Abstract
Intestinal mucosal integrity is dependent on epithelial function and a regulated immune response to injury. Fucosyltransferase VII (Fuc-TVII) is an essential enzyme required for the expression of the functional ligand for E- and P-selectin. Trefoil factor 3 (TFF3) is involved in both protecting the intestinal epithelium against injury as well as aiding in wound repair following injury. The aim of the present study was to assess the interplay between barrier function and leukocyte recruitment in intestinal inflammation. More specifically, we aimed to examine how targeted disruption of Fuc-TVII either in wild-type or TFF3−/− mice would alter their susceptibility to colonic injury. TFF3 and Fuc-TVII double-knockout mice (TFF3/Fuc-TVII−/− mice) were generated by mating TFF3−/− and Fuc-TVII−/− mice. Colitis was induced by administration of dextran sodium sulfate (DSS) (2.5% wt/vol) in the drinking water. Changes in baseline body weight, diarrhea, and fecal blood were assessed daily. Upon euthanasia, extents of colonic inflammation were assessed macroscopically, microscopically, and through quantification of myeloperoxidase (MPO) activity. Colonic lymphocyte subpopulations were assessed at 6 days after administration of DSS by flow cytometry and immunohistochemistry. No baseline intestinal inflammation was found in TFF3/Fuc-TVII−/−, TFF3−/−, Fuc-TVII−/−, or wild-type mice. Loss of Fuc-TVII resulted in a reduction in disease severity whereas TFF3−/− mice were markedly more susceptible to DSS-induced colitis. Remarkably, the loss of Fuc-TVII in TFF3−/− mice markedly decreased the severity of DSS-induced colitis as evidenced by reduced weight loss, diarrhea, decreased colonic MPO levels and improved survival. Furthermore, the loss of TFF3 resulted in increased severity of spontaneous colitis in IL-2/β-microglobulin-deficient mice. These studies highlight the importance of the interplay between factors involved in the innate immune response, mucosal barrier function, and genes involved in regulating leukocyte recruitment and other aspects of the immune response.
Keywords: trefoil factor family 3
inflammatory bowel disease (IBD) is characterized by chronic gastrointestinal inflammation of unknown etiology (16, 34). It has been hypothesized that the development of IBD results from altered epithelial barrier function and a dysregulated immune response to intestinal microbes in genetically susceptible individuals (50). The maintenance of the mucosal barrier integrity is believed to be fundamental in preventing the induction of dysregulated immunity induced by microbes and other luminal components. The importance of the mucosal barrier in the pathogenesis of IBD is supported by the findings that intestinal permeability is altered in some first-degree relatives with Crohn's disease and that intestinal permeability precedes a flare of IBD (9, 17, 51). We have previously shown that trefoil factor (TFF) peptides contribute to mucosal barrier function by protecting the gastrointestinal mucosa from injury and promoting repair after an injury has occurred (2, 13, 14, 23, 35). The TFF proteins are classified into TFF1 (formerly pS2), TFF2 (formerly SP) and TFF3 (formerly ITF). The TFF proteins are highly protease-resistant and are secreted onto the mucosal surface. TFF1 is synthesized primarily in gastric mucosal pit cells, whereas TFF2 is produced both by gastric mucous neck cells and by duodenal submucosal Brunner's glands (19). Despite the normal restriction of TFF1 and TFF2 expression to the proximal gastrointestinal tract, TFF3 is abundantly expressed in the small and large intestine where it is secreted by goblet cells (45). Targeted deletion of the gene encoding TFF3 results in markedly increased susceptibility to colonic injury, which can be reversed by exogenous administration of TFF3 (23).
A large number of studies have been carried out to determine how dysregulated mucosal immune responses contribute to the pathogenesis of IBD (33). The mucosal immune system is poised to detect bacteria and antigens at the mucosal surface. Migration of innate immune cells, such as neutrophils, macrophages, and dendritic cells, into target mucosal tissues plays a pivotal role in mucosal immune response. The recruitment of leukocytes into the intestinal mucosa is dependent on the expression of numerous cytokines, adhesion molecules, and chemokines (44). Emigration of leukocytes from the blood is initiated by the capture of leukocytes from the bloodstream followed by their rolling along the endothelial cell surface. These processes are mediated by a group of glycoprotein adhesion molecules called selectins, which bind to carbohydrate determinants on selectin ligands. The function of selectin ligands is closely linked to the appropriate posttranslational glycosylation including fucosylation, sialylation, or sulfation. Furthermore, the addition of fucose to the GlcNAc of core 2 decorated O-glycans in α1,3 linkage [α(1,3) fucosylation] is a key step for synthesis of functional selectin ligands. To date, six α(1,3) fucosyltransferases (Fuc-T) have been identified. Among them, Fuc-TVII plays a crucial for selectin-dependent leukocyte recruitment and lymphocyte homing (22, 49) and expression is restricted to endothelial cells and leukocytes (18, 40, 43). Selectin ligand expression is absent in Fuc-TVII−/− mice as a consequence of disabled fucosylation of the ligand glycan structure. Consequently, leukocyte homing is substantially attenuated in Fuc-TVII−/− mice (22).
The goal of the present studies was to assess how genes involved in the inmate immune response, barrier function, and other aspects of immune regulation may interact to regulate both intestinal inflammation and homeostasis. Although many have hypothesized that IBD results from some form of environmental signal or insult, across a defective mucosal barrier, in individuals that have alterations in genes that regulate innate and/or adaptive immune responses, this paradigm has not been well assessed experimentally. To this end, we have assessed whether Fuc-TVII-dependent leukocyte recruitment impacts the development of dextran sodium sulfate (DSS)-induced colitis in mice deficient in the barrier protective molecule TFF3. To further define to the interactions of barrier function and immune regulation in mediating intestinal inflammation and homeostasis, we assessed the impact of the loss of TFF3 in a immune-mediated model of spontaneous colitis.
MATERIALS AND METHODS
Materials.
