Abstract
Endoplasmic reticulum (ER) stress-mediated apoptosis is a key feature of hepatocyte cytotoxicity by saturated free fatty acids (FFA). This lipoapoptosis is dependent, in part, on the transcriptional upregulation of the BH3-only protein PUMA (p53 upregulated modulator of apoptosis). Although the activator protein (AP)-1 complex facilitates PUMA expression by saturated FFA, the transcription factor CAAT/enhancer binding homologous protein (CHOP) is also induced by ER stress and promotes apoptosis. To integrate the role of these two transcription factors in ER stress-induced apoptosis, we examined the relative contribution of CHOP and AP-1 in mediating PUMA induction by saturated FFA. Our results demonstrate that short-hairpin RNA-targeted knockdown of CHOP attenuates palmitate-induced apoptosis in Huh-7 cells. Loss of CHOP induction also reduced the increase in PUMA mRNA and protein levels as well as Bax activation by palmitate. No functional CHOP binding sites were identified in the PUMA promoter sequence. Rather, we observed that CHOP physically interacts with the AP-1 complex protein c-Jun upon palmitate treatment, and a CHOP:phosphorylated c-Jun heteromeric complex binds to the AP-1 consensus binding sequence within the PUMA promoter region. Finally, loss of function studies suggest that both transcription factors are necessary for maximal PUMA induction. Collectively, these data suggest that CHOP and AP-1 cooperatively mediate PUMA induction during hepatocyte lipoapoptosis.
Keywords: BH3-only proteins, c-Jun, endoplasmic reticulum stress, hepatic steatosis, nonalcoholic steatohepatitis, CAAT/enhancer binding homologous protein, activator protein-1, p53 upregulated modulator of apoptosis
nonalcoholic fatty liver disease (NAFLD) is often a hepatic manifestation of systemic insulin resistance accompanying the metabolic syndrome (3). This syndrome is characterized by elevated serum free fatty acids (FFA) and hepatic lipid accumulation. A subset of patients with NAFLD develop severe hepatic lipotoxicity, known as nonalcoholic steatohepatitis (NASH), which can progress to end-stage liver disease (1, 9, 30). Circulating FFA, which are disproportionately elevated in NASH compared with NAFLD (27), are taken up by the liver and reesterified into neutral triglycerides. Excessive hepatocyte accumulation of nonesterified FFA overwhelms the capacity for their incorporation into neutral triglycerides or oxidation, resulting in cellular cell death by apoptosis, a process referred to as lipoapoptosis (16, 36). Indeed nonesterified FFA, in particular long-chain saturated fatty acids, are toxic to liver cells (4, 6, 20, 21, 37–39), and both increased serum FFA levels and hepatocyte apoptosis characterize NASH (10, 14).
Endoplasmic reticulum (ER) stress-induced apoptosis has been implicated in hepatocyte lipoapoptosis by saturated FFA (2, 38, 39), and induction of ER-stress responses is observed in both rodent and human steatohepatitis (28, 37). ER stress by saturated FFA upregulates the proapoptotic activities of the BH3-only proteins Bim and PUMA (2, 4, 6, 20), and both Bim and PUMA contribute to lipoapoptosis by saturated FFA in liver cells (2, 4, 6, 20). These two BH3-only proteins participate in processes triggering the mitochondrial pathway of apoptosis (15, 41). The upregulation of PUMA and Bim by saturated FFA-induced ER stress is transcriptionally mediated (2, 4, 6). Further insights into these transcriptional processes may help provide therapeutic strategies to ameliorate lipoapoptosis.
Saturated FFA-induced ER stress results in the induction of the transcription factor CCAAT/enhancer binding homologous protein (CHOP) in liver cells (2, 38, 39). CHOP has been implicated as a key mediator of ER stress-induced cell death in various cell types including murine fibroblasts (43), lymphocytes (26) and pancreatic β-cells (8, 22, 24). Indeed, CHOP knockdown confers protection against lipoapoptosis in pancreatic β-cells (8) and reduces hepatocyte apoptosis in alcohol-induced liver injury (13). CHOP can transcriptionally regulate the expression of several death signaling molecules, including the death receptor DR5 and Bim (29, 40). However, a potential role for CHOP in PUMA expression has not been explored. In contrast, we have implicated activator protein (AP)-1 as a key transcription factor complex driving PUMA expression downstream of ER stress in lipotoxicity (6). Emerging data suggest that AP-1 and CHOP may cooperatively regulate the transcription of AP-1 target genes (35). On the basis of this information, PUMA expression may be regulated by both of these two transcription factors.
In the present study, we explored the potential role of CHOP in mediating PUMA upregulation by saturated FFA. The results implicate CHOP as a mediator of PUMA induction in this model. These observations were further integrated with our prior observations that AP-1 promotes PUMA induction by demonstrating that CHOP binds to phosphorylated c-Jun and enhances the AP-1-dependent PUMA expression. These data provide further mechanistic insights linking ER stress to PUMA induction during FFA-mediated lipotoxicity.
MATERIALS AND METHODS
Cells.
Huh-7 cells, a human hepatoma cell line, were cultured in DMEM containing glucose (25 mM), 100,000 U/l penicillin, 100 mg/l streptomycin, and 10% fetal bovine serum.
