Abstract
GTP cyclohydrolase I (GTPCH) is the rate-limiting enzyme for tetrahydrobiopterin (BH4) synthesis. Decreases in GTPCH activity and expression have been shown in late stages of acute cardiac rejection, suggesting a deficit in BH4. We hypothesized that increasing intracellular levels of BH4 by cardiac myocyte-targeted overexpression of GTPCH would diminish acute cardiac allograft rejection. Transgenic mice overexpressing GTPCH in the heart were generated and crossed on C57BL6 background. Wild-type and transgenic mouse donor hearts were transplanted into BALB/c recipient mice. Left ventricular (LV) function, histological rejection, BH4 levels, and inflammatory cytokine gene expression (mRNA) were examined. Expression of human GTPCH was documented by PCR, Western analysis, and function by a significant (P < 0.001) increase in cardiac BH4 levels. GTPCH transgene decreased histological rejection (46%; P < 0.003) and cardiac myocyte injury (eosin autofluorescence; 56%; P < 0.0001) independent of changes in inflammatory cytokine expression or nitric oxide content. GTPCH transgene decreased IL-2 (88%; P < 0.002), IL-1R2 (42%; P < 0.0001), and programmed cell death-1 (67%; P < 0.0001) expression, whereas it increased fms-like tyrosine kinase 3 (156%; P < 0.0001) and stromal-derived factor-1 (2; 190%; P < 0.0001) expression. There was no difference in ejection fraction or fractional shortening; however, LV mass was significantly increased (P < 0.05) only in wild-type grafts. The decreases in LV mass, cardiac injury, and histological rejection support a protective role of cardiac GTPCH overexpression and increased BH4 synthesis in cardiac allografts. The mechanism of the decreased rejection appears related to decreased T cell proliferation and modulation of immune function by higher expression of genes involved in hematopoietic/stromal cell development and recruitment.
Keywords: tetrahydrobiopterin, rejection, stromal-derived factor 1, left ventricular mass, guanosine 5′-triphosphate cyclohydrolase
tetrahydrobiopterin (BH4) is a cofactor for only a few known enzymes including three isoforms of nitric oxide (NO) synthases (NOS), four aromatic amino acid hydroxylases, and glyceryl ether monoxygenase (25, 30). Synthesis of BH4 is regulated by the expression of the key enzyme GTP cyclohydrolase I (GTPCH). The discovery of nuclear localization of GTPCH and other proteins involved in BH4 synthesis (5) coupled with earlier findings that the mitogenic action of BH4 in various cells is independent of changes in catecholamine and NO synthesis (1) suggests potentially new unknown roles of BH4. Thus there is new awareness of the potential regulation of GTPCH/BH4 for a vast array of biological processes far beyond that regulating NO and catecholamine synthesis including cell proliferation, cell division, apoptosis, and immune function.
Previously, we reported in rat cardiac allografts that alloimmune activation resulted in increased GTPCH activity and biopterin levels (21). However, with development of advanced rejection the increased levels of pterin synthesis were not maintained. In preliminary studies (unpublished observations), we confirmed in our mouse model of cardiac rejection that BH4 levels increased in mouse allografts compared with nontransplanted donor heart controls, but this fell back below control levels in rejected allografts. This coincided with decreased GTPCH expression. Thus both rat and mouse models of rejection confirm a loss of BH4 synthesis capacity upon rejection of cardiac grafts. This initiated studies to determine the potential benefits of strategies to enhance BH4 levels.
Previous studies have shown that exogenous administration of authentic BH4 or its precursor sepiapterin diminished acute cardiac allograft rejection in mouse or rat transplant models (2, 19). Both studies concluded that the beneficial effects of BH4 or sepiapterin were NO or NOS independent, suggesting alternative mechanisms of action. Indeed, we concluded that the antirejection effect of sepiapterin was rather mediated, at least in part, by an action on immune cell proliferation based upon decreased IL-2 expression.
Previously, we found that adult cardiac myocytes despite expression of GTPCH mRNA and protein have a defective basal and cytokine-stimulated synthesis of BH4 via the de novo synthesis pathway and impaired synthesis via the salvage pathway in contrast with that typically seen in neonatal cardiac myocytes (7, 26). In this context, we surmised that the beneficial effects of exogenous pterin administration on acute cardiac rejection was unlikely mediated via a mechanism of action to significantly change the intracellular BH4 levels within cardiac myocytes per se.
To circumvent the limitation of intrinsic defects in synthesis of BH4 in cardiac myocytes, we developed a mouse model carrying cardiac myocyte-specific expression of human GTPCH transgene. We hypothesized that increasing intracellular levels of BH4 by cardiac myocyte-targeted overexpression of GTPCH in mice would diminish acute cardiac allograft rejection.
METHODS
Cloning of the myosin GTPCH transgene and generation of transgenic mice.
