Abstract
Thiamin is essential for normal cellular functions, and its deficiency leads to a variety of clinical abnormalities. Humans and other mammals obtain the vitamin via intestinal absorption. The intestine is exposed to two sources of thiamin, a dietary and a bacterial (i.e., normal microflora of the large intestine) source. Chronic alcohol consumption is associated with thiamin deficiency, which is caused (in part) by inhibition in intestinal thiamin absorption. However, little is known about the physiological and molecular aspects of the intestinal thiamin uptake process that are affected by chronic alcohol use. To address these issues, we used rats fed an alcohol-liquid diet and human intestinal epithelial HuTu-80 cells chronically exposed to ethanol as model systems. The results showed that chronic alcohol feeding to rats led to a significant inhibition in carrier-mediated thiamin transport across both the jejunal brush-border membrane and basolateral membrane domains. This was associated with a significant reduction in level of expression of thiamin transporter-1 (THTR-1), but not THTR-2, at the protein and mRNA levels. Level of expression of the heterogenous nuclear RNA of THTR-1 in the intestine of alcohol-fed rats was also decreased compared with their pair-fed controls. Chronic alcohol feeding also caused a significant inhibition in carrier-mediated thiamin uptake in rat colon. Studies with HuTu-80 cells chronically exposed to ethanol also showed a significant inhibition in carrier-mediated thiamin uptake. This inhibition was associated with a reduction in level of expression of human THTR-1 and THTR-2 at the protein, mRNA, and transcriptional (promoter activity) levels. These studies demonstrate that chronic alcohol feeding inhibits intestinal thiamin absorption via inhibition of the individual membrane transport event across the polarized absorptive epithelial cells. Furthermore, the inhibition is, at least in part, mediated via transcriptional mechanism(s).
Keywords: vitamin B1, brush-border membrane vesicles, basolateral membrane vesicles, thiamin transporter-1, thiamin transporter-2
TRANSLATIONAL HIGHLIGHTS Recent advances in our understanding of the molecular mechanisms involved in the transport of water-soluble vitamins and their regulation at the transcriptional and posttranscriptional levels have provided an excellent opportunity to address the negative impact of chronic alcohol consumption on micronutrient physiology. The authors took advantage of the recent progress made in the area of intestinal vitamin B1 (thiamin) absorption to delineate the cellular and molecular mechanism(s) involved in the thiamin malabsorption that occurs as a result of chronic alcohol exposure. The knowledge gained will help in the design of effective strategies to optimize thiamin nutrition in chronic alcoholism and in conditions associated with thiamin deficiency and suboptimal levels.
Thiamin (vitamin B1), a water-soluble micronutrient, plays an essential role in normal cellular functions, growth, and development. The vitamin acts as a cofactor for multiple enzymes (transketolase, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched chain ketoacid dehydrogenase) that are involved in a variety of critical metabolic reactions related to energy metabolism (5). Furthermore, because thiamin bridges the glycolytic pathway and the pentose phosphate metabolic pathway, which is a critical step in creating chemical-reducing power in cells, this micronutrient also plays a role in reducing cellular oxidative stress (15). Thiamin deficiency in humans leads to a variety of abnormalities including neurological and cardiovascular disorders (3, 41, 45). On the other hand, optimization of thiamin status may prevent diabetic retinopathy and block tissue damage caused by hyperglycemia of diabetes (14). Thiamin deficiency and suboptimal status represent significant nutritional problems worldwide and are associated with a variety of conditions including chronic alcohol consumption (17, 41–43, 45).
Humans (and all other mammals) cannot synthesize thiamin and thus must obtain the vitamin from exogenous sources via intestinal absorption. Therefore, the intestine plays a central role in regulating body homeostasis of the vitamin. The human intestine mediates absorption from two sources of thiamin: a dietary thiamin (which is absorbed in the small intestine) and a bacterial thiamin (where the vitamin is produced in significant quantities by the normal microflora of the large intestine and is absorbed primarily in that region of the gut). In both the small and large intestine, absorption of thiamin occurs via an efficient and specialized carrier-mediated processes (reviewed in Refs. 32 and 36). Thiamin was found at both membrane domains of the polarized intestinal absorptive cells, i.e., across the intestinal brush-border membrane (BBM) and basolateral membrane (BLM) domains (reviewed in Refs. 32 and 36). Studies from our laboratory have also shown that both thiamin transporter-1 and -2 (THTR-1 and THTR-2), products of the SLC19A2 and SLC19A3 genes, respectively, are involved in intestinal thiamin uptake (27, 33). Furthermore, we have also shown that, whereas THTR-1 is expressed at the both the apical BBM and BLM domains of the intestinal epithelial cells, expression of THTR-2 is restricted to the apical BBM domain only (33, 39).
Chronic alcohol consumption leads to thiamin deficiency, and that inhibition in intestinal thiamin absorption process plays a role in causing this abnormality (9, 17). However, little is known about the physiological and molecular aspects of the intestinal thiamin absorption process that are affected by chronic alcohol consumption. In this study, we investigated the effect of chronic alcohol use on both transport across the small intestinal BBM and/or BLM, whether one or both of the thiamin transport systems (i.e., THTR-1 and THTR-2) are affected, and the mechanism(s) that mediates the effect. We also investigated whether thiamin absorption in the colon is affected by chronic alcohol feeding. We used two models in our investigations: an in vivo model of chronic alcohol feeding to rats (Lieber-Decarli liquid diet) and an in vitro model in which the human-derived intestinal epithelial HuTu-80 cells are chronically exposed to ethanol (46).
MATERIALS AND METHODS
Materials.
[3H]Thiamin (specific activity 20 Ci/mmol; radiochemical purity >99%) was obtained from American Radiolabel (ARC, St. Louis, MO). Nitrocellulose filters (0.45-μm pore size) were purchased from Millipore (Fisher Scientific, Fair Lawn, NJ). Unlabeled thiamin and other chemicals including molecular biology reagents were obtained from commercial vendors (Fisher Scientific and Sigma) and were of analytical grade.
