Abstract
Adrenergic stimulation of isolated guinea pig distal colonic mucosa produced transient Cl− and sustained K+ secretion. Transient short-circuit current (Isc) depended on β2-adrenergic receptors (β2-AdrR), and sustained Isc relies on a β1-AdrR/β2-AdrR complex. Epinephrine (epi) increased cAMP content with a biphasic time course similar to changes in epi-activated Isc (epiIsc). Inhibition of transmembrane adenylyl cyclases (tmACs) reduced peak epiIsc and cAMP to near zero without decreasing sustained epiIsc, consistent with cAMP from tmAC signaling for only Cl− secretion. Inhibition of soluble adenylyl cyclase (sAC) reduced sustained epiIsc and cAMP to near zero without decreasing peak epiIsc or cAMP, consistent with cAMP from sAC signaling for K+ secretion. Sensitivity to phosphodiesterase (PDE) inhibitors and peptide YY (PYY) stimulation further supported separate signaling for the two components. PDE3 or PDE4 inhibitors enhanced peak epiIsc but not sustained epiIsc, consistent with these PDEs as part of the β2-AdrR signaling domain. PYY suppressed peak epiIsc in a pertussis toxin (PTx)-sensitive manner, supporting Gαi-dependent inhibition of tmACs producing cAMP for Cl− secretion. Since PYY or PTx did not alter sustained epiIsc, signaling for K+ secretion occurred via a Gαi-independent mechanism. Presence of multiple sAC variants in colonic epithelial cells was supported by domain-specific antibodies. Responses to specific activators and inhibitors suggested that protein kinase A was not involved in activating peak or sustained components of epiIsc, but the cAMP-dependent guanine nucleotide exchange factor, Epac, may contribute. Thus β-adrenergic activation of electrogenic Cl− and K+ secretion, respectively, required tmAC- and sAC-dependent signaling pathways.
Keywords: epinephrine, norepinephrine, soluble adenylyl cyclase, protein kinase A, Epac
sympathetic stimulation suppresses digestion via norepinephrine (norepi) released from nerves and blood-borne epinephrine (epi). This slowing of digestion occurs by a combination of reduced intestinal motility and ion secretory activity as well as vasoconstriction that limits blood flow to the gut. Together, this inhibition of Cl− secretion that stops fluid flow into the lumen, coupled with reduced blood flow and motility, helps conserve whole body water resources (15, 24). Also, direct sympathetic innervation of the mucosa in the distal colon stimulates a modulatory mode of secretion consisting of sustained electrogenic K+ secretion (73, 74). The generally lower rates of this secretory mode contrast dramatically with the flushing and synergistic modes stimulated by enteric ganglia that lead to rapid fluid accumulation in the lumen. Thus a sympathetic event overrides any enteric Cl− secretory tone and replaces it with electrogenic K+ secretion.
In the distal colon, the sympathetic conversion from electrogenic Cl− secretion to electrogenic K+ secretion involves several features of enteric signaling. The first feature that limits Cl− secretion during a sympathetic event is the long-appreciated inhibition of enteric secretomotor nerves (15, 24). The second feature is the transient nature of Cl− secretory activation via β-adrenergic receptors (β-AdrR). This secretory response peaks at ∼1 min and largely subsides within 5 min (73, 74). Thirdly, adrenergic nerves release neuropeptide Y (NPY) together with norepi, which in guinea pig distal colon directly inhibits Cl− secretion via Y2-neuropeptide receptors (Y2-NpR) (15, 74). And fourth, β-adrenergic activation of enteroendocrine L-cells releases peptide YY (PYY), which also inhibits Cl− secretion via Y2-NpR (52, 74). As is apparent from the combined action of these agonists, the sympathetic response at the distal colonic mucosa is not simply to end secretory activity, but instead it produces almost solely electrogenic K+ secretion.
Clinical manifestations of colonic K+ secretion are apparent in patients with cystic fibrosis and those with acute colonic pseudo-obstruction (aCPO). The lack of CFTR Cl− channels in the apical membrane for cystic fibrosis patients makes normally Cl− secretory stimuli produce primarily electrogenic K+ secretion in the rectal colon (27, 49). The absence of Cl− secretion from these responses illustrates the underlying capacity to produce modulatory secretion. In those aCPO patients having high fecal K+ concentrations (9, 13, 41, 67), hypokalemia likely results from inappropriately prolonged modulatory secretion. The accompanying colonic distension caused by the dominance of sympathetic over parasympathetic influences on gut motility provides a large reservoir for K+ accumulation from the continual K+ secretion, likely activated via a β1-AdrR/β2-AdrR complex (74). Inappropriate switching between the Cl− secretion of the flushing mode and the K+ secretion of the modulatory mode may underlie many types of secretory dysfunction.
The cellular mechanism producing electrogenic K+ secretion closely resembles the standard model of Cl− secretion with the addition of apical membrane K+ channels and basolateral membrane Cl− channels (30, 31, 46, 57). This similarity makes electrogenic K+ secretion and Cl− secretion compatible enough in transport mechanism to occur in the same epithelial cell type. As with any cellular process, a key requirement for physiologically appropriate responses is to have intracellular signaling pathways that can coordinate the activities of all the required elements. β-Adrenergic activation of secretion during a sympathetic event likely occurs as a sequence of activations. Initially, opening of apical membrane Cl− channels and K+ channels allows secretion to begin. Cl− secretory rate exceeds K+ secretion as seen by the upsweep of the transient component. Maintenance of secretion requires entry of Cl− and K+ through the basolateral membrane to match the apical exit, which also supports the electrochemical driving forces needed for apical flow to be out of the cell. Basolateral membrane localized Na+-K+-2Cl− cotransporters serve to bring Cl− and K+ into the cell, thereby increasing intracellular concentration; Na+/K+ pumps also bring K+ into the cell and extrude the Na+ entering via Na+-K+-2Cl− cotransporters. Basolateral K+ channels contribute to cellular K+ balance and creation of a membrane electrical potential difference that drives Cl− exit via apical channels. The unique feature of β-adrenergic activation is the transition from a transient phase of flushing secretion to a sustained phase when only electrogenic K+ secretion is apparent (57, 74). As apical Cl− channels close and produce the fall of the transient component, basolateral membrane Cl− channels would open. Cellular balance is maintained as earlier with the distinction that conductive Cl− exit occurs only at the basolateral membrane. Left largely unexplained are the signaling events that underlie this secretory activation by β-AdrRs.
β-Adrenergic receptors were among the first G protein-coupled receptors (GPCR) to be linked to cAMP production as the means to actuate cellular responses (12). A hallmark of electrogenic Cl− secretion is the involvement of cAMP in the activation of apical membrane Cl− conductance (5). Upon binding of epi (or norepi), β-AdrRs activate Gαs by the exchange of GDP for GTP, which then enhances adenylyl cyclase production of cAMP (43, 63). Phosphodiesterases (PDEs) cleave cAMP into AMP, thereby limiting cAMP buildup and aiding in response termination after cAMP production ends (6). Both β1-AdrR and β2-AdrR are required to produce the sustained K+ secretion of the modulatory response (73, 74). Since the adenylyl cyclase activator forskolin also stimulates a sustained K+ secretion (46), the β1-AdrR/β2-AdrR response likely occurs via cAMP production. Of the 10 mammalian class III adenylyl cyclases, nine have transmembrane domains (tmAC1–9) and one is soluble in the cytoplasm (sAC) (43, 63). Several of the tmACs have been found in colonic epithelial cells (23), but those responsible for specific GPCR signaling are not known. Interestingly, Y2-NpR activation by PYY or NPY inhibits the transient Cl− secretory component of β-adrenergic activation but not the sustained K+ secretory component (74). Since a dominant mode of signaling by Y2-NpR occurs via Gαi inhibition of tmACs (50), distinct ACs must contribute to activation of each secretory component. Similarly, the PDEs associated with each secretory component of β-adrenergic activation may be distinct. Although cAMP appears as a likely signaling molecule, the means by which cAMP promotes modulatory secretion is comparatively undefined.
The present study examined the involvement of cAMP in the β-adrenergic activation of electrogenic Cl− and K+ secretion. As in previous studies from this laboratory (74), secretory responses were studied after suppressing endogenous agonists in the ex vivo mucosa. These procedures allowed a clearer distinction of the features controlling each component of the response. The experiments presented here examined the type of β-AdrR required for activation of transient Cl− secretion, the adenylyl cyclase involvement in each secretory component, the changes in cAMP content during β-adrenergic activation, as well as functional evidence for the cAMP-dependent actuators of Cl− and K+ secretion.
METHODS
Male guinea pigs (500–800 g body wt, Hartley strain, Hilltop Lab Animals, Scottdale, PA) received standard chow and water ad libitum and were housed on site at least 2 wk before experiments. Guinea pigs were euthanized with an animal decapitator (Harvard Apparatus, Holliston, MA) in accordance with a protocol approved by the Wright State University Laboratory Animal Care and Use Committee. Colonic mucosa was isolated as described previously (73). These isolated colonic mucosal sheets were used for protein detection by immunoblot, biochemical assays, and measurement of transepithelial electrical parameters.
Detection of proteins.
Proteins were isolated from colonic mucosa as described previously (47, 73). Briefly, after disruption by sonication in a buffered solution containing protease inhibitors, samples were centrifuged to obtain a membrane sample. Following SDS-PAGE and transfer to polyvinylidene difluoride membranes, incubation with specific primary antibody and then with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) allowed detection of specific proteins. Antibodies for sAC were generously provided by L. R. Levin and J. Buck at the Weill Medical College of Cornell University, New York, NY (monoclonal mouse-anti-sAC, R21, residues 203–216 in exon 5 of human adcy10; 1:1,000) and by P. Huang at the Hong Kong University of Science and Technology, Kowloon, Hong Kong [polyclonal rabbit-anti-sAC, sAC(N-term), residues 1–17 of human adcy10; 1:250].
Assay for cAMP and activated Rap1.
Isolated mucosal sheets were used for assays of cAMP content and Rap1 activation. These sheets were glued apical surface up to stainless steel rings (1.2 mm thick, 2.4-cm OD, 0.64-cm2 opening) with cyanoacrylate glue. The annuli holding the mucosae were positioned vertically by using dental wax within beakers in a temperature-controlled water bath (38°C), and the bathing solution was gassed continuously to provide aeration and mixing. A basal condition was produced, as done for transepithelial electrical measurements. Mucosae were treated with secretagogues and inhibitors, punched from the rings on an ice-cold glass plate, placed in a microcentrifuge tube, and then quick frozen in a dry-ice/isopropanol bath (−78°C). Three mucosae were assigned to each experimental condition and chosen to minimize any variation along the distal colon segment used (∼40 cm). Samples were stored at −80°C until use with cAMP EIA kit (Cayman Chemical, Ann Arbor, MI) or Active Rap1 Pull-Down and Detection kit (Pierce/ThermoFisher Scientific, Waltham, MA). Assays were conducted according to manufacturer's instructions on a pooled sample from the three mucosae for each experimental condition. Acetylation was used in the cAMP assay to increase sensitivity. All cAMP contents were converted to apparent intracellular concentration by using the mucosal surface area (1.5 cm2), estimates for cell height (20 μm), and the ratio of cell number in crypt and surface zones (1:1). Immunoblot membranes were probed for Rap1, stripped, and reprobed with Rap2 antibody from Santa Cruz Biotechnology, Santa Cruz, CA (polyclonal goat-anti-Rap2, V-19, COOH-terminus of human rap2; 0.4 ng/μl), since RalGDS-RBD can be used as an activation probe for Rap2 as well as Rap1 (20).