All chemicals were reagent grade unless otherwise indicated. Spermidine was purchased from Sigma (St. Louis, MO). Fresh proteinase K was purchased from Fisher (Worcester, MA). A Gene Screen Plus membrane was purchased from NENLIFE Science Products (Boston, MA). Taq polymerase and PCR buffer were purchased from Perkin-Elmer-Cetus (Norwalk, CT). DSS (molecular weight 40,000) was purchased from ICN Biomedical (Aurora, OH). SENSA paper was purchased from SmithKline Diagnostics (San Jose, CA). DC protein assay kit was purchased from Bio-Rad (Hercules, CA). Tricine gel was purchased from Novex (San Diego, CA), and polyvinylidene difluoride membrane was purchased from Millipore (Bedford, MA). Horseradish peroxidase-conjugated donkey anti-rabbit secondary antibody was purchased from Amersham Life Science (Arlington Heights, IL). Renaissance chemiluminescence was purchased from DuPont (Boston, MA). Mouse monoclonal antibodies used for flow cytometry or immunohistochemistry including FITC-CD3, FITC-B220, PE-CD4, PE-CD8, PE-F4/80, and PE-NK-1.1 were purchased from BD Biosciences Pharmingen (San Diego, CA).
Animals and construction of TFF3/Fuc-TVII−/− mice.
All animals were of a 129/C57B6 background between 8 and 16 wk of age. TFF3-deficient mice (TFF3−/− mice) have been described previously (23). Mice with targeted disruption of Fuc-TVII gene were produced by insertion of a neomycin phosphotransferase gene under control of the murine phosphoglycerate kinase promoter into a unique Eag site in the 5′ end of the coding region as described previously (4). TFF3/Fuc-TVII−/− mice were generated via breeding of homozygotes. For colitis experiments, animals were matched by age, sex, and body weight for each experiment. All animal experiments were performed in accordance with National Institutes of Health guidelines, and protocols were approved by the Subcommittee on Research Animal Care at the Massachusetts General Hospital and Harvard Medical School.
Determination of TFF3/Fuc-TVII−/− mice.
Southern blot and PCR analysis were used in establishing the TFF3/Fuc-TVII−/− homozygotes, as we have described previously (4, 23). Briefly, DNA was digested with BamH1 and BglII overnight according to the manufacturer's instructions. The digested DNA was then resolved on a 0.7% agarose gel and transferred to a Gene Screen Plus membrane and probed with a 3-kb BglII-Eag fragment. TFF3 genotyping was performed by PCR analysis. In brief, 0.5 μl of the above DNA suspension was added to 50 μl of PCR cocktail containing 1 × PCR buffer, 0.8 μM each primer, 0.2 mM dNTPs, and 1 U Taq polymerase. For TFF3, the primers were 5′-GCAGTGTAACAACCG-TGGTTGCTGC (sense) and 5′-TGACCCTGTGTCATCACC-CTGGC (antisense), and expected product size was 277 bp. For neomycin, the primers were 5′-CGGCTGCTCTGATGCCGCC (sense) and 5′-GCCGGCCACAGTCGATGA-ATCC (antisense); expected product size was 547 bp. Western blotting for TFF3 was performed on colonic mucosal scrapings as we have described previously (28).
Fucosyltransferase assay.
The α1,2/3-fucosyltransferase activity was measured using asialofetuin type-1 as the exogenous acceptor as previously described (5). Briefly, the enzyme activity was carried out in a reaction mixture (total volume of 0.1 ml) that contained 5 mM ATP, 10 mM l-fucose, 25 mM MnCl2, 50 mM NaCl2, 50 mM HEPES (pH 7.0), 60 nM GDP-fucose, 10 nCi GDP-[14C]fucose with 50 μg microsomal fraction of the intestinal mucosal preparation as a source of enzyme. The concentration of GDP-fucose was determined to be sufficient to maintain the enzyme activity at Vmax through the duration of the assay. The assay mixture was incubated for 30 min at 37°C and the incorporation of GDP-[14C]fucose on to asialofetuin was carried out by filtering the reaction mixture through 24 mm GF/B filter. The radioactivity of the filtered material was determined by scintillation counting as described previously (5). Specific positive and negative controls to determine the background incorporation of GDP-[14C]fucose and the enzyme reaction with in 30 min were linear at this concentration of substrates.
Microsomal fraction.
Intestine from each mouse was thoroughly with ice-cold 0.9% NaCl. The mucosa was harvested by scraping on a glass plate sitting on ice with a microscope slide. A 10% mucosal homogenate in 0.1 M Tris·HCl buffer (pH 7.4) was prepared over ice and centrifuged at 1,000 g for 15 min at 4°C to remove nuclei and cellular debris. The supernatant was then centrifuged at 105,000 g for 1 h at 4°C in a Beckman L8–80M ultracentrifuge with a SW41 Ti rotor. The resulting microsomal pellets were resuspended in the homogenization (Tris) buffer containing 1% Triton X-100 and 10% glycerol. Aliquots of microsomal fraction from each mouse were then frozen, stored at −80°C, or used immediately for the fucosyltransferase enzyme assay, as described above (5).
Lectin staining with UEA-1.
Expression of terminal α1,2-fucosyl glycans on glycoproteins and glycolipids on the mucosal surface was measured using FITC-conjugated UEA-1 lectin (Vector Laboratories, Burlingame, CA) on frozen section as described previously (20). The midcolon from each mouse was fixed in 4% paraformaldehyde for 4 h at 4°C, then washed in ice-cold PBS (pH 7.4) containing 30% sucrose. The tissue was embedded in optimal cutting temperature compound before sectioning in cryostat, and frozen sections (6–7 μm thick) were made and blocked with PBS containing 2% BSA before staining with labeled lectin for 1 h (10 μg/ml). Sections were then washed 3 times in ice-cold PBS, mounted using Anti-Fade (Vector Laboratories) and analyzed by confocal microscopy (5). Nonspecific staining was determined with incubation with excess fucose in the reaction mixture as well as staining with Lotus lectin.
Induction and assessment of colitis.
