Abstract
Although in vitro studies show that muscarinic M3 receptors primarily mediate the effects of acetylcholine on gastrointestinal contractility, the muscarinic receptor subtypes regulating gastrointestinal motor activity and transit in humans in vivo are unclear. We hypothesized that muscarinic M3-specific but not nonspecific receptor antagonists would delay gastrointestinal and colonic transit in humans. In this parallel-group study, gastric emptying, small intestinal transit, and colonic transit were assessed by scintigraphy on days 4-6 in 72 healthy subjects (49 women) who received placebo (n = 16), the M3 antagonist darifenacin ER [7.5 mg (n = 20) or 15 mg daily (n = 17)], or the nonspecific antagonist tolterodine [4 mg daily (n = 19)] for 6 days. Bowel habits were recorded by daily diaries. Both doses of darifenacin substantially delayed [P < 0.01 vs. placebo (for both doses), P < 0.01 vs. tolterodine (for 15 mg)] small intestinal transit, i.e., colonic filling at 6 h (placebo [59.6 ± 6.4%, mean ± SE], 7.5 mg ER [34.4 ± 6.1%], 15 mg ER [20.4 ± 6.3%)]. Darifenacin (15 mg) also delayed (P < 0.01 vs. placebo and tolterodine) half-time for ascending colonic emptying [placebo (12.0 ± 1.5 h), 7.5 mg (18.6 ± 1.9 h), 15 mg (22.9 ± 2.6 h)] and colonic transit (geometric center) at 24 [placebo (2.8 ± 0.2), 7.5 mg (2.4 ± 0.2), 15 mg (1.9 ± 0.2)] but not 48 h. Darifenacin did not affect gastric emptying and tolterodine did not affect bowel habits or gastrointestinal transit. With muscarinic antagonists used at clinically approved doses, these findings demonstrate that muscarinic M3 receptors regulate small intestinal and colonic transit in humans; colonic effects are more pronounced in the right than left colon. At doses that affect small and large intestinal transit, M3 antagonists do not affect gastric emptying in humans. The efficacy of darifenacin in diarrhea-predominant irritable bowel syndrome should be evaluated.
Keywords: cholinergic, motility, colonic, small intestine, irritable bowel syndrome
the effects of acetylcholine, which is the primary excitatory neurotransmitter in both the urinary bladder and the gastrointestinal tract, are mediated by M1–5 muscarinic receptors. Although M2 receptors outnumber M3 receptors by a ratio of 3:1 and 4:1 in bladder and gastrointestinal tract smooth muscle, respectively, in vitro studies suggest that M3 receptors are primarily responsible for mediating the excitatory effects of acetylcholine in both organs (1, 17). Muscarinic M1 receptors mediate slow excitatory postsynaptic transmission in the myenteric plexus. In addition, presynaptic M1 receptors in the guinea pig and M2–M4 receptors in the mouse small intestine inhibit acetylcholine release from nerve terminals (14, 20, 24, 30). Thus the net gastrointestinal effects of nonspecific muscarinic antagonists on gastrointestinal motor activity likely reflect a balance between excitatory and inhibitory effects. However, although muscarinic antagonists are widely used to treat gastrointestinal symptoms (e.g., abdominal cramping and diarrhea) and an overactive bladder, the relative contribution of muscarinic receptor subtypes to normal gastrointestinal motor functions in humans has not been studied. This question is important since the magnitude of antimuscarinic effects and muscarinic receptor selectivity for various muscarinic antagonists are organ and species specific (2, 8).
In clinical trials of patients with an overactive bladder, the incidence of constipation is higher with M3 selective muscarinic antagonists, for example trospium chloride (10.9 vs. 5.8% for placebo) (26) and darifenacin [14.8% (7.5 mg daily) and 21.3% (15 mg daily) vs. 6.2% (placebo)] (9) than with nonspecific antagonists, e.g., tolterodine (7%) vs. placebo (4%) (33). These observations suggest that M3-selective muscarinic antagonists have more pronounced effects than nonspecific antagonists on gastrointestinal motor activity in humans. Thus our hypothesis was that a muscarinic M3-selective but not the muscarinic nonspecific receptor antagonist tolterodine would delay gastrointestinal and colonic transit in healthy subjects.
METHODS
Healthy subjects.
