Abstract
Monochloramine (NH2Cl) is a potent, thiol-directed oxidant capable of oxidizing thiol (S-H) residues in a wide variety of proteins. Generated in the stomach by the interaction of bacterial and host products, monochloramine has been shown to dysregulate Ca2+ homeostasis and disrupt mucosal integrity. In this report, we show that monochloramine also leads to disturbances in intracellular free zinc concentration ([Zn2+]i) in the gastric gland of the rabbit and that the increased Zn2+ within the cell causes an independent decrease in cell viability. Changes in [Zn2+]i were measured by using the fluorescent reporter FluoZin-3, whereas cell viability was assessed by measuring the conversion of calcein-AM to fluorescent calcein, an assay that is not affected by intracellular oxidation state. Cell death was confirmed using propidium iodide and YO-PRO-1 dye uptake measurements. Our experiments demonstrate that [Zn2+]i is increased in gastric glands exposed to NH2Cl and that elevated [Zn2+]i decreases cell viability. Chelation of Zn2+ with tetrakis-(2-pyridylmethyl) ethylenediamine decreases the toxicity of NH2Cl, but only when administered concurrently. These findings suggest that the toxic effect of thiol oxidants present during chronic gastritis is partially due to dysregulation of [Zn2+]i early in the process and that zinc chelation can protect, but not rescue, gastric glands exposed to toxic doses of NH2Cl.
Keywords: gastritis, thiol oxidation, zinc
the thiol-directed oxidant monochloramine (NH2Cl) is a product of chronic bacterial gastritis. Generated through the interaction of ammonia (NH4) produced by urealytic bacteria such as Helicobacter pylori and hypochlorous acid (HOCl) that accumulates as part of the host inflammatory response, NH2Cl oxidizes thiol (S-H) residues on a wide variety of intracellular proteins (20, 29, 33). Recently, we reported disturbances in Ca2+ homeostasis in parietal cells of the rabbit gastric gland following exposure to NH2Cl (6, 10). In these in vitro studies in isolated gastric glands, NH2Cl increased cytoplasmic Ca2+ by triggering release of Ca2+ from intracellular stores in the parietal cell and by accelerating influx of Ca2+ from extracellular sources. We monitored intracellular Ca2+ concentration ([Ca2+]i) using a well-described fluorescent reporter, fura 2, under conditions that excluded interference from heavy metal cations.
An increasing body of evidence suggests that metal cations other than Ca2+, notably Zn2+, also play an important role in oxidant-induced injury. This has been demonstrated in a variety of nonepithelial tissues (1, 2, 31, 34, 39), but whether Zn2+ plays a role in oxidant-induced injury of mucosal cells in the stomach is not known. In this article, we describe the direct toxicity of NH2Cl on isolated rabbit gastric glands, a parietal cell-enriched model that recapitulates in vivo function of the gastric antrum, and identify the contribution of NH2Cl-mediated alterations in free intracellular concentrations of zinc ([Zn2+]i) on cell viability following oxidant injury. To monitor cell viability, we used a fluorometric readout, measuring the uptake and conversion of calcein-AM to fluorescent calcein, in a plate reader-based assay. Using this assay, which is perhaps uniquely inert to interference by reactive species, we evaluated Zn2+-dependent and Zn2+-independent components of NH2Cl injury on cell viability.
In addition, we quantified changes in cytoplasmic free Zn2+ in individual parietal cells of isolated gastric glands (24), during exposure to pathologically relevant concentrations of NH2Cl. We used this assay to evaluate the efficacy of antioxidants in preventing, arresting, and reversing these disturbances. These studies utilized the fluorescent Zn2+ reporter FluoZin-3 (9), which has high specificity for Zn2+ and a Kd (∼3 nM) (24) that makes it highly useful for evaluating alterations in a physiologically relevant range (6, 8, 15). Our studies indicate that small increases in [Zn2+]i, in the submicromolar range (2.5 to 250 nM), are associated with acceleration of cell death. In addition, we find that a significant component of NH2Cl-induced injury is attributable to the associated increase in Zn2+ within the gastric epithelial cell. Finally, our studies suggest that prevention of these disturbances in metal cation homeostasis may limit injury caused by thiol-directed oxidants such as monochloramine.
MATERIALS AND METHODS
Gland isolation.
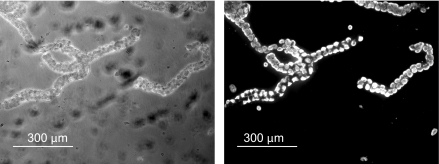
Anesthesia and euthanasia for New Zealand White rabbits were approved according to policies of Harvard Medical School. Gastric glands were harvested as described previously (10, 36). Parietal cell populations were confirmed by immunofluorescent imaging using a mouse monoclonal antibody against the H+/K+-ATPase (ABCAM, no. ab2866) probed by use of a secondary donkey anti-mouse fluorescent antibody (Invitrogen, no. 10037). As shown in Fig. 1, the gland preparation yields a parietal cell-enriched population in a functional gland, including a central lumen. Other cell types present in the gastric mucosa are present in low numbers, with parietal cells accounting for a high percentage of the gland volume (3, 4).
Fig. 1.
Bright-field and immunofluorescence images showing the relative population of parietal cells in a gastric gland. Glands were stained with an antibody directed against the H+-K+-ATPase, which is present in high concentration in the parietal cell (×10).
Dye loading.
FluoZin 3-AM (Invitrogen-Molecular Probes, Eugene, OR) was dissolved in DMSO then loaded (30 min at room temperature) into gastric glands suspended in DMEM containing 100 μM cimetidine, pH 7.4, with dye concentrations between 4 and 8 μM. Subsequently, glands were rinsed several times in dye-free DMEM, mounted on glass coverslips, and transferred to the microscope stage (Nikon TE-2000U, Nikon, Melville, NY), where they were superfused with Ringer solution (145 mM NaCl, 2.5 mM KH2PO4, 1.0 mM MgSO4 or MgCl2, 1 mM CaCl2, 10 mM HEPES, 10 mM glucose, pH 7.4) at room temperature.
Gland permeabilization.