Fatty acid treatment.
Palmitic and oleic acid (Sigma Aldrich, St. Louis, MO) were individually dissolved in isopropyl alcohol at a stock concentration of 40 to 80 mM. FFA were added to DMEM containing 1% bovine serum albumin to assure a physiological ratio between bound and unbound FFA in the medium (32). The concentrations of FFA used in the experiments (400 to 800 μM) were similar to the fasting FFA plasma concentrations observed in human nonalcoholic steatohepatitis (5, 33). The concentration of the vehicle, isopropyl alcohol, was 1% in final incubations.
Quantitative real-time PCR.
Trizol-extracted total RNA was reverse transcribed with Moloney leukemia virus reverse transcriptase and random primers (both from Invitrogen, Carlsbad, CA). Quantification of the complementary DNA template was performed by real-time PCR using SYBR green fluorescence on a LightCycler 480 instrument (Roche Applied Science, Indianapolis, IN) as previously described by us in detail (4). Primers were as follows: human PUMA (NM_001127240): forward 5′-GACGACCTCAACGCACAGTA-3′ and reverse 5′-AGGAGTCCCATGATGAGATTGT-3′ (101 bp); human CHOP (NM_004083): forward 5′-ATGGCAGCTGAGTCATTGCCTTTC-3′ and reverse 5′-AGAAGCAGGGTCAAGAGTGGTGAA-3′ (177 bp); 18S (X03205): forward 5′-CGTTCTTAGTTGGTGGAGCG-3′ and reverse 5′-CGCTGAGCCAGTCAGTGTAG-3′ (212 bp).
Immunoblot analysis.
Whole cell lysates were prepared as previously described (4). For CHOP protein analysis, nuclear cell extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL). Equal amount of protein (50 to 80 μg) was resolved by SDS-PAGE on a 12.5% or 15% acrylamide gel, transferred to nitrocellulose membranes, and incubated with primary antibodies. Membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Biosource International, Camarillo, CA). Bound antibody was visualized using chemiluminescent substrate (ECL; Amersham, Arlington Heights, IL) and was exposed to Kodak X-OMAT film (Eastman Kodak, Rochester, NY).
Quantitation of apoptosis.
Cells were stained with 5 μg/ml 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) for 30 min at 37°C and visualized under fluorescence microscopy (Nikon Eclipse TE200; Nikon, Tokyo, Japan). Apoptotic cells were quantified by counting 300 random cells per study. Cells with the characteristic nuclear changes of chromatin condensation and nuclear fragmentation were considered apoptotic. Apoptosis was expressed as a percentage of total cells counted. For caspase 3/7 activity, cells were plated in 96-well plates. The assay was performed using the commercially available Apo-ONE homogeneous caspase-3/7 assay (Promega, Madison, WI) as previously described by our laboratory (4, 20). Immunocytochemistry of activated Bax was performed using mouse anti-universal Bax antiserum (clone 6A7; Exalpha Biologicals, Watertown, MA) as previously described (6).
Plasmid and transfection.
Huh-7 cells were transfected with 1 μg/ml DNA plasmid [CHOP, MISSION short-hairpin RNA (shRNA) lentiviral plasmid; Sigma] using Lipofect 2000 (Invitrogen). Stably transfected clones were selected in medium containing 1,200 mg/l G418 and screened by CHOP real-time PCR and immunoblot analysis. The S-peptide-tagged c-Jun plasmid was generated as previously described by our laboratory (6). The cDNA encoding the full-length human CHOP open reading frame (NM_004083) was generated by PCR using the following primers: 5′-ACGGGATCCAGCAGCTGAGTCATTGCCTTTC-3′ (forward) and 5′-ACGGAATTCTCATGCTTGGTGCAGATTCACCA-3′ (reverse). BamHI and EcoRI restriction sites were added respectively at the 5′ end of the forward and reverse primer (underlined sequence). PCR-amplified full-length human CHOP was then cloned into BamHI/EcoRI sites of pcDNA3 empty vector, which encodes an S-tag peptide at the NH2 terminus (S-peptide-tagged CHOP plasmid). S-peptide-tagged CHOP plasmid, S-peptide-tagged c-Jun plasmid, or the pcDNA3 empty vector were transfected using FuGENE HD transfection reagent (Roche Applied Science).
S-peptide pulldown.
Huh-7 were cells transiently transfected with S-peptide-tagged CHOP or S-peptide-tagged c-Jun. Following selected treatment paradigms, cells were washed with PBS and lysed at 4°C for 1 h in lysis buffer consisting of 50 mmol/l Tris·HCl (pH 7.4), 150 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l DTT, 1 mmol/l phenylmethylsulfonyl fluoride, 1 tablet Complete protease inhibitors/50 ml (Roche Diagnostics), 1 mmol/l Na3VO4, 100 mmol/l NaF, and 1% (wt/vol) NP40. Protein concentration was determined, and aliquots containing 1 mg protein in 1 ml lysis buffer were incubated with 50 μl S-protein agarose beads (Novagen, San Diego, CA) at 4°C overnight. After six washes with lysis buffer, samples were released from the beads by boiling in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) containing 5% 2-mercaptoethanol. Samples were resolved by SDS-PAGE and transferred to nitrocellulose membranes.