Seventy-two healthy volunteers aged 19 to 58 yr old (mean age, 35 yr; 24 men and 48 women) were recruited by public advertisement. None had an underlying illnesses or previous gastrointestinal surgery (other than appendectomy or cholecystectomy) or used medications other than oral contraceptives and thyroid hormone replacement therapy. Functional gastrointestinal disorders, anxiety, and depression were excluded by validated screening questionnaires, a clinical interview, and a physical examination (5, 36). Seventy subjects completed the study. Of the two remaining subjects, one had medication-related anticholinergic side effects, and logistical constraints precluded participation in another subject.
Drug.
Subjects were randomly assigned, stratified by sex and age (i.e., < and ≥50 yr), to placebo (n = 16), darifenacin 7.5 mg extended release (ER) (n = 20), darifenacin 15 mg ER (n = 17), or tolterodine 4 mg long aching (n = 19), administered once daily for 6 days. Tolterodine is a competitive nonspecific muscarinic receptor antagonist whereas darifenacin is an M3-selective receptor antagonist. These doses are approved by the Food and Drug Administration for treating urinary symptoms. Medication compliance was assessed both by the return of an empty pill bottle at the conclusion of the study and by recording the time the medication was taken in the bowel diary.
After oral administration, both tolterodine and darifenacin are effectively absorbed, highly bound to plasma proteins, and extensively metabolized by CYP2D6 in the liver. Tolterodine is initially metabolized to the pharmacologically active 5-hydroxymethyl metabolite, whose antimuscarinic effects are similar to those of tolterodine (12, 32). Most (∼93%) Caucasian subjects have the cytochrome P-450 enzyme CYP2D6 and are characterized as extensive metabolizers.
Assessments.
Gastric emptying, small intestinal transit, and colonic transit were assessed by established and validated scintigraphic techniques on days 4–6 after starting medication (11). Gastric emptying and small bowel transit were measured by a 99mTc-labeled egg meal. Colonic transit was measured by 111In-labeled charcoal pellets within a capsule coated by methacrylate. Gastric emptying was summarized as the proportion of stomach contents emptied at 2 and at 4 h and by the half-time for gastric emptying. Colonic filling (i.e., the proportion of 99mTc reaching the colon) at 6 h was used to measure orocecal transit (i.e., a surrogate for small bowel transit). Colonic filling is expressed by measuring the proportion of total 99mTc counts at 6 h, corrected for decay and tissue attenuation, which are in the colon, typically in the cecum and ascending colon. Overall colonic transit was summarized as the colonic geometric center (GC) at 4, 24, and 48 h. The GC represents the average of counts in different colonic regions (ascending, transverse, descending, and rectosigmoid colon) and stool, weighted by factors of 1 to 5, respectively, at these time points. Therefore, a higher GC represents faster colonic transit. Ascending colonic emptying was summarized by the half-time (thalf) calculated by linear interpolation of values on the ascending colonic emptying curve. For 6 days before and 6 days after beginning medication, subjects also recorded stool form (Bristol stool scale), completeness of evacuation (yes/no), and ease of defecation (1 being manual disimpaction to 7 being incontinence of stool) for every bowel movement in a bowel diary.
Statistical analysis.
The primary end point was colonic transit as measured by the GC at 24 h (GC24). Secondary end points included the thalf for gastric emptying, colonic filling at 6 h, which is a measure of small intestinal transit, GC of colonic transit at 48 h (GC48), and effects on bowel habits (i.e., stool frequency, form, ease of passage, and incomplete evacuation). Data were analyzed by an analysis of covariance incorporating sex as a covariate along with the main effect term for treatment group. Analysis of posttreatment bowel diaries incorporated baseline symptom scores as covariates. Since the interaction term (sex by treatment) was not significant, the reported results are based on the main effects models. To accommodate an intent-to-treat analysis, subjects with missing data on any end point had their missing values imputed by using the overall mean in subjects with data for that end point. A corresponding adjustment in the residual error degrees of freedom was made by subtracting one degree of freedom for each missing value imputed for a given end point. The analysis incorporated Bonferroni corrections for three comparisons with placebo (i.e., 2 doses of darifenacin and 1 dose of tolterodine) and two comparisons among drugs (i.e., tolterodine vs. each of 2 doses of darifenacin).
The effect of darifenacin on colonic transit in humans is unknown. Therefore, the sample size was estimated from the pooled distribution of colonic transit (i.e., GC24) in healthy subjects in previous studies wherein the mean GC24 was 2.75 and the SD was 1.0. With a sample size of 18 subjects per group, this study had 80% power (with a two-sample t-test and two-sided alpha level of 0.05) to identify a difference of 1.0 unit in the GC24 between any two drugs. Differences greater than one unit are considered to be clinically relevant (16, 34).
