Abstract
Myosin phosphatase target subunit 1 (MYPT1) is the regulatory subunit of myosin light chain phosphatase (MLCP). It plays a critical role in vasodilatation induced by cGMP-elevating agents such as nitric oxide (NO). The present study was performed to determine the role of MYPT1 in the development of tolerance of the pulmonary artery to NO. Incubation of isolated porcine pulmonary arteries for 24 or 48 h with DETA NONOate (DETA NO) significantly reduced protein levels of MYPT1 and the leucine zipper-positive (LZ+) isoform of MYPT1 but not that of PP1cδ. The extent of reduction in total MYPT1 protein level was comparable to that of MYPT1 (LZ+). The decrease in MYPT1 protein caused by 48-h DETA NO incubation was prevented by ODQ, an inhibitor of guanylyl cyclase, and by inhibitors of proteasomes (MG-132 and lactacystin) but was not affected by the inhibitor of protein synthesis, cycloheximide. A reduction in MYPT1 protein was also obtained with 8-bromo-cGMP, but this was prevented by Rp-8-bromo-PET-cGMP [inhibitor of cGMP-dependent protein kinase (PKG)]. Incubation for 48 h with DETA NO also reduced dephosphorylation of myosin light chain and relaxation of the artery in response to DETA NO, which was prevented by MG-132. These results suggest that the reduction in MYPT1 protein contributes to the development of tolerance of pulmonary arteries to NO. This may result from increased degradation of MYPT1 after prolonged PKG activation.
Keywords: myosin light chain phosphatase; guanosine 3′,5′-cyclic monophosphate-dependent protein kinase; vasodilatation
nitric oxide (NO) is the major vasodilator in various vessel types, including pulmonary arteries (12, 19, 40, 41). It acts mainly by activation of soluble guanylyl cyclase (sGC), followed by cGMP elevation and activation of cGMP-dependent protein kinase (PKG) (8–10, 17, 27, 40–42). PKG causes vasodilatation by reducing intracellular Ca2+ concentration and decreasing Ca2+ sensitivity of myofilaments, termed Ca2+ desensitization (30, 44). It is well recognized that Ca2+ desensitization mediated by PKG is through activation of myosin light chain phosphatase (MLCP), which leads to reduced phosphorylation of myosin light chain (MLC) and vasodilatation (18, 22, 45, 47). MLCP is composed of a catalytic subunit (PP1cδ), the myosin phosphatase target subunit 1 (MYPT1), and a subunit with unknown function (M20). MYPT1 may be expressed as isoforms either with or without leucine zipper domain in its COOH terminus [MYPT1 (LZ+) and MYPT1 (LZ−), respectively] (15, 16). Studies show that NO may stimulate MLCP activity in a PKG-dependent manner through phosphorylation of MYPT1 at serine 695 and serine 852 and through interaction between PKG and the leucine zipper motif of MYPT1 (LZ+) (17, 18).
Although NO and its donors are potent vasodilators, their prolonged use often leads to their diminished effectiveness (6, 11, 37, 38, 43, 48). The underlying mechanisms are not well understood, which may include an increased phosphodiesterase activity (23, 29, 34), a decreased sGC activity, (37, 48), and a decreased PKG activity (6, 11, 38, 43). Emerging evidence indicates that MYPT1 is a key downstream target for Ca2+ desensitization caused by NO via cGMP-PKG signaling pathway (18, 20, 22, 44, 45). Therefore, the present study tested the hypothesis that a reduced expression of MYPT1 may occur during prolonged NO exposure and contribute to the diminished vasodilatation to NO. Our present study demonstrates that the protein levels of total MYPT1 and its isoform, the leucine zipper-positive isoform of MYPT1 (15), of porcine pulmonary arteries were reduced and that this was associated with decreased PKG-mediated MLC dephosphorylation and vasodilatation, indicative of the development of NO tolerance. An increased degradation by proteasomes may contribute to the reduced protein level of MYPT1.
MATERIALS AND METHODS
NO tolerance induction.
Fresh porcine lungs were collected from a local slaughterhouse. Fourth-generation pulmonary arteries were dissected and cut into rings in ice-cold modified Krebs-Ringer bicarbonate buffer. Vessel rings were incubated for 24 or 48 h in serum-free Dulbecco's modified Eagle's medium in the presence of solvent or DETA NONOate [DETA NO, a stable NO donor (32)]. To eliminate the possible involvement of endogenous prostanoids and endothelium-derived NO, indomethacin (10−5 M) and nitro-l-arginine (10−4 M) were included in the medium and present throughout the experiments. After incubation, vessel rings were used for Western blotting, MLCP activity assay, and organ chamber studies (6).
Western blotting analysis.