EMSA.
Nuclear extracts from Huh-7 cells transfected with S-peptide-tagged CHOP plasmid were prepared as previously described by our laboratory (6). A sample (20 μg) of nuclear proteins was incubated at room temperature for 20 min in binding buffer [25 mM HEPES pH 7.5, 60 mM KCl, 2.5 mM MgCl2, 0.1 mM EDTA, 5% glycerol, 1 mM phenylmethylsulfonyl fluoride, 0.75 mM dithiothreitol, 0.5 μg/μl poly(dI-dC), and 0.5 μg/μl salmon sperm] with 0.04 pmol of CY 5.5-labeled double-stranded DNA oligonucleotide containing CHOP-C/enhancer binding protein (EBP)α heterodimer binding sequence (underlined sequence) within the promoter region of human PUMA gene (5′-CCCAGGCTAGAGTGCAATGGCACTATCTCG-3′). Protein-DNA complexes were separated from the unbound DNA probe by electrophoresis, and fluorescence was visualized directly in the gel using an Odyssey fluorescent imager (Licor Biosciences, Lincoln, NE). EMSA with CY 5.5-labeled AP-1 probe was performed as previously reported (6).
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assay was performed employing Huh-7 cells (6–10 million cells) transfected with either S-tagged CHOP plasmid or the empty vector using a commercially available assay kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer's instructions with minor modifications. Briefly, Huh-7 cells treated for 4 h with palmitate were fixed with 1% formaldehyde in PBS at room temperature for 10 min. Crosslinking reactions were quenched with 125 mM glycine, cells were lysed, and chromatin was sheared into small and uniform fragments by sonication. Following centrifugation, DNA concentration in the supernatant was quantified by measuring absorbance at 260 nm. Samples containing 30 μg of DNA were diluted 20-fold and precleared with salmon sperm DNA/protein A-agarose slurry (Upstate Biotechnology) at 4°C for 2 h, and the target protein S-tagged CHOP was immunoprecipitated with 80 μl of anti-S-protein agarose beads (Novagen) at 4°C overnight with rotation. Eluted protein-DNA crosslinks were reversed by heating for 6 h at 65°C, the proteins were digested by proteinase K, and the DNA in the ChIP sample was used as the template for quantitative real-time PCR. Primer sets that span PUMA promoter regions were as previously described (6).
Antibodies and reagents.
Antibodies used were obtained from the following sources: anti-PUMA (600-401-987; Rockland Immunochemicals, Gilbertsville, PA); anti-CHOP (sc-7351), anti-Lamin B, anti-phospho-c-Jun (Ser63), anti-c-Jun, and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA); anti-S-peptide was a gift from Dr. Scott Kaufmann (Mayo Clinic, Rochester, MN). Thapsigargin and the JNK inhibitor SP600125 were from Calbiochem (EMD Biosciences, La Jolla, CA); BSA, Bradford reagent, and all other chemicals were from Sigma Aldrich.
Statistical analysis.
All data represent at least three independent experiments and are expressed as the means ± SE. Differences between groups were compared using Student's t-tests and one-way analysis of variance with post hoc Dunnett test, and significance was accepted at P < 0.05.
RESULTS
CHOP contributes to palmitate-induced apoptosis in Huh-7 cells.
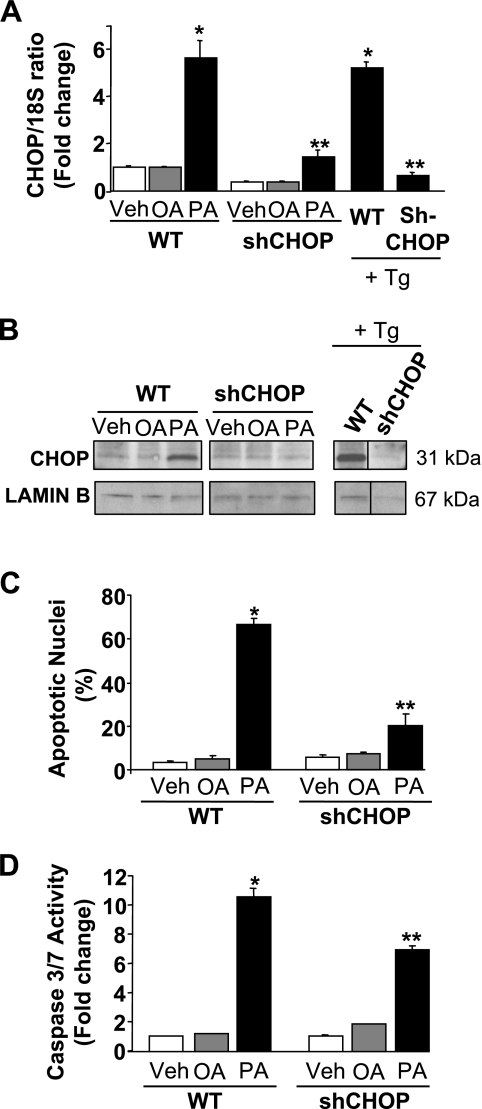
Consistent with prior studies (2, 38, 39), incubation of Huh-7 cells with the saturated FFA palmitate induced a sixfold increase in CHOP mRNA expression (Fig. 1A). Similarly, an increase in nuclear protein levels of CHOP was observed by immunoblot analysis 16 h following treatment with palmitate (Fig. 1B). In contrast, the nontoxic monounsaturated FFA oleate had no effect on CHOP mRNA or protein levels (Fig. 1, A and B). To ascertain whether CHOP is directly involved in saturated FFA-induced apoptosis, Huh-7 cells were stably transfected with a lentivirus construct containing a CHOP-targeted shRNA. The level of CHOP mRNA and protein in cells stably transfected with CHOP shRNA (shCHOP) was efficiently reduced compared with parental wild-type cells (WT) following exposure to palmitate or thapsigargin, a potent pharmacological ER-stress inducer (34) (Fig. 1, A and B). As assessed by both morphological and biochemical criteria (e.g., caspase 3/7 activity), knockdown of CHOP expression attenuated palmitate-induced apoptosis (Fig. 1, C and D). These results suggest that CHOP contributes to hepatocyte lipoapoptosis by palmitate.