To permeabilize the plasma membrane of the cells of the gastric gland, glands were first rinsed and stabilized in Ringer, then superfused with intracellular buffer (ICB: 125 mM KCl, 25 mM NaCl, 0.3 mM CaCl2, 0.5 mM MgCl2, 10 mM HEPES, 0.5 mM Na2 ATP, 0.5 mM EGTA, pH 7.25) containing 10 μM digitonin (6, 17). Digitonin selectively permeabilizes the cell membrane without disrupting the membranes of intracellular organelles (14, 37).
Monitoring [Zn2+]i.
Fluorescence was monitored concurrently in 6–10 individual parietal cells in each isolated gland by using a real-time fluorescence imaging microscope (Nikon TE-2000U, Nikon). Glands loaded with FluoZin 3-AM were excited at 488 nm with emission measurement at 520 nm ± 15 nm. Digital images of glands were captured with a digital charge-coupled device camera (Hamamatsu ORCA-ER, Bridgewater, NJ). Images were processed with image processing software (Universal Imaging, Downington, PA) to yield background-corrected pseudocolor images and quantitative measurements of fluorescence intensity. Images were acquired every 10 s, using the minimum exposure time possible, to minimize photobleaching.
Determination of cell viability.
Cell viability was assessed by monitoring uptake and intracellular conversion of nonfluorescent calcein-AM to fluorescent calcein, using a modification of previously reported methods (1, 2). Gland suspensions (200 μl) were allowed to settle for 10 min in wells (96-well plates) coated with poly-d-lysine (Fisher Scientific) to facilitate adherence to the plate. Wells were gently washed with Ringer, and control or experimental conditions were recapitulated. At the conclusion of the protocol, solutions were replaced with identical solutions containing calcein-AM (8 μM) for 30 min, after which fluorescence from each well [excitation (Ex.) 485 nm, emission (Em.) 528 nm] was recorded.
Other indexes of cell death.
Gastric gland cell death in the presence of monochloramine and in the absence of zinc was measured by use of two additional fluorescent reporters. Glands were incubated in Ringer solution containing varying concentrations of NH2Cl, with or without tetrakis-(2-pyridylmethyl) ethylenediamine (TPEN; 20 μM free concentration), for 1 h. Media was then exchanged for the same solution without NH2Cl. The positive control for apoptosis was a gland preparation incubated with camptothecin (100 μM), whereas positive control for necrosis was glands incubated with hydrogen peroxide (30 mM). To monitor cell death, glands were loaded with the dye propidium iodide (1 μg/ml, Ex. 530 nm, Em. 590 nm; Invitrogen), which cannot permeate the plasma membrane of living cells, and the dye YO-PRO-1 (0.1 μM, Ex. 485 nm, Em. 528 nm; Invitrogen), which can permeate the plasma membrane of dead or apoptotic cells, for 30 min on ice and read in the 96-well fluorimeter.
Additional reagents and methods.
Unless otherwise specified, reagents were obtained from Sigma-Aldrich and were dissolved first in ethanol, then added to Ringer solutions in dilutions of 1:1,000. TPEN was obtained from Molecular Probes (Eugene, OR) and dissolved Ringer solution directly. Dithiothreitol (DTT) and ascorbic acid (VitC) were dissolved in Ringer directly. All solutions were checked for changes in pH and adjusted if necessary to baseline pH. For Ringer and ICB, calculations of free and bound concentrations of Ca2+, Zn2+, TPEN, BAPTA, and EGTA were performed using the internet-based WEBMAXSTANDARD program (http://www.stanford.edu/∼cpatton/webmaxc/webmaxcS.htm).
Data summary and statistical analysis.
Fluorescence measurements were made at discrete time intervals and summarized as means ± SE. For comparison between treatments, unless stated otherwise, measurements in different regions of interest (6–10 cells for each gland) were combined to provide a single integrated value at each time point for each gland. Unless stated otherwise, comparisons were performed by analysis of variance for sequential or multiple measurements with standard statistical software (SigmaStat, Version 3.5, Systat Software, Port Richmond, FL).
RESULTS
Release of Zn2+ from intracellular pools during exposure to NH2Cl.
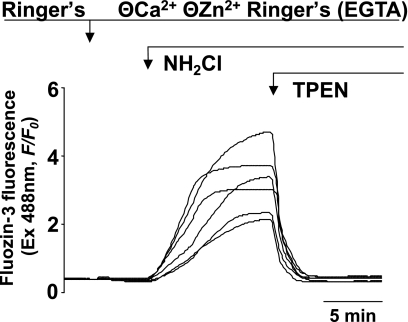
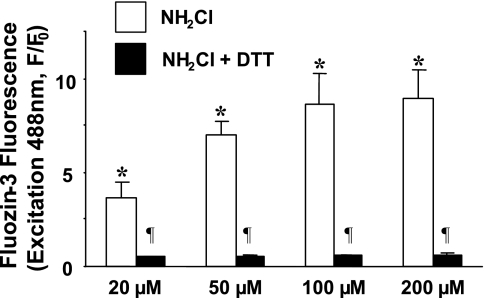
To measure changes in [Zn2+]i in the presence of the thiol oxidant NH2Cl, we used the Zn2+-specific fluorophore FluoZin-3, which we have previously demonstrated to be an effective method of quantifying Zn2+ flux in the cytoplasm of individual gastric glands (24). [Zn2+]i was monitored during exposure of gastric glands to pathologically relevant concentrations of NH2Cl (12). In the absence of extracellular Ca2+ or Zn2+ (Ringer solution containing 0.5 mM EGTA), exposure to 100 μM NH2Cl induced a rapid rise in the FluoZin-3 signal that was abolished when the membrane-permeable zinc chelator TPEN (10 μM) was added to the perfusate. A representative tracing from an individual gland is shown in Fig. 2, and mean values of multiple experiments in Fig. 3, confirming that the effect was the result of increased [Zn2+]i. Exposure to NH2Cl at concentrations as low as 20 μM caused accumulation of labile Zn2+ (concentrations of NH2Cl below 20 μM are unstable in Ringer solution). To confirm that the increase in [Zn2+]i was the result of thiol oxidation by NH2Cl, we added a selective thiol reducer, DTT (1 mM), which eliminated the increase in [Zn2+]i (Fig. 3).
Fig. 2.
