Abstract
Gastric surface pH (pHo) transiently increases in response to focal epithelial damage. The sources of that increase, either from paracellular leakage of interstitial fluid or transcellular acid/base fluxes, have not been determined. Using in vivo microscopy approaches we measured pHo with Cl-NERF, tissue permeability with intravenous fluorescent-dextrans to label interstitial fluid (paracellular leakage), and gastric epithelial intracellular pH (pHi) with SNARF-5F (cellular acid/base fluxes). In response to two-photon photodamage, we found that cell-impermeant dyes entered damaged cells from luminal or tissue compartments, suggesting a possible slow transcellular, but not paracellular, route for increased permeability after damage. Regarding cytosolic acid/base status, we found that damaged cells acidified (6.63 ± 0.03) after photodamage, compared with healthy surface cells both near (7.12 ± 0.06) and far (7.07 ± 0.04) from damage (P < 0.05). This damaged cell acidification was further attenuated with 20 μM intravenous EIPA (6.34 ± 0.05, P < 0.05) but unchanged by addition of 0.5 mM luminal H2DIDS (6.64 ± 0.08, P > 0.05). Raising luminal pH did not realkalinize damaged cells, suggesting that the mechanism of acidification is not attributable to leakiness to luminal protons. Inhibition of apical HCO3− secretion with 0.5 mM luminal H2DIDS or genetic deletion of the solute-like carrier 26A9 (SLC26A9) Cl−/HCO3− exchanger blocked the pHo increase normally observed in control animals but did not compromise repair of damaged tissue. Addition of exogenous PGE2 significantly increased pHo in wild-type, but not SLC26A9 knockout, animals, suggesting that prostaglandin-stimulated HCO3− secretion is fully mediated by SLC26A9. We conclude that cellular HCO3− secretion, likely through SLC26A9, is the dominant mechanism whereby surface pH transiently increases in response to photodamage.
Keywords: photodamage, NAD(P)H, autofluorescence, two-photon microscopy, confocal microscopy, intracellular pH, SNARF-5F, Cl-NERF, permeability, solute-like carrier 26A9
the secretion of bicarbonate (HCO3−) from the surface epithelial cells of the stomach provides a higher pH microenvironment adjacent to the gastric epithelial lining, compared with the bulk lumen. Along with secreted mucus proteins such as MUC5AC and MUC6, this mucus-HCO3− layer acts as a protective barrier, preventing damage and/or proteolytic digestion of the epithelium (14). Measurements of pH within this microenvironment have been made previously. Using pH-sensitive microelectrodes, in vivo surface pH (pHo) has been reported at pH 7 (16, 18), whereas pH-sensitive dyes used by our group reported pHo values of ∼4 (2, 3, 5). Regardless, all previous measurements show that pH directly adjacent to the gastric surface is higher than the pH of the bulk luminal fluid in the fasted stomach.
Previous work has also shown that protective mechanisms, such as prostaglandin secretion via cyclooxygenase-1, are important in maintaining pHo within this microenvironment, as well as protecting the epithelium against microscopic injury (2, 4, 5). Recently, our laboratory has shown that, in response to two-photon photodamage, a transient increase in pHo occurs over the site of damage (19), presumably as a protective mechanism to preserve the intact epithelium not subjected to damage, and/or for efficient repair of the damage. However, the mechanism by which pHo increases, whether through transcellular HCO3− secretion, paracellular leakage of plasma HCO3− into the gastric lumen, or a combination of both pathways, remains undetermined.
Although the molecular mechanism of mammalian gastric surface HCO3− secretion is unknown, the substrate is provided by carbonic anhydrase-mediated CO2 hydrolysis (1) or transported by submucosal capillaries to the epithelium (21). There are several candidate transporters that could mediate the secretion of HCO3− across the apical membrane. The anion exchanger (AE) isoform 4, a member of the solute-like carrier (SLC)4 (Na+-HCO3− cotransporters and Cl−/HCO3− exchangers) family, and SLC26A9, a member of the SLC26A (Cl−/HCO3− exchangers) family, have been found to localize to the apical membrane of gastric surface epithelial cells (26, 27). Because the disulfonic stilbene DIDS is known to inhibit acid-induced gastric surface epithelial HCO3− secretion (17) and SLC26A9 Cl−/HCO3− exchange function, but not AE4 (23, 26), there is expectation that SLC26A9 contributes to gastric HCO3− secretion. It remains unclear whether either protein is involved in regulating gastric pHo and intracellular pH (pHi) regulation during damage and repair processes, although both Na+/H+ exchange and HCO3− secretion have both been shown to be important for gastric epithelial repair (7, 10).
Another hallmark of epithelial damage is disruption in barrier function, leading to an increase in paracellular permeability. In previous models of pervasive gastric surface cell damage, such as absolute ethanol exposure (9, 11–13), the exfoliated epithelium forms a “mucoid cap” over intact healthy tissue, shown to contain mucus, HCO3−, and plasma proteins such as fibrin (6, 24). Additionally, gastric epithelial damage attributable to acid (20), hydrogen peroxide (8), and Helicobacter pylori (25) are all known to increase paracellular permeability by inhibiting protective prostaglandins and tight junction proteins that maintain integrity of the epithelial barrier. Because our model of microscopic damage also produces cell exfoliation (19), it is predicted that paracellular leakage occurs in response to photodamage and may therefore contribute to events such as pHo changes soon after damage.
In this study, we sought to examine the source of the transient pHo increase observed after two-photon photodamage to the gastric epithelium. We have used methods to help us selectively appraise transcellular and paracellular flux of materials in the living animal, in combination with genetic and pharmacological tools.
MATERIALS AND METHODS
Animals.
