Abstract
Glial cell line-derived neurotrophic factor (GDNF) is a factor produced by glial cells that is required for the development of the enteric nervous system. In transgenic mice that overexpress GDNF in the pancreas, GDNF has been shown to enhance β-cell mass and improve glucose control, but the transcriptional and cellular processes involved are not known. In this study we examined the influence of GDNF on the expression of neurogenin3 (Ngn3) and other transcription factors implicated in early β-cell development, as well as on β-cell proliferation during embryonic and early postnatal mouse pancreas development. Embryonic day 15.5 (E15.5) mouse pancreatic tissue when exposed to GDNF for 24 h showed higher Ngn3, pancreatic and duodenal homeobox gene 1 (Pdx1), neuroD1/β2, paired homeobox gene 4 (Pax4), and insulin mRNA expression than tissue exposed to vehicle only. Transgenic expression of GDNF in mouse pancreata was associated with increased numbers of Ngn3-expressing pancreatic cells and higher β-cell mass at embryonic day 18 (E18), as well as higher β-cell proliferation and Pdx1 expression in β-cells at E18 and postnatal day 1. In the HIT-T15 β-cell line, GDNF enhanced the expression of Pax6. This response was, however, blocked in the presence of Pdx1 small interfering RNA (siRNA). Chromatin immunoprecipitation studies using the HIT-T15 β-cell line demonstrated that GDNF can influence Pdx1 gene expression by enhancing the binding of Sox9 and neuroD1/β2 to the Pdx1 promoter. Our data provide evidence of a mechanism by which GDNF influences β-cell development. GDNF could be a potential therapeutic target for the treatment and prevention of diabetes.
Keywords: insulin, embryonic, progenitors, endocrine, enteric
the vertebrate pancreas originates from the foregut as two protrusions of specialized endodermal epithelial cells and goes through three developmental transitions in which there are extensive cell proliferation and differentiation culminating in the formation of the exocrine pancreas (consisting of acinar tissue and ductal epithelium) and endocrine pancreas (consisting of glucagon-secreting α-cells, insulin-secreting β-cells, somatostatin-secreting δ-cells, and pancreatic polypeptide-secreting PP-cells) (20). These waves of cell proliferation and differentiation are driven by signals such as activin-β and fibroblast growth factors that derive from the mesoderm and notochord, and by the sequential expression of transcription factors (10, 15, 18, 27, 29).
Pancreatic and duodenal homeobox gene 1 (Pdx1) is a transcription factor expressed in pancreatic progenitor cells as well as mature β-cells and is essential for the development of all pancreatic cell types and maintenance of β-cell function (16, 37). These progenitors proliferate to enhance the progenitor pool and are maintained in an undifferentiated state through the expression of the transcription factor Sry/HMG box gene 9 (Sox9) and activation of the notch signaling (3, 22, 30). All endocrine cell types derive from Pdx1-positive progenitors that express the basic helix-loop-helix (bHLH) transcription factor neurogenin3 (Ngn3) (15, 19, 28). Genetic gain-and-loss-of-function experiments have shown that the transcription factors neuroD1/β2, the NK-homeodomain genes NKx2.2 and NKx6.1, and paired homeobox genes 4 (Pax4) and 6 (Pax6) that are required for β-cell development are downstream of Ngn3 (19, 27, 28, 31).
Although the pancreas develops in close association with the enteric nervous system, the role that neurotrophic factors play in pancreas development has not been well characterized. Glial cell line-derived neurotrophic factor (GDNF), which belongs to the TGF-β family of growth factors, plays a critical role in the development of the enteric nervous system (2, 33). Our previous studies have shown evidence of expression of GDNF in and around the islets in mice, whereas GDNF transgenic mice that are engineered to overexpress GDNF in glia under the control of the glial fibrillary acidic protein promoter have higher β-cell mass and improved glucose control than their CF1 wild-type (WT) littermates (24). The role that GDNF plays in early β-cell development and the mechanisms involved is, however, not known.
In this study we examined the influence of GDNF on early β-cell development. Our studies demonstrate that GDNF enhances Pdx1, Ngn3, neuroD1/β2, and Pax4 gene expression in embryonic mouse pancreata and β-cell proliferation in embryonic and postnatal mouse pancreata. We also show that GDNF can enhance the binding of the transcription factors neuroD1/β2 and Sox9 to the Pdx1 promoter to stimulate the gene. Our data provide evidence of a mechanism by which GDNF can influence β-cell development.
MATERIALS AND METHODS
Experimental animals.
Embryonic day 15.5 (E15.5) and 18 (E18) and postnatal day 1 and 7 mice were obtained from crossing CF1 WT mice with GDNF transgenic (GDNF-tg) mice that were on a CF1 background and were engineered to overexpress GDNF in glia under the control of the glial fibrillary acidic protein promoter (36). Previous studies in these mice showed increased GDNF expression in the brain and spinal cord and enhanced motor neuron survival during embryonic and postnatal development (36). Mid-day on which a vaginal plug was detected was considered as stage E0.5 for embryo collection (26). The genotypes of the mice were determined by polymerase chain reaction (PCR) using DNA extracted from mouse tail by using the REDExtract-N-Amp Tissue PCR Kit (Sigma-Aldrich, St. Louis, MO) according to recommended procedure. Forward (5′-AGACGCATCACCTCCGCT-3′) and reverse (5′-TGACGTCATCAAACTGGTCAGG-3′) primers designed to amplify the transgene sequence were used. All animal studies were approved by the Emory University Animal Care and Use Committee.