Measurements of intracellular free zinc concentration ([Zn2+]i) in individual gastric parietal cells during exposure to NH2Cl. A representative recording of FluoZin-3 signals in an individual gland is shown. Glands are exposed to 50 μM NH2Cl in Ringer solution with no free Zn2+ of Ca2+ (0.5 mM EGTA). Recording begins with glands perfused by standard Ringer solution containing Ca2+, followed by Zn2+-free Ringer, and then exposure to NH2Cl (10 min). Elimination of the FluoZin-3 signal with the addition of tetrakis-(2-pyridylmethyl) ethylenediamine (TPEN; 20 μM) confirms the role of Zn2+ in FluoZin-3 fluorescence. Each line represents a recording from an individual parietal cell.
Fig. 3.
Response to monochloramine (20, 50, 100, and 200 μM) and abrogation of zinc release by concurrent administration of the thiol-reducing agent DTT (1 mM). Results are expressed as means ± SE, with the y-axis indicating fluorescence intensity normalized to starting values (F/F0). Each column summarizes values obtained in 5–7 glands at the peak of NH2Cl effect. *P < 0.001 compared with Ringer baseline (paired t-test); ¶P < 0.01 compared with NH2Cl alone (unpaired t-test).
Control studies were performed to determine whether increases in FluoZin-3 fluorescence might reflect contributions from dye located within Zn2+-enriched vesicles (38) or organelles (7, 19), rather than changes in [Zn2+]i. The plasma membranes of glands loaded with FluoZin-3 were selectively permeabilized with digitonin and then exposed to 200 μM NH2Cl. Under these conditions (n = 3), minimal increases (<300 fluorescence intensity units, maximum change in F/F0 from 1.0 to 1.5) were observed, confirming that the response to NH2Cl reflects fluorescence signals localized in the cytoplasm (data not shown).
Effect of Zn2+ on cell viability.
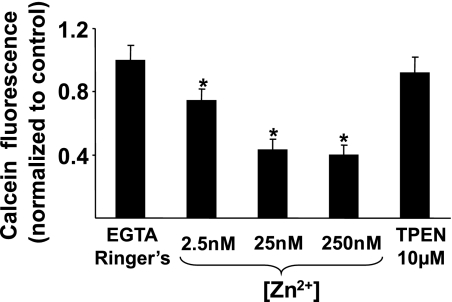
Having shown that NH2Cl causes increases in [Zn2+]i, we next determined whether the viability of gastric glands is altered by short-term increases in [Zn2+]i alone. To deplete cellular stores of Zn2+, glands were incubated at 37°C with Ringer solutions containing no free Zn2+ (Zn2+ and Ca2+ chelated with EGTA 0.5 mM) and the Zn2+ ionophore Na-pyrithione (50 μM). On the basis of the known Kd for zinc of EGTA, we then prepared solutions with total [Zn2+] calculated to achieve free concentrations of 2.5, 25, or 250 nM. These concentrations represent the full range of physiologically plausible [Zn2+]i. After 3 h incubating at these concentrations, glands were processed for calcein-AM uptake and conversion in a semiquantitative measure of cell viability. As shown in Fig. 4, increases in [Zn2+]i led to dose-dependent decreases in viability. Conversely, there was no effect on cell viability during exposure to the avid intracellular zinc chelator TPEN (10 μM). Control studies showed that calcein fluorescence was not altered in solution by the presence of pyrithione, indicating that none of the decreases in calcein fluorescence were due to independent effects of this cationophore. In addition, since calcein fluorescence might be influenced by oxidant-induced changes in [Zn2+]i, we also performed control studies to determine responsiveness of calcein to incremental increases in [Zn2+]i. We found that calcein-loaded glands were not acutely responsive to increases in [Zn2+]i, up to 250 nM (data not shown), indicating that the reporter is not itself sensitive to relevant changes in [Zn2+]i.
Fig. 4.
Effect of [Zn2+] on cell viability. Calcein-AM uptake and conversion to fluorescent calcein was measured in isolated gastric glands exposed to increasing doses of [Zn2+] (2.5, 25, 250 nM) for 3 h before processing. Results are normalized to Ca2+ and Zn2+ depleted (EGTA 0.5 mM) Ringer controls run concurrently and expressed as means ± SE, n = 15 wells in 3 experiments, *P < 0.05 compared with Ringer alone (ANOVA).
Specificity of responses to NH2Cl and effects of NH2Cl precursors and membrane-impermeable chloramines. To determine whether the [Zn2+]i dysregulation observed in gastric glands after exposure to NH2Cl is specific to that compound, we monitored FluoZin-3 fluorescence in isolated glands during exposure to monochloramine precursors NH3 (20 mM), HOCl (200 μM), and H2O2 (200 μM) (28). At these concentrations, no changes in signal were observed for any of the agents (n = 4 experiments each, data not shown), indicating that observed effects were due to NH2Cl alone and not to its precursors.
To determine whether NH2Cl exerts its effect intracellularly or at the interface with the plasma membrane, we exposed gastric glands to taurine-monochloramine (200 μM), a stable, but membrane-impermeant, chloramine species (29). No alterations in FluoZin-3 signals were observed (data not shown), suggesting that the effects of NH2Cl on intracellular [Zn2+] homeostasis are the result of the oxidant acting intracellularly.
Contributions of Zn2+ to NH2Cl-induced cell injury.