Fig. 1.
CAAT/enhancer binding homologous protein (CHOP) knockdown attenuated palmitate-mediated apoptosis. A and B: Huh-7 (wild-type, WT) and Huh-7 cells stably expressing short-hairpin (sh) RNA complementary to CHOP (shCHOP) were incubated with palmitic acid (PA), oleic acid (OA) at 800 μM, or Thapsigargin (Tg) at 450 nM. Vehicle (Veh)-treated cells were used as controls. A: total RNA was extracted 8 h after treatments, and CHOP mRNA expression was quantified by real-time PCR (RT-PC). Fold induction was determined after normalization to 18S. Data represent the means and standard errors of 3 experiments; *P < 0.05, palmitate or thapsigargin-treated cells vs. vehicle-treated cells, **P < 0.01, palmitate or thapsigargin-treated shCHOP vs. palmitate or thapsigargin-treated WT. B: nuclear extracts were prepared 16 h after treatments, and immunoblot analysis was performed for CHOP protein expression. Lamin B was used as a control for protein loading. Images were cut and combined from the same radiograph. C and D: WT Huh-7 and shCHOP Huh-7 were incubated 24 h with PA, OA at 400 μM, or vehicle. C: apoptotic nuclei were counted according to morphological criteria after DAPI staining. D: caspase 3/7 catalytic activity was measured by a fluorogenic assay. Data represent means and standard errors of 3 experiments; *P < 0.01, palmitate-treated cells vs. vehicle-treated cells, **P < 0.01, palmitate-treated shCHOP vs. palmitate-treated WT.
Palmitate-induced PUMA upregulation and Bax activation is CHOP dependent.
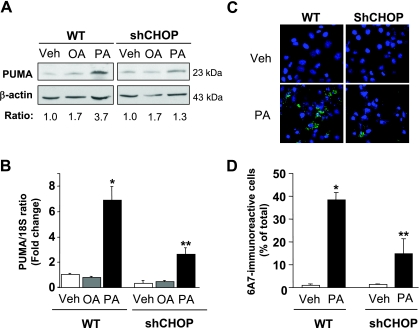
Given that induction of PUMA expression is a critical event contributing to lipoapoptosis (2, 6), we sought to investigate whether ER stress-induced CHOP promotes PUMA expression during palmitate-induced apoptosis. In accord with prior observations (2, 4, 6), increases of cellular PUMA protein levels were observed in Huh-7 cells following treatment with palmitate but not after treatment with oleate (Fig. 2A). More importantly, knockdown of CHOP expression partially attenuates this increase in PUMA upregulation by palmitate (Fig. 2, A and B).
Fig. 2.
CHOP knockdown decreased palmitate-mediated increase in PUMA (p53 upregulated modulator of apoptosis) protein levels and Bax activation. A and B: WT Huh-7 and shCHOP Huh-7 were incubated with palmitic acid and oleic acid at 800 μM. Vehicle -treated cells were used as control. A: whole cell lysates were prepared 16 h after treatments, and immunoblot analysis was performed for PUMA protein expression. β-Actin was used as a control for protein loading. PUMA/β-actin ratio was calculated by densitometry and normalized to vehicle-treated cells. B: total RNA was extracted 8 h after treatments, and PUMA mRNA expression was quantified by real-time PCR. Fold induction was determined after normalization to 18S. Data represent the means and standard errors of 3 experiments; *P < 0.01, palmitate-treated cells vs. vehicle-treated cells, **P < 0.01, palmitate-treated shCHOP vs. palmitate-treated WT. C and D: WT Huh-7 and shCHOP Huh-7 were incubated for 12 h with palmitic acid at 800 μM. Vehicle-treated cells were used as control. Cells were fixed, and Bax activation was assessed using conformation specific antisera (6A7) and immunofluorescence microscopy. C: representative images of 3 independent experiments are depicted. D: 6A7-immunoreactive cells were quantified in 6 random ×40 objective fields for each condition with automated software. Data represent means and standard errors of 3 experiments; *P < 0.05, palmitate-treated cells vs. vehicle-treated cells, **P < 0.01, palmitate-treated shCHOP vs. palmitate-treated WT.