RESULTS
Subject characteristics.
Seventy of 72 subjects completed the study. Data were imputed for two subjects (both darifenacin 7.5 mg) who dropped out prior to the transit study. Consistent with the stratified randomization, the age and sex were evenly distributed among groups (Table 1). The body mass index (BMI) was also not significantly different among groups.
Table 1.
Demographic characteristics
| Parameter | Placebo | Tolterodine | Darifenacin (7.5 mg) | Darifenacin (15 mg) |
|---|---|---|---|---|
| Number | 16 | 19 | 20 | 17 |
| Age | 32.8 ± 1.7 | 34.9 ± 2.4 | 35.8 ± 2.3 | 34.1 ± 2.7 |
| Women | 10 | 13 | 13 | 11 |
| BMI, kg/m2 | 25.1 ± 1 | 26 ± 1 | 28.1 ± 1 | 26.7 ± 0.9 |
All values represent observed mean [95% confidence interval (CI)].
Effects on gastrointestinal transit.
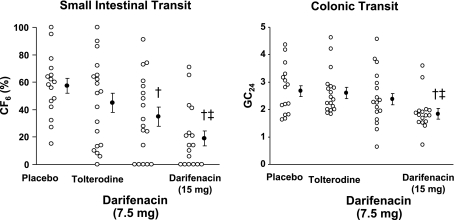
Neither tolterodine nor darifenacin had significant effects on gastric emptying (Table 2) compared with placebo, both doses of darifenacin delayed small bowel transit (P < 0.01); the higher dose also delayed (P ≤ 0.0001) ascending colonic emptying and colonic transit at 24 h (GC24) but not at 48 h (GC48). The higher dose of darifenacin (15 mg) also delayed (P = 0.003) small bowel and colonic transit (GC24) and the ascending colonic emptying thalf vs. tolterodine (Table 2). In contrast, tolterodine did not significantly affect gastric emptying or small intestinal or colonic transit (Table 2, Fig. 1).
Table 2.
Effects of drugs on gastrointestinal transit
| Parameter | Placebo | Tolterodine | Darifenacin (7.5 mg) | Darifenacin (15 mg) |
|---|---|---|---|---|
| Gastric emptying, thalf, min | 125.5 ± 6.7 | 127 ± 6.4 | 115.4 ± 6.4 | 120.4 ± 6.7 |
| Colonic filling at 6 h, % | 59.6 ± 6.4 | 47.1 ± 6 | 34.4 ± 6.1† | 20.4 ± 6.3†‡ |
| Colonic transit | ||||
| Ascending colonic emptying, thalf, h | 12.0 ± 1.5 | 14.0 ± 1.0 | 18.6 ± 1.9* | 22.9 ± 2.6†‡ |
| GC24 | 2.8 ± 0.2 | 2.7 ± 0.2 | 2.4 ± 0.2 | 1.9 ± 0.2†‡ |
| GC48 | 3.8 ± 0.2 | 4 ± 0.2 | 3.6 ± 0.2 | 3.4 ± 0.2 |
All values represent least squares means (95% CI), adjusted for gender and BMI. GC24 and GC48, geometric center of oclonic transit at 24 and 48 h, respectively.
P = 0.02;
P < 0.01 vs. placebo;
P < 0.01 vs. tolterodine.
Fig. 1.
Effect of darifenacin and tolterodine on small intestinal and colonic transit in healthy subjects. Both doses of darifenacin delayed small intestinal transit [i.e., colonic filling at 6 h (CF6)] relative to placebo. The higher dose delayed small intestinal and colonic transit as measured by the GC at 24 h (GC24) compared with placebo and tolterodine. †P < 0.01 vs. placebo, ‡P < 0.01 vs. tolterodine.
The effects on gastrointestinal and colonic transit parameters were correlated. The thalf for ascending colonic emptying was inversely correlated with colonic filling at 6 h (r = −0.46, P < 0.0001), GC24 (r = −0.81, P < 0.0001), and GC48 (r = −0.53, P < 0.0001), implying that a longer ascending colonic emptying time was associated with less colonic filling at 6 h (i.e., slower small intestinal transit) and with slower colonic transit at 24 and 48 h.
Gastric emptying and colonic transit were slower (P < 0.01) in women. The effects of sex were not modified by treatment, i.e., the sex-by-treatment interaction was not significant. Thus the thalf for gastric emptying was 133 ± 4 (means ± SE) min in women and 111 ± 6 min in men. The GC48 for colonic transit was lower, reflecting slower colonic transit, in women (i.e., 3.8 ± 0.1) than in men (i.e., 4.0 ± 0.2).
