5, and C22:6 fatty acid residues. As expected, the disappearance of “oxidizable” CLs was accompanied by the emergence of their oxygenated counterparts as illustrated by a typical fragmentation pattern of the CL molecular ion with m/z 1,499.8 (Fig. 4Aa). Molecular ions of C18:2 (m/z 279.3), C18:1 (m/z 281.3), C20:4 (m/z 303.3), C22:6 (m/z 327.3), and C22:5 (m/z 339.3) along with other ions typical for CL fragmentation were detected in the MS2 spectrum (Fig. 4Ab). A molecular ion with m/z 1,499.8 was represented by three highly unsaturated molecular species: C18:1/C20:4/C20:4/C18:1, C18:0/C18:2/C18:2/C22:6, and C18:1/C18:2/C18:2/C22:5. Oxidized molecular species of CL with m/z 1,528.1, 1,530.1, and 1,532.1 were present in the spectra of the hyperoxic lung. The MS2 analysis showed that these oxidized molecular species originated from the molecular species with m/z 1,495.8, 1,497.8, and 1,499.8, respectively, after addition of two oxygen atoms. Using an MS2 analysis, we identified the peroxidized CLs as monohydroperoxy (C18:1/C18:1/C18:2-OOH/C22:6) and dihydroxy (C18:1/C20:4-OH/C20:4-OH/C18:1 and C18:0/C18:2-OH/C18:2/C22:6-OH; Fig. 4Ba). The molecular ions of C18:2-OH (m/z 295.3), C18:2-OOH (m/z 311.3), C20:4-OH (m/z 319.3), and C22:6-OH (m/z 343.3) were identified after fragmentation of the parent ion with m/z 1,532.1 (Fig. 4Bb). Thus CL oxidation involves less abundant but highly unsaturated molecular species of CL that underwent oxidative modification under hyperoxic conditions. In some of them, however, oxygenation occurred not as expected in the most polyunsaturated acyl groups (C20:4 and C22:6) but in the C18:2 acyl moieties.
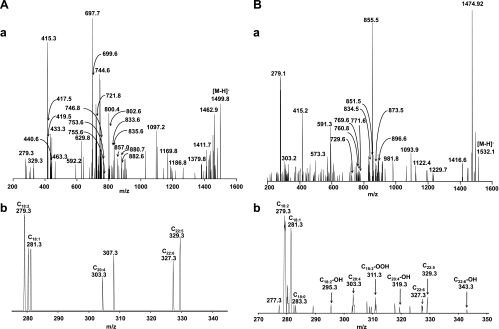
Fig. 4.
ESI-MS2 analysis of CL isolated from control and hyperoxic mouse lungs. Aa: MS2 fragmentation of CL species with m/z 1,499.8 from control lung. Ions [a]− and [b]− with m/z 721.8 corresponding to C18:1/C20:4-PA as well as characteristic daughter [a+136]− and [b+136]− (m/z 857.0); ions [a-(C18:1)]− and [b-(C18:1)]− (m/z 440.6); and [a-(C20:4)]− and [b-(C20:4)]− (m/z 419.5) were formed during fragmentation of the molecular ion with m/z 1,499.5 corresponding to molecular species of CL (C18:1/C20:4/C20:4/C18:1). Fragmentation of molecular species of CL C18:1/C18:2/C18:2/C22:5 revealed the presence of [a]− and [b]− ions with m/z 697.7 and 746.8, mainly corresponding to C18:1/C18:2-PA and C18:2/C22:5-PA. In addition, ions with m/z 753.6 ([a+56]−); 833.6 ([a+136]−); 802.6 ([b+56]−); and 882.6 ([b+136]−) were detected on MS2 spectrum. Moreover, [a]− and [b]− ions with m/z 699.5 and 744.6 corresponded to C18:0/C18:2-PA and C18:2/C22:6-PA. In addition, ions with m/z 755.6 ([a+56] −); 835.6 ([a+136]−); 800.4 ([b+56]−); and 880.7 ([b+136]−) were formed after fragmentation of molecular ion corresponding to molecular species of CL C18:0/C18:2/C18:2/C22:6. b: Part of MS2 spectrum of molecular ion with m/z 1,499.8 shown in the range of m/z 270–350. Molecular ions of C18:1 (m/z 281.3), C18:2 (m/z 279.3), C22:5 (m/z 329.3), and C22:6 (m/z 327.3) were presented in MS2 spectrum as well. Thus MS2 analysis of the singly charged ion (m/z 1,499.8) revealed the copresence of molecular species of CL (C18:1/C20:4/C20:4/C18:1) along with CLs (C18:1/C18:2/C18:2/C22:5 and C18:0/C18:2/C18:2/C22:6). Ba: MS2 fragmentation of CL species with m/z 1,532.1 from hyperoxic lung. Fragments {[a+136+16 (one oxygen)] and [b+136+16 (one oxygen)]} of oxidized molecular species of CL (C18:1/C20:4-OH/C20:4-OH/C18:1) with m/z 873.5 corresponding to [C18:1/C20:4-OH-PA+136] were detected on MS2 spectrum. In addition, fragmentation of CL molecular species C18:1/C18:2-OOH/C18:2/C22:5 resulted in appearance of fragments {[a+32 (two oxygen)]} with m/z 729.6 corresponding to C18:1/C18:2-OOH-PA. Moreover, fragments with m/z 760.8 {[a+16 (one oxygen)]} corresponding to C18:0/C18:2-OH-PA; m/z 896.6 {[a+136+16 (one oxygen)]} corresponding to C18:0/C18:2-OH-PA+136; m/z 771.6 {[b+56+16 (one oxygen)]} corresponding to C18:2/C22:6-OH-PA; and 851.5 {[b+136+16 (one oxygen)]} corresponding to C18:2/C22:6-OH-PA were detected in the MS2 spectrum and originated from a molecular species of CL , C18:0/C18:2-OH/C18:2/C22:6-OH. b: Part of MS2 spectrum of molecular ion with m/z 1,532.1 shown in the range of m/z 270–370. Molecular ions of oxygenated fatty acids C18:2-OH (m/z 295.3), C18:2-OOH (m/z 311.3), C20:4-OH (m/z 319.3), and C22:6-OH (m/z 343.3) alone with nonoxidized fatty acids C18:0 (m/z 283.3), C18:1 (m/z 281.3), C18:2 (m/z 279.3), C22:5 (m/z 329.3), and C22:6 (m/z 327.3) were presented in the MS2 spectrum of CL molecular ion with m/z 1,532.1.