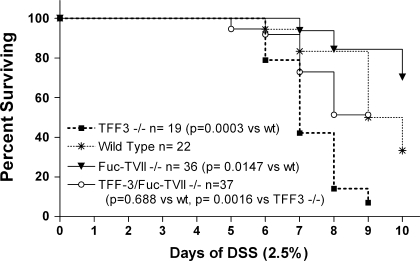
Colitis was induced by 2.5% DSS in drinking water (molecular weight 40,000, ICN Biomedical, Aurora, OH). The severity of diarrhea and fecal blood were assessed daily as previously described (1). Briefly, for the severity of diarrhea, we used a 0–3 scale: 0 = normal, 1 = soft, 2 = very soft but formed, 3 = liquid stool. For fecal blood, fecal pellet was suspended in H2O and was followed by brief centrifugation. The supernatant was added to a 0.5 × 0.5 cm piece of SENSA paper and developed with one drop of SENSA developer solution (SmithKline Diagnostics, San Jose, CA). The intensity of SENSA color change was scored by observers, blinded to animal group and treatment, on a 0–4 scale: 0 = nil, 1 = faintly blue, 2 = moderately blue; 3 = dark blue, 4 = fecal blood visible to the eye. Mice were then euthanized on day 3, day 6, and day 9 after administration of DSS in the drinking water commenced. The entire colon was removed and cut open along the mesenteric border. For microscopic evaluation, colonic segments were fixed in 10% neutral buffered formalin and embedded in paraffin. Colonic sections were stained with hematoxylin and eosin in standard fashion and examined by light microscopy. Histological assessment was performed in a blinded fashion by using the following criteria: 1) percent ulceration was assessed by determining the areas with loss of epithelium vs. areas of intact epithelium; and 2) inflammation was scored as 0 = normal, 1 = increased inflammatory cells in lamina propria, 2 = increased inflammatory cells in submucosa, 3 = dense inflammatory cell mass, 4 = transmural inflammation. Sections from the cecum and from 4 and 2 cm proximal the anus were assessed per animal. Animals were removed from study (euthanized) when body weight exceeded greater than 25% of initial basal body weight. Furthermore, mice were euthanized if they exhibited features of distress as assessed by veterinarian staff even if weight loss had not exceeded the 25% cutoff as per our institutional animal care and use committee (IACUC)-approved animal care protocol. Thus survival (Fig. 4) was assessed by these criteria.
Fig. 4.
Impact of the loss of TFF3 and/or Fuc-TVII on survival in DSS-induced colitis. The Kaplan-Meier method and survival comparisons were performed by using the log-rank or Mantel-Haenszel test. Note that survival was assessed as outlined in materials and methods and animals were removed (euthanized) from the study if they appeared in distress or lost more than 25% of basal body weight as per our institutional animal care and use committee-approved animal care protocol.
Myeloperoxidase assay.
MPO activity, to assess colonic granulocyte infiltration as an index of inflammation, was measured as described previously (8). Briefly, tissue samples from the proximal colon region were removed, rinsed in saline, and then immediately snap frozen in dry ice. Each tissue (50 mg) was homogenized in 1.0 ml of potassium phosphate buffer (50 mM, pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma). The homogenate was centrifuged at 14,000 rpm for 30 min at 4°C. MPO in the supernatant was activated by 0.0005% H2O2 in potassium phosphate buffer (5 mM, pH 6.0) containing 0.5 mM o-dianisidine dihydrochloride (Sigma). A microplate scanner was used to assess absorbance at 460 nm for three separate 30-s intervals. One unit of MPO activity was defined as one micromole of H2O2 broken down to H2O and O−.
Lamina propria leukocyte isolation and flow cytometry.
Leukocytes were extracted from the lamina propria of the colon as previously described (24). For flow cytometry, 2 × 105 colonic lamina propria cells were washed in PBS containing 0.2% BSA and 0.1% sodium azide (Sigma). They were subsequently first incubated with blocking buffer (10% normal mouse serum and 1 μg anti-CD 16/32 mouse antibody) for 20 min at 4°C then incubated with FITC- or Phycoerythin-tagged monoclonal antibodies for 30 min at 4°C. Cells were analyzed using Lysis II software on FACScan (Becton Dickinson, Mountain View, CA). FITC-CD3, FITC-B220, PE-CD4, PE-CD8, and PE-NK-1.1 antibodies were used (BD Biosciences Pharmingen).
Macrophage immunohistochemistry.
Paraffin-embedded sections (5 μm) were deparaffinized in a standard fashion, washed with water, and then heated in a microwave in 10 mM citrate buffer for two cycles of 5 min, washed again with water, incubated in 0.3% H2O2-methanol solution for 10 min, and then washed again with water. Slides were incubated with primary antibody (rabbit monoclonal anti-F4/80 or isotype control, BD Biosciences Pharmingen) in a humidified chamber for 24 h at 4°C. The sections were then rinsed three times in PBS and 0.01% Tween 20 for 10 min prior to incubation with biotinylated donkey anti-rabbit polyclonal antibody (1:500 dilution) for 2 h at room temperature. Vectastain ABC kit (Amersham Life Science) was used, and the slides were developed with a 3-3′-diaminobenzidine solution. Cells were counted and expressed as positive cells per high-powered field.
Animals and construction of TFF3/IL-2/β2m−/−−/− mice.
To further assess the hypothesis that alterations in immune function and barrier function can act synergistically in regulating intestinal homeostasis and inflammation we crossed the TFF3−/− line with mouse line that develops spontaneous colitis IL-2−/− mice that have a loss in β2 microglobulin (IL-2/β2m−/−) (described previously; Refs. 41, 42). These mice lack IL-2 and MHC class I develop spontaneous colitis as young as 8 wk; most animals have active colitis at 25–30 wk. The advantage of this line over the mice deficient in only IL-2 is that they develop less severe colitis and survive longer, allowing for a more detailed assessment of the interaction between these genes (41, 42).
These animals were mated in standard fashion and the resulting offspring were bred to generate mice with a deletion in all of the above three genes (IL-2/β2m/TFF3−/− mice). This was confirmed by PCR and Southern blotting as performed above. Again, all lines were of a 129/C57B6 background and housed as above. Animals were assessed between 25 and 30 wk. Upon euthanasia, the colon was assigned a macroscopic damage score: 0 = normal, 1 = erythema, mild bowel wall thickening, 2 = more pronounced erythema and bowel wall thickening, 3 = presence of erosions/ulcerations and strictures. Bowel wall thickness was assessed via histology (each ocular unit = 100 μm), and inflammation was score as above (0–4 scale). All assessment was done in a blinded fashion.
Statistical analysis.
Data are presented as means ± SE. Parametric data were analyzed by a one-way ANOVA followed by a Dunnett multiple-comparisons posttest. Nonparametric data (scoring) were analyzed by a Kruskal-Wallis test (nonparametric ANOVA) followed by a Dunn's multiple-comparisons posttest. An associated probability (P value) of <0.05 was considered significant. Survival curves were created using the Kaplan-Meier method and survival comparisons were performed using the log-rank or Mantel-Haenszel test to generate a two-tailed P value. All statistical analysis was performed with Graph Pad Instat and Prism 3.0 programs (San Diego, CA).