Tissue lysates were prepared from isolated porcine pulmonary arteries after the above-mentioned treatments. Lysates, each containing 10 μg of protein, were subjected to SDS-PAGE and electrotransferred to polyvinylidene difluoride (PVDF). Preliminary studies demonstrated that the protein level loaded for Western blotting analysis is within the linear range of detection. Nonspecific binding of antibody was blocked by washing with Tris-buffered saline (TBS) buffer containing 10% milk for 1 h. The blot was then subjected to two brief washes with TBS plus 0.5% Tween 20, incubated first with the antibody against MYPT1 (LZ+) (Affinity BioReagents, Golden, CO; 1:40,000 dilution), MYPT1 (BD Biosciences, San Jose, CA; 1:1,000 dilution), or PP1cδ (Upstate Biotechnology, Lake Placid, NY; 1:1,000 dilution) for 1 h and then with a secondary antibody for 40 min. Blots were developed using the chemiluminescent detection method (Amersham ECL). MYPT1 (LZ+), MYPT1, or PP1cδ protein in blots was quantified by densitometry using a Gel Doc 2000 densitometer (Bio-Rad, Hercules, CA) and normalized to scanning signals of actin (Calbiochem, San Diego, CA).
MLC phosphorylation.
Isolated porcine pulmonary arteries were incubated in serum-free Dulbecco's modified Eagle's medium in the presence of solvent or DETA NO (10−4 M) for 48 h as described above. After incubation, vessel rings were repeatedly rinsed and balanced for 30 min in the organ chambers filled with 10 ml of the modified Krebs-Ringer bicarbonate solution maintained at 37 ± 0.5°C and aerated with 95% O2-5% CO2 (pH 7.4). First, a control group of vessel rings was taken out of the organ chamber and frozen in liquid nitrogen. Next, U46619 (3 × 10−7 M) was added to the organ chamber, and after 10 min, a second group of vessels (U46619 group) was taken out and frozen in liquid nitrogen; DETA NO (10−4 M) was then added to the remaining vessels in the organ chamber, and 5 min later, the vessel rings were taken out (DETA NO group) and frozen in liquid nitrogen.
Tissue lysates were prepared from vessel rings treated as described above. The lysates, each containing 20 μg of protein, were subjected to SDS-PAGE and electrotransferred to PVDF. Nonspecific binding of antibody was blocked by washing with TBS buffer containing 10% milk for 1 h. The blot was then subjected to two brief washes with TBS plus 0.5% Tween 20, incubated first with antibody against MLC-p (Sigma, St. Louis, MO; 1:1,000 dilution) or MLC20 (Sigma; 1:1,000 dilution) for 1 h and then with the secondary antibody for 40 min. Blots were developed using the chemiluminescent detection method (Amersham ECL). MLC-p or MLC20 protein present in blots was quantified by densitometry using a Gel Doc 2000 densitometer (Bio-Rad) and normalized to scanning signals of actin (Calbiochem).
Vessel tension study.
Vessel rings obtained following the various treatments as described earlier were repeatedly rinsed and suspended in organ chambers filled with 10 ml of the modified Krebs-Ringer bicarbonate solution maintained at 37 ± 0.5°C and aerated with 95% O2-5% CO2 (pH 7.4). At the beginning of the experiment, each vessel ring was stretched to its optimal resting tension, which was achieved by stepwise stretching until the active contraction of the vessel ring to 100 mM KCl reached a plateau. The optimal resting tension of the porcine pulmonary arteries was ∼1.5 g. After the vessels were brought to their optimal resting tension, 1 h of equilibration was allowed (6).
Drugs.
The following drugs were used (unless otherwise specified, all were obtained from Sigma): 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP), 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP), 2,2′-(hydroxynitrosohydrazono) bis(ethanamine) (DETA NO), indomethacin, nitro-l-arginine, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), β-phenyl-1,N2-etheno-8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Rp isomer (Rp-8-Br-PET-cGMPS; Biolog Life Science Institute, Bremen, Germany), and 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619).
ODQ, U46619, and MG-132 were dissolved in DMSO (final concentration <0.2%). Preliminary experiments showed that DMSO at the concentration used had no effect on contraction to U46619 and on relaxation induced by the NO donor and the cGMP analog. The other drugs were prepared using distilled water.
Data analyses.
Data are means ± SE. Mean values of two groups were compared using one-way ANOVA test, with the Student-Newman-Keuls test used for post hoc testing of multiple comparisons. Statistical significance was accepted when the P value (2 tailed) was <0.05. In all experiments, n represents the number of animals.
RESULTS
Effects of prolonged treatment with DETA NO on protein levels of MLCP subunits.