Cell and tissue culture and transfection.
CF1 E15.5 embryos were obtained according to approved protocol. The embryos were removed and the entire gut including the stomach, pancreatic buds, and small and large bowel were isolated. The pancreatic buds containing β-cell clusters were identified after staining with the pancreatic cell DTZ detection assay kit (Chemicon International, Temecula, CA), teased out from the rest of the gut tissue, split into two groups of at least five pancreatic rudiments per group, and cultured for 24 h at 37°C in a CO2 incubator in Kaighn's modification of Ham's (F-12K) medium (ATCC, Manassas, VA) supplemented with or without 100 ng/ml recombinant rat GDNF obtained as previously described (9).
HIT-T15 hamster β-cells (ATCC) were cultured in F-12K medium supplemented with 10% horse serum (ATCC) and 2.5% fetal bovine serum (ATCC). For GDNF stimulation studies, the cells were serum deprived for 24 h followed by incubation with serum-free F-12K medium alone or serum-free F-12K medium supplemented with 100 ng/ml recombinant rat GDNF. HIT-T15 cells grown in complete medium were transfected with 30 nM Pdx1 small interfering RNA (siRNA) (CAG CUC CCU UUC CCG UGG AUG AAA U) or control siRNA by use of Lipofectamine RNAiMax (Invitrogen). The siRNA was designed based on published Mesocricetus auratus PDX1 mRNA sequence (accession no. U73854.1). The cells were washed three times with F-12K medium without serum (incomplete F-12K medium) 24 h after transfection and cultured for an additional 48 h in incomplete F-12K medium supplemented with or without 100 ng GDNF/ml.
PCR.
GDNF gene expression in embryonic and postnatal mouse pancreata was confirmed by RT-PCR. Total RNA was isolated from frozen sections from mouse pancreata by use of the PicoPure RNA isolation kit (Arcturus, Mountainview, CA) and first-strand cDNA generated with the Sensiscript Reverse Transcription kit (Qiagen, Hilden, Germany). Analyses of transcription factor gene expression in cultured E15.5 mouse embryonic pancreatic tissue were performed by real-time PCR. Total RNA was isolated from cultured tissue by using the RNeasy Mini kit (Qiagen) and reverse transcribed with the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The integrity of isolated RNA was checked on an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany) equipped with Agilent's RNA 6000 Nano Chips. RNA integrity numbers (RINs) were generated by using the 2100 Bioanalyzer Expert software (Agilent Technologies), and samples with a RIN of 6.0 or higher were used for downstream assays whereas those with lower RINs were discarded. Real-time PCR reactions were set up by using Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and thermal cycling was performed on an iCycler (Bio-Rad) equipped with an iCycler real-time PCR detection system. The sequences of the oligonucleotide primers used are presented in Table 1. All primers were designed such that at least one primer in each pair spanned an intron to prevent it from priming on genomic DNA. The inability of these primers to amplify genomic DNA was confirmed by PCR. GAPDH was used as an endogenous control for RT-PCR experiments to analyze the expression of GDNF in pancreatic tissue whereas β2-microglobulin was used as an endogenous control in real-time PCR analyses of gene expression in embryonic and postnatal mouse pancreata.
Table 1.
Oligonucleotide primer sequences
| Oligo Name | Oligo Sequence | Temperature | Start Position | Location | Product Size, bp | Genomic Size, bp | Gene Accession No. |
|---|---|---|---|---|---|---|---|
| Ngn3_FW | TTTGAGTCGGGAGAACTAGGATGG | 57.7°C | 220 | Exon 1–2 | 118 | 0–251 | NM_009719.6 |
| Ngn3_RV | TTGGAACTGAGCACTTCGTGGT | 58.6°C | 337 | Exon 2 | |||
| NeuroD1_FW | AGGAATTCGCCCACGCAGAA | 59.3°C | 4 | Exon 1 | 101 | 0–1,603 | NM_010894 |
| NeuroD1_RV | TGGTCATGTTTCCACTTCCTGTTGT | 58.5°C | 104 | Exon 2–1 | |||
| Insulin_FW | TCAAGCAGCACCTTTGTGGT | 57.2°C | 150 | 127 | 0–615 | NM_008387.3 | |
| Insulin_RV | AGCTCCAGTTGTGCCACTTGT | 59.1°C | 276 | ||||
| Pax4_FW | AGCTGTGTTGGCTCCAGTTCTT | 58.8°C | 641 | Exon 6 | 144 | 0–370 | NM_011038.1 |
| Pax4_RV | TGCCCACGCTGAAACTCTTTCT | 58.9°C | 784 | Exon 7–6 | |||
| Pdx1_FW | CAGTGGGCAGGAGGTGCTTA | 59.2°C | 508 | Exon 1–2 | 157 | 3,889 | NM_008814.3 |
| Pdx1_RV | AGTTCAACATCACTGCCAGCTC | 57.3°C | 664 | Exon 2 | |||
| β2-Microglobulin_FW | ACCGGCCTGTATGCTATCCAGAAA | 97 | Exon 1–2 | 124 | NM_009735.3 | ||
| β2-Microglobulin_RV | TTTCAATGTGAGGCGGGTGGAA | 220 | Exon 2 | ||||
| GDNF_FW | TTATGGGATGTCGTGGCTGT | 56.3°C | 137 | 618 | 19,199 | NM_010275.2 | |
| GDNF_ RV | ACACCGTTTAGCGGAATGCT | 57.3°C | 754 | ||||
| GAPDH_FW | TTGTGATGGGTGTGAACCACGA | 59.0°C | 168 | ||||
| GAPDH_RV | TCTTCTGGGTGGCAGTGATGG | 59.3°C |
ChIP assays.