Identification and quantitative analysis of oxidized molecular species of PS in the hyperoxic mouse lung.
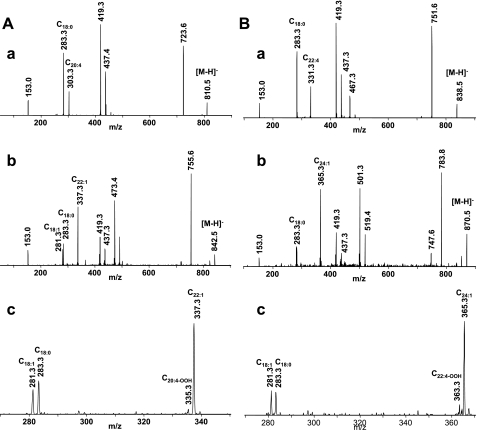
A comparative LC/ESI-MS analysis of PS isolated from normal and hyperoxic lungs detected the decrease in intensity of two molecular ions with m/z 810.5 (C18:0/C20:4) and 838.5 (C18:0/C22:4; Fig. 3B). Accordingly, the quantitative assessment of PS from the hyperoxic lung revealed that the amounts of PS-C18:0/C20:4 and PS-C18:0/C22:4 were reduced as well and were 135.0 ± 3.6 and 50.9 ± 2.4 vs. 162.2 ± 7.7 and 69.0 ± 4.1 pmol/nmol in control lung (Fig. 3C). It is likely that these two molecular species were oxidized in the mouse lung under hyperoxic conditions. To characterize the oxidation products, PS was isolated from hyperoxic lung, separated by 2D-HPLC, and utilized for direct infusion ESI-MS experiments. The results of a fragmentation experiment (MS2 spectra) using nonoxidized PS with m/z 810.5 and oxidized PS with m/z 842.5 are presented in Fig. 5, Aa and Bb, respectively. Daughter ions at m/z 283.3 and 303.3 corresponding to C18:0 and C20:4 along with other ions typical for PS fragmentation were detectable in the MS2 spectrum of the molecular ion with m/z 810.5 (Fig. 5Aa). MS2 fragmentation of the molecular ion with m/z 842.5 showed two overlapping molecular species. One of them is nonoxidized PS containing C18:1 (m/z 281.3) and C22:1 (m/z 337.3) fatty acids (Fig. 5Ab). Another one likely corresponds to a monohydroperoxy C18:0/C20:4-OOH or dihydroxy C18:0/C20:4–2OH molecular species originating from a molecular species with m/z 810.5 containing C20:4 in the sn-2 position. The molecular ions of C18:0 (m/z 283.3) and C20:4-OOH (m/z 335.3) fatty acids were detected in the MS2 spectrum (Fig. 5A, b and c). In addition, we performed the MS2 analysis of nonoxidized PS with m/z 838.5 (Fig. 5Ba) and an oxidized PS molecular species with m/z 870.5 (Fig. 5Bb). Daughter ions with m/z 283.3 and m/z 331.3 were detected in the MS2 spectrum of the molecular ion with m/z 838.5 and corresponded to C18:0 and C22:4 fatty acids. The fragmentation pattern of molecular ion with m/z 870.5 revealed the presence of a molecular ion with m/z 363.3 along with an ion with m/z 283.3 corresponding to C22:4 containing two oxygens and C18:0 fatty acids, respectively (Fig. 5B, b and c). Thus the detailed analysis of oxidized PS molecular species with m/z 842.5 and 870.5 confirmed that they originated from a molecular species of PS containing fatty acids with four double bonds (m/z 810.5 and 838.5) after the addition of two oxygens and contain hydroperoxy-C20:4 (C20:4-OOH) and hydroperoxy-C22:4 (C22:4-OOH) or dihydroxy-C20:4 (C20:4–2OH) and dihydroxy-C22:4 (C22:4–2OH) fatty acids, respectively. Accumulation of a hydroxy- and hydroperoxy-molecular species of PS originating from PS-C18:0/C22:6 was also observed (data not shown). Notably, hyperoxia-induced oxidation of PS occurred only in acyl groups with four and six double bonds, in contrast to CL peroxidation where C18:2 fatty acid residues were involved in the oxidative peroxidation along with acyl moieties containing four and six double bonds. No oxidation products in the other major phospholipid classes such as PC, PE, and PI were detected by ESI-MS, in spite of the fact that these phospholipids contain highly unsaturated fatty acid residues (67).
