Abstract
Cholangiocarcinomas arise after the neoplastic transformation of the cholangiocytes that line the intra- and extrahepatic biliary epithelium. Symptoms usually do not present until late in the course of the disease, at which time they are relatively resistant to chemotherapeutic agents and as such are difficult to treat and display a poor prognosis. Because of the relative rarity of this disease, the overall volume of research into the molecular pathophysiology associated with this disease is small compared with other more prevalent tumors. However, the incidence of this devastating cancer is on the rise and renewed efforts to understand the pathogenesis of cholangiocarcinoma is needed to design novel therapeutic strategies to combat this disease. This review summarizes the recent advances into our knowledge and understanding of cholangiocarcinoma and highlights potential novel therapeutic strategies that may prove useful to treat this deadly disease.
Keywords: gastrointestinal hormones, growth factors, inflammation, neuroendocrine hormones
General Background
Cholangiocarcinoma arises from the neoplastic transformation of cholangiocytes and can be either intrahepatic, perihilar, or distal extrahepatic tumors (3). Typically, cholangiocarcinomas are adenocarcinomas and have a poor prognosis and limited treatment options. This is due at least in part to the late presentation of symptoms and the relative resistance to current treatment options (81).
The incidence of both intra- and extrahepatic cholangiocarcinoma is typically more prevalent in Asian countries (74). The mortality rates for intrahepatic cholangiocarcinoma have increased since the 1970s, whereas deaths from extrahepatic cholangiocarcinoma have declined in most countries (74). There is a slight preponderance for cholangiocarcinoma in men (93), and the incidence in both sexes increases with age (74).
Risk Factors
Cholangiocarcinoma occurs with a varying frequency in different regions of the world. This can be explained in part by the distribution of risk factors in geographic regions and ethnic groups (7). The common link between these regional risk factors seems to involve chronic inflammation and biliary irritation (38).
The prevalence of cholangiocarcinoma in Asian countries shares a relationship with infections such as liver flukes, hepatitis B, and hepatitis C (7). In contrast, ∼90% of patients diagnosed with cholangiocarcinoma in Western countries do not have any recognized risk factors (7). However, the remaining 10% of cases are associated with certain risk factors. Apart from factors related to chronic inflammation, both intra- and extrahepatic cholangiocarcinomas are well-known complications of primary sclerosing cholangitis (19). Other known risk factors include obesity, hepatolithiasis, bacterial infection, and/or bile stasis-related chronic cholangitis (14, 15, 19).
Epigenetic Changes
Neoplastic transformation of normal cells into their malignant counterparts often requires a series of genetic changes. These changes can range from “simple” mutations in the genes themselves, that ultimately lead to loss-of-function or gain-of-function changes in key genes that are responsible for the control of apoptosis and cell cycle progression respectively, to more complex changes in nonprotein factors (e.g., RNA and DNA) that regulate the control of specific gene expression (18, 48, 49). These more complex changes can be classified as either genetic or epigenetic changes (18, 48, 49). Recent data suggest that both genetic and epigenetic changes are required for transformation, promotion, and progression of cholangiocarcinoma (58–60, 88, 90, 91, 102).
Hypermethylation.
A large number of genes are regulated by hypermethylation in cholangiocarcinoma. The functions of these genes range from tumor suppressors, cell cycle inhibitors, DNA repair, as well as regulators of cell adhesion and invasion (84). The most well-characterized epigenetic change with respect to cell cycle inhibitors is the p16INK4a gene, which has been described in up to 83% of cholangiocarcinomas (88, 102). This gene is responsible for binding to the cyclin-dependent kinase 4 (CDK4) and inhibits its ability to interact with cyclin D1 (63). In the absence of p16INK4a activity, such as after promoter methylation, CDK4 binds to cyclin D1, which subsequently leads to unchecked entry into the S phase of the cell cycle (63). There also appears to be increased incidence of hypermethylation of the related p14ARF occurring in 25% of cholangiocarcinoma samples studied (91). Many other cell cycle entry inhibitors have been shown to be hypermethylated, including p16INK4b (50% of tumors studied) (102) and 14-3-3 sigma (59.5% of tumors studied) (54).
In addition, the expression of many tumor suppressor genes are repressed in cholangiocarcinomas (90). The most striking of these is Semaphorin3B, which was found to be methylated in 100% of the cholangiocarcinoma cases studied (90). RassF1A (102) and p73 (102) are also hypermethylated and suppressed in 65.3 and 36.1% of cholangiocarcinoma cases studied, respectively.
Several DNA repair genes have been shown to be hypermethylated in cholangiocarcinoma. Hypermethylation of the hMLH1 mismatch repair gene promoter has been shown to occur in up to 23.6% of cholangiocarcinomas (102), which has previously been revealed to lead to microsatellite instability in other tumor types (31). Another gene, O6-methylguanine-DNA methyltransferase (MGMT), is silenced in up to 33% of cholangiocarcinoma tumors studied (102). This gene is an important suicide enzyme involved in the defense against O6-alkylating endogenous metabolites and environmental carcinogens. Interestingly, transcriptional repression of MGMT was associated with the accumulation of GC-AT transitional mutations in the p53 gene and, to a lesser extent, the k-ras gene in cholangiocarcinoma (51, 102). Lastly, the glutathione S-transferase P1 (GSTP1) gene, which inactivates electrophilic carcinogens by conjugation with glutathione, is hypermethylated in cholangiocarcinoma, occurring in 6–34% of cases studied (54, 102).
Lastly, hypermethylation of E-cadherin, a calcium-dependent cell adhesion molecule that suppresses metastatic processes and tumor cell invasion (25, 69, 82), has been demonstrated in up to 48% of cholangiocarcinoma samples studied (50, 54, 91, 102). Downregulation of this gene by epigenetic changes has been reported in other cancers (25), and that reexpression can be induced by treatment with a demethylating agent in cholangiocarcinoma (26).
A summary of the incidence of the above-mentioned hypermethylation event is found in Table 1.
Table 1.