Experiments used C57BL/6J mice (Jackson, Bar Harbor, ME) and SLC26A9 wild-type (WT) and knockout (KO) littermates, bred in house. WT mice with the same genetic background as the A9 KO were used in 16,16-dimethyl-PGE2 studies. All animals were used for experiments at 2–6 mo of age, were fed a standard rodent chow diet, and had free access to water. The surgical preparation for these experiments has been previously described (2, 4, 19). Briefly, mice were anesthetized with Ketaject (50 mg/kg ip; Phoenix Scientific, St. Joseph, MO) and Inactin (100 mg/kg ip; Sigma, St. Louis, MO), and then a cannula (polyethylene tubing, PE-90; Becton Dickinson, Lincoln Park, NJ) was inserted in the trachea to facilitate breathing. The stomachs were then exteriorized through a midline abdominal incision and everted (inside-out) to expose the gastric corpus mucosa for imaging. Animals were placed supine in a 37°C chamber on the stage of an inverted two-photon/confocal microscope (Zeiss LSM 510 NLO; Carl Zeiss, Jena, Germany), with a portion of the mucosa protruding into the chamber and facing a Zeiss C-Apo X40 water immersion objective lens. All experimental procedures have been approved by the Animal Care and Use Committee of the University of Cincinnati.
Drugs/perfusate solutions.
The exposed gastric epithelium was continuously superfused at 0.2 ml/min (KD Scientific push/pull syringe pump), with lightly buffered pH 3, pH 5 (150 mM NaCl + 4 mM homopiperazine-N,N′-bis-2-(ethanesulfonic acid) or pH 7 (150 mM NaCl + 4 mM HEPES) solutions. In some experiments, EIPA (12 mg/kg) was injected intravenously (iv) to block Na+/H+ exchange. In other experiments, SITS (0.1 mM) or H2DIDS (0.5 mM; Invitrogen, Eugene, OR) was added to the perfusate to block anion transport. To measure tissue permeability, fluorescein-dextran [70 kDa, 4 mg/kg, excitation (EX) 488 nm, emission (EM) 535–590 nm, Invitrogen] or Alexa-647-dextran (10 kDa, 4 mg/kg, EX 633 nm, EM > 650 nm, Invitrogen) was injected iv. In other experiments, dmPGE2 (0.1 mg/kg) was added topically to the exposed gastric mucosa for 5 min to stimulate gastric HCO3− secretion. All drugs were solubilized in H2O or saline and purchased from Sigma unless noted.
RNA extraction and real-time PCR.
Total RNA was extracted from SLC26A9 WT (n = 4) and KO (n = 4) mouse stomachs using TRI reagent (Sigma). RNA concentration was measured using a NanoDrop Spectrometer (Thermo Scientific, Wilmington, DE), and 1 μg RNA was reverse-transcribed to cDNA using random primers (Promega, Madison, WI) or iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Primers were designed for mouse AE4 on the basis of its GenBank expressed sequence tag (NM_172830.2), confirmed for specificity using a Basic Local Alignment Search Tool (BLAST) and production of a single amplicon of expected size by conventional PCR (120 bp), and used for real-time PCR amplification with Sybr Green as reporter. Forward primer was 5′-AGGTGTCTAAGGACCCTCTGC-3′, and reverse primer was 5′-CGCCCATGTCTAGCTTTTCC-3′. GAPDH was used as a normalization control (5′-AACGACCCCTTCATTGAC-3′ forward primer, 5′-TCCACGACATACTCAGCAC-3′ reverse primer).
In vivo confocal imaging of pH.
For pHi measurements, the gastric epithelium was topically loaded with 5 μM SNARF-5F 5-(and-6)-carboxylic acid-AM acetate (SNARF-5F-AM; EX 543 nm, EM 550–600, and 620–680 nm; Invitrogen) for 30 min. SNARF-5F-AM was diluted from a 5 mM DMSO stock solution with pH 7 buffered saline (150 mM NaCl + 20 mM HEPES). For pHo experiments, the cell impermeant pH indicator Cl-NERF (10 μM, EX 514 and 458 nm, EM >530 nm; Invitrogen) was added to the pH 3 perfusate. Cl-NERF was also used to assess tissue permeability in response to damage.
Live tissue microscopy and creation of two-photon microlesions.
Following 20-min animal equilibration on the microscope stage, the gastric epithelium was imaged with the use of the endogenous fluorophore NAD(P)H as a marker of healthy cell metabolism (720 nm EX, 435–485 nm EM). Structural appearance of the epithelium was monitored by images of 720 nm confocal reflectance. Two-photon imaging in this system was advantageous, in that it allowed switching from low-power (70 mW), nondestructive imaging of NAD(P)H, to high-power (350 mW) photodamage to a select group of two to three cells, as reported previously (19).
Briefly, a small region (2–3 cells, ∼200 μm2) of the gastric epithelium was subjected to photodamage by 720-nm light, using a femtosecond pulsed titanium sapphire laser (Mai Tai, Spectra-Physics, CA) to repetitively scan the targeted region for 5–8 s. The induction of photodamage was monitored by the rapid loss of cellular NAD(P)H autofluorescence within the targeted region, and time course imaging (up to 60 min) evaluated the progress of damage and repair, measuring reflectance, NAD(P)H, and a ratiometric pH indicator at each time point. Using new regions of undamaged tissue, multiple independent cycles of photodamage and repair were induced in each animal. We have previously reported that the tissue response to each microlesion is not different in initial vs. later lesions (19).
Image analysis.
Images were analyzed using MetaMorph software (Molecular Devices, Downingtown, PA). The area of epithelial cells that lost NAD(P)H over time was used to quantify damage area (19).
To measure pHo or pHi, background-corrected ratio images of Cl-NERF or SNARF-5F fluorescence were used, respectively (19). For pHo measurements, an 11-μm2 region was measured directly adjacent to the tissue site of damage, and average fluorescence intensity was measured within that region. For pHi measurements, regions of the gastric surface were evaluated that included separately 1) epithelial cells near the damaged area (5–10 μm), 2) cells far from the damaged area (100–150 μm), or 3) cells in the damaged area. Ratio values were converted to pH, using a standard curve of the relevant pure dye in solution.
Statistics.
Data are presented as means ± SE and were analyzed using Student's t-test or one- or two-way ANOVA (GraphPad Prism 4 software), with Bonferroni's post hoc test. P < 0.05 was considered significant.
RESULTS
The goal of this study was to determine whether paracellular leakage pathways and/or transcellular acid/base transport were involved in regulating surface pH after photodamage to the gastric epithelium. Using an assay to monitor flux of plasma constituents across the epithelium, we first asked whether photodamage increased paracellular permeability, potentially contributing to changes in gastric surface pH.