Transepithelial current measurement.
Isolated mucosal sheets were used for measurement of transepithelial current and conductance as described previously (47). Mucosal sheets were mounted in Ussing chambers (0.64 cm2 aperture), supported on the serosal face by Nuclepore filters (∼10 μm thick, 5-μm pore diameter; Whatman, Clifton, NJ). Bathing solutions (10 ml) were circulated by gas lift through water-jacketed reservoirs (38°C). Standard Ringer's solution contained (in mM) 145 Na+, 5.0 K+, 2.0 Ca2+, 1.2 Mg2+, 125 Cl−, 25 HCO3−, 4.0 H(3−X)PO4X−, 10 d-glucose. Solutions were continually gassed with 95% O2 and 5% CO2, which maintained solution pH at 7.4. Low Cl− solutions were made by substituting NaCl and KCl with gluconate salts and increasing Ca2+ to 8 mM to compensate for buffering by gluconate−. Automatic voltage clamps (Physiologic Instruments, San Diego, CA) permitted measurement of short-circuit current (Isc) and calculation of transepithelial conductance (Gt) from current responses to voltage pulses imposed across the mucosa (± 5 mV, 3-s duration, 60-s intervals). Isc was referred to as positive for cation flow across the epithelium from mucosal to serosal side.
Mucosal responses to physiological secretagogues and to inhibitors were examined after producing a quiescent basal condition. This basal state was produced by suppressing the neural and paracrine activators persisting in the isolated colonic mucosa (74). The mucosal preparation removes influences from nerves in the underlying muscle layers. Compounds released into the bathing solutions were reduced in concentration (∼8,000-fold) by replacing the solutions three times after mounting the mucosa. Prostanoid production within the isolated mucosa was suppressed by the cyclooxygenase-1 inhibitor SC560 (1 μM) and the cyclooxygenase-2 inhibitor CAY10404 (1 μM) added to both bathing solutions. The action of PYY and NPY released from mucosal cells was inhibited by adding the Y2-NpR antagonist BIIE0246 (1 μM) to the serosal bath. Amiloride (10 μM) was added to the mucosal bath to inhibit electrogenic Na+ absorption. Sequential addition of secretagogues (epi, prostaglandin-E2, carbachol) was used to examine the range of secretory response from modulatory to flushing to synergistic. Prior addition of epi does not alter subsequent PGE2 responses (32, 33, 47), and combined addition of PGE2 and carbachol (CCh) produces the synergistic mode of secretion (74).
Bicarbonate (bicarb)-free solutions were made by replacing HCO3− with either HEPES or Bis-Tris propane (BTP). HEPES Ringer's solution contained (in mM) 142 Na+, 5.0 K+, 2.0 Ca2+, 1.2 Mg2+, 143 Cl−, 4.0 H(3−X)PO4X−, 10 HEPES, 10 d-glucose. Bis-Tris propane Ringer's solution contained (in mM) 141 Na+, 5.0 K+, 2.0 Ca2+, 1.2 Mg2+, 151 Cl−, 4.0 H(3−X)PO4X−, 5 Bis-Tris propane, 10 d-glucose. The solutions were titrated to a pH of 7.4 with NaOH or HCl and continually gassed with atmosphere or 100% O2. The influence of bicarb was examined by replacing the bathing solutions with a bicarb-free Ringer, after an initial equilibration of the mucosae in standard Ringer bubbled with 95% O2-5% CO2 for 30 min. Within 5 min of the transition to bicarb-free Ringer, Gt began increasing and reached a peak approximately threefold higher than control over ∼30 min. This conductance crisis resolved over the next ∼60 min with a return to near control levels. The amount of the Gt change and the duration generally was less when the bicarb-free solution was bubbled with 100% O2 rather than atmosphere, but subsequent secretory responses were not discernibly different. Results with HEPES Ringer (n = 4) compared with BTP Ringer (n = 5) were not noticeably different and were combined.
The inhibitor of soluble adenylyl cyclase, KH7, was generously provided by L. R. Levin and J. Buck (Weill Medical College of Cornell University). SC560, CAY10404, prostaglandin-E2, and H89 were obtained from Cayman Chemical (Ann Arbor, MI); BIIE0246, CGP20712A, ICI-118551, myristoylated PKI 14–22 amide, KT5720 from Tocris Bioscience (Ellisville, MO); PYY from Bachem Americas (Torrance, CA); epi from Hospira (Lake Forest IL); pertussis toxin from List Biological Laboratories (Campbell, CA); 8-Br-adenosine-3′,5′-cyclic-monophosphate (8Br-cAMP), 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8CPT2M-cAMP), 8-(4-methoxyphenylthio)-2′-O-methyl-cAMP (8MPT2M-cAMP), N6-benzoyl-cAMP (6Bnz-cAMP), 8-piperidino-cAMP (8Pip-cAMP), 8-Br-adenosine-3′,5′-cyclic-monophosphorothioate-Rp-isomer (Rp-8Br-cAMPS) from BioLog Life Science Institute (Bremen, Germany). All other chemicals were obtained from Sigma Chemical (St. Louis, MO). Drugs were added in small volumes from concentrated stock solutions.
Data analysis.
Responses of Isc and Gt to secretagogues and antagonists were obtained from adjacent mucosae in each colon to permit direct comparisons. Recordings of Isc were digitized at 10-s intervals to examine the secretory time course. Concentration responses of Isc and Gt were fit by Henri-Michaelis-Menten binding curves via a nonlinear least-squares procedure. Results were reported as means and SE with the number of animals (n) indicated. Statistical comparisons were made by using a two-tailed Student's t-test for paired responses, with significant difference accepted at P < 0.05.
RESULTS
β-Adrenergic activation of secretion.
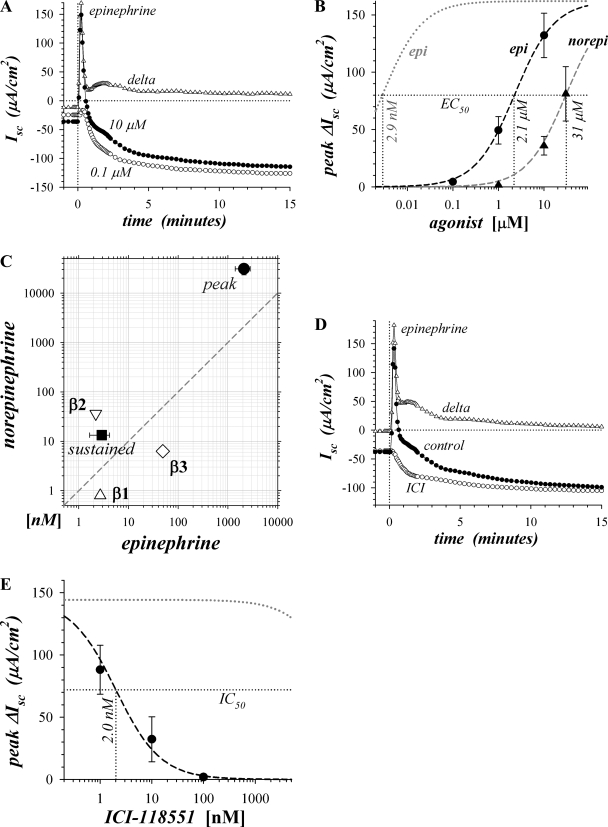
Epi activation of ion secretion includes a transient and a sustained component associated with Cl− secretion and K+ secretion, respectively (57, 73, 74). Epi activation at 10 μM produced a rapid rise in Isc to a peak in 15–30 s, with a small second peak apparent at 60–90 s, followed by the sustained negative Isc (Fig. 1A). Activation of the transient component was minimal at 0.1 μM epi even though sustained secretion was near maximal. The concentration dependence for peak ΔIsc activation (ΔIsc, difference between stimulated and basal Isc) indicated a ∼700-fold lower sensitivity to epi (Fig. 1B) compared with the previously determined sensitivity of the sustained component (74). Preference for epi over norepi in activating peak ΔIsc was consistent with β2-AdrR involvement, but with an overall lower sensitivity (Fig. 1C). β-Adrenergic activation of the sustained component requires both β1- and β2-adrenergic receptor types as indicated by synergistic antagonism with CGP20712A (β1-AdrR) and ICI-118551 (β2-AdrR) (73, 74). In contrast, ICI-118551 alone reduced peak epi-activated ΔIsc (epiΔIsc) to near zero (Fig. 1D), but CGP20712A (1.0 μM) did not reduce this peak epiΔIsc associated with Cl− secretion (data not shown). The low Kd for ICI-118551 antagonism (Fig. 1E) was consistent with a response acting solely via β2-AdrR (3).
Fig. 1.
Activation of transient secretion was sensitive to β2-adrenergic antagonism. Isolated mucosae were stimulated by epinephrine (epi) or norepinephrine (norepi) addition to the serosal bath from the standard basal condition with electrogenic secretion monitored by short-circuit current (Isc) (see methods). A: Isc was measured in adjacent mucosae during activation by epi at low and high concentration, 0.1 μM (○) and 10 μM (●), respectively. The difference between high and low concentration activation illustrated the transient component (delta, △). B: responses at 3 concentrations of either epi (●) or norepi (▲) were measured in adjacent mucosae. The resulting concentration dependences of peak ΔIsc (ΔIsc = difference between stimulated and basal Isc) were fit by Henri-Michaelis-Menton kinetics with a single binding site (dashed line; maxΔIsc, maximal ΔIsc; epiEC50 and norepiEC50, half-maximal effective concentrations for epi and norepi, respectively): epiEC50 = 2.1 ± 0.6 μM, maxΔIsc = +162 ± 15 μA/cm2, n = 4; norepiEC50 = 30.9 ± 7.5 μM, maxΔIsc = +186 ± 20 μA/cm2, n = 3. For comparison, a fit is shown (dotted gray line) using maxΔIsc together with the epiEC50 obtained previously for sustained secretion (74). C: EC50 values of the peak ΔIsc responses to epi and norepi (●) were compared with those for the sustained Isc response, all in the presence of BIIE0246 (■; Ref. 74) and the 3 β-adrenergic receptors (△, ▽, ◊; Ref. 61). D: Isc was measured during activation by epi (3 μM) for a control mucosa (●) and an adjacent mucosa treated with the β2 adrenergic receptor (β2-AdrR)-selective antagonist ICI-118551 (0.1 μM) added 20 min prior to stimulation (○). The difference between control activation and activation with ICI-118551 illustrated the transient component (delta, △). E: 3 adjacent mucosae were treated with ICI-118551 20 min prior to stimulation with epi (3 μM); a fourth adjacent mucosa served as an epi activation control. A fit to the concentration dependence of epi-activated ΔIsc (epiΔIsc; n = 4, dashed line) together with the epi activation control without antagonist (+144 ± 25 μA/cm2) provided an IC50 for ICI-118551 of 2.0 ± 0.2 nM. The calculated Kd was 4.7 ± 1.4 nM, assuming competitive antagonism. For comparison a fit is shown (dotted gray line) using the same Kd as obtained previously for sustained secretion, 131 ± 23 nM (74).
Dependence of ion secretion on soluble adenylyl cyclase.