HIT-T15 cells grown to 80–90% confluency in incomplete F-12K medium in six-well plates were washed three times with serum-free F-12K medium and cultured for 24 h in serum-free F-12K medium. The medium was replaced with fresh serum-free F-12K medium or serum-free F-12K medium supplemented with 100 ng GDNF/ml, and the cells were grown for a further 24 h. The cells were fixed for 10 min with 1% paraformaldehyde, and chromatin immunoprecipitation (ChIP) assays were performed according to recommended procedure with the EZ-ChIP Chromatin Immunoprecipitation kit (Millipore, Temecula, CA). Sonicated chromatin were incubated for 24 h at 4°C with 1:100 dilutions of rabbit antibodies to Pdx1 (Millipore), NeuroD1 (Abcam, Cambridge, MA), and Sox9 (Santa Cruz Biotechnology, Santa Cruz, CA). A rabbit antibody to Myc-Tag (Cell Signaling) was used as a negative control IgG. PCR to amplify immunoprecipitated DNA was performed using primer pairs 5′-TGGATTCAGAGCGGAAATGCGT and 5′-CCCAGGATGTTTGCTTAGCACTTCT, and 5′-TCGCTCATAACCGTGTCACCAA and 5′-TGCCAGTGATCACCGTGTTTCT. The primers amplify portions of the mouse Pdx1 promoter that extent, respectively, from nt −2613 to −2084 and nt −1976 to −1279 upstream of the mouse Pdx1 gene transcription start site (accession no. NT_039324.7). These regions contain, respectively, potential neuroD1 and Sox9, and Pdx1 transcription factor binding sites as determined by use of the Transcription Element Search System software (TESS; Computational Biology and Informatics Laboratory, University of Pennsylvania); they lie within an area of the mouse Pdx1 promoter that has been shown to be required for β-cell-specific transcription of the Pdx1 gene (14).
Immunofluorescence microscopy.
Pancreata isolated from mouse embryos and postnatal day 1 and 7 mice were either fixed in 10% formalin solution and embedded in paraffin by standard techniques or frozen in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA). Paraffin sections (5 μm thick) were dewaxed according to suggested protocols (Cell Signaling Technologies, Danvers, MA) and antigen retrieval achieved by boiling for 10 min at 125°C in 10 mM sodium citrate (pH 6.0) in a decloaking chamber (Biocare Medical, Concord, CA). Frozen sections (7 μm thick) and HIT-T15 cells were fixed for 15 min in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and permeabilized for 5 min with 1% Igepal CA-630 (Sigma) in phosphate-buffered saline (pH 7.5). Blocking was performed for 1 h at room temperature in 3% bovine serum albumin in phosphate-buffered saline containing 0.02% Triton X-100 (Sigma). Immunostaining was performed as previously described (24) with use of the following antibodies: mouse anti-Ngn3 (Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa department of Biology, Iowa City, IA), guinea pig anti-insulin (Zymed Laboratories, San Francisco, CA), mouse anti-Ki67 (Novocastra, Newcastle, UK), and rabbit anti-hairy enhancer-of-split (Hes)-1 (Millipore) at 1:100 dilution, rabbit anti-Pdx1 (Upstate Cell Signaling Solutions, Lake Placid, NY) at 1:200 dilution, and Alexa Fluor 488 and 594 donkey anti-mouse, anti-guinea pig and anti-rabbit IgG (Molecular Probes, Eugene, OR) secondary antibodies at 1:500 dilution. Primary and secondary antibodies for single labeling and antibody cocktails for double-labeling were prepared at their appropriate dilution in blocking buffer. Incubations with primary antibodies were performed overnight at 4°C in a humidified chamber, whereas incubations with secondary antibodies were for 1 h at room temperature. Nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI) was achieved by adding 300 nM DAPI (Invitrogen) to the second phosphate-buffered saline wash buffer after secondary antibody incubation.
Scoring procedure.
Frozen sections from pancreatic tissues from E18 GDNF-tg mouse embryos and WT littermates were double labeled with antibodies to Ngn3 and insulin and nuclei counterstained with DAPI. Images were taken from at least three randomly selected fields from each section with use of a ×40 objective and the number of Ngn3-positive cells as well as the total number of cells in each field determined. The proportion of Ngn3-positive cells was then expressed as a percentage of the total number of cells in each field.
Ki67 and Pdx1 scores were obtained from pancreas sections double labeled with antibodies to Ki67 and insulin and Pdx1 and insulin, respectively, and nuclei were counterstained with DAPI. Images of all the islets in four sections (separated by 200 μm) from each mouse were taken and the number of Ki67 and Pdx1-positive β-cells as well as the total numbers of β-cells in each islet were determined. The numbers of Ki67- and Pdx1-positive β-cells were then expressed as a percentage of all β-cells in an islet.
Relative β-cell area.