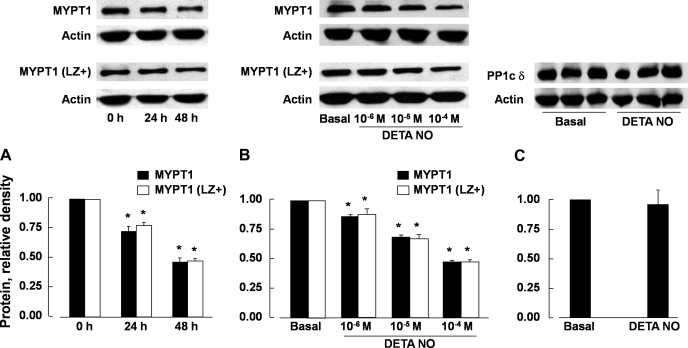
Protein levels of total MYPT1 and MYPT1 (LZ+) in porcine pulmonary arteries that were incubated for 24 and 48 h with DETA NO (10−4 M) were reduced in a time-dependent (Fig. 1A) and concentration-dependent manner (Fig. 1B). MYPT1 (LZ+) and total MYPT1 levels were decreased to a similar degree (Fig. 1, A and B). The expression of PP1cδ was not significantly affected (Fig. 1C).
Fig. 1.
Protein levels of total myosin phosphatase target subunit 1 (MYPT1) and the leucine zipper-positive (LZ+) isoform of MYPT1 [MYPT1 (LZ+)] of porcine pulmonary arteries were reduced to a similar extent following incubation with DETA NONOate (DETA NO; 10−4 M) for 24 and 48 h (A) and incubation for 48 h with different concentrations of the nitric oxide (NO) donor (B). Incubation with DETA NO (10−4 M) for 48 h had no effect on the expression of PP1cδ protein (C). Top: Western blots; bottom, summaries of densitometric scanning of MYPT1 or PP1cδ protein normalized to actin. Data are means ± SE; n = 4–6 for each group. *P < 0.05, significantly different from values at 0 h (A) or basal values (B).
cGMP-PKG pathway dependency of DETA NO-induced reduction in MYPT1 protein.
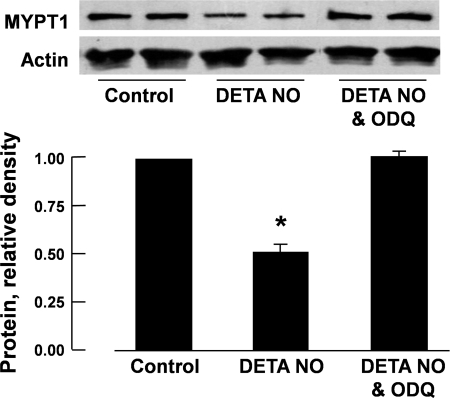
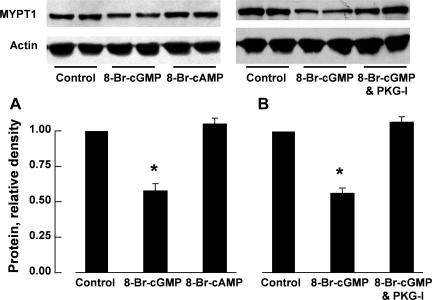
The reduction in MYPT1 protein levels in porcine pulmonary arteries induced by 48-h incubation with DETA NO was prevented when ODQ (3 × 10−5 M), a special inhibitor of sGC (13), was coincubated (Fig. 2). A similar reduction in MYPT1 protein was also observed when the arteries were incubated for 48 h with 8-Br-cGMP [10−4 M, a cell-permeable analog of cGMP (31)] but not with 8-Br-cAMP [10−4 M, a cell-permeable analog of cAMP (31)] (Fig. 3).
Fig. 2.
Reduction in protein levels of MYPT1 of porcine pulmonary arteries caused by incubation with DETA NO (10−4 M) was prevented when 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ; 3 × 10−5 M) was presented in the incubation medium. Top, Western blots; bottom, summaries of densitometric scanning of MYPT1 protein normalized to actin. Data are means ± SE; n = 4–6 for each group. *P < 0.05, significantly different from control.
Fig. 3.
Protein levels of MYPT1 in porcine pulmonary arteries were reduced by 48-h incubation with 8-bromo-cGMP (8-Br-cGMP; 10−4 M) but not by 8-Br-cAMP (10−4 M; A). The effect of 8-Br-cGMP was inhibited by Rp-Br-PET-cGMPS (PKG-I; 3 × 10−5 M; B). Top, Western blots; bottom, summaries of densitometric scanning of MYPT1 protein normalized to actin. Data are means ± SE; n = 4–5 for each group. *P < 0.05, significantly different from control.