Activation of the proapoptotic protein Bax, a known mediator of mitochondrial dysfunction, represents a pivotal step in hepatocyte lipoapoptosis (2, 6, 20), and PUMA can directly activate Bax, which leads to mitochondrial dysfunction and cell death (11, 41, 42). Therefore, we next sough to determine whether the decrease in PUMA cellular levels observed in shCHOP-transfected cells was sufficient to prevent Bax activation. Activated Bax in response to palmitate treatment was detected by immunofluorescence using the 6A7 monoclonal antibody, an antibody that specifically recognizes the active conformation of Bax (12). Knockdown of CHOP expression reduced Bax activation following treatment with palmitate (Fig. 2, C and D). Taken together, these data suggest that palmitate-mediated induction of PUMA, with subsequent activation of Bax, is downstream of CHOP expression.
Palmitate promotes CHOP association with the AP-1 complex protein c-Jun.
To ascertain whether CHOP may directly upregulate PUMA transcription, the human PUMA gene sequence was examined to identify potential consensus CHOP binding sites. No such binding sites were detected within the PUMA promoter sequence, but a perfect CHOP-C/EBPα heterodimer binding site (TGCAAT) was identified at +451/+456 nucleotides from the transcriptional start codon in the exon 1B of the PUMA gene. However, EMSA studies failed to detect an interaction between CHOP and the putative CHOP-C/EBPα heterodimer binding site (data not shown), making this an unlikely site regulating PUMA transcription during lipoapoptosis.
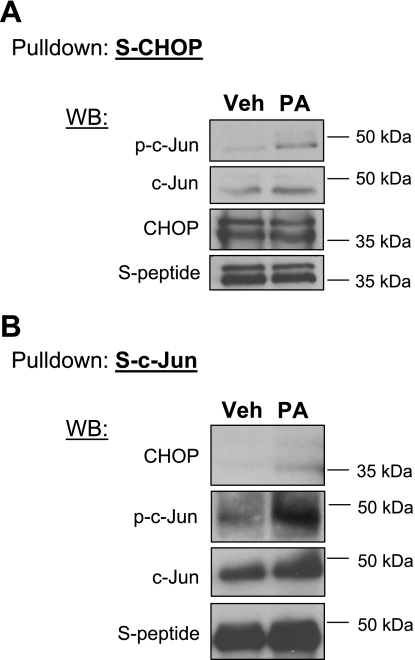
CHOP can also directly interact with members of the AP-1 family of transcription factor, such as c-Jun, and promote transcription of AP-1 target genes (35). We recently identified an AP-1 binding site within the PUMA promoter as a critical regulatory site for PUMA transcription by phosphorylated c-Jun during lipotoxic insults (6). Therefore, we posited that CHOP may contribute to the AP-1 transcriptional activation of PUMA by binding to the AP-1 complex protein c-Jun. Pull-down experiments were performed to determine whether CHOP interacts with active phosphorylated c-Jun. Huh-7 cells were transfected with S-peptide-tagged CHOP plasmid; protein complexes associated with S-peptide-tagged CHOP, following its pull down, were examined by immunoblot analysis. Upon treatment with palmitate, active phosphorylated c-Jun was found associating with S-peptide-tagged CHOP (Fig. 3A), suggesting a direct physical interaction between the two transcription factors. Following palmitate treatment, binding of CHOP to S-peptide-tagged c-Jun was also observed by performing the reciprocal experiment using Huh-7 cells transfected with S-peptide-tagged c-Jun plasmid (Fig. 3B). These results indicate that, following palmitate treatment, CHOP is capable of forming protein complexes containing phosphorylated c-Jun.
Fig. 3.
S-peptide-tagged CHOP interacts with c-Jun following palmitate treatment. A: Huh-7 cells were transiently transfected for 24 h with an S-peptide-tagged CHOP (S-CHOP). Next, cells were treated with palmitic acid at 800 μM for 8 h. Vehicle was used as control. S-peptide-tagged CHOP was pulled down from whole cell lysate as described in materials and methods, and immunoblot analysis was performed for S-peptide, CHOP, phosphorylated c-Jun (p-c-Jun) and c-Jun. B: Huh-7 cells were transiently transfected for 24 h with an S-peptide-tagged c-Jun (S-c-Jun) and treated as described above. S-peptide-tagged c-Jun was pulled down, and immunoblot analysis was performed for S-peptide, CHOP, phosphorylated c-Jun, and c-Jun.
The CHOP:phosphorylated c-Jun protein complex binds to the PUMA promoter.