Paracellular permeability.
Mice were injected intravenously with Alexa-647 dextran (10 kDa) or fluorescein-dextran (70 kDa) to load the gastric vasculature with a fluorescent plasma. Figure 1 confirms the vascular labeling and distribution of each dextran in the gastric mucosa. Whereas the 70-kDa dextran remained more tightly restricted to gastric blood vessels (Fig. 1B), the 10-kDa dextran appeared to permeate the interstitial tissue (Fig. 1A). When photodamage was induced at the gastric surface, there was a gradual accumulation of both dextrans at the site of damage (shown for the fluorescein-dextran in Fig. 2, A and B), but no measurable egress was seen into the luminal spaces adjacent to the damage where surface pH changes are normally observed, or into healthy cells far from the damage (Fig. 2B). In control experiments without dextrans, photodamage did not increase autofluorescence within the damaged area at wavelengths used to image either Alexa-647 or fluorescein (data not shown).
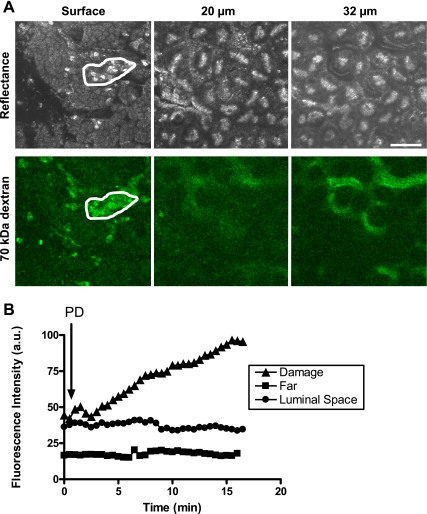
Fig. 1.
Fluorescent-dextran labeling of gastric blood vessels. The gastric epithelium of anesthetized mice was surgically exposed, and Alexa-647-dextran (10 kDa, 4 mg/kg) (A) or fluorescein-dextran (70 kDa, 4 mg/kg) (B) was injected via tail vein. Top images in each set are confocal reflectance, and bottom images are respective fluorescence images, focusing into tissue 16 or 28 μm as indicated, ∼15 min after dextran injection. Representative images from 4 experiments are shown. Scale bar = 50 μm.
Fig. 2.
Effect of photodamage on plasma leakage to gastric lumen. Mice were intravenously injected with Alexa-647-dextran (4 mg/kg, n = 3) or fluorescein-dextran (4 mg/kg, n = 3) and were placed on the microscope stage following surgical exposure of the gastric mucosa. A: representative confocal reflectance and fluorescein-dextran images showing that, after photodamage (PD), fluorescein-dextran accumulates in the damaged area (white region), and submucosal vessels are still brightly labeled. Scale bar = 50 μm. B: representative time course from a single image series showing the intensity of fluorescein-dextran within the damaged area (▲), in control cells far from the damage (■), or in luminal spaces adjacent to damage (●).
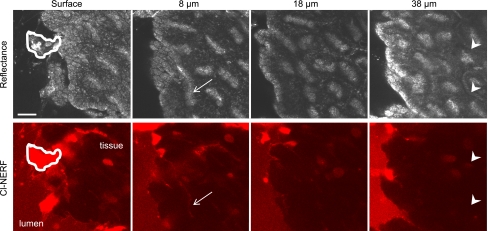
To extend these findings, we also examined permeation in the opposite direction. We tested whether a cell-impermeant luminal dye (Cl-NERF) could permeate into tissue beyond the damaged area, which would indicate disruption of barrier function and/or damage to blood vessels. In these experiments, photodamage was induced at the gastric surface, and following repair of the epithelium, confocal z-stacks were collected (through the area of damage) to examine Cl-NERF fluorescence deep into the tissue (40 μm), as well as in gastric blood vessels. Figure 3 shows representative reflectance and Cl-NERF images, with the exfoliated damaged area indicated at the gastric surface (white region). The damaged area became highly permeable to Cl-NERF, in accordance with our previous findings (19). Focusing 8 μm from the surface, some Cl-NERF fluorescence is visible, but it appears to be restricted to the cell layer directly beneath the damaged area/repaired epithelium, as well as in the openings to gastric pits (white arrows), which have direct access to luminal Cl-NERF even before photodamage. However, at deeper tissue depths, Cl-NERF fluorescence is not detectable in the vasculature (white arrowheads) or surrounding tissue. Therefore, local damage to the epithelium does not appear to cause gross changes in tissue and vessel permeability that cause significant accumulation of extracellular molecules in the spaces above or below the epithelial layer.
Fig. 3.
Effect of photodamage on permeability of tissue to luminal Cl-NERF. Following surgical exposure of the gastric mucosa, mice were placed on the microscope stage, and tissue was superfused with 10 μM Cl-NERF added to the luminal perfusate. After photodamage, bright Cl-NERF is visible in the damaged cells (white region) but does not permeate deeper into tissue (arrowheads). Just below the surface (8 μm), Cl-NERF labeling of gastric pit openings is visible (arrow). Scale bar = 25 μm.
Measuring gastric epithelial pHi in vivo.