Four 5-μm pancreas sections (separated by 200 μm) per mouse were used to assess β-cell mass as previously described (4). Briefly, after staining of β-cells for insulin, each section was analyzed on a Zeiss Axioskop 2 plus fluorescent microscope (Carl Zeiss Werk, Gottingen, Germany) mounted with an AxioCam MRc 5 camera and images of the entire section were taken with the aid of the Axiovision (Rel 4.5) software (Carl Zeiss Imaging System). The total area covered by β-cells and the total area of pancreatic tissue in each section were determined by use of the Image J version 1.42j software (National Institutes of Health). The β-cell area expressed as a percentage of the total pancreas area was then calculated.
Western blotting.
Western blotting was performed as previously described (23) with use of the following antibodies: mouse anti-β-actin (Sigma, St. Louis, MO), and rabbit anti-Pdx1 (Upstate Cell Signaling Solutions) and α-tubulin (Chemicon International) were used at 1:5,000 dilution; mouse anti-GAPDH and anti-Pax6 (Chemicon International) at 1:100 and 1:2,000 dilution, respectively, and guinea pig anti-insulin (Zymed Laboratories) were used at 1:1,000 dilution. Horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG (Cell Signaling Technologies) were used at 1:3,000 dilution. A semiquantitative measurement of band density was performed with Scion Image for Windows software.
Statistical analysis.
All statistical analyses were conducted using the GraphPad Prism software version 3.00 for Windows (GraphPad Software, San Diego CA). Data were tested for normality and subjected to t-tests or one-way ANOVA with Tukey's posttest.
RESULTS
GDNF enhances expression of genes influencing β-cell development in mouse embryo pancreata.
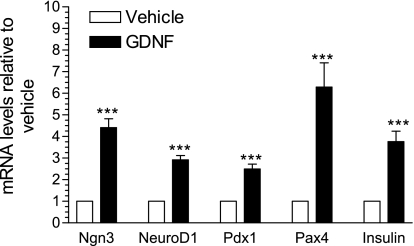
The developmental period from about E13.5 to E16.5 in the mouse is characterized by intense pancreatic endocrine cell differentiation (20) and major changes in the expression pattern of several endocrine progenitor cell markers (20, 32). To investigate whether GDNF could exert its influence during this growth phase, the expression of mRNA for markers of pancreatic endocrine progenitors in E15.5 WT mouse embryos pancreas rudiments cultured for 24 h in F-12K medium supplemented with or without GDNF was assessed by real-time PCR. Ngn3, neuroD1/β2, Pdx1, and Pax4 (4.4 ± 0.4, 2.9 ± 0.2, 2.5 ± 0.2, and 6.3 ± 1.1-fold, respectively) mRNA expression was higher in rudiments cultured in the presence of GDNF than in vehicle only (P < 0.001, Fig. 1). Rudiments cultured in GDNF also showed 2.8 ± 0.5-fold higher insulin gene expression than those cultured in vehicle only (Fig. 1).
Fig. 1.
Glial cell-derived neurotrophic factor (GDNF) enhances endocrine progenitor cell marker gene expression in mouse embryo pancreata. Real-time PCR analysis of neurogenin3 (Ngn3), NeuroD1, pancreatic and duodenal homeobox gene 1 (Pdx1), paired homeobox gene 4 (Pax4), and insulin gene expression in embryonic day 15.5 (E15.5) mouse embryo pancreata cultured for 24 h in vehicle or GDNF. Plotted are means ± SE. ***P < 0.001, N = 5 embryos per group.
Expression of GDNF mRNA is higher in pancreata from embryonic and postnatal day 1 and 7 GDNF transgenic mice.
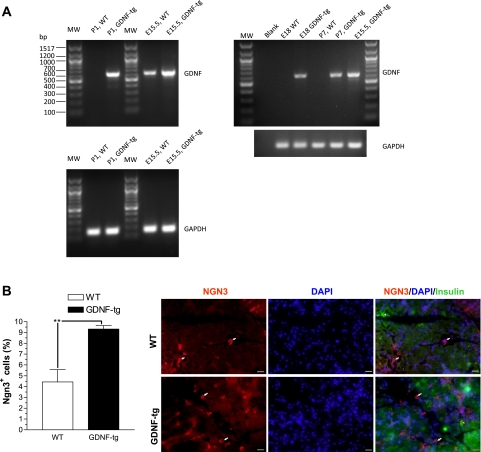
To compare the levels of GDNF gene expression in the developing pancreas between WT and GDNF-tg littermates, total RNA was isolated from pancreata from E15.5, E18, and postnatal day 1 and 7 mice and the presence of GDNF transcripts confirmed by RT-PCR using a set of primers that amplify a 600-bp fragment from GDNF mRNA. Although high expression of GDNF mRNA was observed in pancreata from GDNF-tg mice at all time points, significantly lower expression was observed in pancreata from E15.5 WT mouse embryos, and none in pancreata from E18 and postnatal day 1 and 7 WT mice (Fig. 2A).
Fig. 2.
GDNF transgenic (GDNF-tg) mouse embryos have more Ngn3-expressing pancreatic cells than wild-type (WT) littermates. A: analysis of GDNF and GAPDH (loading control) gene expression in pancreata from embryonic day 15.5 (E15.5) and 18 (E18) and postnatal day 1 (P1) and 7 (P7) GDNF-tg mice and WT littermates. MW, 100-bp molecular weight marker. B: pancreas sections from E18 GDNF-tg mice and WT littermates immunofluorescently stained for Ngn3 (red) with insulin (green) and 4′,6-diamidino-2-phenylindole (DAPI; blue) nuclear staining. Arrows show representative Ngn3+ cells. Scale 20 μm. Histogram shows a plot of Ngn3+ cell frequency expressed as a percentage of all the cells/field. Plotted are means ± SE (**P < 0.01), N = 3 mice.