RESULTS
Generation and characterization of TFF3/Fuc-TVII−/− mice.
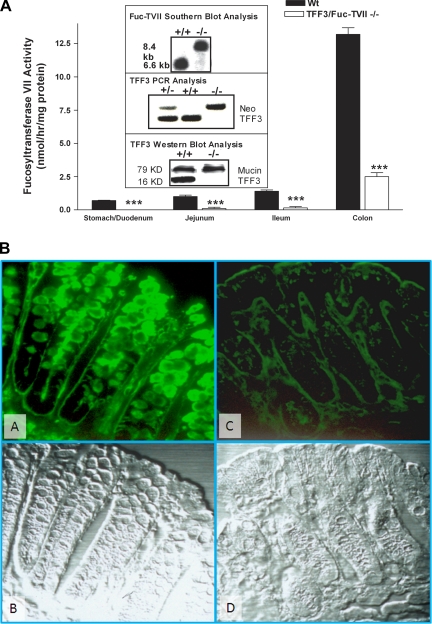
A TFF3/Fuc-TVII−/− murine line was successfully generated as confirmed by Southern blot analysis for Fuc-TVII and by PCR and Western blot analysis for TFF3 (Fig. 1A). The TFF3/Fuc-TVII−/− mice were viable and reproduced normally. There was no significant difference in body weight among untreated mice including wild-type, TFF3−/−, Fuc-TVII−/−, and TFF3/Fuc-TVII−/− mice. Assessment of untreated animals failed to demonstrate any signs of intestinal inflammation, diarrhea, or intestinal bleeding.
Fig. 1.
Characterization of TFF3/Fuc-TVII−/− mice. A: deletion of the TFF3 and Fuc-TVII genes was confirmed by Southern blot and PCR analysis. Loss of TFF3 protein expression was confirmed by Western blotting of colonic mucosal tissue. Loss of fucosyltransferase activity was confirmed in the stomach, duodenum, jejunum, ileum, and colon (***P < 0.001 compared with wild-type). Some fucosyltransferase activity was noted in the colon and likely represents a cross-reaction with other fucosyltransferase species. B: fucosylated glycan expression in the colon of the wild-type (A and B) and TFF3/Fuc-TVII−/− mice (C and D). UEA1 lectin binding of the fucosylated glycans was very high in the wild-type mice (A), and the expression was compromised in the mutant mice (C). B and D: Nomarski image of the same section of the colon, demonstrating normal morphology of the tissue. Wt, wild-type.
Fucosyltransferase activity in the TFF3/Fuc-TVII−/− line was markedly decreased in the stomach, duodenum, jejunum, and ileum (Fig. 1A). Colonic fucosyltransferase activity was also significantly (P < 0.001) reduced in the TFF3/Fuc-TVII−/− murine line with a mean activity level in full-thickness colonic sections being 2.5 ± 0.3 nmol·h−1·mg−1 protein compared with 13.2 ± 0.5 nmol·h−1·mg−1 protein wild-type mice. As expected, there was an increasing gradient of fucosyltransferase activity was observed in the wild-type mice, with the highest levels in the colon. Colonic fucosyltransferase activity in TFF3−/− was not different from that of wild-type animals (14.4 ± 1.8 nmol·h−1·mg−1 vs. 13.2 ± 0.5 nmol·h−1·mg−1), and the Fuc-TVII−/− mice had similar reductions in colonic fucosyltransferase activity as the TFF3/Fuc-TVII−/− line (2.1 ± 0.6 nmol·h−1·mg−1 vs. 2.5 ± 0.3 nmol·h−1·mg−1).
Decreased fucosyltransferase activity was also accompanied by corresponding decreases in expression of fucosylated glycans on the mucosal surface measured as reduced binding by Ulex europaeus I lectin in TFF3/Fuc-TVII−/− mice (Fig. 1B). These differences were most pronounced in the colon, where the Fuc-T activities were highest in the wild-type mice. Expression of fucosylated glycans was the greatest on the epithelial cell surface and in goblet cells (Fig. 1B). In the TFF3/Fuc-TVII−/− mice, the level of fucosylated glycan expression was significantly reduced both on the epithelial surface and within the goblet cells. Similar to that seen with fucosyltransferase activity, there were no significant differences in TFF3 expression between untreated Fuc-TVII−/− and untreated type mice (data not shown). Figure 1B, bottom, shows the Nomarski image of the same section of the colon, demonstrating normal morphology of the colon in the wild-type mice and in the mutant mice. Similarly, hematoxylin and eosin (H&E) staining failed to reveal any underlying architectural changes or evidence of inflammation in untreated mice from all four lines studied (wild-type, TFF3−/−, Fuc-TVII−/−, and TFF3/Fuc-TVII−/−) (data not shown; see Fig. 2D for MPO levels in untreated lines).
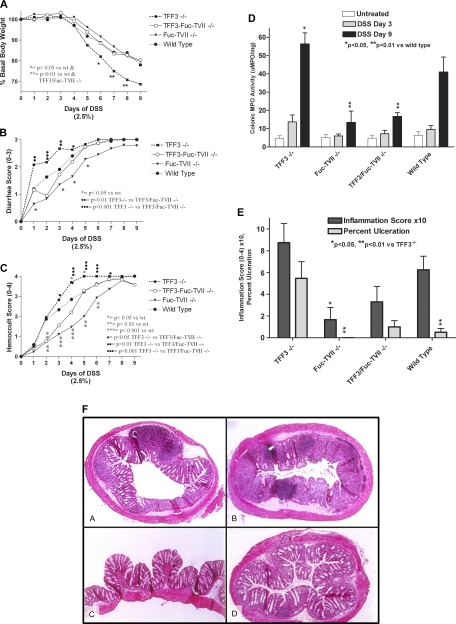
Fig. 2.