Summary of genes that are silenced by hypermethylation in CCA
| Gene Name | Function | Incidence in CCA | Reference |
|---|---|---|---|
| 14-3-3 sigma | Cell cycle regulator | 59.5% | 54 |
| E-cadherin | Cell adhesion | 43% | 50, 54, 91, 102 |
| EGFR | Growth Factor | ND | 96 |
| GSTP1 | Inactivation of carcinogens | 6–34% | 54, 102 |
| hMLH1 | DNA mismatch repair | 25% | 102 |
| MGMT | Methyl transferase | 11–33% | 54, 102 |
| p14ARF | Cell cycle regulator | 38% | 102 |
| p15INK4b | Cell cycle regulator | 12–50% | 50, 102 |
| p16INK4a | Cell cycle regulator | 14–50% | 50, 54, 102 |
| p73 | Tumor suppressor | 36% | 102 |
| RassF1A | Tumor suppressor | 26–65% | 50, 102 |
| Semaphorin3B | Tumor suppressor | 100% | 90 |
| SOCS-3 | Inhibits inflammation | ND | 46 |
CCA, cholangiocancinoma; ND, not determined.
microRNA.
Our knowledge concerning the changes in microRNAs (miR) in cholangiocarcinoma is still premature. Meng et al. (58) showed that miR-141 was highly overexpressed in malignant cholangiocytes. Using a bioinformatics approach, a predicted target of miR-141 was the CLOCK gene, which regulates circadian rhythms and can act as a tumor suppressor (58). Inhibiting miR-141 effectively increased CLOCK protein expression in cholangiocarcinoma cells (58). Another microRNA species that was overexpressed in cholangiocarcinoma was miR-200b (58). The target gene for this was predicted to be the protein tyrosine phosphatase, nonreceptor type 12 (PTPN12), the dysregulation of which may contribute to tumor cell survival and oncogenesis (58). Similarly, the expression of miR-21 was overexpressed in cholangiocarcinoma, which effectively blocks the expression of the tumor suppressor gene PTEN (58).
Conversely, other microRNA species have been identified as being downregulated in cholangiocarcinoma compared with nonmalignant cholangiocytes. miR-29b expression was suppressed in the cholangiocarcinoma cell line KMCH as well as in ∼33% of human cholangiocarcinoma samples (64). Enforced miR-29b overexpression in cholangiocarcinoma cells effectively reduced the expression of Mcl-1, an antiapoptotic protein of the Bcl-2 family (64), and sensitized cholangiocarcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand cytotoxicity, suggesting that the suppression of miR-29b expression allows Mcl-1 overexpression that can ultimately lead to the resistance of cholangiocarcinoma to cell death (64). Another microRNA that is downregulated in cholangiocarcinoma is miR-370 (60) that is under tight epigenetic regulation by hypermethylation (60). One of the targets for miR-370 is the oncogene mitogen-activated protein kinase kinase kinase 8 (MAP3K8) (60); thus MAP3K8 is upregulated in cholangiocarcinoma cell lines as well as in tumor cell xenografts in vivo (60).
Association with Inflammation
Chronic inflammation is a recognized risk factor for the development of cholangiocarcinoma (3, 81). Indeed, sustained overexpression of the cytokine interleukin-6 (IL-6) plays an integral role in cholangiocarcinoma biology (85, 103). This aberrant overexpression of IL-6 is a consequence of the epigenetic silencing of the suppressor of cytokine signaling 3 (SOCS-3) (46). SOCS-3 promoter methylation was observed in a subset of cholangiocarcinoma samples and cholangiocarcinoma cell lines (46). Enforced overexpression of SOCS-3 in these cell lines effectively reduced the IL-6-mediated signal transduction cascade (46), therefore the loss of this negative regulator of IL-6 in cholangiocarcinoma may contribute to increased expression and activity of inflammatory molecules seen in cholangiocarcinoma.
A downstream consequence of aberrant IL-6 expression may be the further hypermethylation of the promoter regions of a number of critical target genes in cholangiocarcinoma. IL-6 has been show to regulate the enzyme activity of one of the DNA methyltransferases responsible for the hypermethylation of promoter regions (44). In cholangiocarcinoma cells, IL-6 overexpression resulted in the altered promoter methylation of a number of genes including the epidermal growth factor receptor (EGFR) (96). EGFR promoter methylation was decreased and gene and protein expression were increased by IL-6 (96), suggesting that the epigenetic regulation of gene expression by the inflated IL-6 expression seen in cholangiocarcinoma can contribute to tumor progression by altering the expression of growth regulatory pathways, such as those involving EGFR, caspase 8, and survivin in a manner that promotes survival and growth of the tumor cell (96).
Role of Growth Factors in Cholangiocarcinoma Growth
Immunohistochemical studies have shown (100) that the overexpression of NGF-β and VEGF-C occurred in ∼57.1 and 46.4% of cholangiocarcinoma samples, respectively. A number of human cholangiocarcinoma cell lines and samples express VEGF-A and VEGF receptors (VEGFRs) and the angiogenic factors angiopoietin-1, -2, and thrombospondin-1 (4, 86). Also, estrogens modulate cholangiocarcinoma growth. For example, tamoxifen (estrogen receptor antagonist) inhibits cholangiocarcinoma growth through calmodulin targeting of protein kinase B and cellular-FLICE inhibitory protein (75). An interaction between VEGF and estrogens in the regulation of cholangiocarcinoma growth has been defined in vivo and in vitro (55). Specifically, estrogens have been shown to stimulate the growth of human cholangiocarcinoma by inducing the expression and secretion of VEGF (55).
Other studies have shown that intrahepatic and extrahepatic cholangiocarcinoma samples overexpress EGFR and VEGF (42, 104). The studies have also shown that 1) EGFR expression is associated with cholangiocarcinoma progression and 2) VEGF expression regulates metastasis in cholangiocarcinoma (104). In support of this notion, inhibition of VEGFR and EGFR signaling with vandetanib (ZD6474, a tyrosine kinase inhibitor) can be an important approach for the management of cholangiocarcinoma that lack KRAS mutations and/or have EGFR amplification (105). Indeed, cholangiocarcinoma cell lines possessing KRAS mutations were resistant to vandetanib therapy (105). A recent study has also shown that the dual epidermal growth factor receptor and ErbB-2 tyrosine kinase inhibitor NVP-AEE788 are more efficient than the epidermal growth factor receptor inhibitors gefitinib and erlotinib in the regression of biliary tract cancers (97).