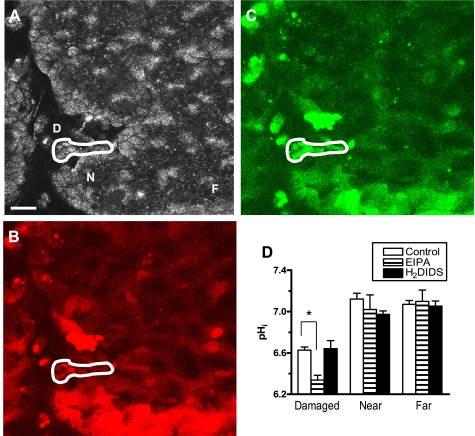
Next, we asked whether photodamage activated acid/base fluxes in the gastric surface cells, as a potential hallmark for activation of transcellular HCO3− transport. We developed an in vivo assay for pHi measurements of the mouse gastric epithelium. After establishing a successful dye-loading protocol using SNARF-5F-AM (see materials and methods), we quantified pHi in epithelial cells before and after photodamage under different ion transport inhibitory conditions. Because of dye leakage, experiments measuring pHi longer than ∼20 min were not routinely possible. Using 720-nm confocal reflectance and SNARF-5F fluorescence images, successful imaging of the gastric epithelium with SNARF-5F-AM was achieved, with some heterogeneity in loading of individual cells (Fig. 4, A–C). Under resting conditions (before photodamage), surface cell pHi was similar in control, EIPA-, and H2DIDS-treated mice (Fig. 4D). H2DIDS was used instead of DIDS because it is nonfluorescent and would therefore not interfere with our image collection. Treatment of mice with another anion transport inhibitor, 0.1 mM luminal SITS, also did not affect resting surface cell pHi (7.11 ± 0.05, n = 4). After photodamage was induced, pHi was measured 10 min postdamage in damaged, near, and far cells, in the same microscopic field of view. In these experiments, the damaged cell area is defined as those cells that have lost NAD(P)H autofluorescence (cell viability) but retain confocal reflectance (19). Near cells are those adjacent to damaged cells, and far cells are those 100–150 μm from the site of damage. Figure 5 shows confocal reflectance (Fig. 5A) and SNARF-5F fluorescence images (Fig. 5, B and C), with indications for damaged (D), near (N), and far (F) cell locations. As shown, damaged cells retain the pHi indicator. As shown in the compiled results of Fig. 5D, the damaged cells acidified (6.63 ± 0.03, P < 0.05) compared with healthy near and far cells, which maintained similar predamage pHi. It was noted that some cells not subjected to photodamage were shed from the monolayer and also are acidic (data not shown). These potentially interesting shed cells were not analyzed in the present study. H2DIDS (6.64 ± 0.08, P > 0.05) had no effect on damaged cell acidification compared with control, but EIPA treatment caused a further damaged cell acidification (6.34 ± 0.05, P < 0.05), suggesting that Na+/H+ exchange was activated to extrude H+ from the damaged cells.
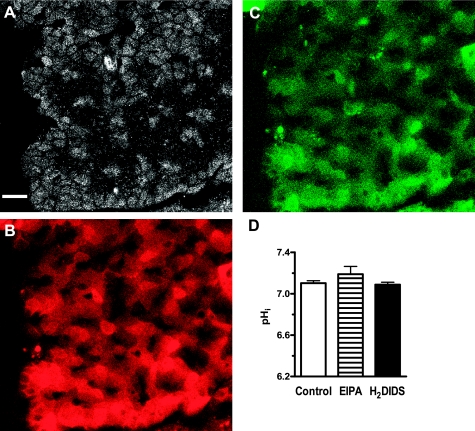
Fig. 4.
Resting intracellular pH of gastric surface cells after treatment with inhibitors of Na+/H+ exchange or HCO3− transport. Confocal reflectance (A) and SNARF-5F fluorescence images of 620–680 nm (B) and 550–600 nm (C) emission illustrate loading of gastric surface cells in vivo. Bar = 25 μm. D: resting intracellular pH (pHi) in control (n = 12), EIPA- (n = 12, 12 mg/kg iv), and H2DIDS-treated (n = 15, 0.5 mM in perfusate) mice. Values represent mean ± SE.
Fig. 5.
Gastric surface intracellular pH response to photodamage. Values are reported for damaged (D) cells, compared with healthy near (N) and far (F) cells. Confocal reflectance (A) and SNARF-5F fluorescence images of 620–680 nm (B) and 550–600 nm (C) emission illustrate dye retention in damaged cell areas (white region). Bar = 25 μm. D: pHi was measured in control (n = 3), EIPA- (n = 4), and H2DIDS-treated (n = 5) mice 10 min after photodamage. Values represent mean ± SE. *P < 0.05 vs. control.
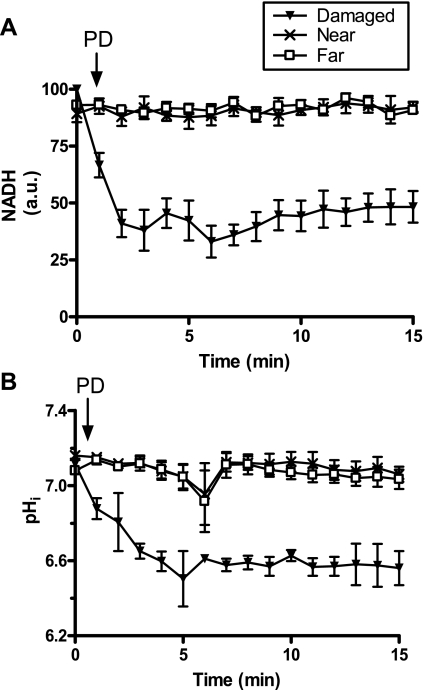
One consequence of photodamage to the gastric epithelium is rapid loss of cellular NAD(P)H within the damaged cell area (19). Therefore, we questioned whether loss of NAD(P)H and intracellular acidification occurred simultaneously. We measured NAD(P)H fluorescence and pHi in the damaged, near, and far cell types over a short 15-min time course. As shown in Fig. 6A, the induction of photodamage caused a rapid loss of NAD(P)H fluorescence within the damaged area, while near and far cells remained healthy. We found that maximal loss of NAD(P)H preceded maximal acidification (Fig. 6B), although both measures were already different from control values by 1 min after photodamage. Results suggest that both events initiate in similar time frames as a rapid response to damage, but the changes in pHi are not directly caused by H+ ions released in the cytosol from NAD(P)H loss.
Fig. 6.
Correlation of damaged cell acidification with loss of NAD(P)H autofluorescence. The gastric epithelium was loaded with SNARF-5F 5-(and-6)-carboxylic acid-AM acetate (SNARF-5F-AM) and NAD(P)H (A), and pHi (B) levels were measured before and after photodamage throughout a 15 min time course. Means ± SE, 4 experiments per group.
Luminal pH and cell permeability.