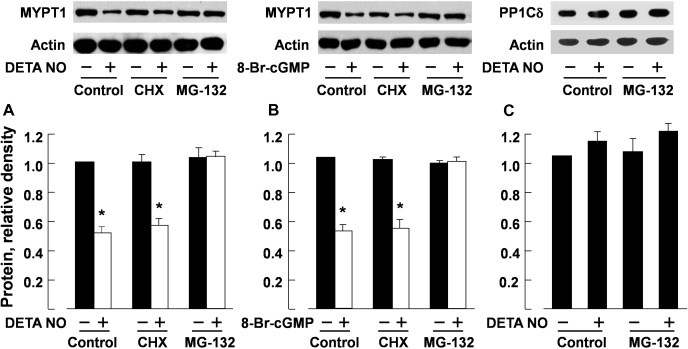
Effect of inhibitors of protein synthesis and of proteasomes on the reduction in MYPT1 induced by incubation with DETA NO and 8-Br-cGMP.
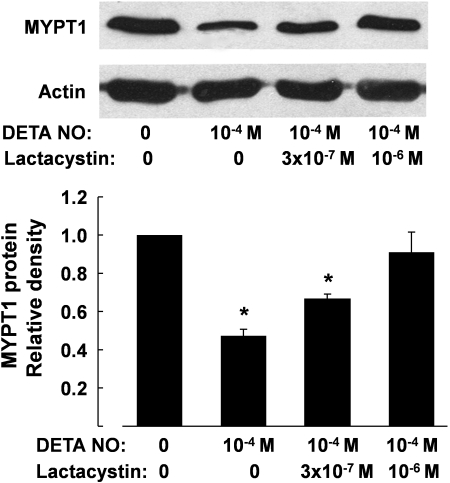
Cycloheximide (10−4 M), an inhibitor of protein synthesis (33), had no significant effect on the reduction in MYPT1 protein levels induced by 48-h incubation with DETA NO (10−4 M) or 8-Br-cGMP (10−4 M). The effects of incubation with DETA NO and cGMP analog on the expression of MYPT1 were prevented when MG-132 (3 × 10−6 M), a proteasome inhibitor (25), was coincubated. MG-132 or MG-132 plus DETA NO had no effect on the protein level of PP1cδ (Fig. 4). The decrease in MYPT1 protein caused by DETA NO (10−4 M) was also prevented by lactacystin (10−6 M), a structurally different inhibitor of proteasomes (7) (Fig. 5).
Fig. 4.
Reduction of MYPT1 protein in porcine pulmonary arteries following 48-h incubation with DETA NO (10−4 M; A) or 8-Br-cGMP (10−4 M; B) was not affected by cycloheximide (CHX; 10−4 M) but was prevented by MG-132 (10−5 M). Incubation with DETA NO (10−4 M) or MG-132 (10−5 M) for 48 h had no effect on the expression of PP1cδ protein (C). Top, Western blots; bottom, summaries of densitometric scanning of MYPT1 or PP1cδ protein normalized to actin. Data are means ± SE; n = 4–6 for each group. * P < 0.05, significantly different from basal values.
Fig. 5.
Reduction of MYPT1 protein in porcine pulmonary arteries following 48-h incubation with DETA NO (10−4 M) was prevented by lactacystin (10−6 M). Top, Western blots; bottom, summaries of densitometric scanning of MYPT1 protein normalized to actin. Data are means ± SE; n = 4 for each group. *P < 0.05, significantly different from solvent group.
MLC phosphorylation.
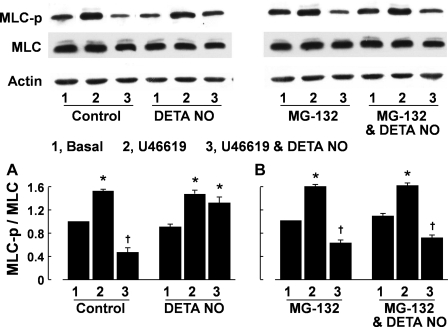
After 48-h incubation with solvent, the administration of U46619 (3 × 10−7 M) caused a significant increase in phosphorylation of MLC in the pulmonary arteries, as indicated by the ratio of phosphorylated MLC over total MLC. The phosphorylation of MLC evoked by U46619 was significantly inhibited by DETA NO (10−5 M). In arteries preincubated for 48 h with DETA NO (10−4 M), subsequent stimulation with U46619 (3 × 10−7 M) caused a similar degree of MLC phosphorylation. However, the inhibitory effect of DETA NO (10−5 M) on U46619-induced MLC phosphorylation was blunted (Fig. 6A). MLC phosphorylation of the arteries in response to U46619 and U46619 plus DETA NO in vessels preincubated with MG-132 (3 × 10−6 M) was not significantly different from those preincubated with solvent alone. However, the reduced MLC dephosphorylation in response to DETA NO preincubation was prevented when MG-132 was present in the preincubation medium (Fig. 6B).
Fig. 6.