Chronic alcohol feeding of rats.
Male Wistar rats (Charles River, Wilmington, MA) weighing ∼120 g (∼14 wk old) were housed at the Animal Core of the NIAA-funded Southern California Research Center for Alcoholic Liver and Pancreatic Disease (ALPD) and Cirrhosis at the University of Southern California. The experimental protocols were approved by animal use committee of the Veterans Affairs at Long Beach and the University of Southern California. Rats were fed Lieber-Decarli (21) ethanol liquid diet (ethanol provides 36% of total calories) (Bio Serv, Frenchtown, NJ); control rats were pair fed the same liquid diet but without ethanol (maltose-dextrin replaced ethanol isocalorically). After 2, 4, and 6 wk of chronic alcohol feeding, rats were euthanized, and the jejunum was removed and used for isolation of BBM vesicles (BBMV) and BLM vesicles (BLMV). A portion of the jejunum was also collected and stored at −80°C in TRIzol (Invitrogen, Carlsbad, CA) for subsequent determination of mRNA and heterogenous nuclear (hnRNA) levels of thiamin transporters.
Preparation of rat jejunal BBMV and BLMV and transport studies.
After euthanasia of rats with ketamine, the jejunum was removed and flushed with ice-cold saline solution, and the mucosa was scraped. BBMV were then isolated on the same day from alcohol-fed rats and their pair-fed controls by a divalent cation (Mg2+) chelation method, while BLMV were isolated by a Percoll-gradient differential centrifugation method. Both methods have been previously described and widely used by our laboratory and others (6, 7, 9, 18, 35, 38). Freshly isolated BBMV and BLMV were preloaded with a buffer of (in mM) 280 mannitol and 20 Tris·HCl in pH 5.5. Uptake studies were performed by incubating vesicles [10-s initial rate (6, 7, 44) in a buffer of 100 mM NaCl, 80 mM mannitol 20 mM HEPES, pH 7.4, in presence of 0.5 μCi (0.25 μM) 3H-thiamin]. Transport studies were performed at 37°C using a rapid-filtration technique as described previously (16).
Preparation of colonic sheets and uptake.
Colonic sheets were prepared and used in uptake investigations as described by us previously (10). Briefly, the colon was removed from alcohol-fed rats and their pair-fed controls, and flushed with an ice-cold saline solution, cut open longitudinally, and two 1-cm pieces (sheets) were then prepared from the mid portion and immediately incubated in Krebs-Ringer buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, 10 MES, pH 7.4) in presence of 0.5 μCi (25 nM) 3H-thiamin. Incubation was performed at 37°C for 5 min (initial rate; data not shown). Reaction was terminated by removal of the incubation solution and immediate addition of 5 ml of ice-cold Krebs-Ringer buffer. The colonic sheets were then washed again with ice-cold Krebs-Ringer buffer, and digested with NaOH, neutralized with 10 N HCl, and radioactivity was counted in a Beckman Coulter LS6500 multi-purpose liquid scintillation counter.
Real-time PCR analysis.
Total RNA (5 μg) was isolated from jejunum of alcohol-fed rats and their pair-fed controls and primed with oligo-dT primers to synthesize first-strand cDNA (Superscript First-Strand synthesis RT-PCR kit; Invitrogen). To amplify the coding region of rat THTR-1, THTR-2, and β-actin, we used the following gene-specific primers for real-time PCR study (THTR-1: forward 5′-GCTGTCATCTACAATGGCGG-3′, reverse 5′-GATGTACACTGCAGCAGCAATC-3′; THTR-2: forward 5′-CGTGATACTCTGCTTGTTCGG-3′, reverse 5′-GGTAAGAGTACGTCCAAACAGG-3′; and β-actin: forward 5′-GTCAGGTCATCACTATCGGC-3′, reverse 5′-CATGGATGCCACAGGATTCC-3′). Real-time PCR conditions were as described previously (28, 33), and data were normalized to β-actin and then quantified using a relative relationship method supplied by the vendor (Bio-Rad, Hercules, CA) (22). The real-time PCR analysis of human THTR-1 and THTR-2 expression in human intestinal epithelial HuTu-80 was carried out using gene-specific primers for the hTHTR-1 (forward, 5′- AGCCAGACCGTCTCCTTGTA- 3′; reverse, 5′- TAGAGAGGGCCCACCACAC- 3′), hTHTR-2 (forward, 5′-TTCCTGGATTTACCCCACTG-3′), and β-actin (forward, 5′-AGCCAGACCGTCTCCTTGTA-3′; reverse, 5′-TAGAGAGGGCCCACCACAC-3′) as described by us previously (23).
Western blot analysis.
Western blot analysis was performed on purified BBM and BLM proteins isolated from the jejunum of alcohol-fed and control pair-fed rats. Thirty to sixty micrograms of BBM or BLM proteins were resolved onto premade 4–12% Bis-Tris mini-gel (Invitrogen) as described before (23, 25, 33, 34). After electrophoresis, proteins were electro-blotted onto polyvinylidene difluoride membrane (Bio-Rad) and then blocked with a PBS solution containing 5% dried milk (Bio-Rad) for 1 h at room temperature. The membranes were then incubated with either THTR-1- or THTR-2-specific polyclonal antibodies. The antibodies were raised in rabbits against the KKCRKQEDPNSSPQ and EPYLQEPRDVSTKE peptides, which correspond to amino acids 481–494 and 468–481 of the THTR-1 and THTR-2, respectively, by a commercial vendor (Thermo Fisher Scientific, Huntsville, AL). To establish specificity of the antibodies, membranes were also incubated (1 h, room temperature) with THTR-1 and THTR-2 antibodies pretreated with synthetic antigenic peptides (Thermo Fisher Scientific) as described previously (23, 33). Western blotting with membranous preparation of the human intestinal epithelial HuTu-80 cells was performed using specific polyclonal antibodies against human THTR-1 and THTR-2 as described by us previously (23, 33).