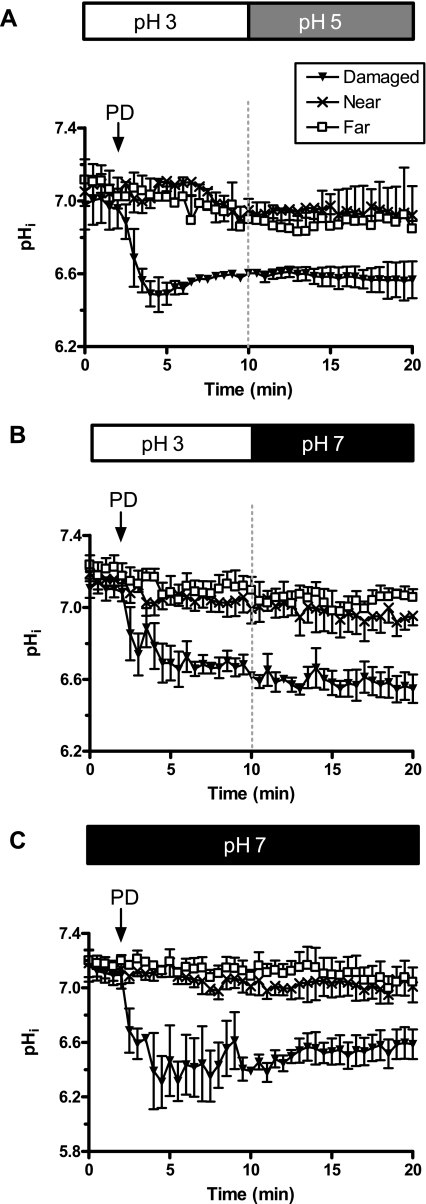
Results in Figs 2 and 3 suggest that damaged cells become permeable to at least some extracellular molecules. Therefore, we asked whether the pH of the luminal perfusate influenced damaged cell pHi. In these experiments, photodamage was induced, and after 10 min the luminal perfusate was changed from pH 3 to either pH 5 (Fig. 7A) or pH 7 (Fig. 7B). As shown in Fig. 7, pHi of the damaged cells was not altered after luminal pH was increased to pH 5 or pH 7. The healthy near and far cells were also unaffected by changes in luminal pH.
Fig. 7.
Influence of luminal pH on gastric surface intracellular pH. The gastric epithelium was loaded with SNARF-5F, and pHi was measured before and after photodamage throughout a 20-min time course. From time 0 until 10 min, the tissue was superfused with pH 3 lightly buffered saline and, at 10 min, was switched to either pH 5 (A) or pH 7 (B) lightly buffered saline. C: in other experiments, the tissue was superfused with only pH 7 lightly buffered saline. Data represent means ± SE from 4 experiments per group.
It is possible that the damaged cells in Fig. 7, A and B, could not realkalinize following luminal pH increase because they restored proton impermeability within the first 10 min after damage. Therefore, we conducted experiments where the gastric epithelium was superfused with pH 7 perfusate even at the time of damage. In this condition, damaged cell acidification was similar (Fig. 7C). In statistical comparisons (one-way ANOVA) made 20 min after damage, the damaged cell pHi values were similar in all three experiments, so we conclude that luminal protons are not contributing to the observed changes in pHi of damaged cells.
Cl−/HCO3− exchange, intracellular pH, and surface pH.
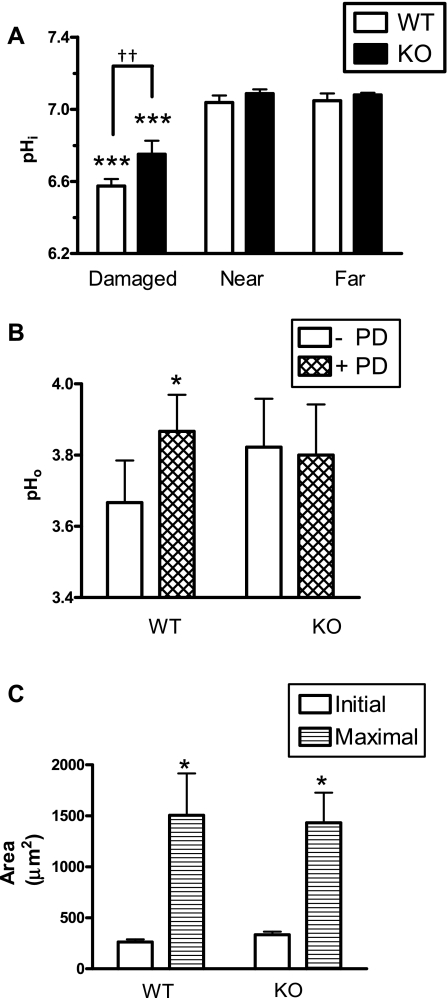
The SLC26A9 protein has been reported to function as a Cl−/HCO3− exchanger and to localize to the apical membrane of gastric surface epithelial cells (27). To ask whether this transport contributes to HCO3− secretion in this cell type, we measured pHi in the gastric epithelium of SLC26A9 WT and KO littermates before and after photodamage. Before photodamage, surface epithelial pHi was similar in WT (7.1 ± 0.06, n = 4) and KO (7.1 ± 0.03, n = 3) animals. After photodamage, only the damaged cells of both genotypes acidified (Fig. 8A). Damaged cell pHi in KO mice was significantly higher compared with WT mice (6.75 ± 0.13 vs. 6.58 ± 0.08, P < 0.01), suggesting that acid/base fluxes in damaged cells of KO mice are altered compared with WT. We measured surface pH (pHo) in both SLC26A9 WT and KO animals (Fig. 8B) and found that, whereas resting pHo was similar in WT (3.67 ± 0.12, n = 9) and KO (3.8 ± 0.15, n = 9) animals, the transient rise in pHo in response to photodamage that occurred in the WT animals was absent in the KO animals. To determine whether the resting pHo in the SLC26A9 KO animals was being maintained by enhanced expression of another surface epithelial Cl−/HCO3− exchanger, semiquantitative real-time PCR was performed, checking for AE4 expression. We found that AE4 mRNA levels were similar in stomachs from the SLC26A9 WT (524 ± 181-fold lower than GAPDH, n = 4) and KO (456 ± 250-fold lower than GAPDH, n = 4), suggesting that increased levels of AE4 are unlikely to explain observations in the SLC26A9 KO animal.