The 0.7-kb human GTPCH cDNA under the control of a 935-bp fragment of the mouse myosin heavy chain gene promoter was kindly provided by Drs. Keith Channon and Nicholas Alp. Details of the development of cardiac-specific GTPCH transgenic mice such as cloning, construct development, and pronuclear injection of oocytes are provided in supplemental material (supplement Fig. 1; all supplemental material can be found with the online version of this article).
Transplant surgery model.
All experimental procedures and surgery were in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals. Specific protocols were approved by the local Institutional Animal Care and Use Committee. Donor hearts from wild-type and cardiac-specific GTPCH transgenic mice on a C57BL/6 background (H-2b) were transplanted heterotopically to BALB/c (H-2d) recipient mice as described (6) with the modification that excised donor hearts were placed in Aedesta organ and tissue medium (Cell Preservations Solutions, Columbia, SC).
Biochemical analysis.
Tissue BH4 and 7,8-dihydropterin (BH2) analysis were performed by high-performance liquid chromatography (HPLC) with electrochemical detection as described (26, 31). Samples were analyzed on an HPLC system (ESA Biosciences CoulArray system, Model 582 and 542) using an analytical Polar-RP column eluted with argon saturated 50 mM phosphate buffer (pH 2.6). NO was determined by the chemiluminescence assay of NOx (nitrate + nitrite) on a NO analyzer as previously described (7, 19). Blood was collected in heparin, and plasma was obtained immediately by centrifugation. An aliquot of plasma (∼200 μl) was deproteinized on a Centricon 10,000 MW cut-off filters at 12,000 rpm for 45 min at 4°C. Eluants (100 μl) were injected onto an analytical C18 column (Synergi HydroRP; Phenomenex) and isocratically eluted with 0.1% trifluoroacetic acid (1 ml/min). Neopterin and biopterin were detected by fluorescence using 350 nm excitation and 440 nm emission wavelengths. Standards were prepared in 10 mM sodium hydroxide and used at 10–500 nM concentration range.
Histological rejection scoring.
Formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin. Histological rejection was scored by two individuals and averaged using a modified six-point ISHLT linear scoring system to allow statistical analysis (20, 24). Scoring consisted of: 0, no rejection; 1, focal, perivascular, or interstitial infiltrate without necrosis; 2, diffuse, but sparse infiltrate; 3, a single focus of aggressive infiltrate and/or focal myocyte damage; 4, multifocal aggressive infiltrate and/or myocyte damage; 5, diffuse inflammatory process/necrosis; and 6, diffuse, aggressive polymorphic infiltrate with edema, hemorrhage, vasculitis, and necrosis.
Eosin autofluorescence.
Eosin autofluorescence of paraffin-embedded tissue sections stained with hematoxylin and eosin has been used as an early marker of myocardial injury due to ischemia (28). We used this technique to determine the extent of myocyte injury in acute rejection of wild-type and GTPCH transgenic allografts. Fluorescence was detected as described (11, 22). A total of 4 to 5 independent 400× fields were captured for each allograft. Fluorescence scores for each field were determined independently by two individuals, and the results were averaged for each allograft sample. Scoring was based on a score of 0 equal to that seen in nontransplanted hearts and a maximum score of 3 equal to the fluorescence of erythrocytes used as an internal control for each allograft sample.
Echocardiography.
Echocardiography was performed using a Vivid 7 electrocardiograph (General Electric, Waukesha, WI) and 11-MHz M12L linear array transducer as described (19). Images were analyzed using EchoPAC software with Q analysis (General Electric, Waukesha, WI).
PCR.
Total cellular RNA was isolated from graft biopsies, and complimentary DNA was synthesized from 1 µg of total RNA as described (19). Primers and conditions used for these analyses are summarized (supplemental Table 1). PCR reactions were performed in a 25-μl volume using GoTaq Green Master Mix (Promega, Madison, WI). The PCR product was resolved by 1% TAE-agarose gel electrophoresis. Densitometric analysis was performed using Alpha Imager (Alpha Innotech, San Leandro, CA). Additional studies on gene expression of alloimmune biomarkers of rejection were performed using real-time quantitative PCR as described (9). Primer sequences and conditions are described (supplemental Table 2). The relative mRNA level was determined as 2[(Ct/β-actin − CT/gene of interest)].
Statistical analysis.
Data were expressed as means ± SE. Statistical analysis was performed by one-way ANOVA with Student-Newman-Keuls test or Student's t-test for comparisons, where appropriate. Statistical significance was set at P < 0.05.
RESULTS
Characterization of mouse phenotype.
Cardiac myocyte-specific overexpression of GTPCH did not cause any apparent abnormality in breeding patterns, litter size, or general health of the animals. Cardiac morphology due to transgene expression appeared normal. Heart weight and heart weight-to-body weight ratios were not different between wild-type and transgenic mice (Fig. 1, A and B). Echocardiographic analysis at 1 mo of age revealed that GTPCH transgenic mice displayed similar LV mass (not shown) and normal LV function with no differences in ejection fraction or fractional shortening (Fig. 2, A and B). Additional echocardiographic analysis in mice without transplantation revealed no difference between GTPCH transgenic and wild-type mice for heart rate, LV end-systolic and end-diastolic volume, fractional shortening, ejection fraction, isovolumic contraction and relaxation time, myocardial performance index, and ejection time (supplemental Table 3).