Myosin light chain (MLC) phosphorylation [judged as the ratio of phosphorylated MLC to total MLC (MLC-p/MLC)] in porcine pulmonary arteries evoked by U46619 (3 × 10−7 M) was inhibited by DETA NO (10−4 M) in arteries preincubated for 48 h with solvent but not with DETA NO (10−5 M). The effect of preincubation with DETA NO on MLC phosphorylation was prevented by MG-132 (3 × 10−6 M). The MLC-p/MLC values at basal and after U46619 were determined using tissues taken before and 10 min after administration of the thromboxane A2 mimetic, respectively. The MLC-p/MLC values for U46619 plus DETA NO were obtained from tissues treated with U46619 for 10 min followed by DETA NO (10−4 M) for 5 min. Top, Western blots (lane 1, basal value; lane 2, U46619; lane 3, U46619 plus DETA NO); bottom, summaries of densitometric scanning of MLC-p and MLC proteins expressed as MLC-p/MLC. Data are means ± SE; n = 4 for each group. *P < 0.05, significant difference between basal and U46619-treated arteries. †P < 0.05, significant difference between vessels treated with U46619 and those treated with U46619 plus DETA NO.
Vessel tension study.
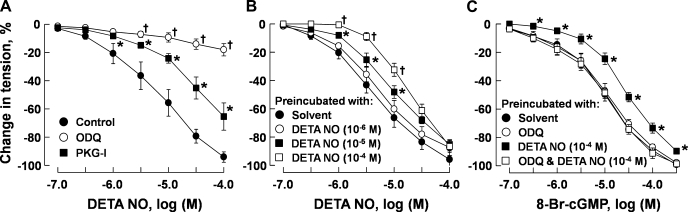
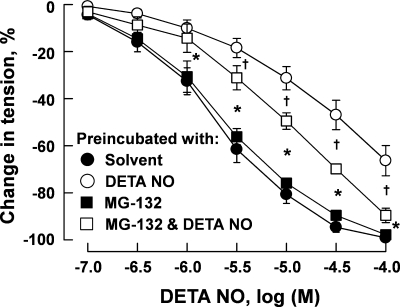
Relaxant response measurements in porcine pulmonary arteries were conducted in vessels preconstricted with U46619 (3 × 10−7 M) to a similar tension level (range: 3.96 ± 0.51–4.74 ± 0.63 g, n = 4–6 for each group, P > 0.05). DETA NO caused a concentration-dependent relaxation of the arteries that was nearly abolished by ODQ (3 × 10−5 M) and markedly inhibited by Rp-8-Br-PET-cGMPS (3 × 10−5 M) (Fig. 7A). Preincubation with DETA NO attenuated subsequent relaxation of the arteries to this NO donor (Fig. 7B) in a concentration-dependent manner. Preincubation with DETA NO (10−6 M) also attenuated the subsequent relaxation of the arteries to 8-Br-cGMP. This effect was prevented when DETA NO was coincubated with ODQ (3 × 10−5 M). Preincubation with ODQ alone had no significant effect on relaxation induced by 8-Br-cGMP (Fig. 7C). In pulmonary arteries, preincubation for 48 h with MG-132 (3 × 10−6 M) had no significant effect on relaxation induced by DETA NO. The attenuation of relaxant response to DETA NO after 48-h preincubation with this NO donor was prevented when MG-132 was included in the preincubation medium (Fig. 8).
Fig. 7.
A: acute responses in porcine pulmonary arteries induced by DETA NO in the presence of solvent, ODQ (3 × 10−5 M), and PKG-I (3 × 10−5 M). The vessels were preconstricted with U46619 (3 × 10−7 M) to similar tension levels. B and C: effects of 48-h incubation with DETA NO on relaxation of porcine pulmonary arteries to DETA NO and 8-Br-cGMP, respectively. After 48-h incubation, the vessels were repeatedly rinsed and preconstricted with U46619 (3 × 10−7 M) to similar tension levels before the effect of DETA NO or 8-Br-cGMP was tested. Data are means ± SE; n = 5–6 for each group. *P < 0.05, significantly different from control. †P < 0.05, significantly different from vessels treated with PKG-I (A) or from vessels-treated with DETA NO at 10−5 M (B).
Fig. 8.
Relaxation of porcine pulmonary artery to DETA NO after 48-h incubation with this nitrovasodilator (10−4 M) in the presence and absence of MG-132 (3 × 10−6 M). After incubation, the vessels were repeatedly rinsed and preconstricted with U46619 (3 × 10−7 M) to similar tension levels before the effect of DETA NO was tested. Data are means ± SE; n = 4–6 for each group. *P < 0.05, significant difference between vessels treated with DETA NO and those treated with solvent or MG-132. †P < 0.05, significant difference between vessels treated with DETA NO and those treated with MG-132 plus DETA NO.