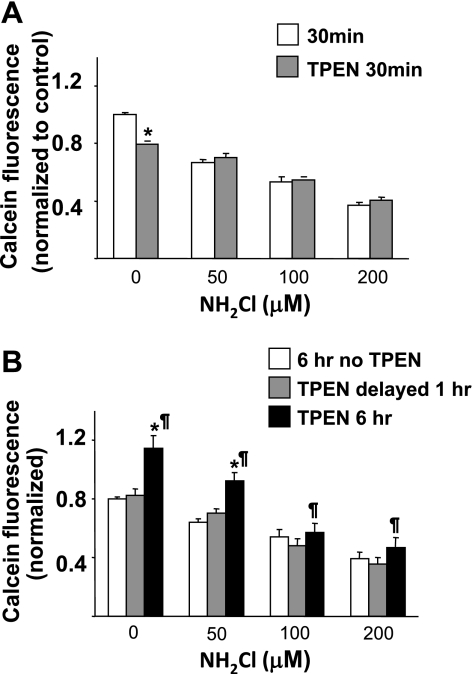
To quantify the degree of cell injury caused by NH2Cl that is attributable to Zn2+, glands were equilibrated with Ringer solutions then exposed to varying concentrations of NH2Cl (0 μM, 50 μM, 100 μM, 200 μM), in the presence or absence of the zinc chelator TPEN (20 μM). After a brief (30 min) exposure to NH2Cl, gland viability decreased in a dose-dependent, TPEN-independent manner, as shown in Fig. 5A. This suggests that the initial toxicity of NH2Cl occurs through a zinc-independent pathway. In contrast, during a 6-h exposure to the oxidant (Fig. 5B), the concurrent presence of TPEN appeared to be protective; that is, calcein fluorescence was higher when TPEN was present at the beginning and during the entire 6 h incubation. This protective effect was not observed if addition of TPEN was delayed until the end of the first hour of exposure to NH2Cl. The toxic effect of NH2Cl appears to overwhelm the protective effects of Zn2+ chelation at higher doses (100 and 200 μM). These observations indicate that viability of the cells of the gastric gland over a prolonged time course is influenced by the release of Zn2+ within the first hour of exposure to NH2Cl, although the effect of increased [Zn2+]i on viability may not manifest until several hours after exposure to NH2Cl or elevated [Zn2+]i and the effect may be overwhelmed by high doses of NH2Cl.
Fig. 5.
Relative contributions of Zn2+ and monochloramine to decreased cell viability. Calcein-AM uptake and conversion were measured in glands exposed to varying doses of NH2Cl, in the presence or absence of TPEN (20 μM). A: glands were exposed to NH2Cl alone (white bars) or concurrently with TPEN (shaded bars) for 30 min. B: glands were exposed to NH2Cl for 1 h then Ringer alone for 5 h (white bars), NH2Cl for 1 h then Ringer with TPEN for 5 h (light gray bars), or NH2Cl in the presence of TPEN for 6 h (dark gray bars). Each column represents 20 wells of glands in 4 separate preparations. Results are normalized to 30 min incubation in Ringer alone, as in A, and expressed as means ± SE, *P < 0.05 compared with Ringer or NH2Cl control, ¶P < 0.05 comparison of TPEN delayed vs. TPEN 6 h (ANOVA).
Effect of NH2Cl on gland viability.
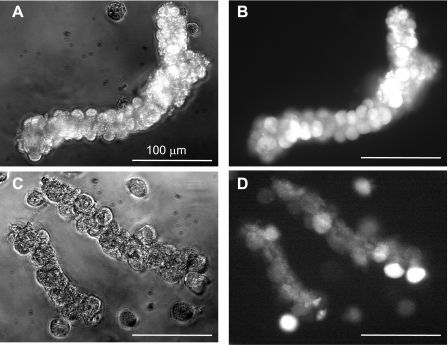
As shown in Fig. 6, the morphology of glands exposed to NH2Cl for 1 h then allowed to equilibrate in Ringer for 5 h was generally preserved, although the fluorescence of calcein was highly variable among individual cells within each gland. During exposure to higher concentrations of NH2Cl, gland morphology was less well preserved but calcein fluorescence was seen in individual cells. Calcein fluorescence was not observed in cells that were distorted or clearly disrupted. These observations confirm that calcein-AM uptake and conversion to calcein can monitor integrity of the gland and the viability of individual cells within isolated gastric glands. Given that gland preparation yields an enriched population of parietal cells, the bulk of the observed signal from any given gland represents parietal cell viability. In addition, the distinctive large, round parietal cells were individually identified and monitored in in vivo fluorescence studies. These experiments demonstrate that there is a window of time after exposure to NH2Cl when gland morphology is preserved, even though individual cells may have lost important functional properties, such as the intracellular esterases that cleave calcein-AM.
Fig. 6.
Morphology and esterase activity of gastric glands exposed to NH2Cl. Bright-field (A and C) and fluorescence images (B and D) of calcein-AM uptake and conversion were obtained in gastric parietal cells incubated in Ringer alone for 6 h (A and B) or in Ringer + NH2Cl 50 μM (C and D) for 1 h followed by Ringer for 5 h. Glands were then loaded with calcein-AM (5 μM) for 30 min, washed, and imaged at ×30.
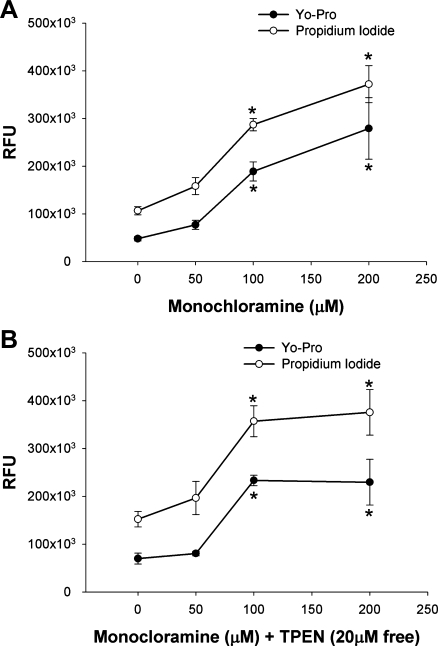
To confirm the cellular toxicity of monochloramine, we next utilized two additional fluorescent markers of cell death. Gastric glands were incubated with NH2Cl (50, 100, and 200 μM) for 1 h and allowed to recover for 2 h in Ringer solution before being stained with YO-PRO-1 and propidium iodide. YO-PRO-1 is a proprietary monomeric cyanine nucleic acid stain that is able to selectively traverse the degraded plasma membrane of apoptotic cells (18). The membrane-impermeant nuclear dye propidium iodide is an established marker of necrosis (35). Dyes did not fluoresce when exposed to NH2Cl in vitro and were responsive to positive controls for apoptosis (camptothecin 100 μM) and necrosis (hydrogen peroxide 30 mM, data not shown).
As shown in Fig. 7A, gastric glands exposed to NH2Cl demonstrated an equivalent proportion of staining with both cell toxicity assays, irrespective of the dose of monochloramine, even as total cell death increased with increasing doses. Likewise, as shown in Fig. 7B, the addition of the zinc chelator TPEN did not alter the relative proportion of staining by each dye in response to NH2Cl. However, it is interesting to note that in the absence of free Zn2+, cell death appears to reach a plateau at the 100 μM dose of NH2Cl.
Fig. 7.