GDNF-tg mouse embryos have more Ngn3-positive pancreatic cells.
Ngn3 expression in the developing pancreas marks a key step in the development of endocrine lineage cells including β-cells. To examine how GDNF might influence the number of β-cell precursors in vivo, sections from pancreatic tissues from E18 GDNF-tg mouse embryos and WT littermates were labeled with antibodies to Ngn3 and insulin and nuclei counterstained with DAPI (Fig. 2B). The percentage of Ngn3-positive cells in each section were then determined. The number of Ngn3-positive (Ngn3+) cells in pancreata from GDNF-tg mice (9.3 ± 0.3, N = 4) was significantly (P < 0.01) higher than that in pancreata from WT littermates (4.4 ± 1.1, N = 3) (Fig. 2B).
GDNF enhances β-cell proliferation in E18 and postnatal day 1 mouse pancreata.
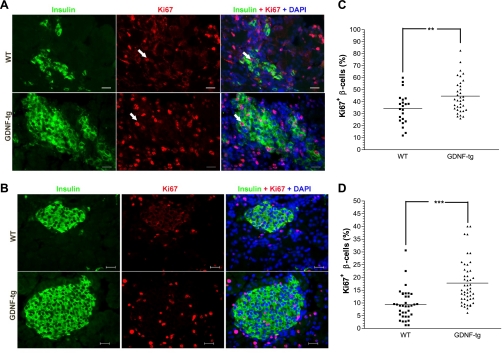
To further investigate the mechanisms by which GDNF could influence β-cell development, pancreata from E18 and postnatal day 1 GDNF-tg mice and WT littermates were analyzed for β-cell proliferation by staining for the Ki67 cell proliferation marker and insulin with DAPI nuclear counterstaining (Fig. 3, A and B). Higher percentages of β-cells in each islet that were positive for Ki67 were observed in pancreata from GDNF-tg mice than from WT littermates at both E18 (44.7 ± 2.4 vs. 34.3 ± 2.8%) (Fig. 3C) and postnatal day 1 (17.8 ± 1.3 vs. 9.3 ± 0.1%) (Fig. 3D).
Fig. 3.
GDNF enhances β-cell proliferation in E18 and postnatal day 1 mouse pancreata. Representative images of pancreas sections from E18 (A) and postnatal day 1 (B) GDNF-tg mice and WT littermates stained for Ki67 (red) and insulin (green) with DAPI nuclear staining. Scale bar: 20 μm. C: scatter chart showing percentages of Ki67+ β-cells in E18 WT and GDNF-tg mouse pancreata. Each point represents an islet. Horizontal lines show group means. **P < 0.01, N = 3 WT and 6 GDNF-tg embryos. D: scatter chart showing percentages of Ki67+ β-cells in postnatal day 1 WT and GDNF-tg mouse pancreata. Each point represents an islet. Horizontal lines show group means. ***P < 0.001, N = 3 WT and 5 GDNF-tg mice.
GDNF-tg mouse embryos have higher relative β-cell area than WT littermates.
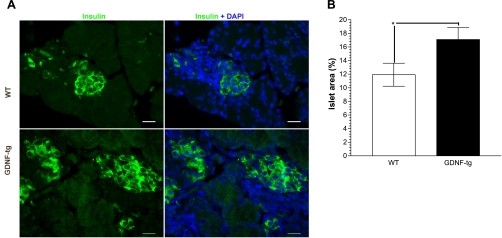
To assess the impact of the higher β-cell proliferation rates observed in GDNF-tg mice, pancreata isolated from E18 GDNF-tg mice and WT littermates were stained for insulin (Fig. 4A) and β-cell areas expressed as a percentage of total pancreatic tissue area determined. As seen in Fig. 4B, GDNF-tg mouse embryos had a significantly higher β-cell area (17.09 ± 1.76%) than WT littermates (11.91 ± 1.69%) (P < 0.05).
Fig. 4.
GDNF-tg mouse embryos have higher β-cell area than WT littermates. A: insulin staining (green) with DAPI (blue) nuclear staining in pancreata from E18 GDNF-tg mouse embryos and WT littermates. β-Cell relative area was assessed as described in methods. Scale bar: 20 μm. B: plot of β-cell area. Plotted are means ± SE. *P < 0.05, N = 3 WT and 6 GDNF-tg embryos.
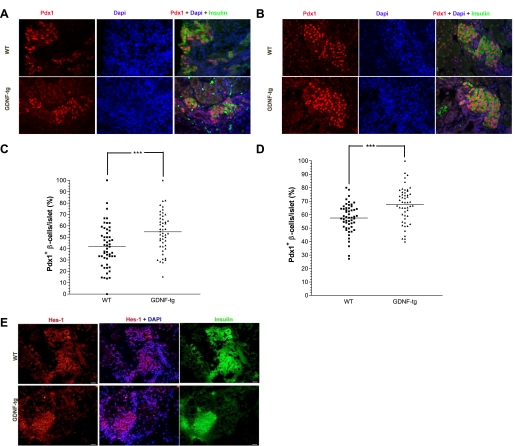
GDNF-tg mouse embryos have more Pdx1-positive β-cells.