Furthermore, ZD1839 (IRESSA), an orally active, selective inhibitor of (EGFR) tyrosine kinase has clinical activity against cholangiocarcinoma by stabilizing p27Kip1 and enhancing radiosensitivity in cholangiocarcinoma cell lines (101). In another study, the cholangiocarcinoma cell line, HuCCT-1, was treated with gefitinib (ZD1839, Iressa), a selective blocker of the EGFR, and CI-1040, a selective inhibitor of the mitogen extracellular regulated kinase [mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase ½]. This study showed that HuCCT-1 cells were resistant to gefitinib and CI-1040 alone, but susceptible to the combination of these drugs both in vitro and in vivo (43). Also, overexpression and functional interaction between transforming growth factor-β 1 and VEGF contributes to the “angiogenic switch” and the development of malignant phenotypes in human cholangiocarcinoma samples and cell lines (8). Cross talk between the prostaglandin E receptor 1 receptor and EGFR signaling synergistically promotes cholangiocarcinoma growth and invasion (41). Moreover, a subset of cholangiocarcinoma patients has been shown to have response-predicting EGFR mutations. Thus EGFR kinase inhibitors can be important in the management of cholangiocarcinoma (40).
Regulation of Cholangiocarcinoma Growth by Gastrointestinal Hormones
In situ immunohistochemical studies have shown the distribution of secretin receptors in the human liver with the expression of this receptor in biliary tract and cholangiocarcinoma but not in hepatocytes or hepatocellular carcinoma (52). Onori et al. (72) have shown that secretin inhibits cholangiocarcinoma growth via dysregulation of the cyclic adenosine monophosphate (cAMP)-dependent signaling mechanisms of secretin receptor. Gastrin inhibits the growth of cholangiocarcinoma cells lines by Ca2+-dependent protein kinase C-α activation of apoptosis (47). Cholecystokinin increases the synthesis and release of carcinoembryonic antigen from the cholangiocarcinoma cell line, SLU-132, a mechanism that induces a decrease in the growth of this cholangiocarcinoma cell line in the nude mouse (45). Manipulation of endogenous cholecystokinin levels may be an important approach for the autocrine regulation of the growth of human cholangiocarcinoma (27).
Neuroendocrine Endocrine Regulation of Cholangiocarcinoma Growth
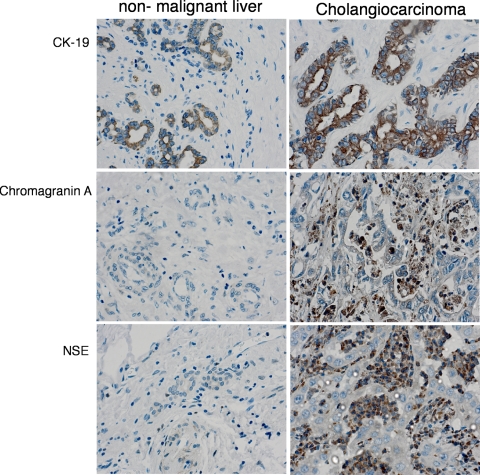
Cholangiocarcinoma displays characteristic features of neuroendocrine tumors such as the expression of chromagranin A and neuron-specific enolase as seen in Fig. 1 (2). The neurotransmitter dopamine is overproduced and secreted at increasingly high levels in cholangiocarcinoma (16). This study demonstrated that the enzymes that regulate dopamine synthesis are dysregulated in cholangiocarcinoma compared with normal cholangiocytes, causing cancerous cells to produce increased levels of dopamine (16). The study has also shown that blockage of dopamine production and secretion induces a decrease in cholangiocarcinoma growth in vitro and in vivo (16). A similar study has shown that the rate-limiting enzyme responsible for serotonin metabolism, tryptophan hydroxylase I, is upregulated and the enzyme responsible for degradation is markedly suppressed (2). Blocking tryptophan hydroxylase I proved to decrease tumor cell growth in models of cell culture and xenograft tumors (2).
Fig. 1.
Cholangiocarcinoma display features of a neuroendocrine tumor. Immunohistochemical analysis of biopsy samples from nonmalignant liver and cholangiocarcinoma tumors were performed by using antibodies against CK-19 (cholangiocyte marker) and chromagranin A and neuron-specific enolase (NSE) (neuroendocrine markers). Representative photomicrographs of the immunoreactivity are shown (magnification ×40). Reproduced from Alpini et al. (2) with permission from American Association of Cancer Research.
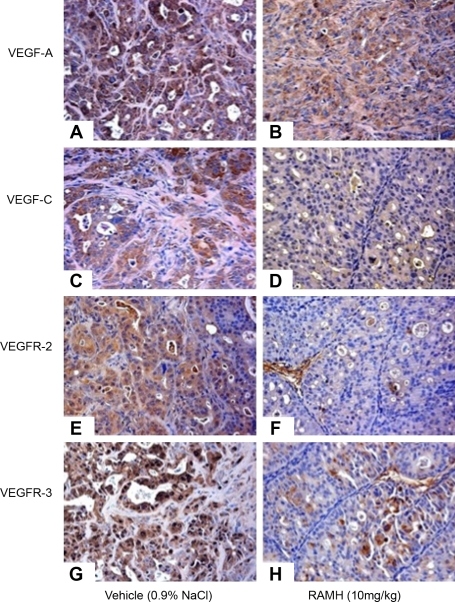
The H3 histamine receptor agonist, RAMH, had been shown to inhibit cholangiocarcinoma growth (34). Treatment with RAMH induced a decrease in all cholangiocarcinoma cell lines but had no effect in normal cholangiocytes (34). RAMH inhibition of cholangiocarcinoma growth was associated with an increase in intracellular IP3 levels and protein kinase C-α translocation and a decrease in the expression of VEGF-A and VEGF-C and its receptors, VEGFR-2 and VEGFR-3 (34). Representative immunohistochemistry images of this expression are found in Fig. 2.