Effects on bowel habits.
The Bristol stool form scale score was lower (P < 0.01), reflecting harder stools, in subjects who received darifenacin 15 mg (2.8 ± 0.2) than those who received tolterodine (3.6 ± 0.2) (Table 3). Otherwise, stool frequency, ease of stool passage, and the sense of incomplete evacuation were not significantly different among groups.
Table 3.
Effects of drugs on bowel habits
| Parameter | Placebo | Tolterodine | Darifenacin (7.5 mg) | Darifenacin (15 mg) |
|---|---|---|---|---|
| Number of bowel movements/ day | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Bristol stool form score | 3.1 ± 0.2 | 3.6 ± 0.2 | 3.3 ± 0.2 | 2.8 ± 0.2‡ |
| Ease of passage | 3.9 ± 0.1 | 3.9 ± 0.1 | 3.9 ± 0.1 | 3.8 ± 0.1 |
| Incomplete evacuation* | 10 ± 4 | 7 ± 4 | 3 ± 4 | 17 ± 4 |
All values represent least squares mean (95% CI), adjusted for gender and BMI.
Proportion (%) of bowel movements with incomplete evacuation.
P < 0.01 vs. tolterodine.
The BMI was associated with the GC24 (r = 0.26, P = 0.03) and GC48 for colonic transit (r = 0.32, P = 0.008). Moreover, stool frequency (r = 0.34, P = 0.004), stool form (r = 0.37, P = 0.002), and ease of passage (r = 0.28, P = 0.02) were all associated with GC24. However, the sense of incomplete evacuation was not associated with colonic transit.
DISCUSSION
Although muscarinic antagonists have been demonstrated to inhibit contractility and are often used to treat abdominal pain and diarrhea, their effects on gastrointestinal transit in humans are poorly understood. This is the first controlled study to compare the effects of nonspecific and relatively selective M3 receptor antagonists on gastrointestinal transit in humans. A lower dose of darifenacin delayed small intestinal transit whereas a higher dose also delayed ascending colonic emptying and colonic transit, demonstrating that muscarinic M3 receptors regulate small intestinal and colonic transit in humans. Darifenacin delayed GC24 and ascending colonic emptying but not GC48, suggesting a more pronounced effect on right than left colonic motor functions. In contrast to darifenacin, tolterodine, which has a comparable affinity for all five muscarinic receptors in the urinary bladder, did not delay gastrointestinal or colonic transit, confirming a previous study (3).
The observed effects of darifenacin are consistent with in vitro and in vivo studies in animals, suggesting that muscarinic receptor antagonists inhibit peristalsis and that, among muscarinic receptors, the M3 subtype is primarily responsible for regulating gastrointestinal motility (15). There are three potential explanations for why darifenacin but not tolterodine delayed small intestinal and colonic transit. First, darifenacin is a more potent competitive antagonist than tolterodine at M3 receptors (15). Second, tolterodine may offset the effects of antagonizing excitatory receptors by also blocking the inhibitory effects of muscarinic stimulation on motility, either by blocking presynaptic muscarinic receptors that inhibit acetylcholine release, which are of the M1, M2, or M4 variety (14, 20, 30), or by blocking M1 receptors on nonadrenergic-noncholinergic inhibitory pathways (13, 27). In the human colon, M2 receptors are located presynaptically on nerve fibers, suggesting they autoregulate acetylcholine release (18). Indeed, the M1 antagonist pirenzepine facilitated peristalsis in the guinea pig small intestine, perhaps by withdrawing tonic M1 receptor-mediated inhibition of acetylcholine release from circular muscle (14, 28). Third, tolterodine binds relatively selectively to muscarinic receptors in the urinary bladder (23). Perhaps this also explains why atropine, which is also a nonspecific muscarinic antagonist but in contrast to tolterodine binds to colonic muscarinic receptors, delays orocecal and colonic transit in humans and dogs, respectively (6, 10). Atropine also has a higher affinity for M3 than for M2 receptors (15).
In the stomach, M3 receptors are located not only on gastric smooth muscle but also increase pacemaker frequency via effects on interstitial cells of Cajal in the murine gastric fundus and antrum (21). Atropine reduced antral motor activity and delayed gastric emptying in humans (25, 35). In contrast, neither tolterodine nor darifenacin delayed gastric emptying, suggesting perhaps that compensatory mechanisms preserve gastric emptying despite antagonism of M3 receptors, as exemplified by the observation that contractile responses to carbachol but not gastric emptying were inhibited in muscarinic M2 and separately M3 knockout mice (22).