Fig. 5.
ESI-MS2 analysis of PS isolated from control and hyperoxic mouse lungs. Aa: MS2 fragmentation of PS species with m/z 810.5 from control lung. b: MS2 fragmentation of oxidized PS molecular species with m/z 842.5 from hyperoxic lung. c: Part of MS2 spectrum of molecular ion with m/z 842.5 shown in the range of m/z 270–350. The ion with m/z 153.0 corresponded to glycerophosphate without molecule of water, a common ion formed during phospholipid fragmentation. Molecular ions with m/z 283.3 and m/z 303.3 corresponding to C18:0 and C20:4 fatty acids, respectively, were also formed during fragmentation of parent ion with m/z 810.5. Molecular ions with m/z 335.3 corresponding to C20:4 fatty acid with 2 oxygen added was formed during fragmentation of parent ion with m/z 842.5. The loss of serine group of PS yielded the fragments with m/z 723.6 and 751.6. Product ions with m/z 419.3 originated from fragments with m/z 723.6 and 755.5 after loss of arachidonic acid C20:4 and oxidized arachidonic acid C20:4-OOH, respectively. Thus the molecular ion at m/z 810.5 corresponds to molecular species of PS-C18:0/C20:4, whereas the molecular ion with m/z 842.5 corresponds to molecular species of oxidized PS-C18:0/C20:4-OOH. Ba: MS2 fragmentation of PS species with m/z 838.5 from control lung. b: MS2 fragmentation of oxidized PS molecular species with m/z 870.6 from hyperoxic lung. c: Part of MS2 spectrum of molecular ion with m/z 870.5 shown in the range of m/z 270–370. The ions corresponded to glycerophosphate without molecules of water (m/z 153.0) were detected on MS2 spectra. Fragmentation of parent ion with m/z 838.5 results in formation of molecular ions with m/z 283.3 and m/z 331.3 corresponding to C18:0 and C22:4 fatty acids, respectively. MS2 analysis of molecular ion with m/z 870.5 revealed the presence of molecular ions with m/z 363.3 corresponding to C22:4 after addition of 2 oxygens. The molecular ion with m/z 365.3 was formed during fragmentation process of nonoxidized molecular species of PS with m/z 870.5 (PS-C18:1/C24:1) and corresponds to nervonic acid (C24:1). The loss of serine group of PS yielded the fragments with m/z 755.7 and 783.6. Product ion with m/z 419.3 originated from fragments with m/z 751.6 and 783.6 after loss of C22:4 fatty acid and C20:4-OOH, respectively. Therefore, molecular ions at m/z 838.5 and m/z 870.5 correspond to molecular species of PS-C18:0/C22:4 and product of its oxidation C18:0/C22:4-OOH.
Hyperoxia-induced apoptosis in the lung.
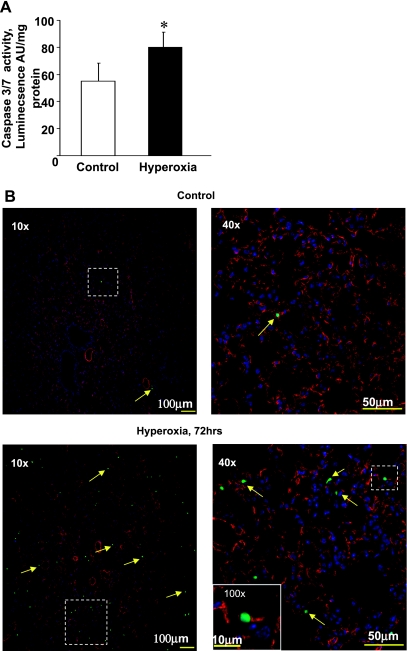
Recently, we demonstrated that the selective oxidation of CL and PS is important for the execution of apoptotic cell death and the clearance of apoptotic cells by macrophages (33, 36). We reasoned that the enhanced oxidation of CL and PS in the hyperoxic lung might be associated with the activation of apoptosis. Measurements of caspase-3 and -7 activity in mouse lung 72 h after hyperoxia revealed a 1.4-fold higher activity vs. controls (P < 0.05; Fig. 6A). Furthermore, histochemical staining for terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling activity, 72 h after hyperoxia, revealed increased numbers of apoptotic (green) cells (indicated by yellow arrows), many of which were derived from CD31 positive (red) endothelial cells (Fig. 6B).
Fig. 6.
Exposure of mice to hyperoxia results in activation of caspase-3 and -7 activity in lung. A: assessment of caspase-3 and -7 activity in mouse lung using Caspase-Glo assay. Open bars are control; closed bars are hyperoxia. AU, arbitrary units. Data are means ± SD. *P < 0.05 vs. control; n = 3 (for control); n = 4 (for hyperoxia). B: exposure of mice to hyperoxia for 72 h results in apoptosis of endothelial cell origin. Lungs were inflated with 2% paraformaldehyde, and frozen sections were used for immunofluorescent detection of apoptosis using an Alexa 488-labeled (green) nick end labeling kit. There were very few apoptotic cells in lungs of animals breathing room air as evident at low (×10) and higher (×40) magnification (top). In contrast, numerous apoptotic cells were apparent (×10 and ×40) in the hyperoxic group and many of these were associated with CD31-positive (e.g., endothelium) cells as was evident in the inset at ×100 (bottom right). Boxed areas at low magnification indicate the subsequent area at high magnification.
Identification and quantitative analysis of phospholipid molecular species in MLEC exposed to hyperoxia.