Fig. 1.
Phenotype characterization of heart weight (A) and heart-to-body weight (B) ratios in wild-type (WT; n = 5) and GTP cyclohydrolase I (GTPCH) transgenic (Tg; n = 4) mice at 18 wk of age.
Fig. 2.
Phenotype characterization using conventional M-mode echocardiography reveals no differences in fractional shortening (A) or ejection fraction (B) in wild-type versus GTPCH transgenic mice.
Validation of functional GTPCH expression.
Expression of human GTPCH mRNA was confirmed in hearts from transgenic mice but not wild-type mice (Fig. 3). Expression of mouse cardiac GTPCH was unaltered by human transgene expression (Fig. 3). Western blot analysis revealed increased expression of GTPCH protein in transgenic versus wild-type mouse hearts (Fig. 4). HPLC analysis revealed significant (P < 0.001) increases in cardiac BH4 levels (Fig. 5A) in GTPCH transgenic mice versus wild-type mice, confirming that transgene expression resulted in increased GTPCH activity. BH4 levels in liver samples were unchanged (Fig. 5B), further attesting the site-specific increase in GTPCH activity due to the transgene expression. Plasma BH4 levels in wild-type and transgenic mice were below the detection limit. Plasma biopterin levels were not significantly different between the two groups (Fig. 5C). Plasma neopterin, a marker of inflammation, was not detected in either group.
Fig. 3.
Documentation of mRNA expression of human GTPCH (hGTPCH-1) in hearts of transgenic versus wild-type control mice and lack of alteration in mRNA expression of mouse GTPCH (mGTPCH-1) versus GAPDH housekeeping gene control.
Fig. 4.
Western blot analysis of human GTPCH protein expression levels in hearts of transgenic versus wild-type control mice.
Fig. 5.
A: increased cardiac tetrahydrobiopterin (BH4) levels but not liver (B) of GTPCH transgenic mice (n = 8) versus wild-type (n = 5). C: plasma biopterin levels were unaltered in GTPCH transgenic mice (n = 6 determinations each).
Cardiac BH4 levels in GTPCH transgenic mice remained unchanged and significantly (P < 0.001) elevated over a range of ages examined between 2 and 12 mo (not shown). Over the same period of time, cardiac BH4 levels in wild-type hearts decreased significantly (P < 0.0001) from 4.64 ± 0.45 (n = 6) to 0.84 ± 0.10 pmol/mg protein (n = 7). Increases in levels of BH2 were also seen in hearts of GTPCH transgenic mice with cardiac BH4-to-BH2 ratios in the range of 1.27 to 1.34, which was not significantly different over 2 and 12 mo of age (not shown).
Cardiac myocyte GTPCH overexpression inhibits cardiac graft rejection.
A total of 40 heart allograft transplantations were performed (n = 19, wild-type; and n = 21, GTPCH transgenic) under similar surgical procedures. The mean ischemic time was similar between wild-type and transgenic grafts (wild-type, 25.5 ± 1.1 min; and transgenic, 25.1 ± 0.5 min). Histological rejection was evaluated at postoperative day 6, which is the time that most grafts retain palpable contractile activity in this specific mouse strain combination model of graft rejection. Histological sections of both wild-type and transgenic mouse heart transplants displayed evidence of inflammatory cell infiltrate. However, the rejection score was significantly (P < 0.003) lower in transgenic versus wild-type allografts (Fig. 6).
Fig. 6.
Decrease in histological rejection score in cardiac allografts of GTPCH transgenic versus wild-type mice (n = 7 each).
As an additional marker of myocardial injury, we determined differences in eosin autofluorescence in tissue sections of transplanted hearts. Eosin autofluorescence was enhanced in cardiac myocytes of individual wild-type allografts (Fig. 7, C and D) compared with transgenic allografts (Fig. 7, E and F) and to nontransplanted hearts (Fig. 7, A and B). The average intensity of the eosin autofluorescence was significantly (P < 0.0006) decreased in allografts from transgenic versus wild-type mice (Fig. 8A). Furthermore, we found that the level of eosin autofluorescence was significantly (P < 0.001) correlated with histological rejection scores (Fig. 8B).
Fig. 7.
Examples showing enhanced eosin autofluorescence in tissue sections of (C and D) wild-type allograft hearts versus (A and B) nontransplanted hearts and (E and F) attenuation in GTPCH transgenic allograft hearts.
Fig. 8.
A: semiquantitative analysis showing decreased intensity of eosin autofluorescence in cardiac allografts of GTPCH transgenic versus wild-type mice (n = 7 each). B: analysis showing a positive relationship between increased eosin autofluorescence and degree of histological rejection.