Impact of the loss of TFF3 and/or Fuc-TVII in dextran sodium sulfate (DSS)-induced colitis. A: body weight throughout the 9 days of DSS exposure expressed as percent of basal body weight. Significantly different from wild-type: *P < 0.05; **P < 0.01. B: diarrhea assessed throughout the 9 days of DSS exposure scored on a 0–3 scale. Significantly different from wild-type: *P < 0.05. Significantly different from TFF−/−: ‥P < 0.01; …P < 0.001. C: fecal blood loss during the 9 days of DSS exposure, (see materials and methods for further details). Significantly different from wild-type: *P < 0.05; **P < 0.01; ***P < 0.001. Significantly different from TFF−/−: ·P < 0.05; ‥P < 0.01; …P < 0.001. D: colonic myeloperoxidase (MPO) levels assessed from tissue isolated prior to DSS (untreated) after 3 or 9 days of DSS exposure (*P < 0.05 compared with wild-type; **P < 0.05 compared with TFF3−/− and wild-type mice; N = 19–37). E: histological scoring of DSS-induced colitis at DSS day 3; inflammation score is 0–4 scale (see materials and methods), and percent ulceration is the percentage of tissue with disruption of the epithelium. F: colonic sections from wild-type (A), TFF3−/− (B), Fuc-TVII−/− (C), and TFF3/Fuc-TVII−/− (D) after 9 days of DSS exposure, stained with hematoxylin and eosin. Parametric ANOVA was performed in A and D, and nonparametric ANOVA was used for B, C, and E, both followed by posttests (see materials and methods).
Assessment of disease activity by clinical parameters observed with wild-type, TFF3−/−, Fuc-TVII−/−, and TFF3/Fuc-TVII−/− mice during DSS treatment.
Loss of TFF3 resulted in more severe colitis as indicated by increased weight loss, more severe diarrhea, increased fecal blood loss, elevated colonic tissue levels of MPO, and more severe injury and inflammation on histological exam (Fig. 2, A–D). The loss of Fuc-TVII−/− resulted in reduction in severity of colitis with lower diarrhea scores, less fecal blood, lower MPO activity levels and less histological injury/inflammation compared with wild-type and the TFF3−/−mice (Fig. 2, A–E). The disruption the Fuc-TVII gene reversed the susceptibility to colitis noted in the TFF3−/− mice. Remarkably, the disease severity in the TFF3/Fuc-TVII−/− mice was significantly less than in the TFF3−/− mice and in many parameters even less than noted in the wild-type mice, including histological changes, colonic tissue MPO activity levels, and fecal blood loss at DSS days 2 and 3 (Fig. 2, A–D). Even by DSS day 3 TFF3−/− had more severe colitis as assessed by hemoccult score (2, B and C) than the other groups of animals but no significant differences were noted in the weight loss or MPO (2A, D). As early as DSS day 3 the loss of Fuc-TVII in TFF3−/− mice partially protected animals from colitis as assessed by diarrhea score (2B) and hemoccult score (2C) and there was a reduction in MPO (2D) and histology scores (2e) but these changes were not significant (P > 0.05).
Assessment of leukocyte populations.
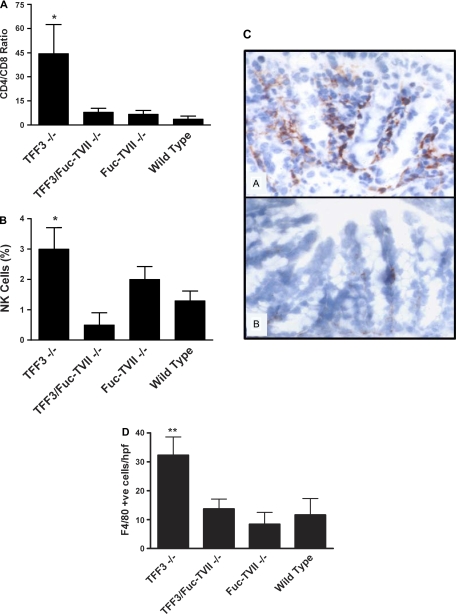
Colonic tissue levels of MPO activity predominately represents the extent of neutrophil infiltration and as noted above these levels were highest in the TFF3−/− and lower in the Fuc-TVII−/− and TFF3/Fuc-TVII−/− (Fig. 2D). Flow cytometry of lamina propria leukocyte populations at day 6 of DSS exposure revealed that the loss of Fuc-TVII in the setting of TFF3 deficiency resulted in a reduction in the CD4-to-CD8 ratio (Fig. 3A) and decreased NK cells (Fig. 3B) compared with the TFF3−/− mice. There were no marked differences in the ratio of alpha/beta to gamma/delta T cells, nor differences in B cell numbers in any of the murine lines assessed (data not shown). Immunohistochemistry revealed a marked reduction in macrophage numbers in the TFF3/Fuc-TVII−/− mice (Fig. 3C, B) compared with the TFF3−/− mice (Fig. 3C, A) on day 6 of DSS 13.7 ± 3.4 cells/high-powered field (hpf) vs. 32.3 ± 6.3 cell/hpf, respectively (P < 0.01) (Fig. 3C, D). No significant differences were noted in macrophage numbers in TFF3/Fuc-TVII−/− compared with wild-type or Fuc-TVII−/− at DSS day 6 (Fig. 3D). No differences in macrophage (F4/80-positive cells) or neutrophils numbers (H&E and MPO) were noted in untreated animals in any of the groups assessed (see Fig. 2D for MPO; only 1–3 F4/80-positive cells/hpf were noted in untreated animals and there were no significant difference between animal lines; data not shown).
Fig. 3.
Analysis of immune cell infiltrate in the colon. Flow cytometry of lamina propria cells populations at day 8 of DSS treatment indicating the CD4-to-CD8 ratio (A) and natural killer (NK) cell populations (B) (*P < 0.05). C–D: immunohistochemical staining of macrophages stained with F4/80 antibody in frozen sections from DSS day 6. In C, A is from a TFF3−/− mouse, and B is from a TFF3/Fuc-Fuc-TVII−/− mouse. The loss of Fuc-Fuc-TVII in the setting of TFF3 deficiency markedly reduced the number of macrophages that infiltrated the colonic lamina propria (**P < 0.01 TFF3−/− vs. Fuc-Fuc-TVII−/−). hpf, High-powered field.
Assessment of impact of the loss of Fuc-TVII on survival.
As noted above, Fuc-TVII−/− mice had less severe DSS-induced colitis than wild-type and TFF3−/− mice and this led to improved survival (Fig. 4). Loss of TFF3 resulted in markedly more severe colitis and increased mortality. Note that survival was assessed as outlined in materials and methods and animals were removed (euthanized) from the study if they appeared in distress or lost more than 25% of basal body weight as per our IACUC-approved animal care protocol. Remarkably, not only did the loss of Fuc-TVII reduce disease severity in TFF3−/− but it actually improved survival to the point that survival rates in the TFF3/Fuc-TVII−/− mice was not significantly different from wild-type mice (Fig. 4).