Semi-quantitative RT-PCR assay of hnRNA.
To investigate the effect of chronic alcohol feeding on transcriptional rate of THTR-1 and THTR-2 genes in rat jejunum, we determined the level of hnRNA in the jejunum of alcohol-fed rats and their pair-fed controls. As it is known, the first product of transcription is primary transcript of hnRNA, which includes introns as well as exons (8). The level of hnRNA can be used as an indicator of gene transcription rate (2, 8, 19). Briefly, total RNA isolated from the jejunum of chronic alcohol-fed rats and their pair-fed controls were used to perform RT-PCR with gene-specific primers that anneal to sequences in the intron/exon boundaries (thus amplifying hnRNA only; Ref. 8). RNA samples were treated with DNAse I (1 μg RNA/unit; Invitrogen) to prevent amplification of genomic DNA. DNAse I-treated RNA was reverse transcribed with the random hexamer (Invitrogen). Semi-quantitative RT-PCR was then performed using the following hnRNA gene-specific primers: THTR-1 (forward, 5′-CACTTTGTACCTGTGTGTG-3′; reverse, 5′- GAATGACAGGCTTGTAACG -3′), THTR-2 (forward, 5′- CTACCGTAACAGGACACAG -3′; reverse, 5′-TGTGAAAATGGAGGCTCAC-3′), and β-actin (forward, 5′- CTGCTCTTTCCCAGATGAG-3′; reverse, 5′- CTCATAGATGGGCACAGTG-3′). A negative control, which constituted a reaction without cDNA template, was run with every assay to establish specificity. The amplified products were then run on 1% agarose gel, and the specific bands were quantified using UN-SCAN-IT gel automated digitizing system, version 6.1 (Silk Scientific, Orem, UT).
Culturing of HuTu-80 cells and chronic alcohol exposure.
The human-derived intestinal (duodenal) epithelial HuTu-80 cells (ATCC, Manassas, VA; passages 10 to 18) were plated at a density of 2 × 105 cells/well onto 12-well plates in MEM media (ATCC) supplemented with 10% FBS and antibiotics. Twenty-four hours later, fresh growth medium containing 200 mM of alcohol (a concentration that simulates the level of alcohol encountered by the intestine in chronic alcohol drinkers; Ref. 26) was introduced, and the plates were kept in an ethanol-saturated incubator for 72 h as described recently with minor modifications (46); in addition to changing the alcohol-containing growth medium every 12 h, studies were designed to minimize ethanol evaporation (46). Uptake studies were performed at 37°C in Krebs-Ringer buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, 10 MES, pH 7.4). Labeled and unlabeled thiamin were added to the incubation medium at the onset of incubation, and uptake was examined during the initial linear period of uptake (5 min; data not shown). The reaction was terminated by the addition of 2 ml of ice-cold Krebs-Ringer buffer followed by immediate aspiration. Cells were then rinsed twice with ice-cold buffer, digested with 1 ml of 1 N NaOH, neutralized with 10 N HCl, and then measured for radioactive content using scintillation counter. Cell digest samples (100 μl each) were taken for protein quantification using a Bio-Rad Dc protein assay kit.
Transfection and reporter gene assay.
SLC19A2 and SLC19A3 full-length promoter-luciferase reporter constructs utilized in this study were generated previously (24, 29). HuTu-80 cells were cotransfected in 12-well plates at less than 80% confluency with 4 μg of each test construct and 100 ng of the Renilla transfection control plasmid Renilla luciferase-thymidine kinase (pRL-TK) (Promega, Madison, WI). Transfection was performed with Lipofectamine reagent (Invitrogen) according to manufacturer's instructions. On the second posttransfection day the cells transfected with SLC19A2 and SLC19A3 were treated with MEM medium containing 200 mM alcohol for 72 h. To ensure proper alcohol effect, the media containing 200 mM alcohol was changed every 12 h. At the end of 72 h of alcohol incubation, Renilla-normalized firefly luciferase activity was determined by using the Dual Luciferase Assay system (Promega).
Statistical analysis.
Uptake data with rat intestinal preparation presented in this paper are means ± SE of at least three separate experiments from different rats and are expressed in femtomoles per milligram of protein per unit of time. Uptakes with the human intestinal epithelial HuTu-80 cells were also means ± SE from at least three separate experiments. Student's t-test was used for statistical analysis. P < 0.05 was considered statistically significant. Carrier-mediated thiamin uptake was determined by subtracting uptake by simple diffusion (determined from the slope of the line between uptake at high pharmacological concentration of 1 mM and point of origin) from total uptake. Protein and RNA determinations as well as promoter assay were run on at least three different occasions using three different preparations.
RESULTS
Effect of chronic alcohol feeding of rats on thiamin transport across jejunal BBM and BLM domains: studies with isolated membrane vesicles.