DISCUSSION
The present study demonstrated that the expression of MYPT1 protein in porcine pulmonary arteries was reduced following prolonged exposure to DETA NO, associated with a reduced relaxation to the NO donor. MYPT1 is the primary target protein for PKG-mediated MLCP activation and Ca2+ desensitization (20, 44, 45, 47). Our results suggest that a reduced MYPT1 protein level may contribute to the attenuated vasodilatation to NO following prolonged exposure to NO. Previous studies have shown that alternations in sGC (37, 48) and phosphodiesterase (23, 29, 34) and decreased PKG activity (6, 11, 38, 43) may be involved in the development of tolerance to NO. The present study indicates that MYPT1, a downstream target protein of PKG action (44), is likely to be involved. In contrast to MYPT1, we found that PP1cδ, the catalytic subunit of MLCP, was not significantly affected. It has been reported that the expression of PP1cδ protein in rat portal veins remains unchanged during postnatal upregulation of MYPT1 (35).
MYPT1 exists as isoforms with and without a leucine zipper domain in its COOH terminus (15). The presence of leucine zipper is required for PKG to activate MLCP, which leads to MLC dephosphorylation and vasodilatation (18, 26, 45, 47). The abundance of MYPT1 (LZ+) has been shown to be closely correlated to the relaxation mediated by cGMP-elevating agents such as NO (2, 22, 28, 35, 49). In our study, protein levels of both total MYPT1 and MYPT1 (LZ+) were reduced to a similar extent. This suggests that MYPT1 (LZ+) is the predominant isoform of the regulatory subunit of MLCP in porcine pulmonary arteries. Studies have shown that heterogeneity exists in the relative abundance of MYPT1 (LZ+) among different vessel types (5, 20, 35, 36).
In the present study, relaxation of porcine pulmonary arteries to DETA NO was nearly abolished by ODQ and markedly inhibited by Rp-8-Br-PET-cGMPS. This suggests that relaxation induced by NO is primarily mediated by cGMP-PKG pathway, which is consistent with previous studies related to nitrovasodilators (10, 12, 17, 19, 27, 40, 41). The reduction of MYPT1 protein expression in pulmonary arteries after 48-h incubation with an NO donor was prevented by ODQ, a specific inhibitor of sGC (13). Furthermore, MYPT1 protein expression could also be reduced by 48-h incubation with 8-Br-cGMP but not with 8-Br-cAMP, an effect that was sensitive to Rp-8-Br-PET-cGMPS, the PKG inhibitor (1). Therefore, the reduction in protein level of MYPT1 may result from a continuous activation of PKG due to the sustained elevation of cGMP induced by NO.
The decrease in protein level of MYPT1 after 48-h incubation with DETA NO and 8-Br-cGMP was not due to decreased synthesis, but rather to increased degradation of MYPT1 by proteasomes, since it was not affected by cycloheximide, an inhibitor of protein synthesis (33), but was prevented by MG-132 and lactacystin, inhibitors of proteasomes (7, 25). The attenuated relaxation of the arteries to DETA NO after 48-h incubation with this NO donor was also prevented by MG-132, supporting the notion that an increased degradation of MYPT1 by proteasomes may contribute to the development of vascular tolerance toward NO.
NO may promote proteasome-dependent degradation by S-nitrosylation of the substrate (24) or by nitration of the substrate via peroxynitrite (4). These mechanisms, however, do not seem to play a major role in the reduction of MYPT1 expression observed in our study. The suppressed MYPT1 expression caused by prolonged exposure to DETA NO was prevented by the inhibitors of sGC and PKG, indicating the enhanced proteasomal degradation resulted from continuous activation of PKG by cGMP, presumably through sustained phosphorylation of MYPT1 (9, 14, 17, 18). Proteasomal degradation occurs after a three-step process of ubiquitination of the substrate utilizing ubiquitin-activating enzyme, ubiquitin-conjugating enzyme, and ubiquitin ligase. The specificity of substrate recognition is provided by the ubiquitin ligases (14). A recent study showed that the degradation of MYPT1 is regulated by its interaction with the E3 ubiquitin ligase seven in absentia homolog 2 (SIAH2) (46). In our study, the concentration of DETA NO needed to suppress MYPT1 expression was lower than that needed to attenuate vasodilatation to subsequent stimulation with DETA NO, indicating that the decrease in MYPT1 expression may not affect vasodilatation until below a threshold level (Figs. 1 and 7).
MLC phosphorylation plays pivotal roles in smooth muscle contraction. MLC is phosphorylated by the Ca2+/calmodulin-activated myosin light chain kinase (MLCK) and by noncanonical Ca2+-independent myosin light chain kinases. Meanwhile, MLC is dephosphorylated by MLCP, which is regulated not only by MYPT1 but also by protein kinase C-potentiated inhibitor protein 17-kDa (CPI-17) and telokin (16, 39, 44). Since MLC dephosphorylation caused by PKG-dependent activation of MLCP is mainly mediated by MYPT1, a reduced expression of MYPT1 would be expected to lead to reduced dephosphorylation of MLC (18). Indeed, the decrease in the protein level of MYPT1 observed in our study after prolonged exposure to the NO donor was associated with a diminished MLC dephosphorylation in response to acute NO stimulus. This effect was prevented by MG-132, suggesting that an augmented proteasome degradation of MYPT1 is involved in the reduced MLC dephosphorylation. A similar phenomenon has also been observed in porcine coronary artery (5).