Cellular toxicity of monochloramine as measured using the selectively membrane-permeable dye YO-PRO-1 (●) and the membrane impermeable dye propidium iodide (○). A: glands were incubated with NH2Cl for 1 h, then allowed to recover in Ringer solution for 2 h before staining. B: glands were coincubated with NH2Cl and TPEN (calculated free concentration 20 μM). RFU, relative fluorescence units. *P < 0.05 vs. Ringer control (ANOVA), N = 5, RFUs are expressed as means ± SE.
Constraint of Zn2+ release by antioxidants.
We next determined whether NH2Cl-induced alterations in [Zn2+]i can be blocked or reversed by exposure to two classes of antioxidant: thiol-reducing agents and oxidant scavengers. We used the potent thiol-reducing agent, DTT, and a common oxidant scavenger, VitC. To better delineate the mechanism of action of the antioxidant therapies used in these experiments, we monitored degradation of 200 μM NH2Cl (absorbance at 242 nm) when mixed with 1 mM DTT or 1 mM VitC in cell-free cuvettes. There was no consumption of NH2Cl when mixed with DTT (6). However, in the presence of VitC, NH2Cl was fully depleted within 10 min. These observations suggest that, as an oxidant scavenger, VitC would neutralize NH2Cl without protecting the actual targets of thiol oxidation, whereas DTT does not neutralize the oxidant but would protect the targets of oxidation or possibly reduce them from the oxidated state.
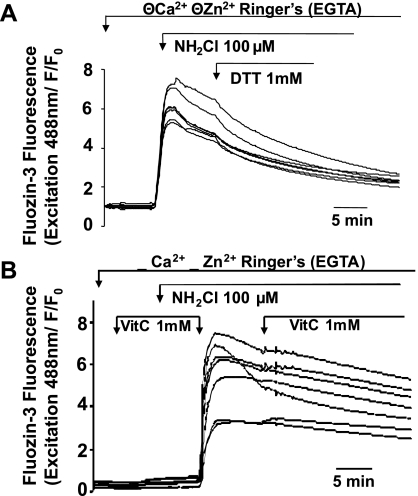
We then evaluated effects of antioxidant strategies on NH2Cl-induced changes in [Zn2+]i. Glands loaded with FluoZin-3 were exposed to NH2Cl in standard Ringer then, within 1 to 2 min of establishing a plateau response, exposed to NH2Cl plus DTT (1 mM) or VitC (1 mM). Figure 3 shows the effect of concurrent administration of NH2Cl and DTT, demonstrating that when administered simultaneously, the antioxidant properties of DTT completely abrogate the release of Zn2+ by NH2Cl. Shown in Fig. 8 are experiments performed in individual glands in which NH2Cl was briefly permitted to act on glands unopposed by DTT or VitC. As shown in Fig. 8A, exposure to DTT appeared to accelerate the downturn in [Zn2+]i that often follows the peak in accumulation caused by NH2Cl. This modest downturn, which occurs almost immediately following addition of DTT and is identified as an acute change in the slope of each tracing that persists for ∼10 min, was observed in four studies, and no greater effects were observed with a higher dose of DTT (1 mM) (n = 3 experiments, data not shown). When VitC was used, no change was detected in the slope of the decrease of Zn2+ signals (n = 3, Fig. 8B). In these experiments, cells were initially coincubated with VitC to establish that VitC does not have an independent effect on [Zn2+]i. Because VitC degrades NH2Cl, medium was then exchanged for a VitC-free solution of NH2Cl to allow the oxidant to work, before VitC was added back to the glands. As shown with DTT, the coadministration of NH2Cl and VitC caused no increase in [Zn2+]i, suggesting that VitC, like DTT, prevents the thiol oxidation of intracellular structures necessary to cause dysregulation of Zn2+.
Fig. 8.
Effectiveness of antioxidants DTT or ascorbic acid (VitC) in arrest and reversal of NH2Cl-induced accumulation of Zn2+ in the cytoplasm, as measured using FluoZin 3-AM. A: gland exposed to NH2Cl in the presence or absence of the thiol-reducing agent DTT. B: gland exposed to NH2Cl, in the presence or absence of VitC. To control for independent effects of VitC on Zn2+ release, VitC is present before addition of oxidant and is then removed to permit effects of NH2Cl before being replaced. Each panel represents a representative experiment (N = 4) and each trace represents an individual parietal cell.
Together these observations suggest that, in contrast to a much higher level of effectiveness in reversing the effects on Ca2+ release, (6) thiol-reducing agents such as DTT and antioxidants such as VitC are only mildly effective in reversing the effects of NH2Cl on [Zn2+]i, despite being able to completely prevent changes in [Zn2+]i when coadministered with the thiol oxidant. Thus disturbances in [Zn2+]i caused by NH2Cl are prevented and arrested but not easily reversed by exposure to different classes of antioxidants.
Effect of antioxidant therapy on cell viability.
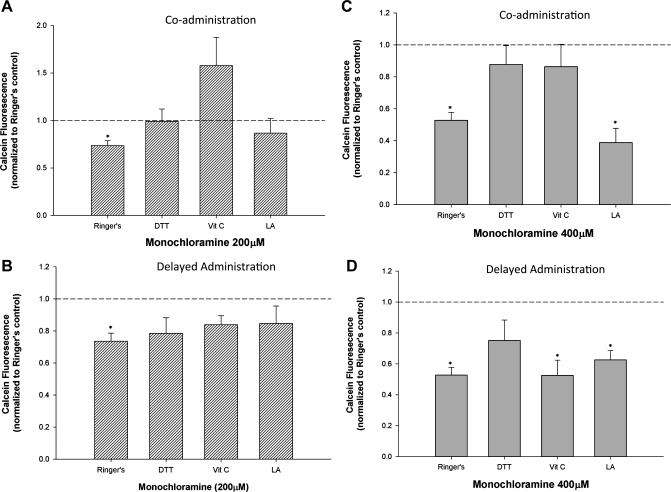
Having shown that two different antioxidant strategies are capable of preventing elevations in [Zn2+]i when coadministered with NH2Cl, and that DTT causes a modest reversal of NH2Cl-induced elevations in [Zn2+]i when applied following oxidant injury, we next sought to determine the role of antioxidant therapies on cell viability. Glands were exposed to NH2Cl (200 μM), and DTT, VitC, or the common nutritional supplement and antioxidant lipoic acid (LA) were added either concurrently or after 1 h of NH2Cl exposure (Fig. 9, A and B). To better demonstrate the degree of protection afforded by antioxidant therapies, a supramaximal dose of NH2Cl (400 μM) was also used (Fig. 9, C and D). After 1 h of NH2Cl exposure, cells were allowed to incubate for an additional 2 h in the antioxidant solution before cell viability was assessed using calcein-AM. As shown in Fig. 9, A and C, concurrent administration of both DTT and VitC, but not LA, abrogate the effect of NH2Cl and preserve cell viability in the face of high doses (400 μM) of the oxidant. This mirrors the effect on [Zn2+]i demonstrated above. Delayed administration of antioxidants resulted in preserved cell viability by all antioxidants at lower NH2Cl doses (200 μM, Fig. 9B), but at high doses only DTT demonstrated a capacity to rescue glands exposed to NH2Cl (Fig. 9D).