Fig. 2.
H3 histamine receptor agonist RAMH (10 mg/kg body wt) administration to mice bearing xenografted cholangiocarcinoma tumors decreased the expression of VEGF-A, VEGF-C, VEGF receptor (VEGFR)-2, and VEGFR-3 compared with vehicle-treated (0.9% NaCl) mice. Original magnification ×20. A, C, E, G: vehicle. B, D, F, H: RAMH. Reproduced from Francis et al. (34) with permission from American Association of Cancer Research.
Other Neurotransmitters
Besides the above-mentioned neuroendocrine factors, there is increasing evidence that cholangiocarcinoma growth can be regulated by a number of other neuroregulatory molecules such as the inhibitory neurotransmitter, GABA (30), various endocannabinoids (23, 24, 32), and opioids (57).
GABA.
Administration of GABA to cholangiocarcinoma cells and in tumors decreased proliferation and migration in a dose-dependent manner (30). This effect was associated with increased intracellular IP3 and cAMP levels and subsequent activation of PKA and dephosphorylation of ERK½ (30).
Endocannabinoids.
We have shown the differential effects of anandamide (AEA) and 2-arachidonyl glycerol (2-AG) on cholangiocarcinoma growth in vitro (24). The growth promoting effects of 2-AG was found to be via a cannabinoid receptor-independent mechanism involving the disruption of lipid raft structures in the cell membrane (24). Conversely, the antiproliferative actions of AEA were via a mechanism involving the stabilization of lipid rafts in the plasma membrane and the recruitment of death receptor complexes (24). Furthermore, we have shown that AEA suppresses tumor growth in vivo using a xenograft model of cholangiocarcinoma (23) and that there was a concomitant activation of the noncanonical Wnt pathway via upregulation of Wnt 5a (23). More recently, we have demonstrated that the antiproliferative actions of AEA are also associated with an increase in Notch 1 expression and activation, whereas the growth-promoting effects of 2-AG can be associated with an increase in Notch 2 expression and activation (32). The cross talk between the Wnt and Notch signaling pathways after AEA treatment and the reliance of these pathways on lipid raft structures are yet to be determined.
Opioids.
Marzioni et al. (57) recently demonstrated a growth promoting effect of μ-opioid receptor (μOR) activation on cholangiocarcinoma. This effect was in contrast to the antiproliferative effects of opioids on hyperplastic cholangiocytes (56), suggesting that the endogenous opioid system is somehow dysregulated during cholangiocyte-to-cholangiocarcinoma transformation. Associated with these growth promoting effects of μOR activation was an increase in ERK½, PI3-kinase and Ca2+-dependent CamKIIα pathways (57).
Other Regulatory Peptides
Leptin.
Leptin is a hormone that is produced by the adipose tissue to regulate caloric homeostasis, is increased in obese patients (95), and stimulates the growth of a number of tumors (35). It has been proposed that the increased production of such a hormone by adipose tissue could explain the well-known correlation between obesity and increased incidence of various types of tumors (35). Administration of leptin increased the proliferation and the metastatic potential of cholangiocarcinoma cells in vitro through signal transducers and activators of transcription 3-dependent activation of ERK ½ (28). Leptin increased the growth and migration and was antiapoptotic for cholangiocarcinoma cells (28). Moreover, the loss of leptin function reduced the development and the growth of cholangiocarcinoma in an experimental carcinogenesis model induced by thioacetamide administration to rats (28).
Endothelins.
Endothelins (ET) are vasoactive peptides that exert their effects through either ETA or ETB receptors (5). These receptors are overexpressed in cholangiocarcinoma cells (29). Administration of ET-1 to cholangiocarcinoma cells inhibited proliferation both in vitro and in vivo.
Microenvironment
Neoplastic epithelial cells coexist with a biologically complex stroma composed of various types of stromal cells as well as extracellular matrix, both of which create the complexity of the tumor microenvironment (73). Mouse models of tumorigenesis have revealed that stromal cells, in particular inflammatory cells, vascular cells, and fibroblasts, actively support tumor growth (11, 17, 70, 92). The role of the microenvironment in the regulation of cholangiocarcinoma growth, metastasis, and progression is largely unknown; however, what is known is summarized below.
Stromal fibroblasts.
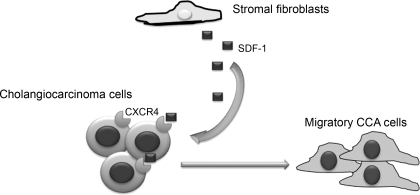
Under normal physiological conditions, fibroblasts have a low proliferative index and only secrete factors needed to maintain normal tissue homeostasis (6, 94). Indeed, normal fibroblasts provide biochemical cues that constrain epithelial tumor cells within their basement membrane (6, 94). In contrast, when homeostasis is disrupted during tissue injury, stromal cells rapidly and reversibly alter their phenotype and proliferation rate (94). However, during tumorigenesis, the fibroblastic wound healing machinery lacks the regulatory mechanisms to revert to normal homeostasis (94). The inability to downregulate the wound healing response affects stromal dynamics. Tumor-dependent changes in signaling and plasticity of the stroma trigger a continuum of alterations yielding a “primed” stroma that can support and incite tumor initiation or progression (94). Fibroblast activation protein is expressed by tumor-associated fibroblasts and fibroblasts involved in wound healing but not in normal fibroblasts (78). In cholangiocarcinoma, stromal fibroblasts have been shown to play an important role in migration and invasion of tumor cells (67, 68). Invasion of cholangiocarcinoma cells has been shown to increase after incubation with the supernatant from fibroblast cultures (67). The secreted factor thought to be involved in this process is stromal-derived factor-1 (68), which is released from stromal fibroblasts and stimulates invasion and migration of the cholangiocarcinoma cells via interaction with the chemokine receptor CXCR4 (68). A schematic diagram of this can be found in Fig. 3.