Echocardiography.
LV mass was significantly increased in wild-type allografts versus nontransplanted wild-type control hearts (Fig. 9). In contrast, LV mass was not significantly increased in transgenic allografts versus nontransplanted, transgenic control hearts. However, there was no difference in function as determined by fractional shortening, ejection fraction, and LV internal diameter (in systole and diastole) between transgenic and wild-type allografts (supplemental Fig. 2).
Fig. 9.
Echocardiographic analysis reveals increased left ventricular (LV) mass in cardiac allografts of wild-type versus nontransplanted wild-type controls and attenuation in GTPCH transgenic allografts (n = 5 to 6, nontransplanted groups; n = 7 to 8, transplanted groups). Tx, transplants.
NO content, inflammatory cytokine, and rejection biomarker gene expression.
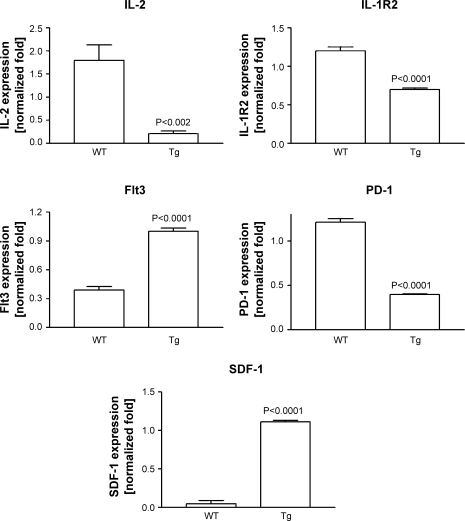
Cardiac NOx content was not different between wild-type and transgenic allografts (i.e., 0.507 ± 0.139 vs. 0.527 ± 0.125 nmol/mg protein, respectively; n = 6 each). Furthermore, the expression of inducible NOS was not different between wild-type and transgenic allografts (supplemental Fig. 3). Expression of inflammatory cytokines is known to be increased in acute cardiac rejection (20). However, the expression level of TNF-α, IFN-γ, IL-10, or IL-6 was not significantly different between wild-type and transgenic allografts (supplemental Fig. 3). In addition to the more classical measures of inflammatory cytokines, we also examined gene profilers of rejection recently determined from human transplant patients in the Cardiac Allograft Rejection Gene Expression Observational (CARGO) study (12, 13). Expression of mRNA for IL-2 was ∼1.8-fold in wild-type allografts but was significantly (P < 0.002) lower and near the limits of detection in transgenic allografts (Fig. 10). IL-1R2 expression was significantly (P < 0.0001) lower in transgenic versus wild-type allografts. Fms-like tyrosine kinase 3 (Flt3) and stromal-derived factor-1 (SDF-1) expression was significantly (P < 0.0001) higher and expression of programmed cell death-1 (PD-1) significantly (P < 0.0001) lower in transgenic versus wild-type allografts (Fig. 10).
Fig. 10.
Selective gene biomarker profiling for rejection showing differential gene expression in cardiac allografts of wild-type versus GTPCH transgenic mice (n = 3 to 4 each). Flt3, fms-like tyrosine kinase 3; PD-1, programmed cell death-1; SDF-1, stromal-derived factor-1.
DISCUSSION
The major finding of the study is that cardiac myocyte-specific expression of human GTPCH resulting in functional GTPCH enzymatic activity and production of BH4 was cardioprotective in a murine model of acute cardiac allograft rejection. This cardioprotective effect was manifested by decreased histological rejection, decreased eosin autofluorescence, decreased expression of rejection-specific biomarkers, and echocardiographic evidence of attenuation of increases in LV mass.
The beneficial effect of increased BH4 synthesis via genetic manipulation further supports the notion that BH4 and derivatives exert a cardioprotective effect in cardiac transplant rejection. The magnitude of the decreased rejection seen in the GTPCH transgenic mice was similar to that previously reported by our laboratory by exogenous treatment of rat cardiac allografts with sepiapterin, a substrate for BH4 synthesis (19).
The cardioprotective benefits of GTPCH overexpression appear to be independent of significant increases in NO production. This is in agreement with earlier observations that treatment with exogenous authentic BH4 or sepiapterin in mouse or rat heart transplant models did not increase tissue NO markers (2, 19). This could be explained by the finding that BH4-to-BH2 ratios do not follow the increased BH4 synthesis due to coincidental accumulation of BH2. In this context, NOS binds both BH4 and BH2 but only the former promotes NO synthesis. Previously, we found that treatment of isolated cardiac myocytes in vitro with BH4 or sepiapterin also caused significant accumulation in BH2 (7). The high BH2 concentrations could be explained by the absence of dihydrofolate reductase in adult but not neonatal cardiac myocytes. Cardiac-specific overexpression of the GTPCH transgenic mouse would circumvent the dependence of the dihydrofolate reductase gene in the salvage pathway of BH4 synthesis. However, the studies performed herein with the transgenic strategy now reveal the contribution of other intrinsic factor(s) regulating intracellular BH4-to-BH2 levels. Based on the known high rate of reaction of BH4 with heme protein (4), a plausible hypothesis is that the high heme protein concentration present in cardiac myocytes coupled with high rates of BH4 synthesis as a consequence to GTPCH overexpression may favor the formation of BH2 from BH4. Further studies will be needed to unravel this complex chemistry.