Fig. 9.
Effectiveness of antioxidants in prevention and reversal of NH2Cl-induced cell death. Each panel shows calcein-AM uptake and conversion in glands exposed to 200 (A and B) or 400 μM NH2Cl (C and D). Fluorescence values are normalized to a Ringer solution baseline (1.0). A and C: glands were coincubated in Ringer containing NH2Cl and DTT, VitC, or lipoic acid (LA) for 1 h, then with the antioxidant alone for 2 h. B and D: glands were incubated in Ringer containing NH2Cl alone for 1 h, then in DTT, VitC, or LA for 2 h. *P < 0.05 vs. Ringer control, N = 8 for all experiments.
DISCUSSION
Our studies indicate that exposure to pathologically relevant concentrations of NH2Cl causes accumulation of labile Zn2+ in the cytoplasm of the gastric parietal cell, in a parietal-cell enriched rabbit gastric gland model. Labile Zn2+ is liberated from proteins in the cytoplasm that are susceptible to thiol oxidation (21). These effects can be prevented by exposure to thiol-reducing agents such as DTT, or oxidant scavengers such as VitC. Oxidant scavengers such as VitC can arrest responses to accumulating oxidant, although they have little capacity to reverse thiol oxidant induced disturbances. Strategies that protect the thiol group from oxidation, including dithiothreitol and N-acetyl cysteine, also arrest such disturbances in Zn2+ homeostasis. However, the capacity for reversal is less robust than that observed for oxidant-induced disturbances in Ca2+ homeostasis that we have previously reported (36).
NH2Cl is the product of NH4 and HOCl. Ammonia is present in abundance in the gastric mucosa of individuals infected with H. pylori, a bacterium known to cause gastritis and ulceration. H. pylori exhibit uniquely high urease activity, with a Michaelis constant (Km) of 0.8 mM (22). In studies of isolated bacteria exposed to a physiologically relevant dose of urea (1.4 mM, with normal serum concentration 1.7–3.4 mM), NH4 concentrations of up to 724 μM were reported within 2 h (32). In turn, HOCl is produced in the extracellular space by neutrophil-secreted myeloperoxidase (MPO), which catalyzes a reaction between hydrogen peroxide (H2O2), H+, and Cl−. Grisham et al. (12) have demonstrated that 107 neutrophils, the number present in 1 g of inflamed intestinal mucosa, can generate HOCl at concentrations of 0.6 mM. Because NH2Cl is lipophilic and short lived in cells, direct measurement of tissue concentrations is not feasible. However, the concentration of NH2Cl can be estimated on the basis of NH4 and HOCl concentrations, with NH4 acting as the limiting reagent in most biological systems, including the gastric mucosa (13). These calculations suggest that concentrations in excess of 500 μM are possible in the setting of H. pylori gastritis.
To our knowledge, these are the first demonstrations that disturbances in [Zn2+]i are a consequence of the accumulation of reactive species directed at thiol groups, as demonstrated by prevention of Zn2+ accumulation with antioxidant and thiol-reducing pretreatment, in a primary preparation of gastric epithelial cells. Also noteworthy is the observation that neither HOCl nor peroxide, both precursors to the formation of NH2Cl, elicited disturbances in [Zn2+]i. Changes in [Zn2+]i have been reported in cultured myocytes in response to HOCl (34), which has also been shown to interact with thiol groups in other in vitro settings (28, 29). These considerations emphasize that such oxidants might elicit different responses in different cell types, depending on their content of antioxidant machinery and metallothioneins, as well as the distribution and function of varying intracellular compartments.
This variable response is illustrated by a comparison to our previously reported studies of epithelial cells in isolated colon crypts (6). In the present report, it appears that NH2Cl induces a much more robust release of Zn2+ in the gastric parietal cell than was observed in intestinal epithelia. In cells of the colon crypt (6), exposure to NH2Cl over the range 50 to 200 μM elicited increases in F/F0 for FluoZin-3 from 1.0 to a peak 1.5 to 3.0, corresponding to an increase in [Zn2+]i from <1 nM to no more than 3–4 nM. In the studies reported in this manuscript, exposure of the parietal cell to concentrations of NH2Cl as low as 20 μM lead to increases in F/F0 of 3 to 4 (Fig. 2). On the basis of previously published work by our laboratory, this degree of change in fluorescence corresponds to an increase of [Zn2+]i to ∼5 nM (24). Exposure to the highest concentrations of NH2Cl elicited increases beyond the dynamic range of the dye, meaning intracellular concentrations of at least 10 nM (Fig. 3). Most likely these findings reflect a more concentrated store of thiol-rich metalloproteins (metallothioneins) that bind Zn2+ and release it in response to oxidative stress. However, they might also reflect release from subcellular compartments enriched in Zn2+.
Intracellular compartments that might contribute to the accumulation of Zn2+ in the cytoplasm include mitochondria and endoplasmic reticulum (9, 16, 30). However, because they, like the cytoplasm, harbor the esterases that cleave the acetoxymethylester moiety in FluoZin 3-AM to render the active dye, FluoZin-3 would be expected to accumulate and report [Zn2+] in these compartments. Our characterization of Zn2+ signals suggests that levels of labile Zn2+ are in fact quite low in noncytosol compartments under control conditions. In addition, almost all of the NH2Cl-induced accumulation of Zn2+ within the cell is washed out when the cell membrane is selectively permeabilized with digitonin (10), which further argues that the signal is largely attributable to accumulation in the cytoplasm. These findings do not exclude release from structures or proteins that bind Zn2+ tightly within the cytoplasm, but they do argue against release of free or labile Zn2+ within these compartments. In addition, our studies do not specifically address release of labile Zn2+ from acidic secretory or lysosomal compartments. Indeed, recent studies in our laboratory suggest that Zn2+ is sequestered in the acid-secreting tubulovesicle compartment of the parietal cell (10, 24).