Fig. 8.
Intracellular pH, surface pH, and damage progression measured in solute-like carrier 26A9 (SLC26A9) wild-type (WT) and knockout (KO) animals. A: gastric epithelium of SLC26A9 WT (n = 4) or KO (n = 3) mice was loaded with SNARF-5F-AM, and pHi was measured before and after photodamage in damaged, near, and far cells in the same microscopic field. B: the gastric mucosa of WT (n = 9) or KO (n = 9) mice was superfused with pH 3 perfusate, and surface pH (pHo) was measured before and after photodamage. C: damage sizes were measured as those cells lacking NAD(P)H but retaining confocal reflectance. Initial size was measured immediately after photodamage, and maximal damage was evaluated over the first 10 min after photodamage. Means ± SE, *P < 0.05 vs. initial size; ***P < 0.001 vs. near cells; ††P < 0.01.
We also measured damage progression and epithelial repair in both WT (n = 3) and KO (n = 4) animals. Comparing between genotypes, there was no significant difference in initial damage sizes, damage expansion to maximal sizes (Fig. 8C), or time to repair damage [17 ± 5 min (WT) vs. 12 ± 1 min (KO)].
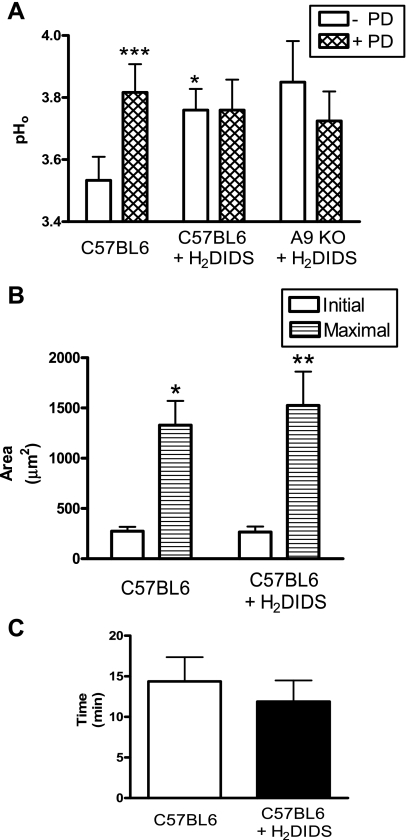
It is known that SLC26A9 is a DIDS-sensitive transporter (27). Therefore, we conducted complementary experiments in normal B6 mice, measuring gastric pHo before and after photodamage in the presence of 0.5 mM H2DIDS added to the pH 3 luminal perfusate. Before photodamage, resting pHo was significantly higher in the presence vs. absence of H2DIDS (Fig. 9A). After photodamage, surface pH transiently and significantly increased in control animals (n = 6, P < 0.001), but H2DIDS blocked the surface pH increase (n = 5). Adding H2DIDS to the A9 KO animals (n = 4) had no effect on surface pH before (3.8 ± 0.15 vs. 3.9 ± 0.13, P > 0.05) or after photodamage (Fig. 9A). Treatment with H2DIDS had no effect on damage progression or the timing of epithelial repair (Fig. 9, B and C). These results suggest that H2DIDS acts to block gastric HCO3− secretion by blocking SLC26A9, but another transporter that is H2DIDS-insensitive can contribute to resting pHo (before damage).
Fig. 9.
Surface pH, damage progression, and epithelial repair measured during HCO3− transport inhibition with H2DIDS. A: extracellular gastric surface pH (pHo) was measured before (open bar) and after (hatched bar) photodamage, using C57BL/6 mice in pH 3 perfusate without (control, n = 6) or with 0.5 mM H2DIDS (n = 5) in the pH 3 perfusate. SLC26A9 KO (n = 4) were also examined in the presence of H2DIDS. B: initial size was measured immediately after photodamage, and maximal damage was evaluated over the first 10 min after photodamage. C: epithelial repair was evaluated as the time taken for the damage size to return to or below initial size. Means ± SE, *P < 0.05 and ***P < 0.001 vs. C57BL6 (- PD); *P < 0.05 and **P < 0.01 vs. initial size.
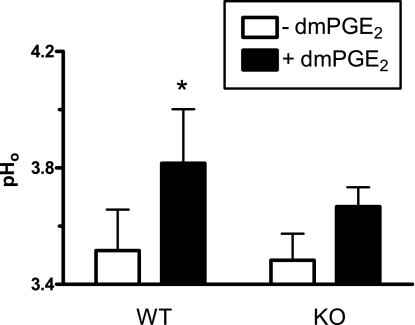
Prostaglandins can increase gastric surface HCO3− secretion. To test whether this regulated secretion was mediated by SLC26A9, we asked whether A9 KO animals could secrete HCO3− in response to exogenous PGE2 (using dmPGE2 with increased stability). Figure 10 shows gastric pHo measurements before and after dmPGE2 treatment, in both WT and KO animals. In WT animals, pHo significantly increased in response to dmPGE2 treatment (P < 0.05, n = 6). In KO animals, pHo was not significantly altered by dmPGE2 (n = 6). Further statistical analysis (one-sample t-test vs. zero) showed that, in KO animals, the change in pHo after dmPGE2 treatment (ΔpHo) was not significantly greater than zero, suggesting that SLC26A9 fully mediates dmPGE2-stimulated HCO3− secretion.
Fig. 10.
Surface pH measured in SLC26A9 WT and KO animals after exogenous 16,16-dimethyl-PGE2 (dmPGE2) treatment. Following initial pHo measurements (− dmPGE2), pHo was recorded 5 min after adding 0.1 mg/kg dmPGE2 topically to the gastric mucosa (+ dmPGE2). Means ± SE of n = 6 animals in each condition. *P < 0.05 − dmPGE2.