Assuming that the endothelium is a critical target of hyperoxic lung injury (16, 76), we performed oxidative lipidomics studies using MLEC isolated from mouse lung. We detected no significant changes in the phospholipid composition in MLEC exposed to hyperoxia (Fig. 7A). Similar to our results on phospholipid oxidation in the lung, hyperoxia caused the oxidation of two phospholipids, CL and PS (Fig. 7B). Hyperoxia-induced oxidation of CL in MLEC was very robust. In MLEC exposed to hyperoxia, the content of CL-OOH was estimated as 91.8 ± 0.9 vs. 5.3 ± 0.6 pmol/nmol of CL control nonexposed MLEC. The hyperoxia-induced accumulation of PS-OOH in MLEC (35.5 ± 0.3 vs. 13.7 ± 4.6 pmol PS-OOH per nmol of PS) was also significantly higher in MLEC than in hyperoxic lung. The hyperoxia-induced increases in the contents of CL-OOH and PS-OOH were 86.5 and 21.8 pmol/nmol CL and PS, respectively. No significant oxidation in the other major phospholipid classes such as PC, PE, and PI was found.
Fig. 7.
Phospholipid composition and accumulation of phospholipid hydroperoxides in mouse pulmonary endothelial cells (MLEC) exposed to hyperoxia. A: phospholipid composition of control and hyperoxic MLEC. MLEC were exposed to hyperoxia (95% O2-5% CO2 for 72 h in a 37°C). Lipids were extracted and separated by 2D-HPTLC, spots of phospholipids were scraped, and lipid phosphorus was determined. B: accumulation of PL-OOH in MLEC exposed to hyperoxia. Lipids were extracted and separated by 2D-HPTLC. Phospholipid hydroperoxides were detected using Amplex Red protocol. Open bars are control; closed bars are hyperoxia. Data are means ± SD. *P < 0.05 vs. control; n = 3.
Similar to the lung, molecular species of CL containing C18:2 were dominant in MLEC with two additional molecular species (compared with the lung) with m/z 1,453.8 and 1,455.8 and corresponding to C18:2/C18:1/C18:1/C18:1 and C18:1/C18:1/C18:1/C18:1, respectively (Table 1). We found no differences in the pattern of PS molecular species between the whole lung and MLEC. The same molecular species of PS were detected on MS spectra obtained from both whole lung and MLEC. However, PS in MLEC was significantly enriched with molecular species at m/z 788.5 and 836.5 corresponding to PS-C18:0/C18:1 and PS-C18:0/C22:5, respectively. In addition, the amounts of two molecular species of PS-C18:0/C20:4 (m/z 810.5) and PS-C18:1/C22:6 (m/z 832.5) were significantly lower in MLEC compared with the lung. Three molecular species containing the unsaturated fatty acids C18:0/C20:4, C18:0/C22:6, C18:0/C22:5 along with the highly saturated C18:0/C18:0 were most abundant in MLEC (Table 2).
Identification and quantitative analysis of oxidized molecular species of phospholipids in MLEC.
Similar to the lung, oxidation of only two phospholipids, CL and PS, was detected in MLEC after exposure to hyperoxia (Fig. 8). Decreased intensity of PS molecular ions corresponding to molecular species with C22:6, C22:5, and C22:4 in the sn-2 position was detected in LC/MS spectra of PS of hyperoxic cells. Specifically, the contents of PS-C18:0/C22:6 (m/z 834.5), PS-C22:5/C18:2 (m/z 836.5), and PS-C18:0/C22:4 (m/z 838.5) in hyperoxia-exposed cells were 81.4 ± 4.3, 136.3 ± 2.8, and 69.3 ± 6.8 vs. 131.8 ± 11.0, 166.7 ± 8.9, and 97.7 ± 5.1 pmol/nmol in PS and control cells, respectively (Fig. 8B). Quantitative MS analysis of CL in MLEC exposed to hyperoxia revealed significantly lower amounts of molecular species with m/z 1,473.8, 1,475.8, 1,495.8, 1,497.8, and 1,499.8 (Fig. 8A). In addition, a significant decrease of the amounts of CL molecular species with m/z 1,423.8 (C16:1/C18:2/C18:2/C18:2), 1,425.8 (C16:1/C18:2/C18:1/C18:1 or C16:0/C18:2/C18:2/C18:1), and 1,447.8 (C18:2/C18:2/C18:2/C18:2) was also observed in MLEC after exposure to hyperoxia. Notably, a 2.5-fold activation of caspase-3 and -7 occurred in MLEC exposed to hyperoxia for 72 h (Fig. 9). We noted a similar twofold activation of caspase-3 and -7 in MLEC that were grown in room air and then placed in hyperoxia for 72 h (data not shown).
Fig. 8.
Oxidation of CL and PS in MLEC exposed to hyperoxia. Quantitative assessments of CL (A) and PS (B) molecular species in MLEC. Open bars are control; closed bars are hyperoxia. Data are means ± SD. *P < 0.05 vs. control; n = 3.
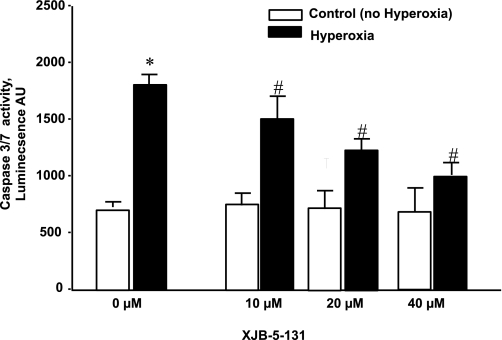
Fig. 9.