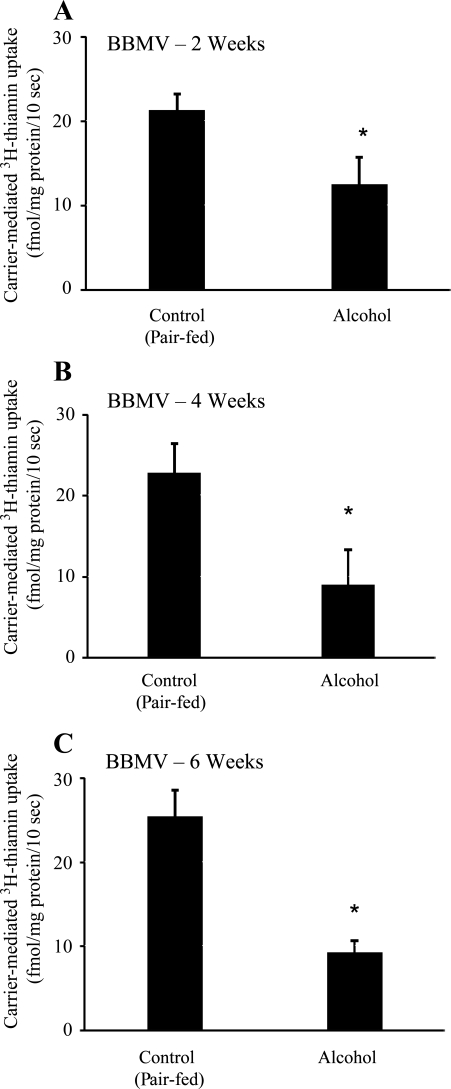
The effect of chronic alcohol feeding of rats for different periods of time (2, 4, and 6 wk) on the entry of a physiological concentration of thiamin (0.25 μM), i.e., transport across the apical BBM of the polarized intestinal epithelial cells, was examined using purified jejunal BBMV. Vesicles were isolated simultaneously from alcohol-fed rats and their pair-fed controls and were used for uptake studies on the day of isolation (see materials and methods). The results showed a significant (P < 0.01 for all) inhibition in carrier-mediated thiamin uptake in intestinal BBMV of chronic alcohol-fed rats compared with their pair-fed controls with the inhibition being evident in 2 wk after the initiation of the alcohol feeding regimen (Fig. 1, A–C).
Fig. 1.
Effect of chronic alcohol feeding of rats for different periods on carrier-mediated thiamin uptake by jejunal brush-border membrane vesicles (BBMV). A, B and C: carrier-mediated uptake of 3H-thiamin (0.25 μM) by jejunal BBMV of rats fed alcohol liquid diet for 2, 4 and 6 wk, respectively, was examined and compared with uptake by BBMV of pair-fed controls. Data are means ± SE of at least 3 separate uptake determinations from multiple sets of rats. *P < 0.01.
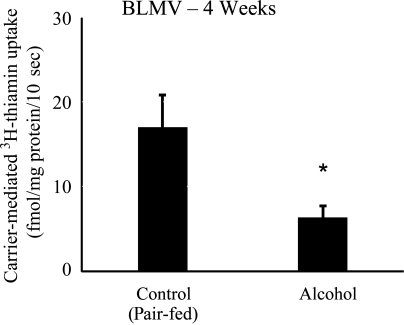
We also examined the effect of chronic alcohol feeding (for 4 wk; standard feeding period used in all subsequent studies) on the exit of thiamin out of the polarized enterocytes, i.e., transport across the BLM, using isolated jejunal BLMV. The results again showed a significant (P < 0.05) inhibition in carrier-mediated thiamin uptake in the chronic alcohol-fed rats compared with their pair-fed controls (Fig. 2).
Fig. 2.
Effect of chronic alcohol feeding of rats on carrier-mediated thiamin uptake by jejunal basolateral membrane vesicles (BLMV). Carrier-mediated 3H-thiamin (0.25 μM) uptake by jejunal BLMV of rats fed alcohol liquid diet for 4 wk compared with their pair-fed controls. Data are means ± SE of at least 3 separate uptake determinations from multiple sets of rats. *P < 0.01.
Effect of chronic alcohol feeding on level of expression of THTR-1 and THTR-2 proteins at the jejunal BBM and BLM domains.
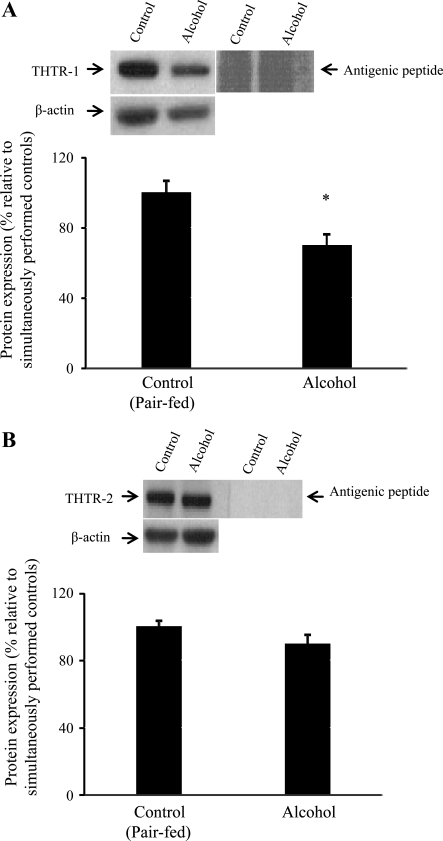
In this study, we examined the effect of chronic alcohol feeding on the level of expression of THTR-1 and THTR-2 proteins at the BBM and BLM domains of rat jejunum. This was performed by Western blot analysis using polyclonal antibodies against the THTR-1 and THTR-2 and purified BBM and BLM preparations isolated from the jejunum of rats chronically fed alcohol liquid diet and their pair-fed controls. Specificity of the rat antibodies was established by treating with antigenic peptides (antigenic peptide sequence is described in materials and methods). The results showed that chronic alcohol consumption leads to a significant (P < 0.05) decrease in the level of expression of the THTR-1 protein at the apical BBM (Fig. 3A). No difference in the level of expression of THTR-2 protein, however, was observed in BBM preparations isolated from alcohol-fed rats and their pair-fed controls (Fig. 3B).
Fig. 3.
Effect of chronic alcohol feeding of rats on level of expression of thiamin transporter-1 (THTR-1) and THTR-2 proteins at the intestinal BBM. Western blot analysis was done using intestinal BBM proteins (60 μg) isolated from chronic alcohol-fed rats (4 wk) and their pair-fed controls and were resolved on 4–12% SDS-PAGE and electroblotted onto polyvinylidene difluoride membrane as described in materials and methods. A: blots were incubated with rabbit polyclonal THTR-1 antibody (left) or anti-THTR-1 antibodies pretreated with the antigenic peptides (right). B: blots were incubated with rabbit polyclonal THTR-2 antibody (left) or anti-THTR-2 antibodies pretreated with the antigenic peptides (right). Data are means ± SE of at least 3 separate sets of experiments and were normalized related to β-actin protein expression. *P < 0.05.