DISCUSSION
Because of its exposure to a harsh acidic lumen, the gastric epithelium has multiple lines of defense that protect it from endogenous and exogenous damaging agents. It is known that the first line of defense, the preepithelial mucus-HCO3− barrier, is a microenvironment in which pH directly adjacent to the gastric surface can be higher than pH of the bulk lumen. Because we and others have shown that surface pH is dynamically controlled (3, 5) and increases in response to epithelial damage (19), the goal of this study was to identify whether paracellular and/or transcellular mechanisms cause surface pH changes in response to microscopic epithelial damage.
Paracellular vs. transcellular bicarbonate flux.
In response to extensive surface damage (e.g., acidified aspirin or ethanol), others have observed that the exfoliated cell contents form a mucoid cap over the mucosa that contains not only dead cells and mucus, but also plasma components and fibrin (9, 11–13). The presence of macromolecules from blood suggests that these models have an extensive paracellular leak of materials as a result of damage, and this could explain the raise in pH noted in both these models and our model of microscopic damage. In contrast, our measures of paracellular flux from lumen to tissue or tissue to lumen failed to detect a major pathway for flow of macromolecules in the region where microscopic cell damage had occurred (Figs. 2 and 3). The utilized fluorescent dyes are certainly larger than the HCO3− anion and would therefore not be predicted to be a reliable indicator of any diffusional flow of HCO3− via paracellular routes. However, given the large distances that must be traversed from blood to lumen, diffusion is unlikely to be a major contributor. In contrast, the dyes should be a reliable reporter of any macroscopic fluxes of tissue extracellular fluids into the lumen. The assays did detect increased accumulation of the normally impermeant substances within damaged cells, suggesting that enhanced transcellular flow could be one route for enhanced flux of materials across a damaged cell monolayer. However, we conclude that such compromises in cellular permeability must be brief, as damaged cells are able to sustain impermeability to luminal protons (Fig. 7) as well as retain SNARF dye (Fig. 5) during the course of cell exfoliation and epithelial repair. We conclude that the macroscopic flux of materials between or through damaged gastric surface cells is a negligible route for egress of tissue components such as HCO3− or any of the macromolecules in the tissue fluids.
Our results provide evidence that the cellular mechanisms of HCO3− secretion are a major contributor to the increase in surface pH (pHo) seen after microscopic damage. This pHo change is diminished by luminal H2DIDS in WT mice or by genetic deletion of the SLC26A9 Cl−/HCO3− exchanger (Figs 8 and 9). Furthermore, H2DIDS has no additional effect in the SLC26A9 knockout mouse (Figs. 8 and 9), and dmPGE2 is ineffective at restoring the pHo increase to significant levels in the KO mouse (Fig. 10). The simplest explanation of these pHo observations is that the dmPGE2-stimulated HCO3− secretion is mediated by H2DIDS-inhibitable SLC26A9, which is activated in response to damage. This is consistent with prior observations that gastric SLC26A9 is activated during acid-induced HCO3− secretion (27) although the molecular mechanism of SLC26A9 activation in either of these conditions remains unknown. One major limitation of our model of gastric damage is that it has been impossible to date to identify the microscopic region of damage (maximum ∼20 cells) for immunohistochemical or microdissection analyses; therefore, we have been limited to functional readouts. The cell acidification noted in our study is most likely not the primary stimulus for alkali extrusion by SLC26A9 unless the activation of basolateral Na+/H+ exchange is somehow linked via a regulatory pathway to SLC26A9 activity in an attempt to balance cytosolic pH. The specific signaling pathways regulating SLC26A9, and the response to damage, remain to be determined.
Even in the presence of H2DIDS, or SLC26A9 KO, the resting surface pH remains higher than the perfusate pH. This suggests that multiple routes exist for cellular HCO3− secretion (e.g., the DIDS-insensitive AE4 apical Cl−/HCO3− exchanger may also contribute). Analysis of AE4 protein expression by Western blot was unsuccessful (data not shown), but real-time PCR suggests that AE4 mRNA abundance in both the SLC26A9 WT and KO stomach are similar. Alternatively, it is possible that increased secretions from pit or gland cells also explain surface pH observations in the SLC26A9 KO, because pH in the extracellular milieu will integrate the net acid/base transport from multiple cells in the mucosa. Future work will be needed to define the contribution of specific cell types and membrane transporters to surface pH homeostasis in the SLC26A9 KO model.
Cellular source of transcellular bicarbonate flux.
Measurements of intracellular pH can help identify cells that have altered acid/base transport in response to damage. In this report, we compared the acid/base status of surface cells that were damaged and that would be exfoliated from the monolayer, and compared their response to healthy cells either very far from the hurt cells or directly adjacent to the hurt cells. We observed that photodamage caused a selective acidification of only the damaged cells and that these cells activated Na+/H+ exchange, but not any H2DIDS-sensitive acid/base transport in response to damage (Fig. 5). Inhibitor studies also suggested that, at normal resting pHi, neither of these transporters was active (Fig. 4). It is possible that compensatory fluxes were activated in the presence of H2DIDS that confounded results or that the surface pH increase may come from a cell type that was not analyzed in this study (e.g., mucus cells in the gastric pit or neck region). Because the SLC26A9 knockout had similar resting pHi as wild-type mice, but a higher pH in damaged cells (Fig. 8), there is some support for a role of SLC26A9 in the surface cell that may not have been revealed by measurements of pHi after H2DIDS.
The mechanism of damaged cell acidification is still unclear. Results suggest that it is not due to uptake of luminal H+ (Fig. 7). Simultaneous measurements of NAD(P)H autofluorescence and pHi showed that damaged cells lost NAD(P)H before becoming maximally acidified, suggesting that acidification could be a direct result of laser damage, but is unlikely to be attributable to H+ released from NAD(P)H consumption or destruction. Previously, high-power, near-infrared (two-photon) laser irradiation has been shown to cause production of reactive oxygen species (ROS), leading to apoptosis, a process associated with cell acidification (22). In our gastric damage model, we speculate that damage proceeds via NAD(P)H absorption of light energy, membrane disruption, and consequent production of cytotoxic ROS (19), leading to the spread of damage to adjacent cells. Because our model involves the destruction of cellular (cytosolic + mitochondrial) NAD(P)H, leading to irreversible cellular damage, it may result in increased NAD(P)H oxidation, whereby intracellular [H+] would increase.