We performed further studies to determine whether oxidant-induced increases in [Zn2+]i would influence cell viability. Assays of cell viability are not necessarily straightforward in a complex epithelial preparation such as the gastric gland, particularly when the effects of oxidants or other forms of chemical injury that can directly affect the assay reagents are under study. For example, classical methods for monitoring injury included measurement of leakage of cytoplasm enzymes such as lactate dehydrogenase (LDH). In vitro, however, we observed that LDH is inactivated by direct exposure to NH2Cl (unpublished observations), almost certainly because the active site of this enzyme (as with many other dehydrogenases) depend on the redox state of thiol residues (23, 27). Similarly, methods used to measure markers of apoptosis, such as measures of caspase function or immunoblot for cleaved caspase, may be unreliable in the setting of thiol oxidants. Caspase proteins are thiol rich, and we have previously demonstrated that both monochloramine and zinc are capable of modulating caspase-3 function, possibly through direct effects on the active site of the enzyme (20). Other methods of determining cell viability, such as measuring exclusion of Trypan blue or uptake of crystal violet (5, 25), are time intensive, requiring visual discrimination of intact glands and individual cells. Moreover, such assays do not take into account the proportion of gland structures and cells that become so distorted or damaged that uptake of reporters is not possible. The advantage of the calcein assay for cell viability is that, so long as conditions of equal loading are assured, it provides a measure of the loss of function of the cell population. This property is not shared by dyes that depend on selective cellular uptake of apoptosis and necrosis markers, such as YO-PRO or propidium iodide, which provide no information about the proportion of viable cells present.
In this study we monitored viability utilizing a timed uptake of calcein-AM and its conversion to fluorescent calcein. Use of a fluorescence-based method, such as uptake of calcein-AM, simplifies the counting of viable cells as well as the quantification of effects. In preliminary studies we confirmed that calcein fluorescence is not influenced directly by NH2Cl or its precursors (HOCL, NH3), by thiol-reducing agents (DTT) or oxidant scavengers (VitC), by changes in [Ca2+]i up to 1 mM and [Zn2+]i up to 250 nM, or by exposure to TPEN.
With respect to indexes of cell viability, we observed a protective response to TPEN, but only if the chelator is provided concurrently with the oxidant. This suggests that cell injury caused by NH2Cl is amplified by the concurrent release of Zn2+. However, there was no effect of post-NH2Cl administration of TPEN on cell viability, indicating that the effects of oxidant-induced increases in [Zn2+]i are not reversible once the Zn2+ has been released. One interpretation of these findings is that release of Zn2+ enhances NH2Cl-induced toxicity to the gland, either because the Zn2+ levels themselves are so high or because of the precipitous timing of release to the cytoplasm. In either case, it appears that a transient increase in [Zn2+]i early in the course of oxidant-induced cell injury sets in motion a cascade that quickly becomes impervious to reduction of [Zn2+]i by TPEN.
Antioxidant therapy is a logical treatment for monochloramine toxicity. We tested a number of antioxidant strategies to both prevent and rescue cells exposed to toxic doses of NH2Cl. As with [Zn2+]i, concurrent exposure to both DTT and VitC prevented the predicted effects of NH2Cl, suggesting that both DTT and VitC are capable of preventing or repairing thiol oxidative damage (Fig. 9). Unlike DTT, VitC is not capable of reversing prior oxidative damage, suggesting that the protective effects of the two antioxidants are different. As we have demonstrated that VitC is capable of directly degrading NH2Cl in vitro, it is possible that the protective effects of VitC in cells is the result of its destroying NH2Cl either within or outside of the cell, in effect detoxifying the cellular milieu rather than repairing oxidative damage to cellular structures. In contrast, DTT has no independent effect on NH2Cl and is capable of reversing prior oxidative injury, suggesting that it acts directly on cellular machinery to reduce oxidized thiol groups. Control experiments demonstrated that DTT does degrade NH2Cl in vitro, supporting prior studies, by our laboratory and others, that suggest that DTT acts to reduce thiol groups within the cell that are targeted by NH2Cl (6, 26, 36). A third compound, lipoic acid, which is available as a “neutriceutical” and marketed for its antioxidant effect, had no effect on cell viability when exposed concurrently or after NH2Cl injury, suggesting that not all antioxidants or thiol-reducing agents are capable of preventing or repairing thiol oxidative damage in the gastric mucosa.
It is not known whether accelerated death of freshly isolated glands is mediated by endogenous production of thiol-directed oxidants. However, it has been recognized that successful culture of isolated human gastric glands for periods beyond 24 h requires an initial step in processing of tissue that includes the incubation in high concentrations of a thiol-reducing agent (DTT 1 mM) (40). Thiol-directed oxidative stress may thus play an important role in the process of anoikis, the process by which apoptosis is initiated after cells or complex cellular structures are detached from their native microenvironment (11). Our observations suggest that Zn2+ may be critical for preservation of cell viability as an integrated process. Further studies will be required to determine, in vivo, the implications of oxidant-induced release of Zn2+ in mucosal tissues of the gastrointestinal tract.