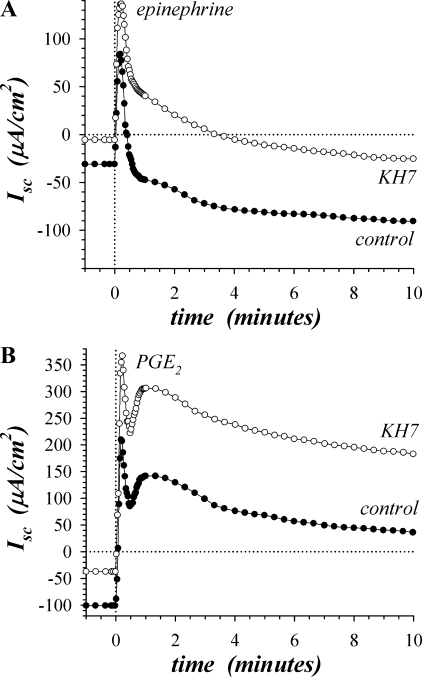
Involvement of cAMP produced by β-adrenergic activation of sAC was examined by using KH7, an inhibitor selective for sAC in preference over tmAC (34). Addition of KH7 (30 μM) during the basal condition significantly reduced Isc toward zero by 25.5 ± 2.8 μA/cm2 (n = 4, P < 0.05), consistent with inhibiting basal K+ secretion. Epi activation produced the early peak Isc, with KH7 increasing the peak by 63.4 ± 10.4 μA/cm2 (n = 4, P < 0.05) compared with the control peak (Fig. 2A, Table 1). KH7 also made the sustained plateau of epiIsc (at 20–30 min) more positive by 68.0 ± 5.5 μA/cm2 (Fig. 2A, Table 1) and decreased Gt by 0.71 ± 0.21 mS/cm2 (n = 4, P < 0.05), consistent with inhibiting K+ secretion. Low Cl− bathing solutions attenuated the transient epiIsc in control and KH7-treated mucosae (data not shown), consistent with Cl− secretion as the source of the epiIsc. Also, low Cl− bathing solutions eliminated the sustained Isc as well as the inhibitory action of KH7 (data not shown), consistent with the Cl− dependence of K+ secretion (31). Given the uniform positive shift of the Isc during epi activation, the primary action of KH7 was to inhibit K+ secretion 68 ± 6%, leaving the transient Cl− secretory component unaltered.
Fig. 2.
Inhibition of soluble adenylyl cyclase decreased sustained secretion. Isolated mucosae were stimulated sequentially by epi (5 μM) and PGE2 (3 μM), from the standard basal condition. A: Isc was measured in adjacent mucosae during activation by epi with 1 mucosa pretreated for 30 min with KH7 (○, 30 μM). B: Isc was measured in adjacent mucosae (as in A), ∼30 min after epi stimulation, during activation by PGE2 with 1 mucosa pretreated with KH7 (○, 30 μM). The responses were representative of 4 similar experiments (see Table 1).
Table 1.
Dependence of secretagog responses on sAC: inhibition by KH7
| 1st Peak | 2nd Peak | Sustained | |
|---|---|---|---|
| Epi | +19.2 ± 27.1 | −107.7 ± 6.2 | |
| Epi + KH7 | +82.6 ± 34.4* | −39.7 ± 9.0* | |
| PGE2 | +220.3 ± 7.0 | +169.3 ± 13.1 | +17.7 ± 28.7 |
| PGE2 + KH7 | +363.9 ± 13.2* | +307.0 ± 27.2* | +115.2 ± 15.9* |
Short-circuit current (Isc) values (μA/cm2) are means ± SE, n = 4, stimulated as in Fig 2. sAC, soluble adenylyl cyclase; epi, epinephrine.
Responses with KH7 (30 μM) significantly different from control (P < 0.05).
Addition of PGE2 (3 μM) to epi-activated mucosae stimulated a positive change in Isc consistent with Cl− secretion (Fig. 2B, Table 1). The time course of PGE2 (3 μM) activation included an early transient at 15–30 s followed by a second peak at 60–90 s and then a decline to a sustained plateau, a timing similar to β2-AdrR-dependent activation (Fig. 1D). KH7 (30 μM) significantly increased the Isc at these three points in the PGE2 response compared with control, with the first peak 143.6 ± 15.2 μA/cm2 higher, the second peak 137.8 ± 22.1 μA/cm2 higher, and the plateau (20–30 min) 97.5 ± 19.9 μA/cm2 higher (n = 4, P < 0.05). The difference in Gt between the KH7 and control conditions during plateau PGE2 activation was not significant (+0.57 ± 0.55 mS/cm2, n = 4, P < 0.05). Similar to epi activation, the primary action of KH7 during PGE2 activation likely was to inhibit ∼100 μA/cm2 of K+ secretion. Cholinergic activation by CCh (10 μM) in the presence of epi and PGE2 exhibited a large positive Isc, consistent with Cl− secretion composed of two early peaks followed by a plateau. KH7 treatment did not alter Isc significantly at any of these times (data not shown, n = 4, P < 0.05).
Dependence of ion secretion on transmembrane adenylyl cyclase.
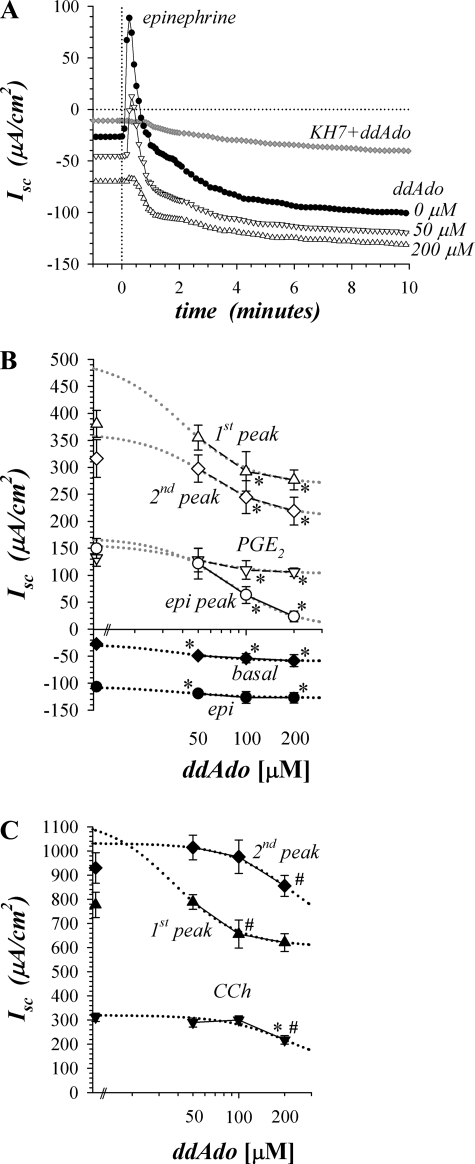
Involvement of cAMP produced by β-adrenergic activation of tmAC was examined by using 2′,5′-dideoxyadenosine (ddAdo), an inhibitor selective for tmAC in preference to sAC (39, 43). Addition of ddAdo (200 μM) during the basal condition significantly decreased Isc by −23.2 ± 5.5 μA/cm2 and increased Gt by 0.44 ± 0.14 mS/cm2 (n = 4, P < 0.05), consistent with stimulating basal K+ secretion (Fig. 3, A and B). ddAdo inhibited the epi-activated peak Isc in a concentration-dependent manner as expected for an action on Cl− secretion (Fig. 3B, Table 2). The sustained epi response had a larger negative Isc in the presence of ddAdo together with a decrease in Gt by 1.19 ± 0.15 mS/cm2 (n = 4, P < 0.05), consistent with suppressing the tail of this Cl− secretory transient. Combining ddAdo (200 μM) with KH7 (30 μM) inhibited both the transient and sustained components (Fig. 3A), supporting the concept that cAMP from these two sources sufficed to complete β-adrenergic activation of secretion.
Fig. 3.
Inhibition of transmembrane adenylyl cyclase decreased transient secretion. Isolated mucosae were stimulated sequentially by epi (5 μM), PGE2 (3 μM), and carbachol (CCh; 10 μM), from the standard basal condition as in Fig. 2. A: Isc was measured in adjacent mucosae during activation by epi after pretreatment for 20 min with 2′,5′-dideoxyadenosine (ddAdo: ●, 0 μM; △, 50 μM; ▽, 200 μM). A mucosa from a different animal was pretreated for 30 min with both KH7 (30 μM) and ddAdo (200 μM) prior to epi stimulation (♦). B: concentration dependence of ddAdo action (n = 4) is shown during activation by epi (○, peak; ●, sustained) and PGE2 (△, first peak; ◊, second peak; ▽, plateau), as well as basal Isc (♦). The peaks shown were calculated as the ΔIsc from the previous condition, either basal or epi-stimulated, the plateau PGE2 response (▽) also is shown as ΔIsc for comparison with the peak epi response (○). Those responses significantly different from control are indicated by an asterisk (*P < 0.05). The points near the y-axis are the responses in the absence of ddAdo. A fit of Henri-Michaelis-Menton kinetics with 2 cooperative binding sites (dotted lines) was made to the concentration dependences (see Table 2). C: concentration dependence of ddAdo action (n = 4) is shown during activation by CCh (▲, first peak; ♦, second peak; ▼, plateau). Significantly different from control (*P < 0.05) and from the next lower concentration (#P < 0.05).
Table 2.
Dependence of secretagog responses on tmAC: fit parameters for concentration dependence of inhibition by ddAdo
| IC50 | Isens | Iresis | |
|---|---|---|---|
| Basal | 36 ± 6 | +32 ± 8* | −59 ± 11* |
| Epi sustained | 38 ± 4 | +21 ± 5* | −127 ± 11* |
| ΔEpi peak | 77 ± 7 | +166 ± 34* | +3 ± 8 |
| ΔPGE2 1st peak | 39 ± 8 | +233 ± 59* | +268 ± 25* |
| ΔPGE2 2nd peak | 58 ± 7 | +155 ± 40* | +208 ± 19* |
| ΔPGE2 sustained | 46 ± 8 | +54 ± 11* | +103 ± 11* |
| CCh 1st peak | 37 ± 6 | +527 ± 146* | +604 ± 52* |
| CCh 2nd peak | 227 ± 42 | +405 ± 87* | +627 ± 35* |
| CCh sustained | 220 ± 16 | +225 ± 22* | +95 ± 12* |
The fit parameters (IC50, μM; Isc, μA/cm2) are shown for concentration dependence of ddAdo (means ± SE, n = 4), from experiments in Fig 3. The Isc components of the response sensitive to (Isens) and resistant to (Iresis) inhibition by 2′,5′-dideoxyadenosine (ddAdo) are shown, Itotal = Isens + Iresis.
Significantly different from zero, P < 0.05.
ddAdo reduced PGE2-activated Isc during the peaks and at the plateau level (Fig. 3B). ddAdo (200 μM) also significantly reduced the PGE2-activated plateau Gt by 0.94 ± 0.30 mS/cm2 (n = 4, P < 0.05) as expected for suppression of the secretory response. The concentration dependence for ddAdo was fit best with a two-site cooperative model that suggested an interaction between catalytic domains of two tmAC proteins. The EC50 values clustered between 36 and 77 μM (Table 2), consistent with a common mechanism for ddAdo action during secretagogue activation. Although ddAdo inhibited 98 ± 6% of the transient β-adrenergic response, for the PGE2 response only 46 ± 5% of the first peak, 43 ± 9% of the second peak, and 34 ± 8% of the plateau were inhibited (Fig. 3B, Table 2), suggesting the presence of signaling agents in addition to cAMP during PGE2 activation. ddAdo inhibited cholinergic activation primarily during the first transient (47 ± 4%) that peaked at 10–15 s (Fig. 3C, Table 2). The second peak and plateau of the CCh response were sensitive only at the highest ddAdo concentration suggesting the possibility of a different mechanism for this inhibition.