In humans cardiac graft rejection is associated with increased LV mass (8, 16). In the case of acute rejection, increases in LV mass likely result from alloimmune-induced increase in inflammatory cell infiltrate and the subsequent tissue injury. Indeed increases in LV mass correlate with serum markers of global cardiac myocyte damage in cardiac transplant patients (17). In our previous studies, the effects of exogenous sepiapterin to inhibit increases in LV mass could have been attributed to effects of BH4 acting on multiple cell types both circulating and fixed to the graft (19). Our finding of increased LV mass in allografts of wild-type mice but not transgenic mice, which displayed decreased rejection, support the notion that targeted increase in BH4 synthesis within cardiac myocytes can have significant cardioprotective effects on inhibiting LV remodeling in cardiac transplants. This agrees with the notion that the antihypertrophic effect of exogenous BH4 in the pressure-overloaded model was due to effects on fibroblasts or myocytes since LV mass was unaltered in endothelial cell GTPCH transgenic mice (15).
Our finding that indexes of fractional shortening, ejection fraction, and LV systolic and diastolic dysfunction were not useful to discriminate differences in rejection between wild-type versus transgenic cardiac allografts agrees with previous findings following sepiapterin treatment in our rat allograft recipients. Such conventional echocardiographic indexes of systolic function are challenging to interpret in the heterotopically transplanted heart due to the influences of mechanical unloading, which occurs even in the absence of alloimmune activation in isogeneic grafts (34). For this reason, LV mass is a more useful echocardiographic indicator of rejection in this model.
In cardiac transplant rejection, rejection scores typically take into consideration various factors including extent and pattern of inflammatory cell infiltration, myocyte damage, necrosis, edema, presence of hemorrhage, and vasculitis. In this context low histological scores are associated with cell infiltrate but without cardiac myocyte damage. As an independent measure of cardiac myocyte injury per se, we also examined eosin autofluorescence. Eosin autofluorescence of paraffin-embedded tissue sections has been used previously as an early marker of myocardial and renal ischemia-reperfusion injury that is not normally apparent upon examination under traditional light microscopy (11, 22, 28). In our rat model of cardiac allograft (G. M. Pieper and I. A. Ionova, unpublished observations), we found significant increases in eosin autofluorescence within cardiac myocytes of untreated allografts versus isograft controls or versus native hearts of allograft recipients, which was blocked by treatment with cyclosporine. Collectively, these observations suggest that enhanced eosin autofluorescence may be a useful marker of myocyte injury in cardiac transplant rejection. Indeed, in the present mouse heart transplant study, we found a significant correlation between the level of eosin autofluorescence and the histological rejection score. The additional finding that eosin autofluorescence was significantly lower in the GTPCH transgenic versus wild-type allografts is consistent with the notion of decreased myocardial injury. We cannot exclude the possibility that the decreased eosin autofluorescence seen in our transgenic allografts might arise from a decrease in post-transplant, ischemia-reperfusion injury, which is a potential risk factor to graft survival and/or rejection. However, differences in eosin autofluorescence cannot be explained by differences in ischemic times, which were similar between each donor group.
Inflammatory cytokines are increased in graft rejection although there is disagreement whether transcript levels correlate with graft rejection (3). Our finding that intragraft expression of several inflammatory cytokines were unaltered by GTPCH transgene indicates that these genes do not discriminate levels of rejection between the two groups in agreement with previous findings using a pharmacological treatment strategy of enhancing cardiac BH4 levels in mice and rats (2, 19). This was also true of IL-10, which we deemed important to examine in this model due to the Th2-dominant cytokine rejection pattern seen in C57BL/6 donor to BALB/c recipient mouse cardiac allograft rejection (29).
Recently, gene profiling via AlloMap analysis of blood samples from human cardiac transplant recipients identified 11 new candidate genes, which either increase or decrease with future rejection (12, 13). In the present study, we examined a few of the most predictive candidate markers including IL-1R2, Flt3, and PD-1. Although AlloMap gene profiler biomarkers of human cardiac rejection were determined on peripheral blood samples, we utilized similar gene profiling of intragraft samples to potentially discriminate rejection between our two experimental groups. This approach was taken considering that in the present study model cardiac myocyte-targeted GTPCH expression results in cell-specific production of increased BH4, and we wanted to determine the influence of BH4 within cardiac myocytes on intragraft gene expression as a mechanism of inhibition of graft rejection. Analysis of intragraft biomarkers was made possible by studies showing that both intragraft and splenic expression correlated with rejection in rat cardiac allografts (9).