Assessment of the loss of barrier protective genes in a spontaneous murine model of colitis.
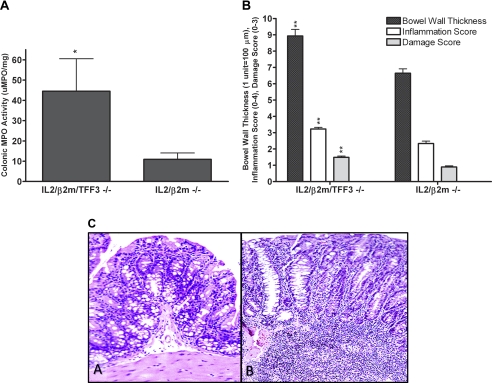
The TFF3−/− line was mated to a murine line that develops spontaneous colitis the IL-2/β2m−/− mice (41, 42). Ten animals from each line were assessed at 25–30 wk of age, and clearly the loss of TFF3 in this model of spontaneous colitis resulted in more severe disease. Five of ten IL-2/β2m/TFF3−/− animals developed rectal prolapse compared 2 of 10 of the IL-2/β2m−/−. The IL-2/β2m/TFF3−/− had more severe colonic inflammation and injury than the IL-2/β2m −/− as assessed colonic tissue levels of MPO activity and by histological assessment (Fig. 5, A–C). No marked differences were noted in average body weight or survival between the two groups.
Fig. 5.
Impact of the loss of TFF3 in an immune-mediated model of spontaneous colitis. IL-2/β2m−/− mice were mated with TFF3−/− mice. IL-2/β2m/TFF3−/− line was confirmed as described previously (41, 42) and above. Ten animals from both IL-2/β2m/TFF3−/− and IL-2/β2m−/− were followed to 25–30 wk prior to euthanasia. A: colonic tissues levels of MPO activity (*P < 0.05). B: bowel wall thickness and inflammation and damage scores in mice euthanized at 25–30 wk of age (**P < 0.01). C: histology of IL-2/β2m−/− (A) and IL-2/β2m/TFF3−/− (B); both animals were euthanized at week 25.
In summary, the loss of Fuc-TVII reduced the severity of DSS-induced colitis and abrogated the marked increased susceptibility to colitis noted in the TFF3-deficient mice. The reduction in disease severity in the Fuc-TVII−/− mice was associated with a markedly enhanced survival. Furthermore, the disruption the Fuc-TVII gene in the setting of TFF3 deficiency again reduced disease severity and enhanced survival. Thus, impairing leukocyte recruitment by disrupting the Fuc-TVII gene markedly reduces DSS-induced colitis and reverses the susceptibility to colitis noted in the TFF−/− murine line with impaired barrier function. Further evidence of a synergistic interaction between barrier protective genes and genes involved in immune regulation was found when we assessed the impact of the loss of the barrier protective gene TFF3 in a spontaneous, immune-mediated, model of colitis.
DISCUSSION
TFF3 plays an important role in epithelial protection and repair from injury (23). Mice lacking TFF3 are more susceptible to intestinal injury, have impaired mucosal healing, and exhibit increased mortality following oral administration of DSS. The recruitment of activated leukocytes into the lamina propria is a key event in the initiation of mucosal inflammation. In the present study we have shown for the first time that deletion of Fuc-TVII reduces the severity of DSS-induced colitis and attenuates the increased susceptibility to DSS-induced colitis observed in mice deficient in TFF3. It is apparent from our data that the reduced severity of colitis appears to be mediated principally by a decrease in leukocyte recruitment. The primary step in leukocyte recruitment to the lamina propria is emigration of leukocytes from the blood. This process begins with increased leukocyte-endothelial cell interactions resulting in the capture of leukocytes from the bloodstream followed by rolling along the endothelial surface. These processes are mediated by three different selectins: P-, E-, and L-selectin. Adhesive interactions between these selectins and their respective ligands mediate a fundamental step in leukocyte emigration from the blood (48). L-selectin is expressed on most types of leukocytes including all myeloid cells, naïve T and B cells and some activated T cells and memory T cells. In contrast, P- and E-selectin are expressed on activated endothelial cells. Therefore, upregulation of P-, E- selectin and the ligands for L-selectin in endothelial cells in concert with the upregulation of L-selectin and ligands for P- and E-selectin in leukocytes generates the necessary machinery to enable the leukocyte-endothelial cell interactions that precede extravasation (44). It was reported that Fuc-TVII is expressed not only in leukocytes but also in murine high endothelial cells of secondary lymphoid organs (43). Intravital microscopy studies revealed that Fuc-TVII−/− mice fail to exhibit the normal leukocyte-endothelial cell interactions, which manifests in a absence of leukocyte rolling in inflamed venules of the ear and the cremaster muscle (22).
Although steroids and immunosuppressive agents are the classical medical treatments for IBD, several biological agents targeting specific disease mechanisms have recently been considered for the treatment of IBD (7, 25). These agents include monoclonal antibodies, recombinant proteins, and antisense oligonucleotides. Major targets for these agents are inflammatory cytokines and their receptors and adhesion molecules. Although anti-TNF-α antibodies, such as infliximab, appear safe and efficacious, the same characteristics of other biological therapies remain to be evaluated. Since upregulation of both of P- and E-selectin has been reported in patients with active IBD (11, 12, 15), selectin blockade appears an attractive approach in IBD management. Although P-selectin blockade is effective in attenuating ischemia-reperfusion injury and indomethacin-induced gastropathy, anti-P-selectin antibody treatment did not provide significant protection in trinitrobenzene sulfonic acid-induced colitis, despite abrogating leukocyte adhesion (39). Similarly, selective E-selectin blockade failed to provide protection in a cotton-top tamarin model of colitis (37). However, anti-α4-integrin antibodies have been found to be effective in patients with IBD but have been removed from the market because of rare central nervous system infections (21, 47). In the present studies we found that deletion of Fuc-TVII, an enzyme that is crucial for the synthesis of selectin ligands, attenuated DSS-induced colitis both in wild-type and TFF3−/− mice. Our data suggest that Fuc-TVII could represent another possible therapeutic target in the management of IBD.