Role of surface pH change in restitution.
Lastly, our results show that the transient surface pH increase observed after photodamage is not necessary for epithelial repair. SLC26A9 KO mice and H2DIDS-treated mice repair damage normally despite surface pH increase inhibition in both conditions. Because we observed the trend toward a response of the SLC26A9 KO animals to dmPGE2, it is possible that a second HCO3− secretory route is more important for repair of damage, because repair can occur in the absence of SLC26A9-mediated HCO3− secretion. Regardless, because gastric surface pH remained at 3.5–4.0 even under SLC26A9 inhibition, we cannot rule out the possibility that the pathway regulating the absolute value of surface pH, whether through prostaglandins such as PGE2 or another transporter, also regulates repair of damage. Our analyses suggest that the SLC26A9 KO stomach does not have global increases in the apical AE4 anion exchanger but do not rule out increased activity of AE4 independent of increased protein levels. Future studies will seek to elucidate which acid/base secretory pathways regulate this repair process.
In summary, we find that cellular HCO3− secretion, and not paracellular leakage, lead to the raised pH at the gastric surface after microscopic damage. This surface pH increase is fully mediated by the SLC26A9 Cl−/HCO3− exchanger but is not necessary for efficient epithelial repair. We hypothesize that another HCO3−-secretory pathway that operates without need for PGE2 stimulation is more important for repair of microscopic damage.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (R01 DK 54940 and DK 62809).
REFERENCES
- 1.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev 73: 823–857, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner HK, Kirbiyik U, Coskun T, Chu S, Montrose MH. Endogenous cyclo-oxygenase activity regulates mouse gastric surface pH. J Physiol 544: 871–882, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgartner HK, Montrose MH. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface pH. Gastroenterology 126: 774–783, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner HK, Starodub OT, Joehl JS, Tackett L, Montrose MH. Cyclooxygenase 1 is required for pH control at the mouse gastric surface. Gut 53: 1751–1757, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu S, Tanaka S, Kaunitz JD, Montrose MH. Dynamic regulation of gastric surface pH by luminal pH. J Clin Invest 103: 605–612, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feil W, Klimesch S, Karner P, Wenzl E, Starlinger M, Lacy ER, Schiessel R. Importance of an alkaline microenvironment for rapid restitution of the rabbit duodenal mucosa in vitro. Gastroenterology 97: 112–122, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Guttu K, Grong K, Svanes K, Gronbech JE. Gastric mucosal repair and release of HCO3− after damage by 2 M NaCl in cat: role of systemic acid base status. Am J Physiol Gastrointest Liver Physiol 267: G536–G545, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, Oshima T, Tomita T, Kim Y, Matsumoto T, Joh T, Miwa H. Oxidative stress induces gastric epithelial permeability through claudin-3. Biochem Biophys Res Commun 376: 154–157, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Ito S, Lacy ER. Morphology of rat gastric mucosal damage, defense, and restitution in the presence of luminal ethanol. Gastroenterology 88: 250–260, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Joutsi T, Paimela H, Bhowmik A, Kiviluoto T, Kivilaakso E. Role of Na(+)-H(+)-antiport in restitution of isolated guinea pig gastric epithelium after superficial injury. Dig Dis Sci 41: 2187–2194, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Lacy ER. Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol 10, Suppl 1: S72–S77, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Lacy ER, Ito S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology 83: 619–625, 1982 [PubMed] [Google Scholar]
- 13.Lacy ER, Ito S. Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab Invest 51: 573–583, 1984 [PubMed] [Google Scholar]
- 14.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 135: 41–60, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Machen TE, Paradiso AM. Regulation of intracellular pH in the stomach. Annu Rev Physiol 49: 19–33, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Phillipson M, Atuma C, Henriksnas J, Holm L. The importance of mucus layers and bicarbonate transport in preservation of gastric juxtamucosal pH. Am J Physiol Gastrointest Liver Physiol 282: G211–G219, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Rossmann H, Alper SL, Nader M, Wang Z, Gregor M, Seidler U. Three 5′-variant mRNAs of anion exchanger AE2 in stomach and intestine of mouse, rabbit, and rat. Ann NY Acad Sci 915: 81–91, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Schade C, Flemstrom G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology 107: 180–188, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Starodub OT, Demitrack ES, Baumgartner HK, Montrose MH. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol 294: C223–C232, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Takezono Y, Joh T, Oshima T, Suzuki H, Seno K, Yokoyama Y, Alexander JS, Itoh M. Role of prostaglandins in maintaining gastric mucus-cell permeability against acid exposure. J Lab Clin Med 143: 52–58, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Teorell T. The acid-base balance of the secreting isolated gastric mucosa. J Physiol 114: 267–276, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tirlapur UK, Konig K, Peuckert C, Krieg R, Halbhuber KJ. Femtosecond near-infrared laser pulses elicit generation of reactive oxygen species in mammalian cells leading to apoptosis-like death. Exp Cell Res 263: 88–97, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Tsuganezawa H, Kobayashi K, Iyori M, Araki T, Koizumi A, Watanabe S, Kaneko A, Fukao T, Monkawa T, Yoshida T, Kim DK, Kanai Y, Endou H, Hayashi M, Saruta T. A new member of the HCO3(−) transporter superfamily is an apical anion exchanger of beta-intercalated cells in the kidney. J Biol Chem 276: 8180–8189, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Wallace JL, McKnight GW. The mucoid cap over superficial gastric damage in the rat. A high-pH microenvironment dissipated by nonsteroidal antiinflammatory drugs and endothelin. Gastroenterology 99: 295–304, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM., Jr Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136: 236–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Barone S, Petrovic S, Wang Z, Seidler U, Riederer B, Ramaswamy K, Dudeja PK, Shull GE, Soleimani M. Identification of an apical Cl-/HCO3− exchanger in gastric surface mucous and duodenal villus cells. Am J Physiol Gastrointest Liver Physiol 285: G1225–G1234, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Henriksnas J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl−/HCO3− exchange, and is inhibited by NH4+. Am J Physiol Cell Physiol 289: C493–C505, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci USA 105: 17955–17960, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]