Decreased blood expression of IL-1R2 was the strongest predictor of human cardiac graft rejection. Interestingly, we found lower intragraft IL-1R2 expression in GTPCH transgenic allografts versus wild-type controls. A reason that this was not predictive of rejection due to transgene expression in this experimental model is unknown. One possibility is that IL-1R2 is a corticosteroid-responsive gene that would be sensitive as a biomarker of rejection in human transplant patients, which received steroids in the normal course of immune suppression but would not necessarily be a good biomarker because of lack of steroid exposure in the present experimental mouse transplant model. A second possibility could relate to differences in intragraft versus peripheral blood expression in the case of the human markers. In this context and consistent with our present findings, increased intragraft expression of IL-1R2 was shown to differentiate rejection from nonrejection in rat cardiac allografts (9). These findings are consistent with information showing that immunosuppression using cyclosporine also inhibits the increased expression of intragraft IL-1R2 (14).
Flt3 is expressed in immature hematopoietic cells and is important for development of multipotent stem cells and B-cells. A low expression of Flt3 was moderately predictive of cardiac rejection in humans (13). Consistent with this notion, we found that intragraft expression of Flt3 was significantly lower in wild-type allografts compared with GTPCH transgenic allografts. These findings suggest an enhanced role of hematopoietic cells in the etiology of the protective effects of GTPCH overexpression in cardiac allografts.
PD-1 is normally expressed at low levels in T and B cells and is induced upon T cell activation. Intragraft expression of PD-1 is absent in normal hearts but is increased in untreated mouse heart allografts reflecting enhanced T cell activation within the graft (18). Furthermore, the role of alloimmune activation in increased intragraft expression of PD-1 was confirmed by its inhibition using rapamycin or cyclosporine treatment (18). Independently, immunosuppression with either cyclosporine, tacrolimus, or sirolimus each was shown to suppress intragraft expression of PD-1 in experimental cardiac allograft rejection (14). The role of PD-1 in rejection is considered important since blockade of PD-1 action with fusion protein for PD-1 ligand diminished histological rejection and prolonged graft survival (18). Collectively, these findings are consistent with increased PD-1 expression as a predictor of human graft rejection (9, 13). In this context, we found significantly higher intragraft expression of PD-1 in wild-type versus GTPCH transgenic mice. These results are consistent with the decreased alloimmune-induced rejection seen in GTPCH transgenic mice as a potentially new mechanism by which GTPCH overexpression may be cardioprotective.
SDF-1 (also known as CXCL12) is a chemokine that acts via its receptor, CXC receptor 4, to recruit hematopoietic stem cells. SDF-1 expression in cardiac myocytes is increased following ischemia-reperfusion injury (10). This recruitment may serve as an adaptive response to repair damaged myocardium. Indeed, treatment with mesenchymal stromal cells improves cardiac function and decreases infarct size following myocardial ischemia (10, 27). Likewise under certain conditions, hematopoietic stem cells prolong cardiac allograft survival (32). Our finding of decreased rejection and significantly increased expression of SDF-1 in GTPCH transgenic allografts versus low expression of SDF-1 in wild-type allografts is consistent with other findings that overexpression of SDF-1 increased cardiac allograft survival in a unique rat model of cardiac transplantation with a superimposed coronary artery occlusion performed shortly after transplantation (33).
Since ischemic times before revascularization of grafts were comparable between our wild-type and transgenic mouse donor hearts, we conclude that a difference in ischemia alone cannot account as a differential signal for the altered post-transplant expression of SDF-1 in the transgenic allografts. Nevertheless, our novel findings support a potentially new mechanism by which localizing the increased synthesis of BH4 to the cardiac myocyte can be cardioprotective by increasing chemokine production signaling for progenitor cell recruitment.
Finally, IL-2 is a growth factor for T cell proliferation and expansion. Our finding of significant increases in IL-2 expression in allografts from wild-type mice but low expression near detection limits in allografts of GTPCH transgenic mice is consistent with decreased rejection in this group and supports a role of decreased T cell function. This finding complements the findings of decreased IL-2 expression by treatment of rat cardiac allograft recipients with exogenous sepiapterin (19). This effect of GTPCH transgene expression distinguishes the mechanism of antirejection from classical immunosuppressant drugs such as cyclosporine, which impact both T cell activation as well as T cell proliferation. In this regard, it is well known that cyclosporine blocks the increase in IL-2 as well as inflammatory cytokines such as the T cell activator IFN-γ. However, the antirejection due to GTPCH transgene shares properties with cyclosporine in reducing intragraft IL-1R2 and PD-1.