PYY inhibition of transient β-adrenergic response.
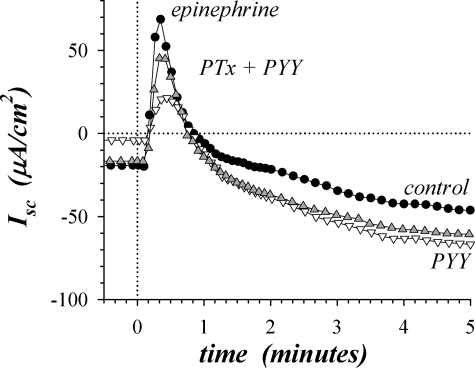
PYY acts through Y2-NpR to inhibit Cl− secretion in the distal colon (17, 74). Since the predominant signaling mode for this receptor occurs via activation of Gαi (50), the resulting inhibition of tmAC would be responsible for inhibiting the Cl− secretory component of β-adrenergic activation. Pretreatment with pertussis toxin (PTx) should inactivate Gαi and block the action of PYY, which was observed as a larger peak epiIsc despite the presence of PYY (Fig. 4). Consistent with the reversal of PYY action on transient epiΔIsc, PTx significantly increased sustained epiIsc in the presence of PYY by +20.4 ± 4.8 μA/cm2 (n = 4, P < 0.05), likely representing a return of the tail of the transient Cl− secretory component. PTx also reversed the PYY inhibition of basal Isc. These results support the presence of a signaling complex for the transient secretory component that included β2-AdrR, Gαs, Y2-NpR, Gαi, and tmAC.
Fig. 4.
Pertussis toxin (PTx) reversed peptide YY (PYY) inhibition of β2-adrenergic response. Isolated mucosae were stimulated by epi (5 μM) from the standard basal condition, and Isc was measured in adjacent mucosae during activation with 1 mucosa in the standard basal condition serving as an activation control (●) and the others pretreated with PYY (▽, 0.3 μM) or PYY and PTx (▲). PTx (1 μg/ml) was added 150 min prior to epi activation and PYY 20 min prior to activation. The responses were representative of 4 similar experiments, with PTx in the presence of PYY significantly increasing peak epiΔIsc by 4.6 ± 1.4-fold compared with PYY treatment alone (P < 0.05).
A portion of the secretory response stimulated by PGE2 was dependent on tmAC (Fig. 3B), suggesting that PYY suppression of this Cl− secretion might act through inhibition of tmAC. In contrast to the epi response, PTx failed to reverse the PYY inhibition of the PGE2 secretory response (data not shown, n = 4, P < 0.05). This result supported the presence of signaling mechanisms for Y2-NpR in addition to activation of Gαi (50). Similarly, PTx failed to reverse the PYY sensitivity of the first peak and plateau responses to CCh (data not shown, n = 4, P < 0.05). However, PTx decreased the second peak of the CCh response by 97.5 ± 24.7 μA/cm2 even though the second peak was insensitive to PYY (n = 4, P < 0.05), suggesting that cAMP had a modest suppressing influence or that Gβγ released together with Gαi contributed to Cl− secretory stimulation.
Influence of PDEs on β-adrenergic activation.
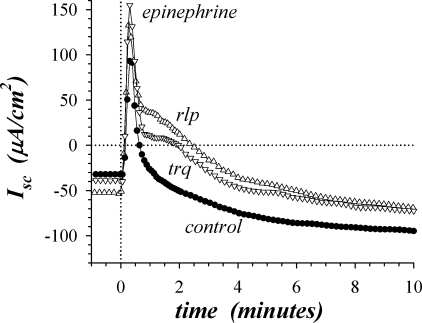
PDEs contribute to regulation of cAMP concentration by mediating an inactivating cleavage to AMP (6). The action of PDEs was tested by using the general inhibiter IBMX and two type-specific inhibitors, trequinsin (PDE3) and rolipram (PDE4). IBMX (100 μM) and rolipram (30 μM) added during the basal condition both led to significantly more negative Isc and increased Gt (−14.1 ± 3.3 μA/cm2, 2.31 ± 0.84 mS/cm2, n = 6, P < 0.05), suggesting a minor stimulation of K+ secretion. Rolipram significantly enhanced the initial epi peak (Fig. 5) by 63.3 ± 1.3 μA/cm2 (n = 3, P < 0.05), whereas neither IBMX nor trequinsin enhanced this peak response significantly. These PDE inhibitors enhanced the smaller second peak in epiIsc by 52.4 ± 9.8 μA/cm2 (n = 9, P < 0.05). Also, elevation of the sustained plateau at 20–30 min to a more positive value by these PDE inhibitors (28.7 ± 3.5 μA/cm2, n = 9, P < 0.05) suggested a prolongation of the Cl− secretory transient. Similarly, the PDE inhibitors increased the plateau of the subsequent PGE2 (3 μM) response (33.5 ± 8.3 μA/cm2, n = 9, P < 0.05), but the peaks were unaltered.
Fig. 5.
Inhibition of phosphodiesterases (PDEs) enhanced β2-adrenergic response. Isolated mucosae were stimulated sequentially by epi (5 μM), PGE2 (3 μM), and CCh (10 μM) from the standard basal condition. Isc was measured in adjacent mucosae during secretagogue activation, with 1 mucosa serving as an activation control (●) and the others pretreated with the phosphodiesterase inhibitors trequinsin (trq, ▽, 3 μM, PDE3) and rolipram (rlp, △, 30 μM, PDE4). The responses were representative of 3 similar experiments. Isc responses during activation by PGE2 and CCh were similar to those in Figs. 2 and 3; only the PGE2 plateau Isc was significantly different from control (higher) and the response to CCh was not significantly different from control (P < 0.05).
Expression of soluble adenylyl cyclase in distal colonic mucosa.
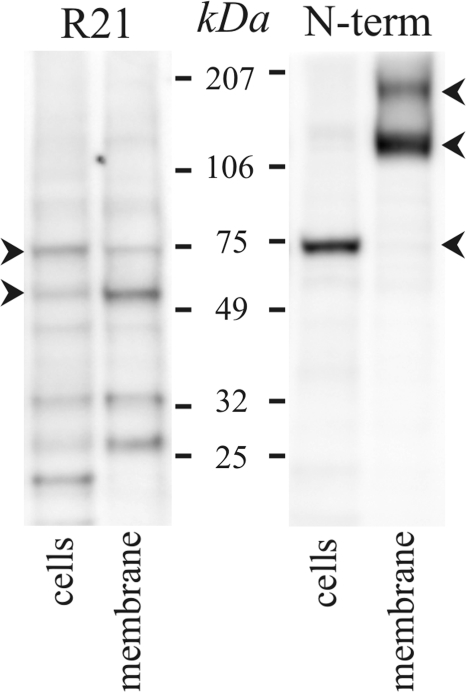
The presence of sAC in colonic epithelial cells was detected by immunoblot (Fig. 6). Several splice forms of sAC have been identified: a full-length form (sAC-fl, ∼180 kDa) and a truncated form (sAC-trnc, ∼50 kDa) containing the NH2-terminal catalytic domains as well as a somatic form (sAC-som, ∼50 kDa) that lacks the initial four exons (22, 25, 40, 70). The R21 antibody recognizes all three of these splice forms (22), and the sAC(N-term) antibody (70) cannot detect sAC-som, which lacks the NH2-terminus. The band at ∼55 kDa detected by the R21 antibody was likely the sAC-som because the sAC(N-term) antibody did not detect a band at this size. The ∼73-kDa band detected by both antibodies was larger than sAC-trnc and smaller than sAC-fl, similar to another possible splice form found in kidney and airway epithelial cells (25, 60). In the membrane fraction the sAC(N-term) antibody detected two bands of higher molecular weight (Fig. 6), the larger consistent with the presence of sAC-fl, but did not detect the ∼73-kDa variant. The ∼120-kDa band may represent a dimer between the ∼73-kDa and ∼55-kDa variants that has the R21 epitope obscured. Also, by using R21 with membranes, the ∼55 kDa variant appeared more prominently than the ∼73 kDa variant. These results support the presence of sAC in colonic epithelial cells.
Fig. 6.
Soluble adenylyl cyclase (sAC) detected in colonic epithelial cells. Protein isolated from distal colonic epithelial cells and membrane fraction was immunoblotted with antibodies against the sAC protein. Immunoreactive bands consistent with forms found in other tissues (22, 25, 29, 40, 51, 60, 70) occurred at 55 and 72 kDa for the R21-sAC antibody and at 73, 121, and 190 kDa for the sAC(N-term) antibody (arrowheads). Bands at ∼35 kDa with R21 are similar in size to those found in endoplasmic reticular fractions (1), and smaller bands may be degradation products. Use of the secondary antibody alone eliminated all bands (data not shown), indicating that the primary antibodies were necessary for the observed results.
Bicarb dependence of secretory activation.
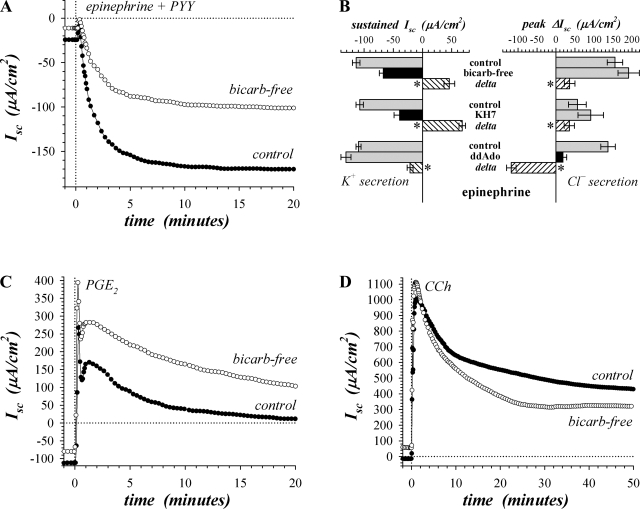
Involvement of sAC in β-adrenergic activation of K+ secretion would confer HCO3− dependence due to the HCO3− sensitivity of sAC. The influence of HCO3− was examined by replacing the bathing solutions with a bicarb-free Ringer (see methods). Epi addition in the bicarb-free condition activated transient and sustained components of Isc as in control with the peak response significantly more positive by 43.0 ± 15.0 μA/cm2 and the sustained level more positive by 53.5 ± 7.3 μA/cm2 (n = 7, P < 0.05), consistent with inhibiting K+ secretion. Suppressing the Cl− secretory transient with PYY highlighted this inhibitory action of bicarb-free solutions on the sustained K+ secretory component (Fig. 7A, Table 3). The similarity in the positive shift compared with control for both bicarb-free and KH7 treatment suggested a common mechanism of action (Fig. 7B), with bicarb-free treatment inhibiting K+ secretion by 38 ± 3% and not inhibiting the transient Cl− secretory component.