Until about E15.5 in mice, Pdx1 expression occurs throughout the developing pancreas but later shifts to β-cells, where it plays a key role in insulin synthesis. To understand how GDNF influences Pdx1 expression, pancreata from E18 and postnatal day 1 GDNF-tg mice and WT littermates were immunofluorescently labeled with antibodies to Pdx1 and insulin followed by DAPI nuclear staining to identify β-cells (Fig. 5, A and B, respectively). The percentage of Pdx1-positive β-cells within each islet was then determined on the basis of intensity of nuclear staining. Islets from E18 and postnatal day 1 GDNF-tg mice had 12.8 ± 3.6 and 9.95 ± 2.4%, respectively, more Pdx1-positive β-cells than islets from WT littermates (Fig. 5, C and D; P < 0.001).
Fig. 5.
Pancreata from E18 and postnatal day 1 GDNF-tg mice have more Pdx1+ β-cells. A: pancreata from E18 GDNF-tg and WT mouse embryos immunofluorescently stained for Pdx1 (red) and insulin (green) with DAPI nuclear staining (blue). B: pancreata from postnatal day 1 GDNF-tg and WT littermates immunofluorescently stained for Pdx1 (red) and insulin (green) with DAPI nuclear staining (blue). Scale bar: 20 μm. C: score of Pdx1+ β-cells in islets from E18 GDNF-tg mouse embryos and WT littermates. Each point represents an islet. Horizontal lines show group means. ***P < 0.001, N = 3 WT and 6 GDNF-tg embryos. D: score of Pdx1+ β-cells in islets from postnatal day 1 GDNF-tg mice and WT littermates. Each point represents an islet. Horizontal lines show group means. ***P < 0.001, N = 3 WT and 5 GDNF-tg mice. E: pancreata from E18 GDNF-tg and WT mouse embryos immunofluorescently stained for Hes-1 (red) and insulin (green) with DAPI nuclear staining (blue). Scale bar: 20 μm.
Hes-1 expression is similar in pancreata from E18 GDNF-tg mice and WT mice.
The notch signaling pathway plays an important role in maintaining the pancreatic progenitor pool, and repression of notch signaling is required for the differentiation of pancreatic endocrine cells from their common Ngn3-expressing progenitor. We assessed the influence of GDNF on notch signaling activation in the pancreas by staining pancreata from E18 GDNF-tg mouse embryos and WT littermates for Hes-1, a downstream effector of notch signaling. Nearly all the cells in the islets, acinar tissue, and ductal tissue in GDNF-tg and WT embryo pancreata showed intense Hes-1 nuclear staining, and there was no difference in the staining pattern between the two groups of mice (Fig. 5E).
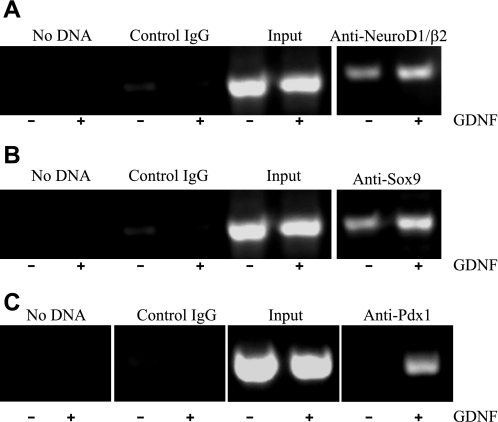
GDNF enhances the binding of Sox9 and neuroD1/β2 to the Pdx1 promoter.
We next examined the effect GDNF on the regulation of the Pdx1 gene. HIT-T15 hamster β-cells were cultured for 24 h in serum-free medium followed by a further 24 h in serum-free medium supplemented with or without 100 ng/ml GDNF. The binding of Sox9, neuroD1/β2, and Pdx1 to the Pdx1 promoter was then analyzed by the ChIP assay using antibodies to Sox9, neuroD1/β2, and Pdx1 and oligonucleotide primer pairs designed to amplify regions of the mouse Pdx1 gene promoter extending from nt −1886 to −1187 and nt −2521 to −1992 upstream of the published S1 transcription start site (14). These regions had been determined by using the Transcription Element Search System online software (TESS; Computational Biology and Informatics Laboratory, University of Pennsylvania) to contain potential Sox9 (nt −1722 to −1708), neuroD1/β2 (nt −1635 to −1627), and Pdx1 (nt −2046 to −1980) binding sites. PCR analyses of products amplified with the primer pair amplifying region −1886 to −1187 of the mouse Pdx1 promoter revealed 1.73 ± 0.01-fold (P < 0.001, N = 4) and 2.1 ± 0.02-fold (P < 0.001, N = 4) higher promoter DNA pull-down from lysates from cells exposed to GDNF by antibodies to neuroD1/β2 (Fig. 6A) and Sox9 (Fig. 6B), respectively, than from cells exposed to vehicle, whereas no DNA pull-down was observed with control IgG. In addition, DNA pull-down was detected using the primer pair amplifying region −2046 to −1980 of the Pdx1 promoter from samples precipitated from lysates from cells exposed to GDNF with anti-Pdx1 antibody whereas none was detected in samples from lysates from cells exposed to vehicle (Fig. 6C). These results suggest the ability of GDNF to promote the binding of transcription factors SOX9 and NeuroD1 to the Pdx1 promoter and result in enhanced Pdx1 transcription.
Fig. 6.