It is interesting however, that decreased IL-2 expression occurred in the transgenic allografts given the cardiac myocyte-specific expression of human GTPCH transgene to increased GTPCH activity and increased BH4 synthesis within cardiac myocytes rather than from exogenous precursors. The observation of decreased SDF-1 in GTPCH transgenic allografts versus wild-type allografts is a significant and important finding in understanding the mechanism by which GTPCH transgene decreased allograft rejection. Thus mesenchymal stem cells, which may be recruited by the dramatically increased SDF-1 expression in our transgenic allografts, may be a key factor to elicit immune suppression that preferentially effects T cell proliferation rather than T cell activation (23). In this context, it is known that T cells stimulated in the presence of mesenchymal stromal cells lock T cells into the G0/G1 phase of the cell cycle (23). Therefore, the mechanism of decreased rejection in GTPCH transgenic allografts can be explained by SDF-1-mediated downregulation of immune function. This impacts T cell proliferation rather than T cell activation. The effect on the former process is a mechanism to explain why inflammatory cytokine markers especially IFN-γ remain elevated but IL-2, a marker of T cell proliferation, is significantly decreased in GTPCH transgenic allografts.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-079837.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The assistance of Chao-Ying Chen for the cardiac transplant surgeries is greatly appreciated. We also thank Dr. Harmut Weiler, the director of the Medical College of Wisconsin transgenic core laboratory, and staff for services in the pronuclear microinjections of oocytes for the development of transgenic mice for this study.
REFERENCES
- 1.Anastasiadis PZ, States JC, Imerman BA, Louie MC, Kuhn DM, Levine RA. Mitogenic effects of tetrahydrobiopterin in PC12 cells. Mol Pharmacol 49: 149–155, 1996 [PubMed] [Google Scholar]
- 2.Brandacher G, Maglione M, Schneeberger S, OBrist P, Thoeni G, Wrulich OA, Werner-Felmayer G, Margreiter R, Werner ER. Tetrahydrobiopterin compounds prolong allograft survival independently of their effect on nitric oxide synthase activity. Transplantation 81: 583–589, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Breinholt JP, Vallejo JG, Gates CM, Clunie SK, Kearney DL, Dreyer WJ, Towbin JA, Bowles NE. Myocardial pro-inflammatory cytokine expression and cellular rejection in pediatric heart transplant recipients. J Heart Lung Transplant 27: 317–324, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Capeillere-Blandin C, Mathieu D, Mansuy D. Reduction of ferric haemoproteins by tetrahydropterins: a kinetic study. Biochem J 392: 583–587, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elzaouk L, Laufs S, Heerklotz D, Leimbacher W, Blau N, Résibois A, Thöny B. Nuclear localization of tetrahydrobiopterin biosynthetic enzymes. Biochim Biophys Acta 1670: 56–68, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa T, Visovatti SH, Hyman MC, Hayasaki T, Pinsky DJ. Heterotopic vascularized murine cardiac transplantation to study graft arteriopathy. Nat Protoc 2: 471–480, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ionova IA, Vasquez-Vivar J, Whitsett J, Herrnreiter A, Medhora M, Cooley BC, Pieper GM. Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am J Physiol Heart Circ Physiol 295: H2178–H2187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawauchi M, Boucek MM, Gundry SR, Kanakriyeh MR, de Begona JA, Razzouk AJ, Bailey LL. Changes in left ventricular mass with rejection after heart transplantation in infants. J Heart Lung Transplant 11: 99–102, 1992 [PubMed] [Google Scholar]
- 9.Khanna AK, Xu J, Uber PA, Burke AP, Baquet C, Mehra MR. Tobacco smoke exposure in either the donor or recipient before transplantation accelerates cardiac allograft rejection, vascular inflammation, and graft loss. Circulation 120: 1814–1821, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Li N, Lu X, Zhao X, Xiang FL, Xenocostas A, Karmazyn M, Feng Q. Endothelial nitric oxide synthase promotes bone marrow stromal cell migration to the ischemic myocardium via upregulation of stromal cell-derived factor-1α. Stem Cells 27: 961–970, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Liang HL, Hilton G, Mortensen J, Regner K, Johnson CP, Nilakantan V. MnTmPyP, a cell-permeable SOD mimetic, reduces oxidative stress and apoptosis following renal ischemia-reperfusion. Am J Physiol Renal Physiol 296: F266–F276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehra MR, Kobashigawa JA, Deng MC, Fang KC, Klingler TM, Lal PG, Rosenberg S, Uber PA, Starling RC, Murali S, Pauly DF, Dedrick R, Walker MG, Zeevi A, Eisen HJ, CARGO investigators Clinical implications and longitudinal alteration of peripheral blood transcriptional signals indicative of future cardiac allograft rejection. J Heart Lung Transplant 27: 297–230, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Mehra MR, Kobashigawa JA, Deng MC, Fang KC, Klingler TM, Lal PG, Rosenberg S, Uber PA, Starling RC, Murali S, Pauly DF, Dedrick R, Walker MG, Zeevi A, Eisen HJ, CARGO investigators Transcriptional signals of T-cell and corticosteroid-sensitive genes are associated with future acute cellular rejection in cardiac allografts. J Heart Lung Transplant 26: 1255–1263, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Mehra MR, Xu J, Uber PA, Baquet C, Khanna A. Intragraft authentication of peripheral transcriptomic molecular biomarkers for cardiac allograft rejection (Abstract). J Heart Lung Transplant 28: S226, 2009 [Google Scholar]