Fig. 7.
Bicarbonate (bicarb)-free conditions suppressed sustained epi response. Isolated mucosae were stimulated sequentially by epi (5 μM), PGE2 (3 μM), and CCh (10 μM). Adjacent mucosae were bathed in standard HCO3−/CO2 buffered solution or bicarb-free solution with the standard basal conditions (see methods). A: Isc was measured during activation by epi in the presence of PYY (0.3 μM) without BIIE0246 to accentuate the sustained response (●, HCO3−/CO2 buffer; ○, bicarb-free). B: transient and sustained responses to epi in bicarb-free conditions (n = 8) are compared with the action of the adenylyl cyclase (AC) inhibitors KH7 (30 μM, n = 4) and ddAdo (200 μM, n = 5), all with BIIE0246 present. Treatments that inhibited compared with control are shown as black bars for emphasis. Paired differences between treated and control conditions provide the delta produced by these treatments (hatched bars); *P < 0.05, deltas significantly different from zero. C: Isc was measured in adjacent mucosae (as in A), ∼30 min after epi stimulation, during activation by PGE2, in the presence of BIIE0246. D: Isc was measured in adjacent mucosae (as in C), ∼30 min after PGE2 stimulation, during activation by CCh. The responses were representative of 7 similar experiments (see Table 3).
Table 3.
Dependence of secretagog responses on HCO3−/CO2 buffer: sensitivity to bicarb-free conditions
| 1st Peak | 2nd Peak | Sustained | |
|---|---|---|---|
| Epi: control | +123.7 ± 21.1 | −137.3 ± 8.3* | |
| Epi: bicarb free | +166.6 ± 29.2† | −83.9 ± 3.0*† | |
| PGE2: control | +265.3 ± 17.4 | +204.0 ± 20.0 | +19.1 ± 13.2 |
| PGE2: bicarb free | +385.1 ± 29.4† | +259.9 ± 23.0† | +56.1 ± 6.8† |
| CCh: control | +747.0 ± 27.7 | +927.1 ± 29.6 | +338.6 ± 28.7 |
| CCh: bicarb free | +842.7 ± 36.5† | +884.0 ± 68.1 | +222.1 ± 22.0† |
Isc values (μA/cm2) are means ± SE, n = 7, stimulated as in Fig 7.
BIIE0246 was present for all responses, except sustained epi in which peptide YY replaced BIIE0246.
Responses in bicarbonate (bicarb)-free conditions significantly different from control (P < 0.05).
Activation with PGE2 (3 μM) in bicarb-free solutions retained the positive change in Isc consistent with stimulation of Cl− secretion (Fig. 7C, Table 3). PGE2 significantly increased the Isc compared with control throughout the response, with the first peak 119.8 ± 15.4 μA/cm2 higher, the second peak 55.9 ± 13.4 μA/cm2 higher, and the plateau 37.1 ± 11.1 μA/cm2 higher (n = 7, P < 0.05). Similar to KH7 action during PGE2 stimulation, the positive shift with bicarb-free treatment likely represented an inhibition of K+ secretion. Activation by CCh (Fig. 7D, Table 3) was unaltered at the highest point by bicarb-free treatment (second peak, P < 0.05) compared with control, but the first peak was significantly higher by 95.7 ± 22.0 μA/cm2. The CCh plateau was significantly lower by 116.5 ± 19.8 μA/cm2 (n = 7, P < 0.05), supporting the concept that the sustained cholinergic response included electrogenic HCO3− secretion, unlike the epi or PGE2 responses.
cAMP dynamics during β-adrenergic activation.
Sensitivity of β-adrenergic activation to inhibitors of adenylyl cyclases indicated that cAMP contributed directly to the signaling required to promote ion flow. During epi stimulation, cAMP content recapitulated the epiIsc time course (Fig. 2A) with a 1.5-fold transient increase in cAMP preceding a sustained elevation of cAMP (Fig. 8A), which supported the link between cAMP and activation of both Cl− and K+ secretion. Forskolin, which activates tmAC (43, 63), produced a supramaximal rise in cAMP content matching its action to stimulate ion secretion (46).
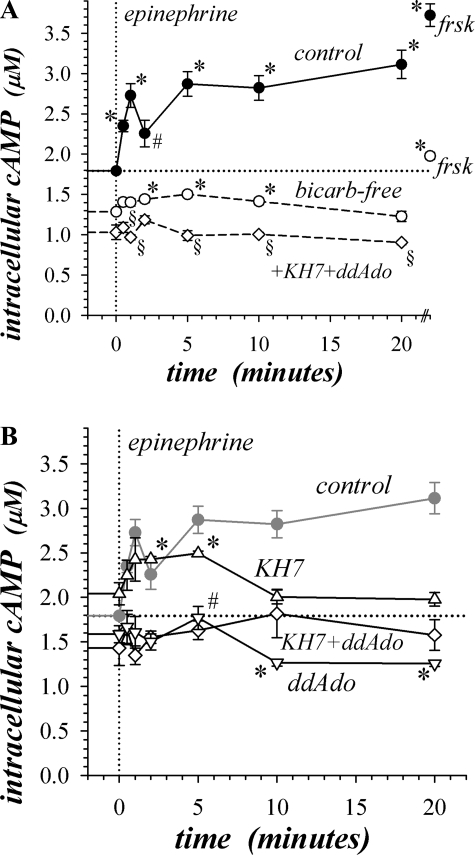
Fig. 8.
Epinephrine stimulated a biphasic increase in cAMP. Isolated mucosae were stimulated by epi (5 μM) from the standard basal condition, and cAMP content was measured by enzyme immunoassay at 7 time points (see methods). Estimates of mucosal histological parameters allowed a conversion to apparent intracellular concentration. A: control (●, n = 4) and bicarb-free (○, n = 4) solutions were used to assess HCO3− dependence. Additional mucosae from the same colon were stimulated with forskolin (frsk; 10 μM) for 5 min as a reference level to compare with epi. The action of AC inhibitors with bicarb-free solutions was assessed by pretreatment for 30 min (◊, 25 μM KH7 + 200 μM ddAdo, n = 4). Control basal cAMP content was 1.79 ± 0.03 μM (n = 17), and bicarb-free basal cAMP content was significantly suppressed (1.28 ± 0.03 μM, n = 4, P < 0.05). *P < 0.05, epi-activated cAMP contents significantly different from basal levels. Control cAMP at 1 min was significantly different from the level at 30 s (P < 0.05), and control cAMP at 2 min epi stimulation was significantly different from the level at 1 or 5 min (#P < 0.05). §Significant differences (P < 0.05) between the cAMP contents in bicarb-free conditions with AC inhibitors and the basal bicarb-free cAMP level without inhibitors. B: action of AC inhibitors in standard solutions was assessed by pretreatment for 30 min (△, 25 μM KH7, n = 3; ▽, 200 μM ddAdo, n = 3; ◊, 25 μM KH7 + 200 μM ddAdo, n = 3). Basal cAMP with KH7 was 2.04 ± 0.12 μM (n = 3), 1.59 ± 0.10 μM (n = 3) with ddAdo, and 1.43 ± 0.20 μM (n = 3) with KH7+ddAdo; the level with KH7 was significantly different from control (P < 0.05). *P < 0.05, cAMP contents significantly different from basal levels. After epi stimulation ddAdo significantly decreased cAMP compared with control at all time points, and KH7 significantly decreased cAMP compared with control at 10 min and longer (P < 0.05). cAMP with ddAdo at 5 min after epi was significantly different from 2 min after epi (#P < 0.05).
KH7 inhibition of sAC significantly increased cAMP in the basal condition, suggesting a suppressive action of cAMP from sAC on tmAC. Inhibiting sAC preserved the initial rise in cAMP during epi activation but reduced the value at longer time compared with control (Fig. 8B), consistent with the action of KH7 on sustained Isc activation (Fig. 2A). The prolongation of the initial cAMP transient supported the possible suppression of tmAC by cAMP from sAC. Inhibition of tmAC with ddAdo eliminated the early cAMP transient (Fig. 8B), consistent with the concurrent loss of the epiIsc transient (Fig. 3A). With ddAdo present, cAMP increased at 5 min, consistent with the independence of sustained epiIsc from tmAC activity. However, at 10 min of epi activation and longer, ddAdo suppressed cAMP content, indicating a supportive influence of tmAC on sustained cAMP production. Combined inhibition of sAC and tmAC suppressed cAMP over the entire epi activation time course, supporting the influence of tmAC on the early cAMP peak and of sAC on the sustained level.
Bicarb-free solutions significantly suppressed basal cAMP, as well as blunting the epi- and forskolin-induced increases in cAMP (Fig. 8A). Even with this suppression, a significant cAMP rise occurred after 2 min of epi stimulation. The peak of the early transient occurred individually at a wider range of times than in control (0.5–2 min), reaching 1.58 ± 0.01 μM. Addition of KH7 and ddAdo eliminated the epi stimulation of cAMP in bicarb-free conditions consistent with the requirement for sAC and tmAC in the β-adrenergic response.
cAMP-dependent actuators of secretory signaling.
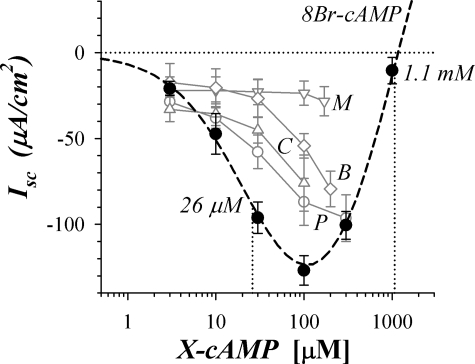
cAMP directly activates protein kinase A (PKA) (64, 65) and the guanine nucleotide exchange factor Epac (10, 26, 35, 59), and either protein could be involved in transducing the cAMP signaling into changes of ion permeability at the apical and basolateral membranes. The cAMP analog 8Br-cAMP has higher lipophilicity and lower susceptibility to PDE degradation than cAMP (55), and when added to the bathing solution it stimulated a negative Isc consistent with K+ secretion (Fig. 9). At concentrations >100 μM, 8Br-cAMP produced a positive change in Isc, suggesting the stimulation of Cl− secretion in addition to the K+ secretion stimulated at lower concentration. This biphasic concentration dependence exactly followed the pattern of stimulation produced by increasing concentrations of forskolin (46). The shape for this concentration dependence suggested further that actuators of K+ secretion were either more sensitive to 8Br-cAMP or simply more accessible to the permeating cAMP analog than the actuators of Cl− secretion. Other analogs of cAMP (35, 55) with preference for PKA (8Pip-cAMP, 6Bnz-cAMP) or Epac (8CPT2M-cAMP, 8MPT2M-cAMP) also were tested for ability to stimulate secretory Isc, and three (8Pip-cAMP, 8CPT2M-cAMP, 6Bnz-cAMP) produced negative Isc at 100 μM (Fig. 9). All of these analogs have higher lipophilicity than 8Br-cAMP (55), suggesting that the lower sensitivity for stimulating Isc occurred because neither PKA nor Epac was involved.