Activation of caspase-3 and -7 in MLEC exposed to hyperoxia. MLEC (15,000 per well) were treated with XJB-5-131 (10–40 uM, for 4 h). After that, the drug was removed and cells were exposed to hyperoxia (95% O2-5% CO2 for 72 h). At the end of the exposure, caspase-3 and -7 activity was measured. *P < 0.05, significantly different from control. #P < 0.05, significantly different from hyperoxia alone. Open bars are control; closed bars are hyperoxia. Data are means ± SD; n = 3.
Mitochondria-targeted nitroxide (XJB-5-131) suppresses hyperoxia-induced CL and PS oxidation in MLEC.
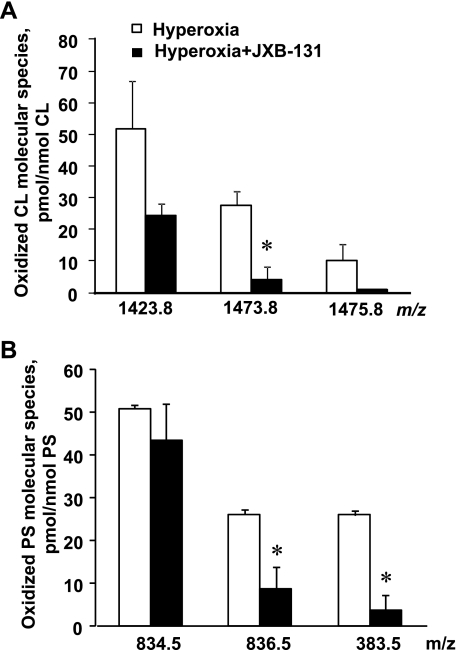
As a mitochondria-specific phospholipid, CL peroxidation occurs in this organelle likely due to the deregulated electron transport and production of reactive oxygen species (45, 52). Recently, we (29, 74) have developed mitochondria-targeted electron scavengers, conjugates of GS with nitroxide radicals (GS-nitroxides), capable of preventing reactive oxygen species production and CL peroxidation in mitochondria. We reasoned that GS-nitroxides may be effective in suppressing hyperoxia-induced phospholipid peroxidation in MLEC. Indeed, we found that GS-nitroxide (XJB-5-131) was able to protect CL (Fig. 10A) in mitochondria; moreover, PS peroxidation was also inhibited in hyperoxic MLEC (Fig. 10B). In addition, XJB-5–131 (10–40 μM) decreased hyperoxia-induced apoptosis in MLEC (as quantified by caspase-3 and -7 activation) in a concentration-dependent manner (Fig. 9).
Fig. 10.
Mitochondria-targeted small-molecule, XJB-5-131, protect both CL (A) and PS (B) against oxidation induced by hyperoxia in MLEC (95% oxygen, 72 h). Open bars are control; closed bars are hyperoxia. Data are means ± SD. *P < 0.05 vs. control; n = 3.
DISCUSSION
Hyperoxic lung injury has generally been assumed to be a “free radical disease” resulting from an imbalance in production and elimination of partially reduced oxygen and nitrogen species (50, 56). The importance of these free radical pathways in mediating hyperoxic lung injury is supported by the following: 1) observations that tolerance (secondary to LPS, cytokines, or hyperoxia itself) is associated with induction of various antioxidants (15, 19, 26, 58); 2) administration of antioxidants ameliorates such injury (13, 47, 61, 73); and 3) overexpression of various forms of SOD protects transgenic mice against hyperoxia (1, 72, 75). The major sources of oxygen radicals, particularly superoxide anion-radical, are the increased electron leakage from mitochondrial respiration (20, 54) and the activation of NAD(P)H oxidases, including NOX family NADPH oxidases (11, 56). Both mechanisms have been confirmed in pulmonary capillary endothelial cells in situ (8, 56). Additionally, superoxide can be produced via one electron oxidation of oxy-ferro-Hb to its ferric form (10), particularly after Hb release from erythrocytes during hyperoxia-induced hemolysis (12, 31).
Hyperoxia also has been known to activate cell death mechanisms in pulmonary endothelial cells (5, 48, 59), whereby both necrotic and apoptotic pathways are considered as important components of acute lung injury (5, 48, 59). Direct evidence for apoptotic vs. necrotic cell death pathways are equivocal: several studies (40, 48, 49, 71) demonstrated increased biomarkers of apoptosis, whereas other workers (5) failed to document apoptosis under hyperoxic conditions. Nonetheless, in the current study we used immunohistochemical (terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling) techniques to reveal hyperoxia-induced apoptosis of endothelial (CD31) origin, and overall, extrinsic (Fas-mediated) and intrinsic (mitochondria-mediated) apoptosis is recognized as a prominent part of hyperoxic acute lung injury (40, 49, 70, 71). The role of mitochondria-dependent cell death signaling in the pathogenesis of hyperoxia-induced lung injury has been intensively discussed recently (9, 48, 59, 71). One of the major hallmarks of apoptosis is caspase cascade activation (13, 37, 77). Both initiator and effector caspases are activated during hyperoxia through an angiopoietin-2-dependent pathway (59). Accordingly, we found 1.4-fold increases in caspase-3 and -7 activity in the hyperoxic lung and prolonged exposure to hyperoxia resulted in endothelial cell apoptosis (Fig. 6), adding to insight into injury and death of endothelial cells in the lung (4, 48). In this regard, it is noteworthy that elements of hyperoxia and apoptosis could be revealed in the twofold activation of caspases-3 and -7 that occurred in MLEC exposed to hyperoxia.