Fig. 3.
Schematic diagram of the cross talk between cholangiocarcinoma cells and stromal fibroblasts. SDF, stromal-derived factor; CCA, cholangiocarcinoma; CXCR4, chemokine receptor 4.
Angiogenesis
The physiological process of the formation of new blood vessels from preexisting blood vessels is termed angiogenesis. A recent immunohistochemical analysis of microvessel density and lymphatic microvessel density revealed that intrahepatic cholangiocarcinoma tumors showed an induction of tumor-associated angiogenesis and lymphangiogenesis (89). Furthermore, tumors with increased microvessel density and lymphatic microvessel density were correlated with a higher recurrence rate, lower 5-yr survival rates, and increased nodal spread influencing patient survival (89). In addition, VEGF-A has been shown to play a role in the neovascularization of extrahepatic cholangiocarcinoma (62). The factors that drive angiogenesis including VEGF have also been shown to have distinct effects on cholangiocarcinoma growth in an autocrine manner (36, 37, 62, 86, 101, 105) as described above.
Novel Treatment Regimes
As mentioned previously, cholangiocarcinoma is relatively resistant to most currently approved chemotherapeutic agents, and as such these treatment options, although perhaps extending the prognosis for a few months, are largely ineffectual in curing this disease. Recently a number of experimental treatment options have come to the forefront. The most promising of these appears to be a multiple kinase inhibitor, sorafenib (98). This has been approved for use as a chemotherapeutic agent for renal cancer (76). Recently, sorafenib was shown to display significant tumor suppression in a rodent model of cholangiocarcinoma (12) and is currently undergoing phase II trials (9).
Another strategy to improve the treatment options of cholangiocarcinoma is to increase the sensitivity of cholangiocarcinoma to common chemotherapeutic agents. A large body of research has focused on plant-derived polyphenols, such as resveratrol (13, 65, 80), caffeic acid (71), tannic acid (66), and green tea polyphenols (53), as therapeutic and chemopreventive agents. Research indicates that these polyphenols may have antioxidant characteristics with potential health benefits including reducing the risk of cancer (83). Caffeic acid, a polyphenol extracted from the propolis of honeybee hives, exerts antiproliferative effects on cholangiocarcinoma (71) and other tumor types (22, 99) in vitro and in vivo by inhibiting the nuclear factor-κB pathway (71). Furthermore, high concentrations of resveratrol have been shown to be antiproliferative in a cholangiocarcinoma cell line (80) as well as in other tumor types (10, 21, 87) through a number of different mechanisms including increased cyclooxygenase 2 expression (87), facilitation of death receptor complex formation (20, 21), or cell cycle arrest (10).
Although it is interesting that these food-derived polyphenols exert antiproliferative effects on tumor growth in their own right, often the concentrations required are prohibitive for these compounds to be considered as viable treatment options. A more rational approach is to study the efficacy of these compounds as adjunct therapies to existing chemotherapeutic strategies. For example, tannic acid and the green tea polyphenol epigallocatechin-gallate have been shown to sensitize cholangiocarcinoma to chemotherapy-induced apoptosis in vitro (53, 66). Furthermore, the green tea polyphenol administered together with gemcitabine not only slowed cholangiocarcinoma tumor progression in a xenograft mouse model, it also decreased tumor volume (53). We have recently shown that resveratrol has a similar sensitizing effect on cholangiocarcinoma to the chemotherapeutic agents gemcitabine, 5-fluorouracil, and mitomycin C, potentially due to the suppression of the xenobiotic metabolizing enzyme cytochrome P-450 1b1 (33).
Another treatment option currently being explored involves the use of a neoadjuvant therapy regime followed by liver transplant (77). Historically, the surgical treatment of cholangiocarcinoma arising in patients with primary sclerosing cholangitis produced disappointing outcomes with no significant improvement of survival over those transplanted patients with primary sclerosing cholangitis alone (1, 39, 61, 79). Recently, the use of various neoadjuvant therapies prior to transplantation has given promising results (77). The established protocol initially involved rigorous patient selection criteria, excluding such parameters as the presence of a mass below the level of the cystic duct and evidence for intrahepatic or extrahepatic metastasis (77). Patients who had transperitoneal biopsy or violation of the bile duct during a prior attempt at surgical resection were also disqualified because peritoneal seeding has been encountered (77). Once selected, the patients commenced the neoadjuvant therapy regime of 4,000–4,500 cGy, administered by external beam radiation, followed by transcatheter radiation (2,000–3,000 cGy) with iridium-192 wires (77). In parallel, 5-fluorouracil was infused during the radiation treatment and followed by oral capecitabine until the day of transplantation (77). Prior to transplantation, the patients underwent a staging abdominal explorative operation to further screen for intrahepatic metastases, or lymph nodes and peritoneal metastases, and if no indication of metastases was present, transplantation was then performed. The results of this treatment regimen have been encouraging, with the 1-, 3-, and 5-yr survival rate after transplantation being higher than that after resection alone (77).
Conclusion and Future Perspectives
From the work described above it is obvious that there are large gaps in our knowledge concerning both the etiology and progression of cholangiocarcinoma. Taken together with the increasing incidence of cholangiocarcinoma worldwide, the lack of effective treatment options is concerning. Increased efforts are needed to design multifaceted approaches to target key features of this complicated and resistant tumor. In addition, research into the mechanism of chemotherapeutic resistance and methods to decrease this resistance in these tumors may prove valuable in designing adjunct therapies.
GRANTS
Portions of these studies were supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, the VA Research Scholar Award, a VA Merit Award and the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK58411 and DK07698 (G. Alpini) and NIH K01 grant award DK078532 and NIH R03 grant award DK088012 (S. DeMorrow).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Because of editorial constrictions, citations are rather illustrative than comprehensive, with a strong emphasis on review articles. We apologize to all investigators whose groundbreaking work could not be cited.