With regard to the effect of chronic alcohol consumption on the level of expression of thiamin transporters at the BLM domain of the polarized enterocytes, the results again showed significant (P < 0.05) reduction in the level of expression of the THTR-1 in alcohol-fed rats compared with their pair-fed controls (Fig. 4A). As previously recognized with human intestinal epithelial cells (33, 39), no expression for THTR-2 was detected in BLM preparations of rat jejunum (Fig. 4B).
Fig. 4.
Effect of chronic alcohol feeding of rats on level of expression of THTR-1 protein at the intestinal BLM. Western blot analysis was performed using intestinal BLM proteins (60 μg). Legend is as in Fig. 3. A: blot shows expression of THTR-1. *P < 0.05. B: blot shows lack of expression of THTR-2 in intestinal BLM.
Effect of chronic alcohol feeding of rats on expression of THTR-1 and THTR-2 at the mRNA and hnRNA levels.
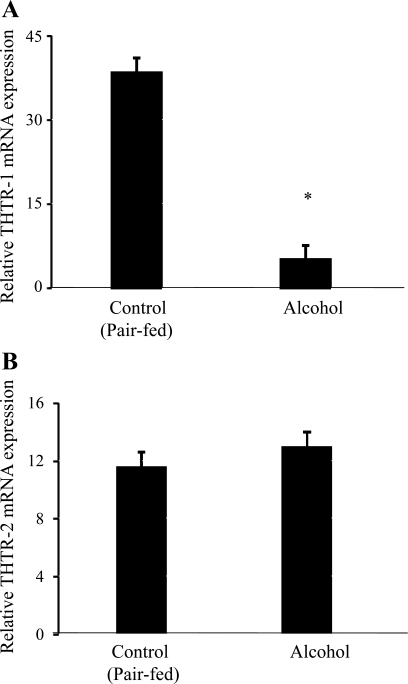
In this study, we used qPCR to examine the effect of chronic alcohol feeding on the level of expression of THTR-1 and THTR-2 mRNA using total RNA isolated from the jejunum of rats fed alcohol chronically and their pair-fed controls. The results showed a significant (P < 0.01) reduction in the level of expression of THTR-1 mRNA (Fig. 5A), but not that of THTR-2 (Fig. 5B), in rats fed alcohol chronically compared with their pair-fed controls.
Fig. 5.
Effect of chronic alcohol feeding of rats on level of expression of THTR-1 and THTR-2 mRNA levels. A and B: real-time quantitative PCR was performed using rat THTR-1 and THTR-2 gene-specific primers (see materials and methods). Data are means ± SE of at least 3 separate sets of experiments and were normalized relative to β-actin and calculated using relative relationship method supplied by manufacturer (Bio-Rad). *P < 0.05.
To further understand the cause of reduction in mRNA level of THTR-1, we investigated the effect of chronic alcohol feeding on level of expression of the hnRNA of THTR-1. Level of hnRNA has been used as an index of transcriptional activity of different genes under different conditions (2, 8, 19). These results showed a significant (P < 0.02) reduction in THTR-1 hnRNA levels in the jejunum of rats fed alcohol chronically compared with their pair-fed controls (0.69 ± 0.3 and 0.95 ± 0.1 for alcohol-fed and pair-fed controls, respectively, in arbitrary units).
Effect of chronic alcohol feeding of rats on colonic uptake of thiamin.
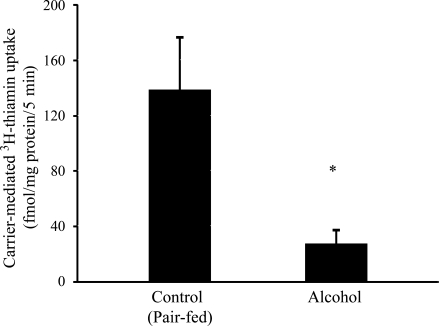
In this study we examined the effect of chronic alcohol feeding of rats on carrier-mediated uptake of a physiological concentration of thiamin (25 nM) using an established colonic sheet procedure as described by us previously (10). Chronic ethanol feeding caused an ∼80% decrease in carrier-mediated thiamin uptake by colonic mucosa (P < 0.03, Fig. 6).
Fig. 6.
Effect of chronic alcohol feeding of rats on carrier-mediated thiamin uptake in the colon. Colonic sheets were prepared as described in materials and methods from chronic alcohol-fed rats (4 wk) and their pair-fed controls. The initial rate of carrier-mediated [3H]thiamin (25 nM) uptake was determined, and data are represented as means ± SE of at least 3 separate experiments. *P < 0.03.
Effect of chronic alcohol exposure on physiological and molecular parameters of thiamin uptake by the human-derived intestinal epithelial HuTu-80 cells.