Execution of the mitochondrial stage of apoptosis has long been associated with the oxidation of CL (27, 36, 51). While the total content of CL was not altered by hyperoxia, significant changes were detected within individual species of CL, e.g., decreased amounts of several oxidizable molecular species of CL were found after hyperoxia. Our previous studies (36) established that CL represents a selective target of oxidative attack, whereby cyt c forms a complex with CL and catalyzes the oxidation, thus facilitating the release of proapoptotic factors, including cyt c, into the cytosol. Here, we demonstrate that hyperoxia-induced apoptosis is accompanied by CL oxidation in MLEC as well as in the lung. The remarkable feature of CL peroxidation during hyperoxia-induced apoptosis is its nonstochastic nature, as evidenced by our oxidative lipidomics MS analyses and identification of the hydroxy and hydroperoxy species of CL formed. The specific character of the peroxidation process has two manifestations: 1) only CL but not other markedly more abundant phospholipids present in mitochondria (PC and PE) were involved in the process, and 2) not all but selected molecular species of oxidizable CL (C18:1/C18:1/C18:2/C22:6, C18:1/C20:4/C20:4/C18:1, and C18:0/C18:2/C18:2/C22:6) were oxygenated, while others with highly oxidizable acyl groups remained nonoxidized. Moreover, in some batches of CL, with molecular species containing both C18:2 and C22:6 unsaturated, oxygenated products were detected in less polyunsaturated C18:2 acyl groups but not in C22:6 acyl moieties. While the mechanisms of selectivity toward CL are likely associated with the catalytic role of cyt c (3, 36) and its high affinity binding to CL, the specificity toward individual molecular species of CL remains to be elucidated. Assuming that high affinity binding with cyt c and catalytic competence of the latter toward CL are the major prerequisites of hyperoxia-induced CL peroxidation, it is conceivable that the selectivity is due to the spatial separation, i.e., inaccessibility of specific CL molecular species confined to the inner leaflet of the mitochondrial inner membrane to cyt c from the intermembrane space.
Cyt c released from mitochondria into the cytosol can bind with PS and cause its peroxidation via the same peroxidase mechanisms to yield hydroxy and hydroperoxy species of PS that have been detected in the hyperoxic lung as well as in MLEC. Similar to CL, the total content of PS in the mouse lung was not changed after exposure to hyperoxia. However, a significant decrease in the amounts of several oxidizable molecular species of PS was detected within individual species of PS. The signaling role of oxidized PS is realized through its participation in facilitating PS externalization and the appearance of PS and its oxidation products on the surface of apoptotic cells (65, 68). Both PS and PS oxidation products are recognition signals for several receptors (22) and subsequent clearance by macrophages (6, 34).
Recently, using pulmonary endothelial cells and LPS as an in vitro model of acute lung injury, we demonstrated that LPS-induced apoptosis in sheep pulmonary artery endothelial cells was accompanied by accumulation of CL and PS oxidation products (63). Moreover, similar CL and PS oxidation products were detected in model systems in the cyt c driven reaction in the presence of H2O2 (63, 66). We speculate that the cyt c driven oxidation of CL and PS is associated with the execution of apoptosis in pulmonary endothelial cells, thus contributing to hyperoxic acute lung injury.
The pulmonary endothelium is the locus of early structural and functional changes in hyperoxic lung injury, and apoptosis of pulmonary endothelium is a contributing factor to the genesis and maintenance of hyperoxic lung injury. Nonetheless, the early molecular pathways that account for hyperoxia-induced pulmonary endothelial cell apoptosis are unknown and therapies for acute lung injury remain entirely palliative. A rational approach may be the delivery of agents selectively to the pulmonary endothelium and, more precisely, to mitochondria to inhibit apoptosis before the point-of-no-return, i.e., release of proapoptotic factors from mitochondria and caspase activation. Inhibition of PS oxidation in cells treated with GS-nitroxide could be due to the ability of the drug to prevent release of cyt c from mitochondria. Our previous work (36) demonstrated that oxidation of CL is essential for the mitochondrial membrane permeabilization and release of cyt c into the cytosol. The released cyt c can interact with PS localized in the inner leaflet of the plasma membrane and form a cyt c/PS peroxidase complex with PS oxidation catalytic competence. Accordingly, GS-nitroxide-dependent prevention of CL oxidation and cyt c release from mitochondria can be responsible for the inhibition of PS oxidation. We suggest that prevention of CL oxidation may be a preferred target for anti-apoptotic strategies to protect pulmonary endothelial cells during acute lung injury. Homologues of GS attached to cyclic nitroxides, GS-Tempo conjugates, showed a high level of effectiveness in the accumulation in mitochondria (30, 74). Indeed, we found that the electron-scavenging activity of GS-nitroxide conjugates was associated with the inhibition of CL and PS oxidation in MLEC exposed to hyperoxia. Importantly, this effect of XJB-5-131 was associated with a decreased hyperoxia-induced apoptosis in pulmonary endothelium (as quantified by caspase-3 and -7 activation). Recently, we noted that XJB-5-131 could inhibit oxidant-mediated apoptosis in intact organs of experimental animals, in vivo (39), and thus future studies will be focused on developing additional mitochondria-targeted strategies to protect the lung against hyperoxia-induced injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-70755, HL-094488, and HL-65697.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1.Ahmed MN, Sulliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia exposed newborn mice. Am J Respir Crit Care Med 167: 400–405, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun 367: 509–515, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry 45: 4998–5009, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci 13: 6653–6661, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, Lee PJ, Geick A, de Fougerolles AR, Elias JA. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med 12: 1286–1293, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borisenko GG, Matsura T, Liu SX, Tyurin VA, Jianfei J, Serinkan FB, Kagan VE. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells–existence of a threshold. Arch Biochem Biophys 413: 41–52, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Böttcher CSF, Van Gent CM, Fries C. A rapid and sensitive sub-micro phosphorus determination. Anal Chim Acta 24: 203–204, 1961. [Google Scholar]