GDNF enhances neuroD1/β2 and Sox9 binding to the Pdx1 promoter. HIT-T15 β-cells were cultured for 24 h in medium supplemented with GDNF (+) or without GDNF (−) and DNA precipitations performed with antibodies to Sox9, neuroD1/β2, Pdx1, or irrelevant IgG (control IgG) as indicated on the top of each gel. Chromatin immunoprecipitation analysis of neuroD1/β2 (A), Sox9 (B), and Pdx1 (C) transcription factor binding to the Pdx1 promoter.
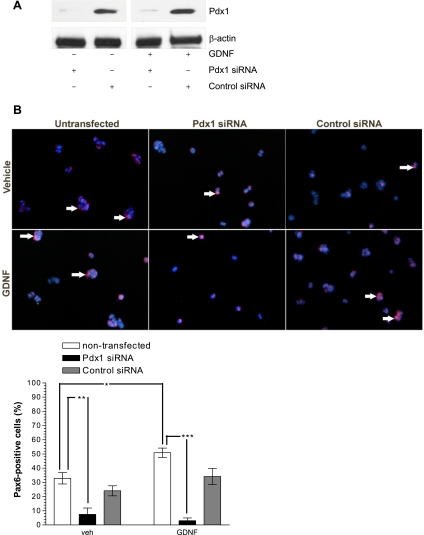
GDNF-stimulated Pax6 expression in β-cells is blocked by knockdown of Pdx1 gene expression.
Pdx1 not only is essential for β-cell development but also plays a critical role in maintenance of differentiated β-cells by influencing insulin gene transcription. Pax6 is a key regulator in the terminal differentiation of the endocrine pancreas and β-cell function. To assess the role of Pdx1 in GDNF signaling in β-cells, Pdx1 gene expression in HIT-T15 hamster β-cells was knocked down by using Pdx1-specific siRNA and the cells were cultured for 48 h in medium supplemented with or without GDNF. The successful knockdown of Pdx1 was confirmed by Western blotting (Fig. 7A) and the expression of Pax6 was analyzed by immunofluorescence staining (Fig. 7B). GDNF increased the levels of PAX6 in the presence of control siRNA as well as in nontransfected cells (P < 0.001) but had no effects on cells transfected with Pdx1 siRNA (50.83 ± 3.35% Pax6-positive, nontransfected, GDNF supplemented vs. 3.0 ± 1.9% Pax6-positive, Pdx1 siRNA transfected, GDNF supplemented) (Fig. 7B).
Fig. 7.
GDNF-stimulated Pax6 expression in β-cells is reduced by knockdown of Pdx1 gene expression. A: Western blot analysis of Pdx1 knockdown with Pdx1 small interfering RNA (siRNA). B: immunofluorescence staining for Pax6 (red) with DAPI nuclear staining (blue) in HIT-T15 cells transfected with Pdx1 or control siRNA and cultured for 48 h in the presence or absence of GDNF. Scale bar: 20 μm. Plotted are means ± SE. ***P < 0.001, **P < 0.01, *P < 0.05. N = 4.
DISCUSSION
Decreased β-cell mass underlies the development and progression of both Type 1 and Type 2 diabetes mellitus in humans. Therefore, understanding how β-cell mass is regulated is imperative for both prevention and treatment (6). In our previous studies we showed that GDNF transgenic mice that are engineered to overexpress GDNF in glia under the control of the glial fibrillary acidic protein promoter have higher GDNF expression in the pancreas and increased β-cell mass and better glucose control than WT littermates (24). In our present study we demonstrate a role for GDNF in early β-cell development. Our data show that GDNF stimulates increased expression of transcription factors including Ngn3, Pdx1, neuroD1/β2b and Pax4 that are involved in early β-cell development, and enhances the number of Ngn3-expressing β-cell precursors during the embryonic stages of pancreas development. Our data also show higher proliferative activity and increased transcription of the insulin gene in islets during embryonic development in mice overexpressing GDNF.
Differentiation of endocrine progenitors into β-cells involves expression of a coordinated set of genes. One of these genes is the bHLH protein Ngn3, which plays an important role in specification of endocrine lineage and is sufficient to trigger endocrine differentiation in pancreatic progenitors and ductal cells (13). Recently, the expression of Ngn3 in the pancreas during embryonic development was shown to exhibit a biphasic pattern with the second wave preceding the second developmental transition (32). Data presented in this study show an ability of GDNF to significantly enhance Ngn3 expression in embryonic mouse pancreata.
In addition to Ngn3, the transcription factor Pdx1 plays an important role in β-cell differentiation. Targeted disruption of Pdx1 has been shown to lead to the development of overt diabetes (1, 5), whereas Pdx1 overexpression is associated with increased β-cell proliferation (12). Similarly, the development of Type 2 diabetes was recently shown to be associated with epigenetic silencing of Pdx1 (17, 25). Data from our study show that GDNF enhances Pdx1 expression during early development, whereas knockdown of Pdx1 expression prevents β-cell differentiation induced by GDNF. Furthermore, GDNF enhances the binding to the Pdx1 promoter of transcription factors involved in β-cell differentiation. Transcriptional regulation of Pdx1 is critical to β-cell development. Thus data from our studies showing enhanced binding of regulators of the Pdx1 promoter in the presence of GDNF suggest a mechanism by which GDNF influences β-cell development.