Fig. 9.
cAMP analogs mimicked sustained β-adrenergic response. Isolated mucosae in the standard basal condition were stimulated by addition of cAMP analogs. Isc responses to cumulative addition of 8-Br-adenosine-3′,5′-cyclic-monophosphate (8Br-cAMP) were measured for 2 adjacent mucosae from each colon (●, n = 3). A fit of Henri-Michaelis-Menton kinetics with 2 independent binding sites (A and B) stimulating separate responses was made to the concentration dependence of Isc (dashed line; maxAΔIsc, maxBΔIsc, AEC50, and BEC50 are maxΔIsc and EC50 for responses A and B, respectively): AEC50 = 26 ± 7 μM, maxAIsc = −195 ± 19 μA/cm2; and BEC50 = 1.1 ± 0.4 mM, maxBIsc = +365 ± 40 μA/cm2. Other cAMP analogs were assessed for concentration-dependent stimulation [○, 8-piperidino-cAMP (8Pip-cAMP; P) n = 2, EC50 ≈ 100 μM; △, 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8CPT2M-cAMP; C), n = 3, EC50 ≈ 270 μM; ◊, N6-benzoyl-cAMP (6Bnz-cAMP; B), n = 2, EC50 ≈ 350 μM; ▽, 8-Br-adenosine-3′,5′-cyclic-monophosphorothioate-Rp-isomer (8MPT2M-cAMP; M), n = 2, EC50 >5,000 μM; range shown for n = 2]. Lipophilicities for the analogs relative to cAMP were 1.8, 8Br-cAMP; 6.0, 6Bnz-cAMP; 12, 8Pip-cAMP; 50, 8MPT2M-cAMP; 70, 8CPT2M-cAMP (Ref. 55).
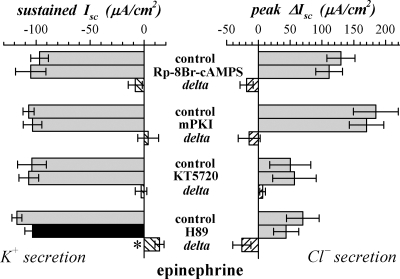
Involvement of PKA in secretory activation was examined further by using several inhibitors with distinct characteristics. Rp-8Br-cAMPS is a competitive inhibitor of PKA, and is ∼30% more lipophilic than 8Br-cAMP (55). The PKI inactivating peptide for PKA is made lipophilic by myristoylation and is the most specific inhibitor for PKA (65). KT5720 is a PKA specific analog of the protein kinase inhibitor staurosporine (19). Pretreatment with these inhibitors did not alter basal Isc nor either the transient or sustained components of adrenergic activation (Fig. 10). H89 inhibits PKA (19), but has the confounding action of antagonism at β-AdrR (54). H89 addition decreased Isc in the basal condition to more negative values and produced a ∼10% inhibition of the sustained response (Fig. 10). Since staurosporine inhibited epi-activated Isc at 0.3 μM (data not shown), a protein kinase may be involved, but the identity was undetermined.
Fig. 10.
Sensitivity of epi stimulation to PKA inhibition. The transient and sustained responses to epi in standard conditions were compared with the epi responses in the presence of the PKA inhibitors Rp-8Br-cAMPS (100 μM, n = 4), mPKI (15 μM, n = 3), KT5720 (10 μM, n = 3), and H89 (10 μM, n = 4). Those conditions that were inhibited compared with control are shown as black bars for emphasis. Paired differences compared with control conditions provide the delta produced by these inhibitors (hatched bars); *P < 0.05, deltas significantly different from zero.
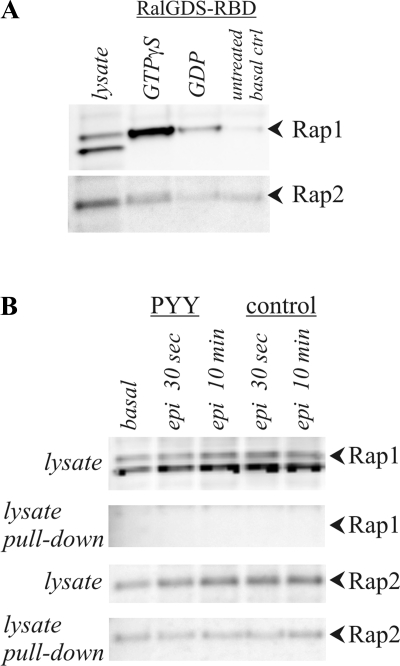
Epac activation of the small GTPases Rap1 or Rap2 (10, 59) could provide a signal for producing epi-stimulated ion secretion. Involvement of Epac/Rap in the β-adrenergic pathways was examined by testing for activation of these small G proteins Rap1 and Rap2 in colonic mucosa stimulated by epi. Epi failed to activate Rap1 during the transient and sustained phases, even though in vitro incubation with GTPγS activated Rap1 in the colonic mucosal cell lysate (Fig. 11). Activated Rap2 was apparent in the basal condition and a small increase with epi may have occurred, but the difference was not statistically discernable. These results support a β-adrenergic activation mechanism that might involve Epac/Rap2 but does not use PKA or Epac/Rap1.
Fig. 11.
Activation status of Rap 1 and Rap2 during epi stimulation. Cell lysates from distal colonic mucosa were used to detect activated Rap via a pull-down assay with GST-coupled binding protein (RalGDS-RBD). Mucosae were incubated in control conditions or with PYY (0.3 μM) replacing BIIE0246. Basal and epi (5 μM)-activated samples were collected. A: Rap1 was present as a double band at ∼27 kDa and ∼29 kDa, and Rap2 was present as a band at ∼28 kDa. Control lysate was treated with either GTPγS or GDP to demonstrate maximal and minimal activation states, respectively. B: lysates of mucosae obtained during basal conditions or after epi activation did not show Rap1 activation, but Rap2 activity was detectable throughout. Similar results for Rap1 and Rap2 were obtained at 1, 2, 5, and 20 min. The responses were representative of 4 similar experiments.
DISCUSSION
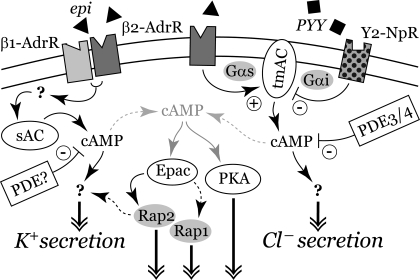
β-Adrenergic receptors transmit sympathetic signals to the ion secretory elements of the colonic epithelial cells such that epi or norepi stimulates a transient Cl− secretion and a sustained K+ secretion (73, 74). This β-adrenergic activated secretion is modified further by the PYY and NPY coreleased in the mucosa during any sympathetic response. The signals for the two secretory components appear to originate from separate receptor complexes with the β-AdrRs in each group activating distinct pathways (Fig. 12). A β2-AdrR complex initiated the Cl− secretory component on the basis of antagonist sensitivity (Fig. 1). This β2-AdrR complex likely included a tmAC-producing cAMP destined to stimulate Cl− secretion (Fig. 3) together with Y2-NpRs that inhibit the tmAC (Fig. 4). Proximity of PDEs also would limit the extent of cAMP emanating from this complex (Fig. 5). In contrast, the sustained-phase K+ secretory response likely involves combined activation of β1-AdrR and β2-AdrR (73, 74) with sAC producing the cAMP that stimulated K+ secretion (Fig. 2).
Fig. 12.
Signaling pathways for epi activation of secretion. The β1- and β2-adrenergic receptors (β1-AdrR, β2-AdrR) and Y2-neuropeptide receptor (Y2-NpR) are shown interacting with transmembrane adenylyl cyclase (tmAC) and sAC. Phosphodiesterases (PDEs) limit buildup of the cAMP, leading to activation of secretion. cAMP from other ACs in the cell also is shown leading to PKA activation as well as to Epac activation of Rap1 and Rap2. Activated Rap2 may enter the signaling pathway for K+ secretion, or possibly that for Cl− secretion. cAMP production via the sAC-dependent pathway would be maximally HCO3− sensitive, since the physiological intracellular HCO3− concentration is near the EC50 for sAC (25, 48). Only with severe hypercapnia would CO2 rise sufficiently to stimulate a large increase in cAMP production via the tmAC pathway (66).
Sympathetic activation of modulatory secretion.
Sympathetic stimulation initiates ion secretion that includes a transient flushing mode together with the sustained modulatory mode (73, 74) by using functionally separate β-AdrRs. The transient Cl− secretion required β2-AdrR as indicated by high sensitivity to the β2 antagonist ICI-118551 (Fig. 1B), similar to that reported for β2-AdrR in other cell types (3) but ∼30-fold more sensitive than for the sustained K+ secretory component (74). Since the combination of ICI-118551 and the β1 antagonist CGP20712A was more effective than either alone at antagonizing sustained K+ secretion (73), a functional β1-AdrR/β2-AdrR oligomer likely activates this response. The transient character of the β2-AdrR-initiated Cl− secretion conforms to the desensitization commonly found for β2-AdrR events, whereas involvement of β1-AdrR in the sustained response conforms to the resistance to desensitization often found with β1-AdrR-mediated events (45, 58). A major consequence of the difference in agonist sensitivity for the two components (Fig. 1C) is that Cl− secretion would be stimulated only at high sympathetic discharge. Since neural NPY and enteroendocrine PYY release also would be most pronounced at these intense levels (24), the transient component often would be severely suppressed. In this way the epithelial response to sympathetic stimulation generally would be limited to the sustained K+ secretory component of β-adrenergic activation.
Epi-stimulated cAMP generation.
As expected for a β-adrenergic response, cAMP increased but with a biphasic time course (Fig. 8) mirroring the two components of the secretory Isc (Fig. 1). Lending further support to the pharmacological evidence for two receptor complexes, each component of the secretory response required different adenylyl cyclases. Loss of both the sustained epiIsc (Fig. 2) and the plateau in cAMP (Fig. 8) with KH7 supported a primary involvement of sAC in activating K+ secretion. Although sAC (adcy10) was designated as soluble (43), because of the lack of transmembrane domains typical of tmACs (adcy1-adcy9), sAC localizes in membrane fractions, presumably via adaptor proteins (76). The membrane localization of sAC in colonic epithelial cells (Fig. 6) would permit a close association with the proposed β1-AdrR/β2-AdrR complex (Fig. 12). Of the several splice variants of sAC presently described (22, 25, 40, 70), two may occur in colonic epithelial cells as indicated by antibodies to distinct domains of sAC (Fig. 6). The larger variant (∼73 kDa) presumably has both catalytic domains but not the full COOH-terminus that regulates activity, and the smaller variant (∼55 kDa) likely represented sAC-som, which also lacks the NH2-terminal catalytic domain. Since cAMP production requires both catalytic domains (43), oligomers of these sAC variants may be involved in β1-AdrR/β2-AdrR signaling as suggested by the larger-sized membrane forms observed (Fig. 6). Although receptor-initiated activation of sAC has been observed in several cell types, heterotrimeric G proteins do not appear to be involved and the receptor-dependent activation mechanisms remain to be determined (11, 29, 43, 56, 62).
Loss of the transient epiIsc with a tmAC inhibitor without diminishing the sustained response (Figs. 3 and 7B) supported selective stimulation of Cl− secretion via β2-AdrR activation of tmACs. Of the nine tmACs (63), seven (tmAC2–7/9) have been detected in colonic epithelial cells (23). tmAC5 and 6 are the most likely candidates for mediating the transient response, because both are sensitive to inhibition by Gαi (14, 63, 71), as was the transient response (Fig. 4). All tmACs are inhibited by ddAdo, but some are more sensitive, with tmAC5 having one of the lower Kd values (39, 42). The fit of ddAdo inhibition to a cooperative two-site model supported cAMP generation via a tmAC dimer in which the catalytic domains interact (Fig. 3), consistent with evidence for tmAC oligomer formation and heterodimerization of adenylyl cyclase catalytic domains (16, 43). The ability of ddAdo to suppress epi-induced increases in cAMP occurring after ∼10 min (Fig. 8B) implied that tmAC activity influenced sAC or that the basal component of cAMP production was reduced.