The effects of darifenacin (15 mg) on intestinal and colonic transit may be clinically relevant. Darifenacin also significantly reduced stool consistency compared with tolterodine; stool characteristics (i.e., consistency, frequency, and ease of passage) were significantly correlated with colonic transit as shown previously (3). The magnitude by which darifenacin delayed small intestinal and colonic transit is, respectively, comparable to and lower than the effect of codeine (30 mg po qid) on these parameters (16). In contrast to codeine, both doses of darifenacin delayed ascending colonic emptying compared with placebo. Current concepts, based on limited data, suggest that patients with chronic constipation who have delayed ascending and transverse colonic emptying also have delayed overall colonic transit (29). However, larger studies are necessary to evaluate whether a subset of patients with chronic constipation have an isolated delay in right colonic emptying.
The effects of darifenacin on gastrointestinal transit are germane since urinary urgency is associated with functional constipation (4). From a therapeutic perspective, the effects of darifenacin on symptoms and gastrointestinal transit in irritable bowel syndrome (IBS), which can be associated with rapid transit, are worthy of further study (7). The darifenacin-induced delay in intestinal and colonic transit in this study is more pronounced than the effects of alosetron in patients with diarrhea-predominant IBS (31). Indeed, zamifenacin, which is also a relatively selective M3 antagonist, reduced the postprandial colonic contractile response in IBS (19).
In summary, these findings, using muscarinic antagonists at clinically approved doses, demonstrate that muscarinic M3 receptors mediate the excitatory effects of acetylcholine on small intestinal and colonic transit in humans. These effects are potentially clinically significant and more pronounced on the right than the left colon. At doses that affect small and large intestinal transit, M3 antagonists do not affect gastric emptying in humans. Further studies evaluating the effects of darifenacin on gastrointestinal and colonic transit in patients with urinary urgency and in diarrhea-predominant IBS are necessary.
GRANTS
This study was supported in part by a grant from Pfizer, and by Grant Number 1 UL1 RR024150* from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
This research was presented in abstract form at the Neurogastroenterology & Motility Joint International Meeting, Lucerne, Switzerland, November, 2008.
REFERENCES
- 1.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 148: 565–578, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alabaster VA. Discovery & development of selective M3 antagonists for clinical use. Life Sci 60: 1053–1060, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE, Andrews CN, Seide B, Baxter K, Guan G, Zinsmeister AR. Effect of a non-specific muscarinic antagonist, tolterodine, on gastrointestinal and colonic transit in humans: a randomized controlled study. Neurogastroenterol Motil 20: 643–648, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Locke GR, McKeon K, Seide B, Schleck C, Zinsmeister AR, Melton L., Jr Differences between painless vs. painful constipation. Am J Gastroenterol 101: 604–612, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, Locke GR, Seide B, Zinsmeister AR. A new questionnaire for constipation and fecal incontinence. Aliment Pharmacol Ther 20: 355–364, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Borody TJ, Quigley EM, Phillips SF, Wienbeck M, Tucker RL, Haddad A, Zinsmeister AR. Effects of morphine and atropine on motility and transit in the human ileum. Gastroenterology 89: 562–570, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 772–781, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmine AA, Brogden RN. Pirenzepine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in peptic ulcer disease and other allied diseases. Drugs 30: 85–126, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Chapple C, Steers W, Norton P, Millard R, Kralidis G, Glavind K, Abrams P. A pooled analysis of three phase III studies to investigate the efficacy, tolerability and safety of darifenacin, a muscarinic M3 selective receptor antagonist, in the treatment of overactive bladder. BJU Int 95: 993–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chiba T, Bharucha AE, Thomforde GM, Kost LJ, Phillips SF. Model of rapid gastrointestinal transit in dogs: effects of muscarinic antagonists and a nitric oxide synthase inhibitor. Neurogastroenterol Motil 14: 535–541, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther 16: 1781–1790, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Darifenacin (Enablex) In: Physicians' Drug Reference (62nd ed.), edited by Borza S, Philippi E. Montvale, NJ: Thomson PDR, 2008, p. 2210–2214 [Google Scholar]