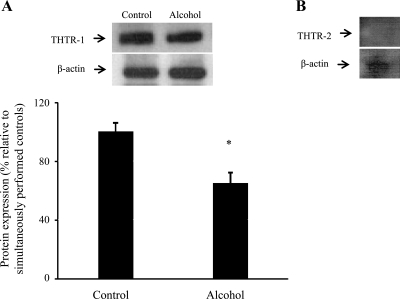
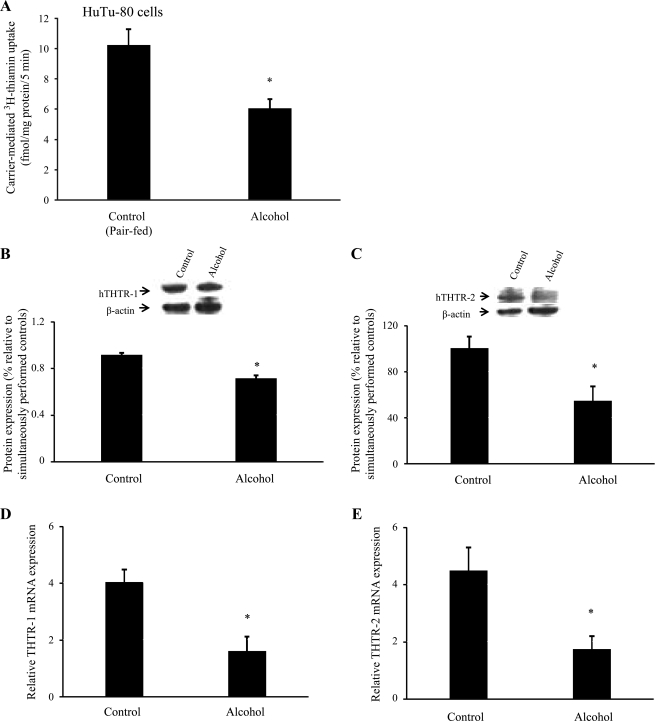
In these investigations, we extended our studies into the human-derived intestinal epithelial HuTu-80 cells and examined the effect of chronic alcohol exposure on physiological and molecular parameters of thiamin uptake by these cells. Chronic alcohol exposure was performed for 72 h as described recently (46). A concentration of ethanol of 200 mM was chosen to mimic the level of ethanol encountered by the human intestine following alcohol ingestion (26). The results showed a significant (P < 0.05) inhibition in carrier-mediated 3H-thiamin uptake in alcohol-exposed cells compared with controls (Fig. 7A). This was associated with a significant (P < 0.05) reduction in the level of expression of hTHTR-1 and hTHTR-2 proteins (Fig. 7, B and C) and for all mRNA levels (Fig. 7, D and E).
Fig. 7.
Effect of chronic alcohol exposure of the human-derived intestinal epithelial HuTu-80 cells on carrier-mediated thiamin uptake and level of expression of THTR-1 and THTR-2 at the protein and mRNA levels. HuTu-80 cells were chronically exposed (72 h) to 200 mM ethanol as described in materials and methods. A: carrier-mediated 3H-thiamin (25 nM) uptake was carried out and compared with control. B and C: Western blot analysis of membranous fraction of HuTu-80 cells run as in Fig. 3. Data are means ± SE of at least 3 separate sets of experiments that were normalized relative to β-actin protein expression. *P < 0.05. D and E: real-time quantitative PCR was performed using hTHTR-1 and hTHTR-2 gene-specific primers (see materials and methods). Data are means ± SE of at least 3 separate sets of experiments and were normalized relative to β-actin and calculated using relative relationship method supplied by manufacturer (Bio-Rad). *P < 0.05.
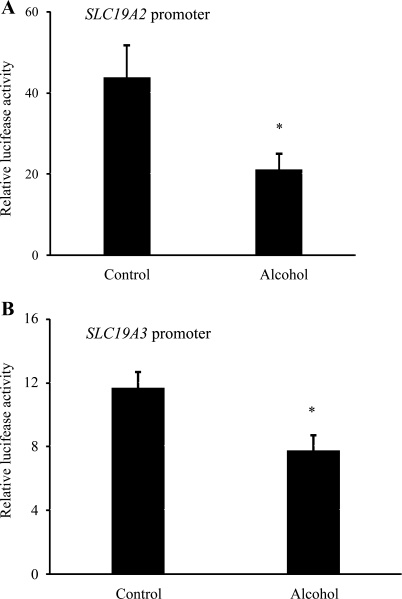
We also examined the effect of chronic alcohol exposure of the human intestinal epithelial HuTu-80 cells on the activity of full-length SLC19A2 (−2,250 to −36 bp) and SLC19A3 (−1,957 to +59 bp) promoters. In these investigations, SLC19A2 and SLC19A3 promoters fused to the Firefly luciferase reporter gene were transiently transfected into HuTu-80 cell lines and chronically exposed to ethanol (200 mM) (26) for 72 h. Luciferase activity was then measured as described by us previously (24, 28, 29). The results showed a significant (P < 0.05) reduction in the activity of the both SLC19A2 (Fig. 8A) and SLC19A3 (Fig. 8B) promoters in cells chronically exposed to alcohol compared with control cells.
Fig. 8.
Effect of chronic alcohol exposure of the human intestinal epithelial HuTu-80 cells on activity of the SLC19A2 and SLC19A3 promoters. A and B: full-length SLC19A2 and SLC19A3 promoters in pGL3-Basic were transfected into HuTu-80 cells. These cells were then subjected to 200 mM ethanol treatment for 72 h followed by determination of luciferase activity. Data are reported as relative luciferase activity normalized to Renilla luciferase activity and presented as means ± SE of at least 3 independent experiments. *P < 0.05.
DISCUSSION
This study was undertaken to determine the physiological and molecular mechanisms by chronic alcohol consumption leading to inhibition in intestinal thiamin absorption. It has been known for some time that chronic alcoholism is associated with thiamin deficiency, which may lead to Wernicke's encephalopathy and Korsakoff's psychosis (30, 41, 45). Although a number of factors may be involved in the pathogenesis of thiamin deficiency in the setting of chronic alcohol intake (e.g., inadequate intake of thiamin, decreased liver storage of the vitamin, urinary losses) (1, 17, 30), an inhibition in intestinal absorption of this vitamin plays an important role in this process given the central role of intestine in regulating normal body homeostasis of thiamin. We used two established models (in vivo and in vitro) of alcohol exposure to achieve our aims: a rat model in which alcohol was provided in a nutritionally adequate liquid diet and involved pair-feeding of controls and human-derived intestinal epithelial HuTu-80 cells chronically exposed to a clinically relevant concentration of alcohol.