REFERENCES
- 1.Abu-Elmagd KM, Malinchoc M, Dickson ER, Fung JJ, Murtaugh PA, Langworthy AL, Demetris AJ, Krom RA, Van Thiel DH, Starzl TE. Efficacy of hepatic transplantation in patients with primary sclerosing cholangitis. Surg Gynecol Obstet 177: 335–344, 1993 [PMC free article] [PubMed] [Google Scholar]
- 2.Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, Onori P, Franchitto A, Coufal M, Frampton G, Alvaro D, Lee SP, Marzioni M, Benedetti A, DeMorrow S. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res 68: 9184–9193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpini G, Prall R, LaRusso NF. The pathobiology of biliary epithelia. The Liver: Biology and Pathobiology (4th ed.), edited by Arias IM. Philadelphia, PA: Lippincott Williams & Wilkins, 2001, p. 421–435 [Google Scholar]
- 4.Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S, Onetti Muda A, Dostal DE, De Santis A, Attili AF, Benedetti A, Gaudio E. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol 169: 877–888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert JD, Juillerat-Jeanneret L. Therapeutic potential of endothelin receptor modulators: lessons from human clinical trials. Expert Opin Ther Targets 13: 1069–1084, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol 15: 329–341, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol 19: 615–617, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Benckert C, Jonas S, Cramer T, Von Marschall Z, Schafer G, Peters M, Wagner K, Radke C, Wiedenmann B, Neuhaus P, Hocker M, Rosewicz S. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res 63: 1083–1092, 2003 [PubMed] [Google Scholar]
- 9.Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, Zironi S, Depenni R, Fontana A, Del Giovane C, Luppi G, Conte P. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 102: 68–72, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J Androl 28: 282–293, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 432: 332–337, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology 50: 1861–1870, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braconi C, Meng F, Swenson E, Khrapenko L, Huang N, Patel T. Candidate therapeutic agents for hepatocellular cancer can be identified from phenotype-associated gene expression signatures. Cancer 115: 3738–3748, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Catalano OA, Sahani DV, Forcione DG, Czermak B, Liu CH, Soricelli A, Arellano RS, Muller PR, Hahn PF. Biliary infections: spectrum of imaging findings and management. Radiographics 29: 2059–2080, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Chen MF. Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol 14: 1144–1149, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Coufal M, Invernizzi P, Gaudio E, Bernuzzi F, Frampton GA, Onori P, Franchitto A, Carpino G, Ramirez JC, Alvaro D, Marzioni M, Battisti G, Benedetti A, DeMorrow S. Increased local dopamine secretion has growth-promoting effects in cholangiocarcinoma. Int J Cancer 126: 2112–2122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 107: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene 25: 6170–6175, 2006 [DOI] [PubMed] [Google Scholar]
- 19.De Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med 341: 1368–1378, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, Gambert P, Cherkaoui-Malki M, Jannin B, Dubrez-Daloz L, Latruffe N, Solary E. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem 278: 41482–41490, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Delmas D, Rebe C, Micheau O, Athias A, Gambert P, Grazide S, Laurent G, Latruffe N, Solary E. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene 23: 8979–8986, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Demestre M, Messerli SM, Celli N, Shahhossini M, Kluwe L, Mautner V, Maruta H. CAPE (caffeic acid phenethyl ester)-based propolis extract (Bio 30) suppresses the growth of human neurofibromatosis (NF) tumor xenografts in mice. Phytother Res 23: 226–230, 2009 [DOI] [PubMed] [Google Scholar]
- 23.DeMorrow S, Francis H, Gaudio E, Venter J, Franchitto A, Kopriva S, Onori P, Mancinelli R, Frampton G, Coufal M, Mitchell BM, Vaculin B, Alpini G. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the non-canonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol 295: G1150–G1158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of fas and fas ligand to lipid rafts. J Biol Chem 282: 13098–13113, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Doki Y, Shiozaki H, Tahara H, Inoue M, Oka H, Iihara K, Kadowaki T, Takeichi M, Mori T. Correlation between E-cadherin expression and invasiveness in vitro in a human esophageal cancer cell line. Cancer Res 53: 3421–3426, 1993 [PubMed] [Google Scholar]
- 26.Endo K, Ashida K, Miyake N, Terada T. E-cadherin gene mutations in human intrahepatic cholangiocarcinoma. J Pathol 193: 310–317, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Evers BM, Gomez G, Townsend CM, Jr, Rajaraman S, Thompson JC. Endogenous cholecystokinin regulates growth of human cholangiocarcinoma. Ann Surg 210: 317–322; discussion 322–313, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fava G, Alpini G, Rychlicki C, Saccomanno S, DeMorrow S, Trozzi L, Candelaresi C, Venter J, Di Sario A, Marzioni M, Bearzi I, Glaser S, Alvaro D, Marucci L, Francis H, Svegliati-Baroni G, Benedetti A. Leptin enhances cholangiocarcinoma cell growth. Cancer Res 68: 6752–6761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fava G, Demorrow S, Gaudio E, Franchitto A, Onori P, Carpino G, Glaser S, Francis H, Coufal M, Marucci L, Alvaro D, Marzioni M, Horst T, Mancinelli R, Benedetti A, Alpini G. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int 29: 1031–1042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T, Taffetani S, Marzioni M, Summers R, Reichenbach R, Alpini G. Gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase ½ pathway. Cancer Res 65: 11437–11446, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Fleisher AS, Esteller M, Tamura G, Rashid A, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Nishizuka S, James SP, Wilson KT, Herman JG, Meltzer SJ. Hypermethylation of the hMLH1 gene promoter is associated with microsatellite instability in early human gastric neoplasia. Oncogene 20: 329–335, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Frampton G, Coufal M, Li H, Ramirez J, DeMorrow S. Opposing actions of endocannabinoids on cholangiocarcinoma growth is via the differential activation of Notch signaling. Exp Cell Res In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frampton G, Lazcano E, Li H, Mohamad A, DeMorrow S. Resveratrol enhances the sensitivity of cholangiocarcinoma to chemotherapeutic agents. Lab Invest In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis H, Onori P, Gaudio E, Franchitto A, DeMorrow S, Venter J, Kopriva S, Carpino G, Mancinelli R, White M, Meng F, Vetuschi A, Sferra R, Alpini G. H3 histamine receptor-mediated activation of protein kinase Calpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res 7: 1704–1713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol 207: 12–22, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Glaser SS, Gaudio E, Alpini G. Vascular factors, angiogenesis and biliary tract disease. Curr Opin Gastroenterol 26: 246–250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology 37: 961–969, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Goss JA, Shackleton CR, Farmer DG, Arnaout WS, Seu P, Markowitz JS, Martin P, Stribling RJ, Goldstein LI, Busuttil RW. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg 225: 472–481; discussion 481–473, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gwak GY, Yoon JH, Shin CM, Ahn YJ, Chung JK, Kim YA, Kim TY, Lee HS. Detection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomas. J Cancer Res Clin Oncol 131: 649–652, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem 280: 24053–24063, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A, Opitz OG. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol 15: 4511–4517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hidalgo M, Amador ML, Jimeno A, Mezzadra H, Patel P, Chan A, Nielsen ME, Maitra A, Altiok S. Assessment of gefitinib- and CI-1040-mediated changes in epidermal growth factor receptor signaling in HuCCT-1 human cholangiocarcinoma by serial fine needle aspiration. Mol Cancer Ther 5: 1895–1903, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Hodge DR, Xiao W, Clausen PA, Heidecker G, Szyf M, Farrar WL. Interleukin-6 regulation of the human DNA methyltransferase (HDNMT) gene in human erythroleukemia cells. J Biol Chem 276: 39508–39511, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Hudd C, Euhus DM, LaRegina MC, Herbold DR, Palmer DC, Johnson FE. Effect of cholecystokinin on human cholangiocarcinoma xenografted into nude mice. Cancer Res 45: 1372–1377, 1985 [PubMed] [Google Scholar]
- 46.Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology 132: 384–396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol 34: 284–291, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Karpf AR. Epigenomic reactivation screening to identify genes silenced by DNA hypermethylation in human cancer. Curr Opin Mol Ther 9: 231–241, 2007 [PubMed] [Google Scholar]
- 49.Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene 25: 6188–6196, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Kim BH, Cho NY, Choi M, Lee S, Jang JJ, Kang GH. Methylation profiles of multiple CpG island loci in extrahepatic cholangiocarcinoma versus those of intrahepatic cholangiocarcinomas. Arch Pathol Lab Med 131: 923–930, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Koga Y, Kitajima Y, Miyoshi A, Sato K, Kitahara K, Soejima H, Miyazaki K. Tumor progression through epigenetic gene silencing of O(6)-methylguanine-DNA methyltransferase in human biliary tract cancers. Ann Surg Oncol 12: 354–363, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Korner M, Hayes GM, Rehmann R, Zimmermann A, Scholz A, Wiedenmann B, Miller LJ, Reubi JC. Secretin receptors in the human liver: expression in biliary tract and cholangiocarcinoma, but not in hepatocytes or hepatocellular carcinoma. J Hepatol 45: 825–835, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Lang M, Henson R, Braconi C, Patel T. Epigallocatechin-gallate modulates chemotherapy-induced apoptosis in human cholangiocarcinoma cells. Liver Int 29: 670–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Kim WH, Jung HY, Yang MH, Kang GH. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol 161: 1015–1022, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, Francis H, Onori P, Franchitto A, Ginanni-Corradini S, Gaudio E, Alvaro D. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis 41: 156–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marzioni M, Alpini G, Saccomanno S, de Minicis S, Glaser S, Francis H, Trozzi L, Venter J, Orlando F, Fava G, Candelaresi C, Macarri G, Benedetti A. Endogenous opioids modulate the growth of the biliary tree in the course of cholestasis. Gastroenterology 130: 1831–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Marzioni M, Invernizzi P, Candelaresi C, Maggioni M, Saccomanno S, Selmi C, Rychlicki C, Agostinelli L, Cassani B, Miozzo M, Pasini S, Fava G, Alpini G, Benedetti A. Human cholangiocarcinoma development is associated with dysregulation of opioidergic modulation of cholangiocyte growth. Dig Liver Dis 41: 523–533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 130: 2113–2129, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Meng F, Henson R, Wehbe-Janek H, Smith H, Ueno Y, Patel T. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem 282: 8256–8264, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene 27: 378–386, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 69: 1633–1637, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Mobius C, Demuth C, Aigner T, Wiedmann M, Wittekind C, Mossner J, Hauss J, Witzigmann H. Evaluation of VEGF A expression and microvascular density as prognostic factors in extrahepatic cholangiocarcinoma. Eur J Surg Oncol 33: 1025–1029, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Moller MB. Molecular control of the cell cycle in cancer: biological and clinical aspects. Dan Med Bull 50: 118–138, 2003 [PubMed] [Google Scholar]
- 64.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 26: 6133–6140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, Reber HA, Pandol SJ. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int J Cancer 98: 761–769, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Naus PJ, Henson R, Bleeker G, Wehbe H, Meng F, Patel T. Tannic acid synergizes the cytotoxicity of chemotherapeutic drugs in human cholangiocarcinoma by modulating drug efflux pathways. J Hepatol 46: 222–229, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohira S, Itatsu K, Sasaki M, Harada K, Sato Y, Zen Y, Ishikawa A, Oda K, Nagasaka T, Nimura Y, Nakanuma Y. Local balance of transforming growth factor-beta1 secreted from cholangiocarcinoma cells and stromal-derived factor-1 secreted from stromal fibroblasts is a factor involved in invasion of cholangiocarcinoma. Pathol Int 56: 381–389, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y, Nakanuma Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol 168: 1155–1168, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S, Takeichi M, Mori T. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res 53: 1696–1701, 1993 [PubMed] [Google Scholar]