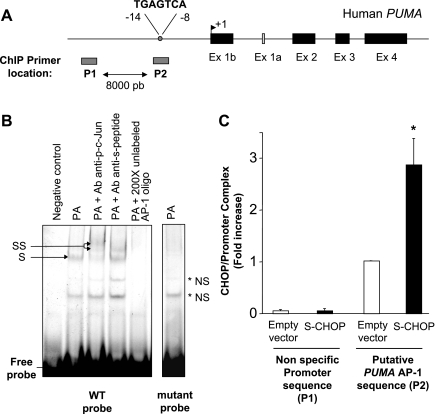
To test whether a CHOP:phosphorylated c-Jun heteromeric complex binds to the AP-1 consensus site within the PUMA promoter (Fig. 4A), an EMSA was performed using a CY 5.5-labeled AP-1 probe (6) and nuclear extracts from Huh-7 cells transfected with S-peptide-tagged CHOP. As previously demonstrated (6), a protein complex binding to the AP-1 sequence of the human PUMA promoter was observed in nuclear extracts obtained from palmitate-treated cells, and this protein complex supershifted when extracts were incubated with anti-phospho-c-Jun antisera (Fig. 4B). More importantly, supershifted complexes were also observed when adding anti-S-peptide antisera, which detect S-peptide-tagged CHOP expressed in the cells (Fig. 4B). Protein binding to the labeled oligonucleotide could be competed away with a 200-fold molar excess of the unlabeled probe. Also, EMSA using the CY 5.5-labeled mutated probe was unable to elicit binding to a larger molecular weight complex (Fig. 4B). To further confirm binding of a CHOP:phosphorylated c-Jun protein complex to the PUMA promoter AP-1 consensus site, a chromatin immunoprecipitation assay was performed. S-peptide-tagged CHOP pull down captured the AP-1 sequence of the human PUMA promoter in nuclear extracts prepared from palmitate-treated Huh-7 cells (Fig. 4C). Binding of S-peptide-tagged CHOP to the AP-1 site of PUMA promoter was specific because it was not detected on an unrelated upstream region (Fig. 4C). These results are consistent with the formation of CHOP:phosphorylated c-Jun heteromeric complex that binds to the AP-1 binding site regulating PUMA transcription during lipoapoptosis.
Fig. 4.
S-peptide-tagged CHOP binds to the activator protein (AP)-1 binding site within PUMA promoter. A: genomic structure of PUMA depicting AP-1 binding site within the promoter region. As indicated by the gray bars, P1 and P2 are the two primer sets used for chromatin immunoprecipitation (ChIP) assays, amplifying, respectively, an upstream nonspecific sequence or the AP-1 sequence within the human PUMA promoter. B: Huh-7 cells were transiently transfected for 24 h with an S-peptide-tagged CHOP. Nuclear protein extracts were isolated 8 h after treatment with palmitic acid at 800 μM. EMSA was performed using CY 5.5-labeled AP-1 probe as described in materials and methods. Antibodies against phospho-c-Jun or S-peptide were used in supershift experiments. Retarded complexes are indicated by arrows (S: shift; SS: supershift, NS: nonspecific band). No specific retarded complexes were observed when adding 200-fold molar excess of the unlabeled probe or when using the CY 5.5-labeled mutated AP-1 probe. CY 5.5-labeled AP-1 probe only with no nuclear extract was used as a negative control. C: Huh-7 cells were transiently transfected for 24 h with an S-peptide-tagged CHOP or the empty vector and then treated with palmitic acid at 800 μM for 4 h. Cells were fixed and lysed, and DNA fragments pulled down with S-peptide-tagged CHOP were subjected to quantitative RT-PCR analysis using the primers P1 or P2 as described above. Data represent the means and standard errors of 3 experiments; *P < 0.01, palmitate-treated S-CHOP vs. palmitate-treated empty vector.
CHOP and AP-1 cooperate to mediate palmitate-induced PUMA expression.
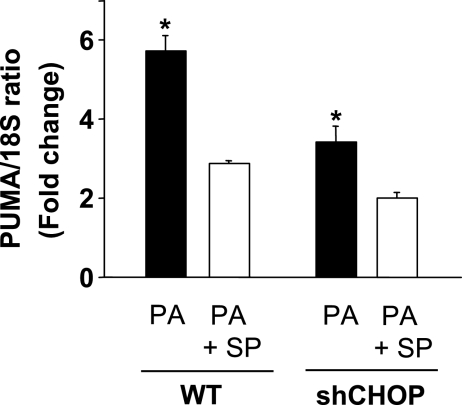
To place the above binding studies into a physiological context, we examined the respective role of CHOP and AP-1 in regulating PUMA mRNA levels during lipoapoptosis. shCHOP-transfected Huh-7 cells or the parental WT cells were concomitantly treated with palmitate and the pharmacological JNK inhibitor SP600125. As previously demonstrated by our laboratory, SP600125 treatment prevented JNK-mediated c-Jun phosphorylation by palmitate (6) and reduced palmitate-induced increase in PUMA mRNA levels in WT Huh-7 cells (Fig. 5). JNK inhibition in the parental WT Huh-7 cells, or knockdown of CHOP, decreases to a similar degree palmitate-mediated PUMA induction. However, concomitant loss of CHOP expression by shRNA plus JNK inhibition by SP600125 was only modestly additive in preventing PUMA mRNA upregulation by palmitate (Fig. 5). These results suggest that AP-1-mediated induction of PUMA transcription also requires CHOP and that these two transcription factors cooperate to induce PUMA expression during palmitate-induced lipoapoptosis.
Fig. 5.