- 13.De Ponti F, Einaudi A, Cosentino M, D'Angelo L, Lecchini S, Frigo GM, Crema A. Differential effects of antimuscarinic agents on intestinal motility in the conscious dog. J Pharmacol Exp Ther 264: 789–794, 1993 [PubMed] [Google Scholar]
- 14.Dietrich C, Kilbinger H. Prejunctional M1 and postjunctional M3 muscarinic receptors in the circular muscle of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol 351: 237–243, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Eglen RM, Choppin A, Watson N. Therapeutic opportunities from muscarinic receptor research. Trends Pharmacol Sci 22: 409–414, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gonenne J, Camilleri M, Ferber I, Burton D, Baxter K, Keyashian K, Foss J, Wallin B, Du W, Zinsmeister AR. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol 3: 784–791, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem 44: 173–202, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Harrington AM, Hutson JM, Southwell BR. Localisation of muscarinic receptor M1-M3 immunoreactivity in human colon (Abstract). Neurogastroenterol Motil 18: 777, 2006 [Google Scholar]
- 19.Houghton LA, Rogers J, Whorwell PJ, Campbell FC, Williams NS, Goka J. Zamifenacin (UK-76, 654) a potent gut M3 selective muscarinic antagonist, reduces colonic motor activity in patients with irritable bowel syndrome. Aliment Pharmacol Ther 11: 561–568, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Kawashima K, Fujimoto K, Suzuki T, Oohata H. Pharmacological differentiation of presynaptic M1 muscarinic receptors modulating acetylcholine release from postsynaptic muscarinic receptors in guinea-pig ileum. Gen Pharmacol 21: 17–21, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Kim TW, Koh SD, Ordog T, Ward SM, Sanders KM. Muscarinic regulation of pacemaker frequency in murine gastric interstitial cells of Cajal. J Physiol 546: 415–425, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitazawa T, Hashiba K, Cao J, Unno T, Komori SI, Yamada M, Wess J, Taneike T. Functional roles of muscarinic M2 and M3 receptors in mouse stomach motility: studies with muscarinic receptor knockout mice. Eur J Pharmacol 554: 212–222, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Maruyama S, Hasuike N, Suzuki K, Yamada S. In vivo characterization of muscarinic receptors in peripheral tissues: evaluation of bladder selectivity of anticholinergic agents to treat overactive bladder. Naunyn Schmiedebergs Arch Pharmacol 377: 463–471, 2008 [DOI] [PubMed] [Google Scholar]
- 24.North RA, Slack BE, Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J Physiol 368: 435–452, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkman HP, Trate DM, Knight LC, Brown KL, Maurer AH, Fisher RS. Cholinergic effects on human gastric motility. Gut 45: 346–354, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudy D, Cline K, Harris R, Goldberg K, Dmochowski R. Multicenter phase III trial studying trospium chloride in patients with overactive bladder. Urology 67: 275–280, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Schiavone A, Sagrada A, Pagani F, Giachetti A. Role of muscarinic receptor subtypes in the regulation of migrating myoelectric complex in the dog. Gastroenterology 96: 116–121, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Schworer H, Kilbinger H. Enhancement of guinea-pig intestinal peristalsis by blockade of muscarinic M1-receptors. Br J Pharmacol 93: 715–720, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stivland T, Camilleri M, Vassallo M, Proano M, Rath D, Brown M, Thomforde G, Pemberton J, Phillips S. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology 101: 107–115, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi T, Fujinami K, Goto H, Fujita A, Taketo MM, Manabe T, Matsui M, Hata F. Roles of M2 and M4 muscarinic receptors in regulating acetylcholine release from myenteric neurons of mouse ileum. J Neurophysiol 93: 2841–2848, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Thumshirn M, Coulie B, Camilleri M, Zinsmeister AR, Burton DD, Van Dyke C. Effects of alosetron on gastrointestinal transit time and rectal sensation in patients with irritable bowel syndrome. Aliment Pharmacol Ther 14: 869–878, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Tolterodine tartarate (Detrol) In: Physicians' Drug Reference (61st ed.), edited by Murray L, O'Hare A. Montvale, NJ: Thomson PDR, 2007, p. 2631–2634 [Google Scholar]
- 33.Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A, Tolterodine Study Group Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology 57: 414–421, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 96: 2671–2676, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Viramontes BE, Kim DY, Camilleri M, Lee JS, Stephens D, Burton DD, Thomforde GM, Klein PD, Zinsmeister AR. Validation of a stable isotope gastric emptying test for normal, accelerated or delayed gastric emptying. Neurogastroenterol Motil 13: 567–574, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370, 1983 [DOI] [PubMed] [Google Scholar]