- 70.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59: 5002–5011, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onori P, DeMorrow S, Gaudio E, Franchitto A, Mancinelli R, Venter J, Kopriva S, Ueno Y, Alvaro D, Savage J, Alpini G, Francis H. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int J Cancer 125: 565–576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onori P, Wise C, Gaudio E, Franchitto A, Francis H, Carpino G, Lee V, Lam I, Miller T, Dostal DE, Glaser SS. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer 127: 43–54, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5: 1597–1601, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2: 10, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pawar P, Ma L, Byon CH, Liu H, Ahn EY, Jhala N, Arnoletti JP, McDonald JM, Chen Y. Molecular mechanisms of tamoxifen therapy for cholangiocarcinoma: role of calmodulin. Clin Cancer Res 15: 1288–1296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O'Dwyer PJ. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24: 2505–2512, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Rea DJ, Rosen CB, Nagorney DM, Heimbach JK, Gores GJ. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am 18: 325–337, ix, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Ozer HL, Schwab M, Albino AP, Old LJ. Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer Res 53: 3327–3335, 1993 [PubMed] [Google Scholar]
- 79.Robles R, Figueras J, Turrion VS, Margarit C, Moya A, Varo E, Calleja J, Valdivieso A, Valdecasas JC, Lopez P, Gomez M, de Vicente E, Loinaz C, Santoyo J, Fleitas M, Bernardos A, Llado L, Ramirez P, Bueno FS, Jaurrieta E, Parrilla P. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg 239: 265–271, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roncoroni L, Elli L, Dolfini E, Erba E, Dogliotti E, Terrani C, Doneda L, Grimoldi MG, Bardella MT. Resveratrol inhibits cell growth in a human cholangiocarcinoma cell line. Liver Int 28: 1426–1436, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology 41: 5–15, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Sommers CL, Thompson EW, Torri JA, Kemler R, Gelmann EP, Byers SW. Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ 2: 365–372, 1991 [PubMed] [Google Scholar]
- 83.Soory M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: potential for therapeutic targeting. Infect Disord Drug Targets 9: 400–414, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Stutes M, Tran S, DeMorrow S. Genetic and epigenetic changes associated with cholangiocarcinoma: from DNA methylation to microRNAs. World J Gastroenterol 13: 6465–6469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sugawara H, Yasoshima M, Katayanagi K, Kono N, Watanabe Y, Harada K, Nakanuma Y. Relationship between interleukin-6 and proliferation and differentiation in cholangiocarcinoma. Histopathology 33: 145–153, 1998 [DOI] [PubMed] [Google Scholar]
- 86.Tang D, Nagano H, Yamamoto H, Wada H, Nakamura M, Kondo M, Ota H, Yoshioka S, Kato H, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Dono K, Wakasa K, Monden M. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-½, thrombospondin-1 and clinicopathological significance. Oncol Rep 15: 525–532, 2006 [PubMed] [Google Scholar]
- 87.Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY. Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells. Mol Cancer Ther 5: 2034–2042, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Tannapfel A, Sommerer F, Benicke M, Weinans L, Katalinic A, Geissler F, Uhlmann D, Hauss J, Wittekind C. Genetic and epigenetic alterations of the INK4a-ARF pathway in cholangiocarcinoma. J Pathol 197: 624–631, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gessner R, Benckert C, Jonas S. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol In Press [DOI] [PubMed] [Google Scholar]
- 90.Tischoff I, Markwarth A, Witzigmann H, Uhlmann D, Hauss J, Mirmohammadsadegh A, Wittekind C, Hengge UR, Tannapfel A. Allele loss and epigenetic inactivation of 3p21.3 in malignant liver tumors. Int J Cancer 115: 684–689, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Tischoff I, Wittekind C, Tannapfel A. Role of epigenetic alterations in cholangiocarcinoma. J Hepatobiliary Pancreat Surg 13: 274–279, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol 11: 97–104, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Tominaga S, Kuroishi T. Biliary tract cancer. Cancer Surv 19–20: 125–137, 1994 [PubMed] [Google Scholar]
- 94.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol 166: 2472–2483, 2001 [PubMed] [Google Scholar]
- 95.Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. Eur J Endocrinol 143: 293–311, 2000 [DOI] [PubMed] [Google Scholar]
- 96.Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res 66: 10517–10524, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Wiedmann M, Feisthammel J, Bluthner T, Tannapfel A, Kamenz T, Kluge A, Mossner J, Caca K. Novel targeted approaches to treating biliary tract cancer: the dual epidermal growth factor receptor and ErbB-2 tyrosine kinase inhibitor NVP-AEE788 is more efficient than the epidermal growth factor receptor inhibitors gefitinib and erlotinib. Anticancer Drugs 17: 783–795, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 7: 3129–3140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu CS, Chen MF, Lee IL, Tung SY. Predictive role of nuclear factor-kappaB activity in gastric cancer: a promising adjuvant approach with caffeic acid phenethyl ester. J Clin Gastroenterol 41: 894–900, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Xu LB, Liu C, Gao GQ, Yu XH, Zhang R, Wang J. Nerve growth factor-beta expression is associated with lymph node metastasis and nerve infiltration in human hilar cholangiocarcinoma. World J Surg 34: 1039–1045, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Yabuuchi S, Katayose Y, Oda A, Mizuma M, Shirasou S, Sasaki T, Yamamoto K, Oikawa M, Rikiyama T, Onogawa T, Yoshida H, Ohtuska H, Motoi F, Egawa S, Unno M. ZD1839 (IRESSA) stabilizes p27Kip1 and enhances radiosensitivity in cholangiocarcinoma cell lines. Anticancer Res 29: 1169–1180, 2009 [PubMed] [Google Scholar]
- 102.Yang B, House MG, Guo M, Herman JG, Clark DP. Promoter methylation profiles of tumor suppressor genes in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol 18: 412–420, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest 78: 89–100, 1998 [PubMed] [Google Scholar]
- 104.Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 98: 418–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoshikawa D, Ojima H, Kokubu A, Ochiya T, Kasai S, Hirohashi S, Shibata T. Vandetanib (ZD6474), an inhibitor of VEGFR and EGFR signalling, as a novel molecular-targeted therapy against cholangiocarcinoma. Br J Cancer 100: 1257–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]