JNK-mediated AP-1 and CHOP cooperate to mediate PUMA upregulation following palmitate treatment. WT Huh-7 and shCHOP Huh-7 were incubated with palmitic acid at 800 μM in the presence of the JNK inhibitor SP600125 (SP) at 50 μM. Total RNA was extracted 8 h after treatment, and PUMA mRNA expression was quantified by real-time PCR and normalized with respect to 18S expression. Results are expressed in fold increase compared with vehicle-treated WT Huh-7 cells (arbitrarily set to 1). Data represent the means and standard errors of 3 experiments; *P < 0.05, palmitate-treated cells in the presence of the JNK inhibitor vs. palmitate-treated cells.
DISCUSSION
These findings provide new mechanistic insights regarding ER stress and PUMA induction during saturated FFA-mediated lipoapoptosis. Our results in Huh-7 cells treated with palmitate to induce lipotoxicity indicate that 1) shRNA-targeted knockdown of CHOP decreases lipoapoptosis; 2) loss of CHOP expression attenuates PUMA upregulation; 3) a CHOP:phosphorylated c-Jun heteromeric complex binds to the AP-1 binding site within PUMA promoter; and 4) CHOP and JNK-activated AP-1 cooperate to mediate PUMA upregulation. These observations suggest that ER stress-associated PUMA upregulation during FFA-mediated hepatocyte apoptosis requires cooperation between CHOP and AP-1.
Impairment of ER function and activation of ER stress responses have been implicated in lipoapoptosis pathways in various cell types including liver cells (2, 7, 22, 24, 26, 38, 39, 43). With regard to lipids, saturated FFA, but not unsaturated FFA, result in an ER stress response with the induction of the transcription factor CHOP in liver cells (2, 39). CHOP is induced downstream of the protein kinase RNA-like ER kinase, one of the three membrane transducers of ER stress responses (23), and prolonged and sustained ER stress with CHOP induction has been implicated as a key mechanism mediating cell death (23). For example, thymocytes or macrophages from CHOP knockout mice are more resistant to thapsigargin-induced apoptosis (29), and CHOP knockdown protects pancreatic β-cells against FFA-induced apoptosis (8). An earlier study has reported the contribution of CHOP in mediating hepatocyte apoptosis in a model of alcohol-induced liver injury (13). Our observations implicate CHOP as a potent mediator of nonalcoholic hepatic lipoapoptosis by demonstrating the resistance of shCHOP-transfected cells to palmitate lipotoxicity.
At the time of finalizing our studies, a report by Pfaffenbach et al. (25) also demonstrated that CHOP deficiency delayed hepatocyte cell death in response to palmitate (400–500 μM) at concentrations similar to those employed in our present study (400–800 μM). On the other hand, at lower concentrations of palmitate, genetic deletion or siRNA knockdown of CHOP did not delay lipoapoptosis in their study (25). However, in our studies, minimal lipoapoptosis is observed at FFA concentrations ≤200 μM (data not shown). The differences between this study and ours may reflect species differences. Indeed, Pfaffenbach et al. (25) employed rodent hepatocytes, whereas we used a human cell line. We note that PUMA upregulation occurs in human NAFLD (6), and, therefore our present studies examining the mechanisms of PUMA upregulation are relevant to human disease.
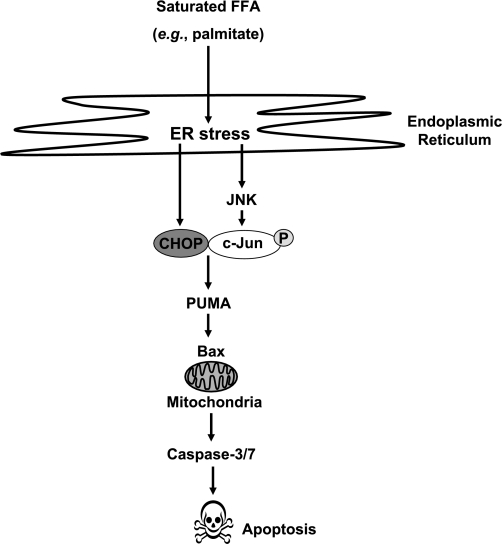
The molecular mechanisms by which CHOP mediates ER stress-associated apoptosis remain incompletely understood. CHOP contributes to the transcriptional regulation of the death receptor DR5 and the BH3-only protein Bim (29, 40), two death signaling molecules upregulated during FFA-induced liver apoptosis (4, 19). Besides Bim, PUMA is also a potent BH3-only protein upregulated by ER stress (17). Hepatic levels of PUMA are increased in patients with NASH, and PUMA contributes to hepatocyte lipoapoptosis (6) by triggering the mitochondrial pathway of apoptosis (11, 15, 42). Herein, we demonstrated that CHOP expression is also requisite for PUMA induction by palmitate. These results are consistent with the concept that PUMA induction is necessary for ER stress-induced apoptosis (31) and can be linked to direct Bax activation, thereby initiating mitochondrial dysfunction (15) (Fig. 6).
Fig. 6.
Proposed model for endoplasmic reticulum (ER) stress-associated lipoapoptosis. Palmitate-mediated ER stress results in the induction of the transcription factors CHOP and phosphorylated-c-Jun containing AP-1 complex. CHOP can bind phosphorylated-c-Jun to form an heteromeric complex, which positively regulates PUMA expression, with subsequent Bax activation, mitochondrial dysfunction, caspase activation, and apoptosis.