Several growth factors including insulin-like growth factors, glucagon-like peptide 1 (GLP-1) and, recently, keratinocyte growth factor have been shown to improve β-cell mass (11, 34). GLP-1, for example, stimulates embryonic stem cells to differentiate into insulin-producing cells, and this stimulation is associated with increased expression of Pdx1 and Ngn3 (35). Moreover, embryonic rudiments from spontaneously diabetic GK rats when treated with IGF-II show improvement in β-cell mass (7). In our studies, addition of GDNF to embryonic pancreatic rudiments accelerated β-cell differentiation in vitro, whereas overexpression of GDNF in embryonic mouse pancreata was associated with increased cell proliferation within the islets, a higher number of Pdx1-positive β-cells per islet, as well as higher β-cell mass. Identification of new factors that can promote the development of insulin-producing cells is critical for the prevention and treatment of diabetes. Studies are ongoing for new sources of diabetes cell therapy (17). Islet cell transplant has been limited owing to shortage of donors. Thus development of methods to enhance the production of β-cells is important in islet transplantation. A recent review highlighted the role of Pdx1 and Ngn3 as important transcriptional therapeutic targets for diabetes (21). In our studies GDNF could enhance the expression of these transcriptional targets. Small molecules that differentiate embryonic stem cells into functional insulin secreting β-cells such as indolactam V have been identified (8). Future studies will focus on the development of an agonist similar to GDNF that can be administered to improve β-cell mass. Understanding how growth factors regulate normal β-cell development will also provide us with new insight on the development of β-cells from embryonic stem cells.
GRANTS
This work was supported by the following grants NIH-KO8 DK067045 (S. Srinivasan), RO3 (S. Srinivasan), Juvenile Diabetes Foundation (S. Srinivasan), VA MERIT award (S. Srinivasan) DK06411 (S. V. Sitaraman) DDRDC (DK064399).
DISCLOSURES
The authors have declared that no conflicts of interest exist.
ACKNOWLEDGMENTS
We thank Dr. Mauricio Rocca and the Emory University Division of Pulmonary Diseases for allowing us use of their histological tissue processing equipment.
REFERENCES
- 1.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 12: 1763–1768, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature 400: 877–881, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest 108: 1631–1638, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher MJ, Simoneau M, Edlund H. The homeodomain-interacting protein kinase 2 regulates insulin promoter factor-1/pancreatic duodenal homeobox-1 transcriptional activity. Endocrinology 150: 87–97, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Calderari S, Gangnerau MN, Thibault M, Meile MJ, Kassis N, Alvarez C, Portha B, Serradas P. Defective IGF2 and IGF1R protein production in embryonic pancreas precedes beta cell mass anomaly in the Goto-Kakizaki rat model of type 2 diabetes. Diabetologia 50: 1463–1471, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5: 258–265, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA 94: 7018–7023, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlund H. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat Rev Genet 3: 524–532, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Egan JM, Bulotta A, Hui H, Perfetti R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev 19: 115–123, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Feanny MA, Fagan SP, Ballian N, Liu SH, Li Z, Wang X, Fisher W, Brunicardi FC, Belaguli NS. PDX-1 expression is associated with islet proliferation in vitro and in vivo. J Surg Res 144: 8–16, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang J, Chakrabarti S, Mirmira R, German M. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc Natl Acad Sci USA 101: 13245–13250, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerrish K, Gannon M, Shih D, Henderson E, Stoffel M, Wright CV, Stein R. Pancreatic beta cell-specific transcription of the pdx-1 gene. The role of conserved upstream control regions and their hepatic nuclear factor 3beta sites. J Biol Chem 275: 3485–3492, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 97: 1607–1611, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Guo T, Hebrok M. Stem cells to pancreatic beta-cells: new sources for diabetes cell therapy. Endocr Rev 30: 214–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart AW, Baeza N, Apelqvist A, Edlund H. Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature 408: 864–868, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes 49: 163–176, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev 28: 685–705, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Miyatsuka T, Matsuoka TA, Kaneto H. Transcription factors as therapeutic targets for diabetes. Expert Opin Ther Targets 12: 1431–1442, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 100: 14920–14925, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mwangi S, Anitha M, Fu H, Sitaraman SV, Srinivasan S. Glial cell line-derived neurotrophic factor-mediated enteric neuronal survival involves glycogen synthase kinase-3beta phosphorylation and coupling with 14-3-3. Neuroscience 143: 241–251, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Mwangi S, Anitha M, Mallikarjun C, Ding X, Hara M, Parsadanian A, Larsen CP, Thule P, Sitaraman SV, Anania F, Srinivasan S. Glial cell line-derived neurotrophic factor increases beta-cell mass and improves glucose tolerance. Gastroenterology 134: 727–737, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 118: 2316–2324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev 11: 1662–1673, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 127: 5533–5540, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127: 3533–3542, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Servitja JM, Ferrer J. Transcriptional networks controlling pancreatic development and beta cell function. Diabetologia 47: 597–613, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA 104: 1865–1870, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 387: 406–409, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn 237: 3270–3279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Boyen GB, Reinshagen M, Steinkamp M, Adler G, Kirsch J. Enteric nervous plasticity and development: dependence on neurotrophic factors. J Gastroenterol 37: 583–588, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S. GLP-1/exendin-4 facilitates beta-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract 73: 107–110, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Yue F, Cui L, Johkura K, Ogiwara N, Sasaki K. Glucagon-like peptide-1 differentiation of primate embryonic stem cells into insulin-producing cells. Tissue Eng 12: 2105–2116, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z, Alam S, Oppenheim RW, Prevette DM, Evenson A, Parsadanian A. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp Neurol 190: 356–372, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13: 103–114, 2007 [DOI] [PubMed] [Google Scholar]