Activity of both tmACs and sAC influenced basal cAMP levels (Fig. 8), producing a concentration of 1.8 μM, similar to basal concentrations in cardiac myocytes (1.2 μM) obtained from a FRET-based cAMP biosensor (38). These basal cAMP levels were high enough to activate PKA strongly and Epac moderately (55), supporting the concept that cytoplasmic compartmentation maintained basal cAMP near the receptor complexes at levels lower than the global cytoplasmic average (2, 37, 38). Subsequent receptor activation would increase cAMP in the region of the receptor complexes and result in cAMP flow into the rest of the cytoplasm such that global cAMP increased (Fig. 12). Thus activated cAMP levels in the region of receptor complexes would be higher than the global measurement, especially if high PDE activity limited cAMP emerging from these regions. If all of the cells in the colonic epithelium did not respond to β-adrenergic activation, then the global measure of the response would be attenuated further from that actually occurring near activated β-AdrRs. The transient and sustained cAMP responses (Fig. 8) were similar to those measured by biosensors in cells with β2-AdrRs or β1-AdrRs. β2-AdrR produces a transient cAMP response with a time for half-activation (τ0.5) of ∼0.5 min and a τ0.5 for decline of ∼2 min (69), whereas a β1-AdrR response remains stable for over 10 min (38).
Increasing CO2 stimulates both tmAC and sAC, whereas HCO3− stimulates only sAC (43, 66). Preferential reduction of sustained epiIsc by removing HCO3−/CO2 while leaving peak epiIsc unaltered (Fig. 7) suggested that sAC sensitivity was higher than that for tmAC. Attenuation of the early cAMP peaks (Fig. 8A) suggested that either sufficient cAMP still arrived to produce near normal transient Cl− secretion or that this cAMP was not crucial for signaling. In contrast, the partial reduction of sustained K+ secretion conformed to the observed reduction in sustained cAMP. With the HCO3− EC50 of 9–11 mM for sAC (25, 48), and assuming an intracellular pH of ∼7.0, the ∼40% reduction in sustained secretion (Fig. 7B) suggested a drop of cell HCO3− from ∼10 to ∼4 mM, consistent with metabolism maintaining cell CO2 at ∼0.5 mM compared with the 1.2 mM level in the standard HCO3−/CO2-buffered conditions. This drop in CO2 may have been insufficient to limit tmAC-dependent cAMP production destined for activating Cl− secretory elements, because a portion of tmAC activity appears CO2 independent and the CO2 EC50 for tmAC (≥6 mM) is much higher than the nominal CO2 level in the cells (66). The partial inhibition of the forskolin response (Fig. 8A) provided support for the extent of CO2 dependence for tmAC in these cells.
The response of cAMP content to epi also could be interpreted to suggest that cAMP is not the primary signal for the observed secretory events. However, since the measured cAMP content tracks the total cellular cAMP, the actual concentrations at the point of production near the tmAC and sAC may have a wider range such that a direct quantitative comparison between Isc and cAMP content will require spatial information concerning production and response (37, 38).
The response to 8Br-cAMP (Fig. 9), which mimics the action of cAMP at many signaling proteins (55), supported the direct dependence of secretion on cAMP. The preferential stimulation of K+ secretion at low concentration and Cl− secretion only at higher concentrations suggested that actuators of K+ secretion were more sensitive to cAMP, or that those for Cl− secretion were relatively isolated from global changes in cAMP. The high levels of cAMP needed for Cl− secretion could be achieved by close localization of the signaling elements as illustrated in the model (Fig. 12). Such receptor signaling complexes have been observed in other cell types (16, 21, 71) and may be a general means to coordinate simultaneous activation of distinct responses.
Termination of cAMP signaling during secretory activation.
Cellular signaling via cAMP occurs as a balance between production by ACs (63) and degradation by PDEs (6). PDE4 and PDE3 likely occurred in the β2-AdrR complex, which signals for Cl− secretion (Fig. 5). PDE inhibition enhanced the second peak of transient epiIsc, which was β2-AdrR dependent (Fig. 1D). Activation of two peaks also occurred in the much smaller β-adrenergic response in T84 cells (72). The lack of any enhancement in sustained secretion suggested that an IBMX/rolipram-insensitive PDE degraded the cAMP produced by sAC in these cells. Insensitivity to IBMX and localization in colonic epithelial cells (6, 18) makes PDE8 and PDE11 candidates for the β1-AdrR/β2-AdrR complex that signals for K+ secretion (Fig. 12). Y2-NpRs also provided a negative regulation of cAMP production from tmAC in the β2-AdrR complex as indicated by sensitivity to PTx (Fig. 4). Proximity of β2-AdrR and Y2-NpR within the signaling complex would allow effective inhibition of the tmACs via Gαi (Fig. 12).
The transition from the basal condition to modulatory secretion involves a concerted activation of the transport proteins required for Cl− and K+ secretion. The basal condition exhibited a negative Isc (Figs. 2–5, 7), consistent with electrogenic K+ secretion that may result from low-level stimulation by agonists remaining in the preparation or through agonist independent activity of receptors such as β1-AdrR and β2-AdrR. Sensitivity of basal Isc to signaling inhibitors had some similarities with epi-stimulated K+ secretion and several differences. Sensitivity to KH7 (Fig. 2A) indicated that both basal and epi-stimulated K+ secretion required sAC activity. For that reason, the tmAC inhibitor ddAdo might be anticipated to do little to basal electrogenic K+ secretion. Instead, ddAdo stimulated basal Isc and Gt (Fig. 3), supporting an inhibitory role for cAMP generated by tmACs. Inhibition of Isc and Gt by KH7 in the presence of ddAdo (Fig. 3A) further supported sAC as the primary activation pathway. Inhibitory influences of cAMP from tmACs also were apparent in the peak responses to PGE2 and CCh, as seen by the consistently higher maxIsc obtained from the ddAdo concentration dependence compared with control stimulation (Fig. 3, B and C).
PDE control of basal and epi-activated K+ secretion differed, with rolipram and IBMX augmenting basal K+ secretion but failing to enhance sustained K+ secretion (Fig. 5). This divergence of action suggested that PDE4 was near the K+ secretory cAMP pool in basal conditions but not after epi activation, or that the K+ secretory rate during epi activation was maximal such that further increases in cAMP were ineffective. Also, in contrast to the insensitivity of epi activated K+ secretion to PYY inhibition (74), basal Isc and Gt decreased with PYY in a PTx-sensitive manner consistent with signaling via Gαi. The simplest interpretations would be either an as-yet-to-be-reported inhibitory action of Gαi on sAC or an involvement of Gαi-sensitive tmAC in the sAC-dependent activation of basal K+ secretion.
cAMP-dependent actuators of ion secretion.
cAMP stimulates three major protein types that actuate further signaling (7, 8, 10, 26, 59, 64): PKA, cAMP-regulated guanine nucleotide exchange factor (Epac), and cyclic nucleotide-gated ion channels (CNG, HCN). PKA phosphorylates numerous proteins including CFTR, a Cl− channel present in the apical membrane of many Cl− secretory epithelial cells (5, 28). For this reason PKA often plays a major role in activation of electrogenic Cl− secretion. Epac activates Rap1 and Rap2, members of the Ras family of small GTPases, leading to increased activity of various cellular functions in many cell types (59). In the colonic tumor cell line T84, forskolin stimulates a PKA-independent Cl− secretion mediated by Epac (36). This Cl− secretion depends on apical Cl− channels other than CFTR, indicating that each cAMP-dependent actuator likely links to specific transport elements. Cyclic nucleotide-gated channels often occur in nerves and muscle (7, 8), but a CNG appears to mediate Na+ absorption in the inner medullary collecting duct of the kidney (68).
Several cAMP analogs with greater lipid solubility and resistance to PDE degradation than cAMP allow extracellular addition to activate PKA and Epac (35, 55). Relative efficacy of these analogs in stimulating secretory Isc aided identification of signaling pathways (Fig. 9). The selectivity sequence for activating sustained K+ secretion was 8Br-cAMP > 8Pip-cAMP > 8CPT2M-cAMP ≥ 6Bnz-cAMP ≫ 8MPT2M-cAMP. Since greater lipid solubility should lead to greater penetration into the cell, the relative lipophilicities of the analogs (55) can adjust for this factor, giving a better estimate of actuator selectivity: 8Br-cAMP > 8Pip-cAMP > 6Bnz-cAMP > 8CPT2M-cAMP ≫ 8MPT2M-cAMP. Differential degradation by PDE would alter the persistence of each analog in the cell also contributing to the observed concentration dependence, and since 6Bnz-cAMP and 8CPT2M-cAMP inhibit PDE activity the most likely effect would be to heighten any efficacy compared with 8Br-cAMP. The relative ineffectiveness of the Epac agonists 8CPT2M-cAMP and 8MPT2M-cAMP and the PKA agonists 8Pip-cAMP and 6Bnz-cAMP suggested that other actuators were involved.
Insensitivity of β-adrenergic activation to PKA inhibitors (Fig. 10) also supported a signaling pathway without a PKA action on either Cl− secretion or K+ secretion (Fig. 12). Each PKA inhibitor was used at concentrations that succeeded at inhibiting responses in cells with PKA as part of the signaling pathway (1, 36, 44, 53). However, each inhibitor exhibits weaknesses that may have limited efficacy in colonic epithelial cells. Rp-8Br-cAMPS competes with cAMP for binding, such that it simply may not have reached concentrations sufficient to effectively antagonize PKA activation. A lack of sufficient intracellular accumulation also may have blunted any action of the peptide inhibitor myristoylated PKI (65). Poor penetration into the cell was unlikely to explain the lack of inhibition by KT5720 or H89 since both are very lipid soluble. Also, the minor inhibition by H89 (Fig. 10) was more likely due to antagonism at βAdrR (54). Thus β-adrenergic activation used a cAMP-dependent actuator other than PKA.
Although Epac can provide a cAMP-dependent activation of the Rap GTPases (10, 26, 59), Epac did not appear to transduce βAdrR-generated cAMP into Rap1 activation for stimulating secretion (Fig. 11). The basal level of activated Rap2 may have obscured any small epi-induced increase in activated Rap2 (Fig. 11), and if this increase was highly localized near the next step in the signaling pathway, then a chain of activation may have occurred. Small changes in Rap2 have been linked to cell signaling (20, 59), and T84 cells have Epac-dependent increases in Rap2 associated with Cl− secretion (36). A small or localized change in Rap2 activity, therefore, would be the most likely response by a known cAMP-dependent signaling actuator, and either the β1-AdrR/β2-AdrR-mediated sustained K+ secretion or the β2-AdrR-mediated transient Cl− secretion might be stimulated (Fig. 12).
Signaling pathways for flushing and synergistic secretion.