- 8.Brueckl C, Kaestle S, Kerem A, Habazetti H, Kromback F, Kuppe H, Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 34: 453–463, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Buccellato LJ, Tso M, Akinci OI, Chandel NS, Budinger GR. Reactive oxygen species are required for hyperoxia-induced Bax activation and cell death in alveolar epithelial cells. J Biol Chem 279: 6753–6760, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Buehler PW, D'Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther 323: 49–60, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 180: 972–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carolla RL, Brubaker LH, Mengel CE. Age of red blood cells destroyed by in vivo hyperoxia. Aerospace Med 45: 1273–1275, 1974 [PubMed] [Google Scholar]
- 13.Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med 167: 57–64, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol 23: 1384–1390, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Comhair SA, Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol 283: L246–L255, 2002 [DOI] [PubMed] [Google Scholar]
- 16.De Souza PM, Lindsay MA. Apoptosis as a therapeutic target for the treatment of lung disease. Curr Opin Pharmacol 5: 232–237, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Fessel JP, Jackson Roberts L. Isofurans: novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension. Antioxid Redox Signal 7: 202–209, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 19.Frank L, Yam J, Roberts JR. The role of endotoxin in protection of adult rats from oxygen-induced lung toxicity. J Clin Invest 61: 269–275, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman BF, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 256: 10986–10092, 1981 [PubMed] [Google Scholar]
- 21.Freeman BF, Young SL, Crapo JD. Liposome mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem 258: 12534–12542, 1983 [PubMed] [Google Scholar]
- 22.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203: 2613–2625, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol 9: 873–879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haworth O, Levy BD. Endogenous lipid mediators in the resolution of airway inflammation. Eur Respir J 30: 980–992, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu FF, Turk J, Rhoades ER, Russell DG, Shi Y, Groisman EA. Structural characterization of cardiolipin by tandem quadrupole and multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom 16: 491–504, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Igbal J, Clerch LB, Hass MA, Frank L, Massaro D. Endotoxin increases lung CuZn superoxide dismutase mRNA: O2 raises enzyme synthesis. Am J Physiol Lung Cell Mol Physiol 257: L61–L64, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Iverson SL, Orrenius S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys 423: 37–46, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Jamieson D, Chance B, Cadenas E, Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol 48: 703–719, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Belikova NA, Hoye AT, Zhao Q, Epperly MW, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int J Radiat Oncol Biol Phys 70: 816–825, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Amoscato AA, Braslau R, Studer A, Fink MP, Greenberger JS, Wipf P, Kagan VE. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther 320: 1050–1060, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Johnson WP, Jefferson D, Mengel CE. In vivo hemolysis due to hyperoxia: role of H2 O2 accumulation. Aerospace Med 43: 943–945, 1972 [PubMed] [Google Scholar]
- 32.Kagan VE. Lipid Peroxidation in Biomembranes. Boca Raton, FL, CRC Press, 1988, p. 1–300 [Google Scholar]
- 33.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med 46: 1439–1453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagan VE, Borisenko GG, Serinkan BF, Tyurina YY, Tyurin VA, Jiang J, Liu SX, Shvedova AA, Fabisiak JP, Uthaisang W, Fadeel B. Appetizing rancidity of apoptotic cells for macrophages: oxidation, externalization, and recognition of phosphatidylserine. Am J Physiol Lung Cell Mol Physiol 285: L1–L17, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang J, Potapovich AI, Kini V, Amoscato AA, Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med 37: 1963–1985, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1: 223–232, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Kuwano K, Yoshimi M, Maeyama T, Hamada N, Yamada M, Nakanishi Y. Apoptosis signaling pathways in lung diseases. Med Chem 1: 49–56, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Macchia L, Hamberg M, Kumlin M, Butterfield JH, Haeggstrom JZ. Arachidonic acid metabolism in the human mast cell line HMC-1: 5-lipoxygenase gene expression and biosynthesis of thromboxane. Biochim Biophys Acta 1257: 58–74, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Macias CA, Chiao JW, Xiao J, Arora DS, Tyurina YY, Delude RL, Wipf P, Kagan VE, Fink MP. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg 245: 305–314, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantell LL, Lee PJ. Signal transduction pathways in hyperoxia-induced lung cell death. Mol Genet Metab 71: 359–370, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med 31: S184–188, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW, Baker PR, Coles B, Coffey MJ, Kuhn H, O'Donnell VB. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem 282: 20151–20163, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care 7: 355–358, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 285: 3451–3461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis 12: 913–922, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 276: L688–L694, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Padmanabhan RV, Gudapaty R, Liemer IE, Schwartz BA, Hoidal JR. Protection against pulmonary oxygen toxicity in rats by intratracheal administration of liposome encapsulated superoxide dismutase or catalase. Am Rev Respir Dis 132: 164–167, 1985 [DOI] [PubMed] [Google Scholar]