In addition to the role of Fuc-TVII in recruitment of leukocytes, this enzyme also appears to contribute to the distinct patterns of recruitment of TH1 or TH2 cells to peripheral inflammatory sites. It was reported that activated TH1 cells interacted with P-selectin and migrated into inflamed tissue, whereas TH2 cells do not (6). Consistent with these findings, expression of Fuc-TVII was found to be upregulated in activated TH1 cells but downregulated in activated TH2 cells (6). IL-12 is responsible for inducing the gene expression of Fuc-TVII in TH1 cells. It is known that an imbalance of the TH1/TH2 immune response plays a central role in the pathogenesis of IBD (27). In this regard, targeting Fuc-TVII may prove effective in certain types of IBD that display overactive TH1-related immune responses. Further studies will be required to determine the effectiveness of Fuc-TVII inhibition in TH1 vs. TH2 inflammatory conditions.
Although several studies have confirmed that T cell subsets can mediate the inflammatory response in DSS-induced colitis, studies have also demonstrated that DSS colitis can be induced in the absence of mature T and B cells as noted in SCID mice (10) and RAG1- and RAG2-deficient mice (20, 38). Thus it appears that the innate immune response plays a critical role in DSS-mediated intestinal injury and inflammation. Neutrophils have been found to be involved in the development of DSS colitis, and depletion of these cells results in less severe disease (26). Furthermore, human studies using the Adacolumn, which results in depletion of neutrophils and monocytes, has been found to effective therapy in ulcerative colitis (46). Our studies further support that the activation of nonlymphoid cells, such as macrophages, may play a central role in disease onset and persistence in DSS colitis. In Okayasu et al.'s (32) initial description of the DSS model they hypothesized that the macrophage played an etiological role in the pathogenesis of colitis. The role of the macrophage and the innate immune response was recently assessed by Ohkawara et al. (29). In this study they found that DSS colitis was associated with increased expression of macrophage migration inhibitory factor (MIF) and that MIF-deficient animals had less severe DSS-induced inflammation (29). This supports an earlier report that anti-MIF antibody treatment reduced the severity of DSS colitis (30). The protective actions of MIF may involve regulation of Toll-like receptor (TLR) 4, since the increase in TLR4 expression that occurred in wild-type mice was absent in MIF −/− mice but could be induced by exogenous MIF (31).
To further confirm our hypothesis that barrier-protective genes may act synergistically with immune regulatory genes, we assessed the impact of the loss of TFF3 in an immune-mediated, spontaneous model of colitis. Clearly, the loss of TFF3 resulted in more severe colitis in IL-2/β2m−/− mice. Further studies are required to fully elucidate the mechanisms at play in this settling but the loss of TFF3 would render animals more susceptible to injury and these animals should have delayed wound repair as has been evidenced in other model systems of TFF3 deficiency (23). One area that has not been fully explored in the present studies is the possible direct interplay of these genes. We did not see changes in TFF3 expression in Fuc-TVII−/− mice (vs. wild-type mice), and there was no difference in Fuc-TVII expression/activity in TFF3−/− mice compared with wild-type animals at baseline. TFF3 expression was not assessed in the IL-2/β2m−/− mice, but one would suspect that it may be increased during the early phases of colitis since TFF3 has been found to be upregulated in forms of intestinal inflammation (3, 23). Although there is no reported direct interplay between these genes, it is possible that fucosyltransferase activity and/or TFF3 expression may alter bacterial colonization and adherence, which may result in an altered intestinal microbiota that could impact intestinal homeostasis. Since Fuc-TVII is mainly expressed on leukocytes and endothelial cells and little message is found within in the intestinal epithelium, it is unlikely that this fucosyltransferase will directly regulate luminal bacteria or TFF3 expression (18, 40). However, in Fig. 1B it is clear that there is a reduction in fucosylated glycans expressed in the epithelium of TVII−/− mice; thus it appears that the loss of Fuc-TVII may indeed impact epithelial fucosylated glycans and further studies are needed to further define the mechanisms underlying this observation. TFF3 has not been found to play a direct role in regulating the intestinal microbiota, Podolsky and colleagues (36) recently reported that TLR2 activation induces synthesis of TFF3 and that exogenous TFF3 rescues TLR2-deficient mice from increased morbidity and mortality during DSS-induced.
In conclusion, we found that loss of the barrier protective molecule TFF3 leads to a profound increase in susceptibility to DSS-induce colitis, and this can be abrogated by reducing Fuc-TVII-dependent leukocyte recruitment. Furthermore, impairment of barrier function, via loss of TFF3, results in more severe intestinal injury and inflammation in a spontaneous, immune-mediated, model of colitis. Clearly, these studies highlight importance of the interactions between genes involved in the innate immune response, barrier function, and other aspects of the immune regulation in maintaining intestinal homeostasis and regulating intestinal inflammation.
GRANTS
This research was funded by a grant-in-aid from the Crohn's and Colitis Foundation of Canada to P. L. Beck. This work was partially supported by National Institutes of Health grants (HD12437, DK70260, and HD059126) to N. N. Nanthakumar. R. J. Xavier is supported by DK043351 and DK83756. P. L. Beck is an Alberta Heritage Foundation for Medical Research (AHFMR) Scholar. E. Ihara and S. A. Hirota are recipients of Canadian Association of Gastroenterology/Canadian Institutes of Health Research fellowships. J. A. MacDonald is an AHFMR Senior Scholar and recipient of a Canada Research Chair (Tier II).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. C. Terhorst and Dr. S. Brandwein for help in generating the IL-2/β2m/TFF3−/− mice.