The ability of CHOP to upregulate target genes is dependent on its heterodimerization with various partners (23). Although C/EPBα is a preferred heterodimerizing partner of CHOP, we failed to identify any functional CHOP-C/EPBα heterodimer binding site within the human PUMA promoter region. Instead we found that, in response to palmitate treatment, CHOP associates with the activated AP-1 complex protein c-Jun to form a heteromeric complex that binds to the AP-1 binding site regulating PUMA transcription during lipoapoptosis (Fig. 6). CHOP, in association with AP-1 members such as c-Jun and fos, has been reported to positively upregulate various AP-1 target genes (35), and this protein-protein interaction requires the leucine zipper domain of CHOP (35). In this context, CHOP can enhance the transcriptional activation of AP-1 by tethering to the AP-1 complex without direct binding of DNA (35), and by this mechanism the transcriptional capacity of CHOP could be extended to a novel set of genes. In our studies we observed that inhibition of either JNK-mediated AP-1 activation or CHOP induction decreased palmitate-induced PUMA expression and that both AP-1 and CHOP were necessary for maximal PUMA transcription by palmitate, possibly by a mechanism involving enhancement of AP-1 transcriptional properties by CHOP.
In summary, our study extends and further integrates present knowledge regarding the mechanisms linking ER stress and PUMA induction during saturated FFA-mediated lipoapoptosis in liver cells. The results suggest that PUMA expression is regulated by both CHOP and AP-1 downstream of palmitate-induced ER stress. In this model, the formation of a CHOP:phosphorylated c-Jun heteromeric complex that binds to the AP-1 binding site in the PUMA promoter is responsible for PUMA transcription during lipoapoptosis. Given the major role of PUMA in mediating lipoapoptosis, insights into the network regulating PUMA expression and/or function should help promote effective therapeutic strategies to minimize liver damage in human fatty liver diseases. For example, microRNA inhibition of CHOP induction could prove useful in these disease processes (18).
GRANTS
This work was supported by NIH Grants DK41876 to G. Gores, DK079875 to J. Mott, and the Mayo Foundation. Support was also provided by the optical microscopy core of the Mayo Center for Cell Signaling in Gastroenterology, NIH Grant DK84567.
DISCLOSURES
The authors have nothing to disclose.
ACKNOWLEDGMENTS
We thank Erin Nystuen-Bungum for excellent secretarial assistance.
REFERENCES
- 1.Adams LA, Lymp JF, Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129: 113–121, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol 52: 586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 17, Suppl: S186–S190, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem 282: 27141–27154, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem 284: 26591–26602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Fan Z, Wang X, Ma C, Bower KA, Shi X, Ke ZJ, Luo J. Brain-derived neurotrophic factor suppresses tunicamycin-induced upregulation of CHOP in neurons. J Neurosci Res 85: 1674–1684, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci 121: 2308–2318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125: 437–443, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S, Juin P. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol 185: 279–290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem 272: 13829–13834, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res 29: 1496–1503, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahraman A, Schlattjan M, Kocabayoglu P, Yildiz-Meziletoglu S, Schlensak M, Fingas CD, Wedemeyer I, Marquitan G, Gieseler RK, Baba HA, Gerken G, Canbay A. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology 51: 92–102, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36: 487–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis 14: 1484–1495, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem 281: 7260–7270, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Sall A, Yang D. MicroRNA: an Emerging Therapeutic Target and Intervention Tool. Int J Mol Sci 9: 978–999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut 56: 1124–1131, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281: 12093–12101, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Masuoka HC, Mott J, Bronk SF, Werneburg NW, Akazawa Y, Kaufmann SH, Gores GJ. Mcl-1 degradation during hepatocyte lipoapoptosis. J Biol Chem 284: 30039–30048, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109: 525–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98: 10845–10850, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab 298: E1027–E1035, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pino SC, O'Sullivan-Murphy B, Lidstone EA, Yang C, Lipson KL, Jurczyk A, diIorio P, Brehm MA, Mordes JP, Greiner DL, Rossini AA, Bortell R. CHOP mediates endoplasmic reticulum stress-induced apoptosis in Gimap5-deficient T cells. PLoS one 4: e5468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46: 1081–1090, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134: 568–576, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ratziu V, Poynard T. Assessing the outcome of nonalcoholic steatohepatitis? It's time to get serious. Hepatology 44: 802–805, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol 162: 587–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res 36: 229–240, 1995 [PubMed] [Google Scholar]
- 33.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA 87: 2466–2470, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ubeda M, Vallejo M, Habener JF. CHOP enhancement of gene transcription by interactions with Jun/Fos AP-1 complex proteins. Mol Cell Biol 19: 7589–7599, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta 1585: 202–212, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147: 943–951, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Wei Y, Wang D, Gentile CL, Pagliassotti MJ. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem 331: 31–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 291: E275–E281, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279: 45495–45502, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Xing D, Liu L. PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-X L during UV-induced apoptosis. Mol Biol Cell 20: 3077–3087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]