- 48.Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann NY Acad Sci 1010: 405–416, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Pagano A, Donati Y, Metrailler I, Barazzone Argiroffo C. Mitochondrial cytochrome c release is a key event in hyperoxia-induced lung injury: protection by cyclosporin A. Am J Physiol Lung Cell Mol Physiol 286: L275–L283, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Pepperl S, Dorger M, Ringel F, Kupatt C, Krombach F. Hyperoxia upregulates the NO pathway in alveolar macrophages in vitro: role of AP-1 and NF-kappaB. Am J Physiol Lung Cell Mol Physiol 280: L905–L913, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Petrosillo G, Casanova G, Matera M, Ruggiero FM, Paradies G. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett 580: 6311–6316, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int 53: 126–131, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5: 494–496, 1970 [DOI] [PubMed] [Google Scholar]
- 54.Sanders SP, Zweier JL, Kuppusamy P, Harrison SJ, Bassett DJ, Gabrielson EW, Sylvester JT. Hyperoxic sheep pulmonary microvascular endothelial cells generate free radicals via mitochondrial electron transport. J Clin Invest 91: 46–52, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shearer GC, Newman JW. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr Atheroscler Rep 11: 403–410, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Singleton PA, Pendyala S, Gorshkova IA, Mambetsariev N, Moitra J, Garcia JG, Natarajan V. Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and reactive oxygen species production in caveolin-enriched microdomains of the endothelium. J Biol Chem 284: 34964–34975, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461: 1287–1291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang G, Berg JT, White JE, Lumb PD, Lee CY, Tsan MF. Protection against oxygen toxicity by tracheal insufflation of endotoxin: role of MnSOD and alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 266: L38–L45, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632–L641, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Tang ZL, Wasserloos K, Liu X, Reynolds IJ, Pitt BR, Croix CM. Roles for metallothionein and zinc in mediating the protective effects of nitric oxide on lipopolysaccharide-induced apoptosis. Mol Cell Biochem 234/235: 211–217, 2002 [PubMed] [Google Scholar]
- 61.Turrens JF, Crapo JD, Freeman BA. Protection against oxygen toxicity by intravenous injection of liposome entrapped catalase and superoxide dismutase. J Clin Invest 73: 87–95, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, Kagan VE. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem 107: 1614–1633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyurin VA, Tyurina YY, Jung MY, Tungekar MA, Wasserloos KJ, Bayir H, Greenberger JS, Kochanek PM, Shvedova AA, Pitt B, Kagan VE. Mass-spectrometric analysis of hydroperoxy- and hydroxy-derivatives of cardiolipin and phosphatidylserine in cells and tissues induced by pro-apoptotic and pro-inflammatory stimuli. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2863–2872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyurin VA, Tyurina YY, Kochanek PM, Hamilton R, DeKosky ST, Greenberger JS, Bayir H, Kagan VE. Oxidative lipidomics of programmed cell death. Methods Enzymol 442: 375–393, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Tyurina YY, Kawai K, Tyurin VA, Liu SX, Kagan VE, Fabisiak JP. The plasma membrane is the site of selective phosphatidylserine oxidation during apoptosis: role of cytochrome C. Antioxid Redox Signal 6: 209–225, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med 44: 299–314, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Tyurina YY, Tyurin VA, Kapralova VI, Amoscato AA, Epperly MW, Greenberger JS, Kagan VE. Mass-spectrometric characterization of phospholipids and their hydroperoxide derivatives in vivo: effects of total body irradiation. Methods Mol Biol 580: 153–183, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Tyurina YY, Tyurin VA, Zhao Q, Djukic M, Quinn PJ, Pitt BR, Kagan VE. Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis? Biochem Biophys Res Commun 324: 1059–1064, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Walton KA, Cole AL, Yeh M, Subbanagounder G, Krutzik SR, Modlin RL, Lucas RM, Nakai J, Smart EJ, Vora DK, Berliner JA. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol 23: 1197–1203, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Ryter SW, Dai C, Tang ZL, Watkins SC, Yin XM, Song R, Choi AM. Necrotic cell death in response to oxidant stress involves the activation of the apoptogenic caspase-8/bid pathway. J Biol Chem 278: 29184–29191, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282: 1718–1726, 2007 [DOI] [PubMed] [Google Scholar]
- 72.White CW, Avraham KB, Shanley PF, Groney Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest 87: 2162–2168, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White CW, Jackson JH, Abuchowski A, Kazo GM, Mimmack RF, Berger EM, Freeman BA, McCord JM, Repine JE. Polyethylene glycol attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J Appl Physiol 66: 584–590, 1989 [DOI] [PubMed] [Google Scholar]
- 74.Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, Kagan VE. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc 127: 12460–12461, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Wispe JR, Warner BB, Clark JC, Dey CR, Neuman J, Glasser SW, Crapo JD, Chang LY, Whitsett JA. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem 267: 23937–23941, 1992 [PubMed] [Google Scholar]
- 76.Xu D, Perez RE, Ekekezie II, Navarro A, Truog WE. Epidermal growth factor-like domain 7 protects endothelial cells from hyperoxia-induced cell death. Am J Physiol Lung Cell Mol Physiol 294: L17–L23, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Zhivotovsky B, Burgess DH, Orrenius S. Proteases in apoptosis. Experientia 52: 968–978, 1996 [DOI] [PubMed] [Google Scholar]