REFERENCES
- 1.Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol 164: 6303–6312, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology 110: 489–497, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126: 796–808, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Beck PL, Xavier R, Lu N, Nanda NN, Dinauer M, Podolsky DK, Seed B. Mechanisms of NSAID-induced gastrointestinal injury defined using mutant mice. Gastroenterology 119: 699–705, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Biol MC, Pintori S, Mathian B, Louisot P. Dietary regulation of intestinal glycosyl-transferase activities: relation between developmental changes and weaning in rats. J Nutr 121: 114–125, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Blander JM, Visintin I, Janeway CA, Jr, Medzhitov R. Alpha(1,3)-fucosyltransferase VII and alpha(2,3)-sialyltransferase IV are up-regulated in activated CD4 T cells and maintained after their differentiation into Th1 and migration into inflammatory sites. J Immunol 163: 3746–3752, 1999 [PubMed] [Google Scholar]
- 7.Blonski W, Lichtenstein GR. Safety of biologic therapy. Inflamm Bowel Dis 13: 769–796, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78: 206–209, 1982 [DOI] [PubMed] [Google Scholar]
- 9.Breslin NP, Nash C, Hilsden RJ, Hershfield NB, Price LM, Meddings JB, Sutherland LR. Intestinal permeability is increased in a proportion of spouses of patients with Crohn's disease. Am J Gastroenterol 96: 2934–2938, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107: 1643–1652, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Fagerstam JP, Whiss PA. Higher platelet P-selectin in male patients with inflammatory bowel disease compared to healthy males. World J Gastroenterol 12: 1270–1272, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagerstam JP, Whiss PA, Strom M, Andersson RG. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm Res 49: 466–472, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, Wang TC. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest 109: 193–204, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Estivariz C, Gu LH, Gu L, Jonas CR, Wallace TM, Pascal RR, Devaney KL, Farrell CL, Jones DP, Podolsky DK, Ziegler TR. Trefoil peptide expression and goblet cell number in rat intestine: effects of KGF and fasting-refeeding. Am J Physiol Regul Integr Comp Physiol 284: R564–R573, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Goggins MG, Goh J, O'Connell MA, Weir DG, Kelleher D, Mahmud N. Soluble adhesion molecules in inflammatory bowel disease. Ir J Med Sci 170: 107–111, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hanauer SB. Inflammatory bowel disease. N Engl J Med 334: 841–848, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology 110: 1395–1403, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Ito H, Hiraiwa N, Sawada-Kasugai M, Akamatsu S, Tachikawa T, Kasai Y, Akiyama S, Ito K, Takagi H, Kannagi R. Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int J Cancer 71: 556–564, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey GP, Oates PS, Wang TC, Babyatsky MW, Brand SJ. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology 106: 336–345, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Kim TW, Seo JN, Suh YH, Park HJ, Kim JH, Kim JY, Oh KI. Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World J Gastroenterol 12: 302–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzarotto F, Carpani M, Chaudhary R, Ghosh S. Novel treatment options for inflammatory bowel disease: targeting alpha 4 integrin. Drugs 66: 1179–1189, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86: 643–653, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274: 262–265, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med 183: 847–856, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura K, Honda K, Mizutani T, Akiho H, Harada N. Novel strategies for the treatment of inflammatory bowel disease: selective inhibition of cytokines and adhesion molecules. World J Gastroenterol 12: 4628–4635, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natsui M, Kawasaki K, Takizawa H, Hayashi SI, Matsuda Y, Sugimura K, Seki K, Narisawa R, Sendo F, Asakura H. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J Gastroenterol Hepatol 12: 801–808, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med 8: 567–573, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Ogata H, Podolsky DK. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am J Physiol Gastrointest Liver Physiol 273: G348–G354, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Ohkawara T, Mitsuyama K, Takeda H, Asaka M, Fujiyama Y, Nishihira J. Lack of macrophage migration inhibitory factor suppresses innate immune response in murine dextran sulfate sodium-induced colitis. Scand J Gastroenterol 43: 1497–1504, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ohkawara T, Nishihira J, Takeda H, Hige S, Kato M, Sugiyama T, Iwanaga T, Nakamura H, Mizue Y, Asaka M. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology 123: 256–270, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Ohkawara T, Takeda H, Miyashita K, Nishiwaki M, Nakayama T, Taniguchi M, Yoshiki T, Tanaka J, Imamura M, Sugiyama T, Asaka M, Nishihira J. Regulation of Toll-like receptor 4 expression in mouse colon by macrophage migration inhibitory factor. Histochem Cell Biol 125: 575–582, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98: 694–702, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Podolsky DK. Inflammatory bowel disease. N Engl J Med 347: 417–429, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Podolsky DK. Inflammatory bowel disease (1). N Engl J Med 325: 928–937, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Podolsky DK. Mechanisms of regulatory peptide action in the gastrointestinal tract: trefoil peptides. J Gastroenterol 35, Suppl12: 69–74, 2000 [PubMed] [Google Scholar]
- 36.Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137: 209–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, deBeaumont M. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest 92: 372–380, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reindl W, Weiss S, Lehr HA, Forster I. Essential crosstalk between myeloid and lymphoid cells for development of chronic colitis in myeloid-specific signal transducer and activator of transcription 3-deficient mice. Immunology 120: 19–27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sans M, Salas A, Soriano A, Prats N, Gironella M, Pizcueta P, Elena M, Anderson DC, Pique JM, Panes J. Differential role of selectins in experimental colitis. Gastroenterology 120: 1162–1172, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Sasaki K, Kurata K, Funayama K, Nagata M, Watanabe E, Ohta S, Hanai N, Nishi T. Expression cloning of a novel alpha 1,3-fucosyltransferase that is involved in biosynthesis of the sialyl Lewis × carbohydrate determinants in leukocytes. J Biol Chem 269: 14730–14737, 1994 [PubMed] [Google Scholar]
- 41.Shah SA, Simpson SJ, Brown LF, Comiskey M, de Jong YP, Allen D, Terhorst C. Development of colonic adenocarcinomas in a mouse model of ulcerative colitis. Inflamm Bowel Dis 4: 196–202, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Simpson SJ, Mizoguchi E, Allen D, Bhan AK, Terhorst C. Evidence that CD4+, but not CD8+ T cells are responsible for murine interleukin-2-deficient colitis. Eur J Immunol 25: 2618–2625, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Smith PL, Gersten KM, Petryniak B, Kelly RJ, Rogers C, Natsuka Y, Alford JA, 3rd, Scheidegger EP, Natsuka S, Lowe JB. Expression of the alpha(1,3)fucosyltransferase Fuc-TVII in lymphoid aggregate high endothelial venules correlates with expression of L-selectin ligands. J Biol Chem 271: 8250–8259, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J 273: 4377–4389, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci USA 88: 11017–11021, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka T, Okanobu H, Yoshimi S, Murakami E, Kogame A, Imagawa H, Numata Y, Kuga Y, Moriya T, Ohya T, Kajiyama G. In patients with ulcerative colitis, adsorptive depletion of granulocytes and monocytes impacts mucosal level of neutrophils and clinically is most effective in steroid naive patients. Dig Liver Dis 40: 731–736, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis 8: 291–300, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 79: 181–213, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, von Andrian UH. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity 12: 665–676, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zamora SA, Hilsden RJ, Meddings JB, Butzner JD, Scott RB, Sutherland LR. Intestinal permeability before and after ibuprofen in families of children with Crohn's disease. Can J Gastroenterol 13: 31–36, 1999 [DOI] [PubMed] [Google Scholar]