The flushing mode of secretion stimulated by PGE2 includes sustained Cl− secretion compared with β-adrenergic activation that only produces transient Cl− secretion (74). The synergistic secretory mode has even higher rates of sustained Cl− secretion. Sensitivity to inhibitors of adenylyl cyclase would be expected because of the association of cAMP with Cl− secretion (4, 5). However, the results were mixed. During the flushing mode (Fig. 2B), the sAC inhibitor KH7 apparently inhibited K+ secretion without altering Cl− secretion, and the HCO3−/CO2 sensitivity of sustained K+ secretion fit with this dependence on sAC (Fig. 7A). Inhibiting tmAC reduced the transient epi response to near zero (Fig. 3, Table 2), but all three components of the flushing and synergistic responses were only partially sensitive, consistent with a substantial contribution by cAMP-independent signaling pathways. Furthermore, insensitivities of flushing and synergistic secretion to PDE inhibitors and PKA inhibitors suggested that the standard model for activating Cl− secretion via cAMP-dependent processes was insufficient to describe these secretory activations.
β-Adrenergic activation of ion secretion in the colonic epithelium has several similarities with β-adrenergic signaling in cardiac muscle cells. In particular, the sequestration of β-AdrR complexes into specialized membrane domains of cardiac cells (38, 75) may be a useful model for the organization of β-AdrRs and signaling components in colonic epithelial cells. Whether the same adaptor proteins are used remains to be determined, but the intricacy of the β-adrenergic response in the colonic epithelium likely will use many similar strategies to produce varied responses to sympathetic input.
GRANTS
This study was supported by a grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK65845).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank L. Levin and J. Buck, Weill Medical College of Cornell University, for helpful discussions.
REFERENCES
- 1.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab 9: 265–276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillie GS. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J 276: 1790–1799, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol 144: 317–322, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett KE, Cohn JA, Huott PA, Wasserman SI, Dharmsathaphorn K. Immune-related intestinal chloride secretion. II. Effect of adenosine on T84 cell line. Am J Physiol Cell Physiol 258: C902–C912, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Barrett KE, Keely SJ. Integrative physiology and pathophysiology of intestinal electrolyte transport. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 2006, p. 1931–1951 [Google Scholar]
- 6.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol 191: 111–136, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Blondon H, Béchade D, Desramé J, Algayres JP. Secretory diarrhoea with high faecal potassium concentrations: a new mechanism of diarrhoea associated with colonic pseudo-obstruction? Report of five patients. Gastroenterol Clin Biol 32: 401–404, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Branham MT, Bustos MA, De Blas GA, Rehmann H, Zarelli VE, Treviño CL, Darszon A, Mayorga LS, Tomes CN. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J Biol Chem 284: 24825–24839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev 46: 121–136, 1994 [PubMed] [Google Scholar]
- 13.Camilleri M, Szarka L. Dysmotility of the small intestine and colon. In: Textbook of Gastroenterology (5th ed.), edited by Yamada T. Hoboken, NJ: Blackwell, 2009, p. 1108–1156 [Google Scholar]
- 14.Chen-Goodspeed M, Lukan AN, Dessauer CW. Modeling of Gαs and Gαi regulation of human type V and VI adenylyl cyclase. J Biol Chem 280: 1808–1816, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cooke HJ, Christofi FL. Enteric neural regulation of mucosal secretion. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 2006, p. 737–762 [Google Scholar]
- 16.Cooper DM, Crossthwaite AJ. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci 27: 426–431, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Cox HM. Peptide YY: a neuroendocrine neighbor of note. Peptides 8: 345–351, 2007 [DOI] [PubMed] [Google Scholar]
- 18.D'Andrea MR, Qiu Y, Haynes-Johnson D, Bhattacharjee S, Kraft P, Lundeen S. Expression of PDE11A in normal and malignant human tissues. J Histochem Cytochem 53: 895–903, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rooij J, Boenink NM, van Triest M, Cool RH, Wittinghofer A, Bos JL. PDZ-GEF1, a guanine nucleotide exchange factor specific for Rap1 and Rap2. J Biol Chem 274: 38125–38130, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Dessauer CW. Adenylyl cyclase–A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76: 935–941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrell J, Ramos L, Tresguerres M, Kamenetsky M, Levin LR, Buck J. Somatic ‘soluble’ adenylyl cyclase isoforms are unaffected in Sacy tm1Lex/Sacy tm1Lex ‘knockout’ mice. PLoS One 3: e3251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman SL, MacNaughton WK. Nitric oxide inhibitable isoforms of adenylate cyclase mediate epithelial secretory dysfunction following exposure to ionising radiation. Gut 53: 214–221, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furness JB. The Enteric Nervous System Malden, MA: Blackwell, 2006, p. 274 [Google Scholar]
- 25.Geng W, Wang Z, Zhang J, Reed BY, Pak CY, Moe OW. Cloning and characterization of the human soluble adenylyl cyclase. Am J Physiol Cell Physiol 288: C1305–C1316, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355–375, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JL, Shapiro AB, Rao MC, Layden TJ. In vivo evidence of altered chloride but not potassium secretion in cystic fibrosis rectal mucosa. Gastroenterology 101: 1012–1019, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem 284: 5774–5783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halm DR. Secretory control of basolateral membrane potassium and chloride channels in colonic crypt cells. Adv Exp Med Biol 559: 119–129, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Halm DR, Frizzell RA. Active K+ transport across rabbit distal colon: relation to Na+ absorption and Cl− secretion. Am J Physiol Cell Physiol 251: C252–C267, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Halm DR, Halm ST. Prostanoids stimulate K+ secretion and Cl− secretion in guinea pig distal colon via distinct pathways. Am J Physiol Gastrointest Liver Physiol 281: G984–G996, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl− and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol 291: C636–C648, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 9: 249–259, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal 20: 10–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoque KM, Woodward OM, van Rossum DB, Zachos NC, Chen L, Leung GPH, Guggino WB, Guggino SE, Tse CM. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J Gen Physiol 135: 43–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iancu RV, Jones SW, Harvey RD. Compartmentation of cAMP signaling in cardiac myocytes: a computational study. Biophys J 92: 3317–3331, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iancu RV, Ramamurthy G, Warrier S, Nikolaev VO, Lohse MJ, Jones SW, Harvey RD. Cytoplasmic cAMP concentrations in intact cardiac myocytes. Am J Physiol Cell Physiol 295: C414–C422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwatsubo K, Minamisawa S, Tsunematsu T, Nakagome M, Toya Y, Tomlinson JE, Umemura S, Scarborough RM, Levy DE, Ishikawa Y. Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J Biol Chem 279: 40938–40945, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J Biol Chem 276: 31698–31708, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Jetmore AB, Timmcke AE, Gathright JB, Jr, Hicks TC, Ray JE, Baker JW. Ogilvie's syndrome: colonoscopic decompression and analysis of predisposing factors. Dis Colon Rectum 35: 1135–1142, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Johnson RA, Désaubry L, Bianchi G, Shoshani I, Lyons E, Jr, Taussig R, Watson PA, Cali JJ, Krupinski J, Pieroni JP, Iyengar R. Isozyme-dependent sensitivity of adenylyl cyclases to P-site-mediated inhibition by adenine nucleosides and nucleoside 3′-polyphosphates. J Biol Chem 272: 8962–8966, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol 362: 623–639, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J Biol Chem 277: 18598–18604, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hébert TE. β1/β2-Adrenergic receptor heterodimerization regulates β2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem 277: 35402–35410, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Halm ST, Halm DR. Secretory activation of basolateral membrane Cl− channels in guinea pig distal colonic crypts. Am J Physiol Cell Physiol 284: C918–C933, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Liao T, Wang L, Halm ST, Lu L, Fyffe REW, Halm DR. The K+ channel KVLQT (Kcnq1) located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl− secretion. Am J Physiol Cell Physiol 289: C564–C575, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase: synergism between calcium and bicarbonate. J Biol Chem 278: 15922–15926, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Mall M, Wissner A, Seydewitz HH, Kühr J, Brandis M, Greger R, Kunzelmann K. Defective cholinergic Cl− secretion and detection of K+ secretion in rectal biopsies from cystic fibrosis patients. Am J Physiol Gastrointest Liver Physiol 278: G617–G624, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 50: 143–150, 1998 [PubMed] [Google Scholar]
- 51.Nunes AR, Monteiro EC, Johnson SM, Gauda EB. Bicarbonate-regulated soluble adenylyl cyclase (sAC) mRNA expression and activity in peripheral chemoreceptors. Adv Exp Med Biol 648: 235–241, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Onaga T, Zabielski R, Kato S. Multiple regulation of peptide YY secretion in the digestive tract. Peptides 23: 279–290, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penn RB, Parent JL, Pronin AN, Panettieri RA, Jr, Benovic JL. Pharmacological inhibition of protein kinases in intact cells: antagonism of beta adrenergic receptor ligand binding by H-89 reveals limitations of usefulness. J Pharmacol Exp Ther 288: 428–437, 1999 [PubMed] [Google Scholar]
- 55.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods 5: 277–278, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol 132: 329–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rechkemmer G, Frizzell RA, Halm DR. Active K+ transport across guinea pig distal colon: action of secretagogues. J Physiol 493: 485–502, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol 10: 819–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol 377: 345–357, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, Levin LR, Conner GE, Fregien N, Salathe M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol 130: 99–109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skeberdis VA. Structure and function of β3-adrenergic receptors. Medicina (Kaunas) 40: 407–413, 2004 [PubMed] [Google Scholar]
- 62.Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem 281: 17253–17258, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2: 168–184, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev 84: 137–167, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Taylor SS, Kim C, Vigil D, Haste NM, Yang J, Wu J, Anand GS. Dynamics of signaling by PKA. Biochim Biophys Acta 1754: 25–37, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Townsend PD, Holliday PM, Fenyk S, Hess KC, Gray MA, Hodgson DR, Cann MJ. Stimulation of mammalian G-protein-responsive adenylyl cyclases by carbon dioxide. J Biol Chem 284: 784–791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Dinter TG, Jr, Fuerst FC, Richardson CT, Ana CA, Polter DE, Fordtran JS, Binder HJ. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology 129: 1268–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Vandorpe DH, Ciampolillo F, Green RB, Stanton BA. Cyclic nucleotide-gated cation channels mediate sodium absorption by IMCD (mIMCD-K2) cells. Am J Physiol Cell Physiol 272: C901–C910, 1997 [DOI] [PubMed] [Google Scholar]
- 69.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. β2-Adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 283: 2949–2961, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Lam CS, Wu F, Wang W, Duan Y, Huang P. Regulation of CFTR channels by HCO3−-sensitive soluble adenylyl cyclase in human airway epithelial cells. Am J Physiol Cell Physiol 289: C1145–C1151, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Yang ZH, Pan A, Ruan YC, Yu HJ, Wu ZL, Xiang H, Zhou WL. Cellular mechanism of adrenalin stimulated chloride secretion via β-adrenoceptor in T84 cells. Cell Biol Int 32: 679–687, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Halm ST, Halm DR. Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: involvement of β1- and β2-adrenergic receptors. Am J Physiol Gastrointest Liver Physiol 297: G269–G277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Halm ST, Halm DR. Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: desensitization via the Y2-neuropeptide receptor. Am J Physiol Gastrointest Liver Physiol 297: G278–G291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng M, Zhu W, Han Q, Xiao RP. Emerging concepts and therapeutic implications of β-adrenergic receptor sub-type signaling. Pharmacol Ther 108: 257–268, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17: 82–84, 2003 [DOI] [PubMed] [Google Scholar]