GRANTS
This study was supported by the American College of Surgeons Resident Research Fellowship (J. E. Kohler), R01 DK069929, T32 DK007754, and intramural support from the Department of Surgery, Brigham and Women's Hospital (D. I. Soybel); Howard Hughes Medical Institute Student Fellowship (H. B. Naik); and Summer Student Research Fellowship from the American Physiological Society (K. Tai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Adler M, Shafer H, Hamilton T, Petrali JP. Cytotoxic actions of the heavy metal chelator TPEN on NG108–15 neuroblastoma-glioma cells. Neurotoxicology 20: 571–582, 1999 [PubMed] [Google Scholar]
- 2.Aoun P, Watson DG, Simpkins JW. Neuroprotective effects of PPARgamma agonists against oxidative insults in HT-22 cells. Eur J Pharmacol 472: 65–71, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Berglindh T, Helander H, Sachs G. Secretion at the parietal cell level—a look at rabbit gastric glands. Scand J Gastroenterol Suppl 55: 7–20, 1979 [PubMed] [Google Scholar]
- 4.Berglindh T, Obrink KJ. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand 96: 150–159, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Carter KJ, Lee HH, Goddard PJ, Yanaka A, Paimela H, Silen W. Cell survival in rabbit gastric glands: effect of extracellular pH, osmolarity, and anoxia. Am J Physiol Gastrointest Liver Physiol 265: G379–G387, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Cima RR, Dubach JM, Wieland AM, Walsh BM, Soybel DI. Intracellular Ca2+ and Zn2+ signals during monochloramine induced oxidative stress in isolated rat colon crypts. Am J Physiol Gastrointest Liver Physiol 290: G250–G261, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem 281: 24085–24089, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Devinney MJ II, Reynolds IJ, Dineley KE. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and fluozin-3. Cell Calcium 37: 225–232, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta cells using a new fluorescent zinc indicator. J Am Chem Soc 124: 776–778, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Gerbino A, Hofer AM, McKay BM, Lau BW, Soybel DI. Divalent cations regulate acidity within the lumen and tubulovesicle compartment of gastric parietal cells. Gastroenterology 126: 182–195, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gilmore AP. Anoikis. Cell Death Differ 12: 1473–1477, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Grisham MB, Gaginella TS, von Ritter C, Tamai H, Be RM, Granger DN. Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, epithelial cell viability. Inflammation 14: 531–542, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Grisham MB, Jefferson MM, Thomas EL. Role of monochloramine in the oxidation of erythrocyte hemoglobin by stimulated neutrophils. J Biol Chem 259: 6757–6765, 1984 [PubMed] [Google Scholar]
- 14.Grubbs RD, Collins SD, Maguire ME. Differential compartmentation of magnesium and calcium in murine S49 lymphoma cells. J Biol Chem 259: 12184–12192, 1984 [PubMed] [Google Scholar]
- 15.Haugland RP. Molecular Probes Product Information and Catalogue Eugene, OR: Molecular Probes, 2003 [Google Scholar]
- 16.Hauser CT, Tsien RY. A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc Natl Acad Sci USA 104: 3693–3697, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofer AM, Machen TE. Direct measurement of free Ca in organelles of gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 267: G442–G451, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Idziorek T, Estaquier J, De Bels F, Ameisen JC. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods 185: 249–258, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Kirschke CP, Huang L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J Biol Chem 278: 4096–4102, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kohler JE, Mathew J, Tai K, Blass AL, Kelly E, Soybel DI. Monochloramine impairs caspase-3 through thiol oxidation and Zn2+ release. J Surg Res 153: 121–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maret W, Jacob C, Vallee BL, Fischer EH. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc Natl Acad Sci USA 96: 1936–1940, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mobley HL, Cortesia MJ, Rosenthal LE, Jones BD. Characterization of urease from Campylobacter pylori. J Clin Microbiol 26: 831–836, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr S, Stamler JS, Brune B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett 348: 223–227, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Naik HB, Beshire MA, Walsh BM, Liu J, Soybel DI. Secretory state regulates Zn2+ transport in the gastric parietal cell of the rabbit. Am J Physiol Cell Physiol 297: C979–C989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura E, Hagen SJ. Role of glutamine and arginase in protection against ammonia-induced cell death in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol 283: G1264–G1275, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Olson CE, Soll AH, Kaplowitz N. Modulating effect of thiol-disulfide status on [14C]aminopyrine accumulation in the isolated parietal cell. J Biol Chem 260: 8020–8025, 1985 [PubMed] [Google Scholar]
- 27.Pamp K, Bramey T, Kirsch M, De Groot H, Petrat F. NAD(H) enhances the Cu(II)-mediated inactivation of lactate dehydrogenase by increasing the accessibility of sulfhydryl groups. Free Radic Res 39: 31–40, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Peskin AV, Winterbourn CC. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic Biol Med 30: 572–579, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Prutz WA. Reactions of hypochlorous acid with biological substrates are activated catalytically by tertiary amines. Arch Biochem Biophys 357: 265–273, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci USA 100: 6157–6162, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am J Physiol Lung Cell Mol Physiol 282: L185–L192, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Miura S, Suematsu M, Fukumura D, Kurose I, Suzuki H, Kai A, Kudoh Y, Ohashi M, Tsuchiya M. Helicobacter pylori-associated ammonia production enhances neutrophil-dependent gastric mucosal cell injury. Am J Physiol Gastrointest Liver Physiol 263: G719–G725, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Tamai H, Kachur JF, Baron DA, Grisham MB, Gaginella TS. Monochloramine, a neutrophil-derived oxidant, stimulates rat colonic secretion. J Pharmacol Exp Ther 257: 887–894, 1991 [PubMed] [Google Scholar]
- 34.Tatsumi T, Fliss H. Hypochlorous acid mobilizes intracellular zinc in isolated rat heart myocytes. J Mol Cell Cardiol 26: 471–479, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Trost LC, Lemasters JJ. A cytotoxicity assay for tumor necrosis factor employing a multiwell fluorescence scanner. Anal Biochem 220: 149–153, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Walsh BM, Naik HB, Dubach JM, Beshire M, Wieland AM, Soybel DI. Thiol-oxidant monochloramine mobilizes intracellular Ca2+ in parietal cells of rabbit gastric glands. Am J Physiol Cell Physiol 293: C1687–C1697, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Weigel PH, Ray DA, Oka JA. Quantitation of intracellular membrane-bound enzymes and receptors in digitonin-permeabilized cells. Anal Biochem 133: 437–449, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Wellenreuther G, Cianci M, Tucoulou R, Meyer-Klaucke W, Haase H. The ligand environment of zinc stored in vesicles. Biochem Biophys Res Commun 380: 198–203, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol 291: C555–C568, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Wroblewski LE, Noble PJ, Pagliocca A, Pritchard DM, Hart CA, Campbell F, Dodson AR, Dockray GJ, Varro A. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci 116: 3017–3026, 2003 [DOI] [PubMed] [Google Scholar]