Our studies with rats revealed that chronic alcohol consumption causes a significant inhibition in both the entry step of thiamin into the polarized enterocytes across the apical BBM and the exit step of the vitamin across the BLM. These findings confirm the previous suggestion that chronic alcohol feeding affects the carrier-mediated process of the intestinal thiamin uptake (17). The effect of alcohol on thiamin transport across the apical BBM was evident as early as 2 wk following the initiation of alcohol feeding. Furthermore, the inhibition of thiamin uptake across the BBM and BLM was associated with a decrease in the level of expression of the THTR-1 protein at both the BBM and BLM; expression of THTR-2 at the apical BBM was not affected. Of note, THTR-2 is only expressed at the BBM and not the BLM, as shown by us previously using confocal imaging and immunological approaches (4, 33) and confirmed in the present study (Fig. 4B). The decrease in protein abundance of THTR-1 protein in alcohol-fed rats was associated with a significant decrease in message level of the transporter. In contrast, THTR-2 protein and mRNA levels were not different in alcohol-fed animals compared with pair-fed controls. These findings demonstrate differential effects of chronic alcohol consumption on expression of thiamin transporters in the intestine, a finding that is in line with previous observations showing differential effects of chronic alcohol feeding on nutrient transport in the gut (11–13) and on gene expression (including those that encode membrane transporters) in other tissues (20). Furthermore, the observation that chronic alcohol feeding affects the expression of THTR-1 but not THTR-2 in the intestine contrasts our recent findings in the kidneys of rats chronically fed alcohol, where decreased levels of expression of both THTR-1 and THTR-2 were observed (40). These findings demonstrate that chronic alcohol feeding exerts a tissue-specific effect on the molecular parameters of thiamin transport. It is interesting to note here that chronic alcohol feeding also affects thiamin metabolism in a tissue-specific manner (31).
The decrease in the level of expression of THTR-1 mRNA in the intestine of rats chronically fed alcohol compared with their pair-fed controls suggests that the inhibition may (at least in part) be transcriptionally mediated. This possibility was tested by determining the level of expression of THTR-1 hnRNA in the jejunum of alcohol-fed rats compared with their pair-fed controls because changes in the level of hnRNA expression could serve as an indicator for changes in the rate of transcription of this gene (2, 8, 19). The results showed that chronic alcohol feeding leads to a significant reduction in the level of THTR-1 hnRNA, thus supporting the possibility that the inhibitory effect of chronic alcohol feeding on intestinal thiamin uptake may (at least in part) be exerted at the transcriptional level. Obviously, this does not exclude the involvement of other mechanisms (e.g., changes in RNA stability), and further studies are needed to address this issue.
As mentioned earlier, the intestinal tract is exposed to two sources of thiamin: a dietary source that is absorbed in the small intestine and a bacterial source where the vitamin is generated by the normal microflora in the large intestine and is absorbed in that region of the gut via an efficient carrier-mediated mechanism (34). Thus in this study we also examined the effect of chronic alcohol feeding to rats on thiamin uptake in the colon and observed a significant inhibition in the uptake process. Thus chronic alcohol use appears to inhibit the absorption of both dietary and bacterial sources of thiamin. Because alcohol is absorbed mainly in the upper small intestine (26), it is reasonable to assume that the colon gets exposed to ethanol from the blood side (37). Also, because colonic bacteria can metabolize some of the ethanol that enters the colon to acetaldehyde (37) and because the colon has a low capacity to metabolize acetaldehyde secondary to a low level of aldehyde dehydrogenase activity (37), it is possible that some of the inhibitory effect of chronic alcohol feeding on colonic thiamin uptake is caused by this alcohol metabolite. Thus chronic alcohol feeding appears to inhibit the absorption of both dietary and bacterial sources of thiamin. The clinical implication of these findings would be that chronic alcohol consumption has a significant deleterious effect on the overall physiology of intestinal thiamin transport.
Our in vitro model, i.e., studies with the human-derived intestinal epithelial HuTu-80 cells chronically exposed to alcohol (26), also showed a significant inhibition in carrier-mediated thiamin uptake. This inhibition, however, was associated with a significant reduction in level of expression of both hTHTR-1 and hTHTR-2 at the protein and mRNA levels. This is unlike what we observed above in rats chronically fed alcohol where only THTR-1 appears to be affected. Whether this is due to differences in the model used (in vivo alcohol feeding vs. in vitro alcohol exposure) and/or due to interspecies difference is not clear and requires further investigations. The reduction in the level of expression of hTHTR-1 and hTHTR-2 appears to again be mediated (at least in part) via transcriptional mechanism(s), as indicated by the significant inhibition in the activity of the SLC19A2 and SLC19A3 promoters in human intestinal epithelial HuTu-80 cells chronically exposed to alcohol compared with control cells. Again, other mechanisms may contribute to the inhibitory effect of chronic alcohol exposure on thiamin uptake by HuTu-80 cells, and further investigations are needed to address this issue.