- 15.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cromaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC, Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin. Circulation 117: 2626–2636, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondillo S, Maccherini M, Galderisi M. Usefulness and limitations of transthoracic echocardiography in heart transplantation recipients. Cardiovasc Ultrasound 6: 2, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran AM, Lipshultz SE, Rifai N, O'Brien P, Mooney H, Perry S, Perez-Atayde A, Lipsitz SR, Colan SD. Non-invasive assessment of rejection in pediatric transplant patients: serologic and echocardiographic prediction of biopsy-proven myocardial rejection. J Heart Lung Transplant 19: 756–764, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Özkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O'Keefe TO, Duong T, Smith T, Gutierrez-Ramos JC, Rottman JB, Coyle AJ, Hancock WW. Programmed death-1 targeting can promote allograft survival. J Immunol 169: 6546–6553, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Pieper GM, Ionova IA, Cooley BC, Migrino RQ, Khanna AK, Whitsett J, Vásquez-Vivar J. Sepiapterin decreases acute rejection and apoptosis in cardiac transplants independently of changes in nitric oxide and inducible nitric-oxide synthase dimerization. J Pharmacol Exp Thera 329: 890–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieper GM, Nilakantan V, Hilton G, Zhou X, Khanna AK, Halligan NLN, Felix CC, Kampalath B, Griffith OW, Hayward MA, Roza AM, Adams MB. Variable efficacy of N6-(1-iminoethyl)-L-lysine in acute cardiac transplant rejection. Am J Physiol Heart Circ Physiol 286: H525–H534, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Pieper GM, Nilakantan V, Halligan NLN, Khanna AK, Hilton G, Vásquez-Vivar J. Nitric oxide formation in acutely rejecting cardiac allografts correlates with GTP cyclohydrolase activity. Biochem J 391: 541–547, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regner KR, Zuk A, Van Why SK, Shames BD, Ryan RP, Falck JR, Manthati VL, McMullen ME, Ledbetter SR, Roman RJ. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int 75: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel G, Schäfer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation 87: S45–S49, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Szabolcs MJ, Sun J, Ma N, Albala A, Sciacca RR, Philips GB, Parkinson J, Edwards N, Cannon PJ. Effects of selective inhibitors of nitric oxide synthase-2 dimerization on acute cardiac allograft rejection. Circulation 106: 2392–2396, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347: 1–16, 2000 [PMC free article] [PubMed] [Google Scholar]
- 26.Vasquez-Vivar J, Whitsett J, Ionova I, Konorev E, Zielonka J, Kalyanaraman B, Shi Y, Pieper GM. Cytokines and lipopolysaccharide induce inducible nitric oxide synthase but not enzyme activity in adult rat cardiomyocytes. Free Radic Biol Med 45: 994–1001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veldkamp CT, Ziarek JJ, Su J, Basnet H, Lennertz R, Weiner JJ, Peterson FC, Baker JE, Volkman BF. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci 18: 1359–1369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Overbeck J, Saraga P, Gardiol D. An autofluorescence method for the diagnosis of early ischaemic myocardial lesions. A systematic study of 732 autopsies, including 182 cases of sudden death. Virchows Arch A Pathol Anat Histopathol 409: 535–542, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Hosiawa KA, Min W, Yang J, Zhang X, Garcia B, Ichim TE, Zhou D, Lian D, Kelvin DJ, Zhong R. Cytokines regulate the pattern of rejection and susceptibility to cyclosporine therapy in different mouse recipient strains after cardiac allografting. J Immunol 171: 3823–3836, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Watschinger K, Keller MA, Hermetter A, Golderer G, Werner-Felmeyer G, Werner ER. Glyceryl ether monooxygenase resembles aromatic amino acid hydoxylases in metal ion and tetrahydrobiopterin dependence. Biol Chem 390: 3–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol 27: 2340–2347, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Jiao C, Zhao S. Role of mesenchymal stem cells in immunological rejection of organ transplantation. Stem Cell Rev 5: 402–409, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Zhao T, Zhang D, Millard RW, Ashraf M, Wang Y. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1α delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol 296: H976–H986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou YW, Bishay R, Feintuch A, Tao K, Golding F, Zhu W, West LJ, Henkelman RM. Morphological and functional evaluation of murine heterotopic cardiac grafts using ultrasound biomicroscopy. Ultrasound Med Biol 33: 870–879, 2007 [DOI] [PubMed] [Google Scholar]