Downregulation of MYPT1 would be expected to lead a decreased MLCP activity and enhanced MLC phosphorylation in response to vasoconstrictors such as U46619. In our present study, this was not the case (Fig. 6). MLCP activity is inhibited not only by MYPT1 but also by CPI-17. In vascular smooth muscle cells, CPI-17 may play a more pronounced role than MYPT1 in inhibiting MLCP activity (15, 16). Therefore, it is possible that a partial reduction in MYPT1 expression occurred in our study may not be sufficient to affect MLC phosphorylation evoked by U46619 at submaximal stimulation (3 × 10−7 M).
Changes in expression of MYPT1 and MYPT1 (LZ+) have been implicated in a number of pathophysiological conditions associated with altered vasodilatation to NO and other nitrovasodilators (2, 3, 21, 28, 36). In a rat model of congestive heart failure, decreased expression of MYPT1 (LZ+) was associated with diminished relaxation of aortas to cGMP (21). Interestingly, in this model, the attenuation of vasodilatation to cGMP was prevented when the reduction in MYPT1 (LZ+) levels was prevented (3). Our present study shows that the blunted vasodilatation responses to NO and cGMP were prevented by inhibition of proteasome-mediated degradation of MYPT1. This information may be of some therapeutic value if we can ameliorate the development of vascular tolerance to NO and nitrovasodilators by preserving the expression of MYPT1 and MYPT1 (LZ+) by preventing proteasomal degradation.
GRANTS
This study was supported in part by National Natural Science Foundation of China Grants 30770789, 30870938, and 30900511; Major National Basic Research Program of China Grant 2006CB503802; and National Heart, Lung, and Blood Institute Grants HL 059435 and HL 075187.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Butt E, Pöhler D, Genieser HG, Huggins JP, Bucher B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br J Pharmacol 116: 3110–3116, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen FC, Brozovich FV. Gene expression profiles of vascular smooth muscle show differential expression of mitogen-activated protein kinase pathways during captopril therapy of heart failure. J Vasc Res 45: 445–45, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Chen FC, Ogut O, Rhee AY, Hoit BD, Brozovich FV. Captopril prevents myosin light chain phosphatase isoform switching to preserve normal cGMP-mediated vasodilatation. J Mol Cell Cardiol 41: 488–495, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Curry-McCoy TV, Osna NA, Donohue TM., Jr Modulation of lysozyme function and degradation after nitration with peroxynitrite. Biochim Biophys Acta 1790: 778–876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou D, Ma H, Zheng X, Ying L, Guo Y, Yu X, Gao Y. Degradation of leucine zipper positive isoform of MYPT1 may contribute to development of nitrate tolerance. Cardiovasc Res 86: 151–159, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Dou D, Zheng X, Qin X, Qi H, Liu L, Raj JU, Gao Y. Role of cGMP-dependent protein kinase in development of tolerance to nitroglycerine in porcine coronary arteries. Br J Pharmacol 153: 497–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268: 726–731, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Francis SH, Corbin JD. Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol 56: 237–272, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Friebe A, Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide 21: 149–156, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Dhanakoti S, Tolsa JF, Raj JU. Role of protein kinase G in nitric oxide and cGMP-induced relaxation of newborn ovine pulmonary veins. J Appl Physiol 87: 993–998, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Dhanakoti S, Trevino EM, Wang X, Sander FC, Portuga AD, Raj JU. Role of cGMP-dependent protein kinase in development of tolerance to nitric oxide in pulmonary veins of newborn lambs. Am J Physiol Lung Cell Mol Physiol 286: L786–L792, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Zhou H, Raj JU. Endothelium-derived nitric oxide plays a larger role in pulmonary veins than in arteries in newborn lambs. Circ Res 76: 559–565, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one. Mol Pharmacol 48: 184–188, 1995 [PubMed] [Google Scholar]
- 14.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Hartshorne DJ, Ito M, Erdödi F. Role of protein phosphatase type 1 in contractile functions: myosin phosphatase. J Biol Chem 279: 37211–37214, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci 104: 109–115, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Huang QQ, Fisher SA, Brozovich FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem 279: 597–603, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res 65: 1–21, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Jaikirshan JK, Katherine MJ, Frank VB, Steven AF. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem 276: 37250–37257, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Karim SM, Rhee AY, Given AM, Faulx MD, Hoit BD, Brozovich FV. Vascular reactivity in heart failure: role of myosin light chain phosphatase. Circ Res 95: 612–618, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem 276: 37250–37257, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Kim D, Rybalkin SD, Pi XC, Wang Y, Zhang C, Münzel T, Beavo JA, Berk BC, Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation 104: 2338–2343, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Lee CM, Kim BY, Li L, Morgan ET. Nitric oxide-dependent proteasomal degradation of cytochrome P450 2B proteins. J Biol Chem 283: 889–898, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol 8: 397–403, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lee E, Hayes DB, Langsetmo K, Sundberg EJ, Tao TC. Interactions between the leucine-zipper motif of cGMP-dependent protein kinase and the C-terminal region of the targeting subunit of myosin light chain phosphatase. J Mol Biol 373: 1198–1212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lincoln TM, Dey N, Sellak H. cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol 91: 1421–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Zhang H, Gokina N, Mandala M, Sato O, Ikebe M, Osol G, Fisher SA. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat l-NAME model of hypertension of pregnancy. Am J Physiol Cell Physiol 294: C564–C571, 2008 [DOI] [PubMed] [Google Scholar]
- 29.MacPherson JD, Gillespie TD, Dunkerley HA, Maurice DH, Bennett BM. Inhibition of phosphodiesterase 5 selectively reverses nitrate tolerance in the venous circulation. J Pharmacol Exp Ther 317: 188–195, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: many roles in cell function. Biochem Biophys Res Commun 369: 149–156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer RBJ, Miller JP. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. Life Sci 14: 1019–1040, 1974 [DOI] [PubMed] [Google Scholar]
- 32.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol 25: 674–678, 1995 [PubMed] [Google Scholar]
- 33.Obrig TG, Culp WJ, McKeehan WL, Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J Biol Chem 246: 174–181, 1971 [PubMed] [Google Scholar]
- 34.Pagani ED, VanAller GS, O'Connor B, Silver PJ. Reversal of nitroglycerin tolerance in vitro by the cGMP-phosphodiesterase inhibitor zaprinast. Eur J Pharmacol 243: 141–147, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Payne MC, Zhang HY, Prosdocimo T, Joyce KM, Koga Y, Ikebe M, Fisher SA. Myosin phosphatase isoform switching in vascular smooth muscle development. J Mol Cell Cardiol 40: 274–282, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Payne MC, Zhang HY, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. Am J Physiol Heart Circ Physiol 286: H1801–H1810, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Perkins WJ, Taniguchi M, Warner DO, Chini EN, Jones KA. Reduction in soluble guanylyl cyclase-specific activity following prolonged treatment of porcine pulmonary artery with nitric oxide. Am J Physiol Lung Cell Mol Physiol 293: L84–L95, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Perkins WJ, Warner DO, Jones KA. Prolonged treatment of porcine pulmonary artery with nitric oxide decreases cGMP sensitivity and cGMP-dependent protein kinase specific activity. Am J Physiol Lung Cell Mol Physiol 296: L121–L129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puetz S, Lubomirov LT, Pfitzer G. Regulation of smooth muscle contraction by small GTPases. Physiology (Bethesda) 24: 342–356, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Qi H, Zheng X, Qin X, Dou D, Xu H, Gao Y. Protein kinase G regulates the basal tension and plays a major role in nitrovasodilator-induced relaxation of porcine coronary veins. Br J Pharmacol 152: 1060–1069, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin X, Zheng X, Qi H, Dou D, Raj JU, Gao Y. cGMP-dependent protein kinase in regulation of basal tone and in nitroglycerin- and nitric-oxide-induced relaxation in porcine coronary artery. Pflügers Arch 454: 913–923, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Schlossmann J, Desch M. cGK substrates. Handb Exp Pharmacol 191: 163–193, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Soff GA, Cornwell TL, Cundiff DL, Gately S, Lincoln TM. Smooth muscle cell expression of type I cyclic GMP-dependent protein kinase is suppressed by continuous exposure to nitrovasodilators, theophylline, cyclic GMP, and cyclic AMP. J Clin Invest 100: 2580–2587, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of Myosin Phosphatase by a Specific Interaction with cGMP- Dependent Protein Kinase I. Science 286: 1583–1587, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Twomey E, Li Y, Lei J, Sodja C, Ribecco-Lutkiewicz M, Smith B, Fang H, Bani-Yaghoub M, McKinnell I, Sikorska M. Regulation of MYPT1 stability by the E3 ubiquitin ligase SIAH2. Exp Cell Res 16: 68–77, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Wu X, Haystead TA, Nakamoto RK, Somlyo AV, Somlyo AP. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. J Biol Chem 273: 11362–11369, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Rikitake Y, Inoue N, Hirata K, Akita H, Yokoyama M. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension 36: 97–102, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res 100: 730–737, 2007 [DOI] [PubMed] [Google Scholar]