In summary, chronic alcohol feeding/exposure inhibits the physiological and molecular parameters of intestinal thiamin uptake process, and that effect is mediated (at least in part) via transcriptional mechanism(s). In addition, the inhibition in thiamin transport appears to be at the level of both the small and large intestine.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK56061 and AA18071 to H. M. Said, and DK71538 to V. S. Subramanian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abe T, Itokawa Y. Effect of ethanol administration on thiamine metabolism and transketolase activity in rats. Int J Vitam Nutr Res 47: 307–314, 1977 [PubMed] [Google Scholar]
- 2.Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139: 434–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berdanier CD. Micronutrients. In: Advanced Nutrition Boca Raton, FL: CRC, 1998, p. 80–88 [Google Scholar]
- 4.Boulware MJ, Subramanian VS, Said HM, Marchant JS. Polarized expression of members of the solute carrier SLC19A gene family of water-soluble multivitamin transporters: implications for physiological function. Biochem J 376: 43–48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusaro G, Rindi G, Sciorelli G. Subcellular distribution of thiamine-pyrophosphokinase and thiamine-pyrophosphatase activities in rat isolated enterocytes. Int J Vitam Nutr Res 47: 99–106, 1977 [PubMed] [Google Scholar]
- 6.Dudeja PK, Tyagi S, Gill R, Said HM. Evidence for a carrier-mediated mechanism for thiamine transport to human jejunal basolateral membrane vesicles. Dig Dis Sci 48: 109–115, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Dudeja PK, Tyagi S, Kavilaveettil RJ, Gill R, Said HM. Mechanism of thiamine uptake by human jejunal brush-border membrane vesicles. Am J Physiol Cell Physiol 281: C786–C792, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Elferink CJ, Reiners JJ., Jr Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Gastaldi G, Casirola D, Ferrari G, Rindi G. Effect of chronic ethanol administration on thiamine transport in microvillous vesicles of rat small intestine. Alcohol 24: 83–89, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Greene HL, Moore MC, Said HM, Ghishan FK, Orth DN. Intestinal glucose transport in suckling rats fed artificial milk with and without added epidermal growth factor. Pediatr Res 21: 404–408, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Hajjar JJ, Baker ER, Renison DM, Gardner PW, Zirin R, Tomicic TK. Effect of ethanol on choline transport in rat jejunum. Am J Physiol Gastrointest Liver Physiol 249: G177–G183, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Hajjar JJ, Tomicic T, Scheig RL. Effect of chronic ethanol consumption on leucine absorption in the rat small intestine. Digestion 22: 170–176, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Hamid A, Wani NA, Rana S, Vaiphei K, Mahmood A, Kaur J. Down-regulation of reduced folate carrier may result in folate malabsorption across intestinal brush border membrane during experimental alcoholism. FEBS J 274: 6317–6328, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9: 294–299, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hazell AS, Butterworth RF. Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol 44: 141–147, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Hopfer U, Nelson K, Perrotto J, Isselbacher KJ. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem 248: 25–32, 1973 [PubMed] [Google Scholar]
- 17.Hoyumpa AM., Jr Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr 33: 2750–2761, 1980 [DOI] [PubMed] [Google Scholar]
- 18.Hunter CK, Treanor LL, Gray JP, Halter SA, Hoyumpa A, Jr, Wilson FA. Effects of ethanol in vitro on rat intestinal brush-border membranes. Biochim Biophys Acta 732: 256–265, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Kohler CU, Roos PH. Focus on the intermediate state: immature mRNA of cytochromes P450–methods and insights. Anal Bioanal Chem 392: 1109–1122, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kubisch CH, Gukovsky I, Lugea A, Pandol SJ, Kuick R, Misek DE, Hanash SM, Logsdon CD. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas 33: 68–76, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Lieber CS, DeCarli LM. The feeding of ethanol in liquid diets. Alcohol Clin Exp Res 10: 550–553, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Nabokina SM, Reidling JC, Said HM. Differentiation-dependent up-regulation of intestinal thiamin uptake: cellular and molecular mechanisms. J Biol Chem 280: 32676–32682, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Nabokina SM, Subramanian VS, Said HM. Comparative analysis of ontogenic changes in renal and intestinal biotin transport in the rat. Am J Physiol Renal Physiol 284: F737–F742, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Rajendram R, Preedy VR. Effect of alcohol consumption on the gut. Dig Dis 23: 214–221, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Reidling JC, Lambrecht N, Kassir M, Said HM. Impaired intestinal vitamin B(1) (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 138: 1802–1809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J Cell Physiol 206: 371–377, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Reidling JC, Subramanian VS, Dudeja PK, Said HM. Expression and promoter analysis of SLC19A2 in the human intestine. Biochim Biophys Acta 1561: 180–187, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Rindi G, Patrini C. Thiamin. In: Encyclopedia of Dietary Supplements, edited by Coates P, Blackman MR, Cragg G, Levine M, Moss J, White J. London, UK: Informa Healthcare, 2005, p. 677–686 [Google Scholar]
- 31.Rindi G, Reggiani C, Patrini C, Laforenza U. Effect of ethanol administration on the in vivo kinetics of thiamine phosphorylation and dephosphorylation in different organs. I. Chronic effects of alcohol. Alcohol 26: 285–301, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Said HM. Recent advances in carrier-mediated intestinal absorption of water-soluble vitamins. Annu Rev Physiol 66: 419–446, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Said HM, Balamurugan K, Subramanian VS, Marchant JS. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am J Physiol Gastrointest Liver Physiol 286: G491–G498, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Said HM, Ortiz A, Subramanian VS, Neufeld EJ, Moyer MP, Dudeja PK. Mechanism of thiamine uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM460. Am J Physiol Gastrointest Liver Physiol 281: G144–G150, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Said HM, Redha R. A carrier-mediated transport for folate in basolateral membrane vesicles of rat small intestine. Biochem J 247: 141–146, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Said HM, Seetharam B. Intestinal absorption of water-soluble vitamins. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR. San Diego, CA: Elsevier, 2006, p. 1791–1825 [Google Scholar]
- 37.Salaspuro M. Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann Med 28: 195–200, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Scalera V, Storelli C, Storelli-Joss C, Haase W, Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J 186: 177–181, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamine transporter-2 in epithelial cells. J Biol Chem 281: 5233–5245, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Subramanian VS, Subramanya BS, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameter of renal thiamin transport. Am J Physiol Renal Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanphaichitr V. Thiamin. In: Modern Nutrition in Health and Disease, edited by Shils ME, Olsen JA, Shike M. New York: Lea and Febiger, 1994, p. 359–375 [Google Scholar]
- 42.Thomson AD, Baker H, Leevy CM. Thiamin absorption in alcoholism. Am J Clin Nutr 21: 537–538, 1968 [Google Scholar]
- 43.Tomasulo PA, Kater RM, Iber FL. Impairment of thiamine absorption in alcoholism. Am J Clin Nutr 21: 1341–1344, 1968 [DOI] [PubMed] [Google Scholar]
- 44.Verri A, Laforenza U, Gastaldi G, Tosco M, Rindi G. Molecular characteristics of small intestinal and renal brush border thiamin transporters in rats. Biochim Biophys Acta 1558: 187–197, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Victor M, Adams RD, Collins GH. The Wernick-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition. Philadelphia, PA: Davis, 1989 [Google Scholar]
- 46.You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008 [DOI] [PubMed] [Google Scholar]