Abstract
Thoracic aortic aneurysms (TAAs) develop as a result of dysregulated extracellular matrix remodeling mediated by several matrix metalloproteinases (MMPs). Membrane type-1 MMP (MT1-MMP) is the prototypical member of a unique family of membrane-bound MMPs, possessing multiple substrates and functions. The present study tested the hypothesis that MT1-MMP expression, abundance, and activity would be elevated during TAA development and that this protease is produced primarily by mesenchymal cells within the thoracic aorta. Descending thoracic aortas were harvested from C57BL/6J mice at multiple time points (2, 4, 8, and 16 wk, n = 15 each) post-TAA induction (0.5M CaCl2, 15 min) and compared with reference controls (n = 15). The expression and abundance of MT1-MMP, MMP-2, and tissue inhibitor of metalloproteinase (TIMP)-2 were assessed by quantitative PCR and immunoblot analysis. MT1-MMP activity was determined by fluorescent peptide assay. MT1-MMP was localized within the aortic wall by immunohistochemistry. MT1-MMP abundance and localization in live animals (8 wk post-TAA induction vs. control) was determined by microultrasound imaging with an MT1-MMP-targeted microbubble contrast agent. Aortic diameter was increased 172 ± 7% at 16 wk post-TAA induction (P < 0.05). MT1-MMP and MMP-2 mRNA levels were elevated at 2 wk post-TAA induction (P < 0.05). MT1-MMP protein abundance increased progressively to a maximum of 178 ± 26% at 16 wk post-TAA induction, whereas MMP-2 and TIMP-2 peaked at 2 wk post-TAA induction (526 ± 93% and 376 ± 48%, respectively, P < 0.05). MT1-MMP colocalized with fibroblasts, and MT1-MMP-targeted contrast binding was elevated in 8-wk TAA-induced mice versus control mice (217 ± 53% vs. 81 ± 8%, P < 0.05). In conclusion, these novel results suggest that MT1-MMP plays a dynamic multifunctional role in TAA development and, therefore, may provide a significant target for therapeutic strategies.
Keywords: fibroblast, vascular remodeling, extracellular matrix, microbubble contrast
thoracic aortic aneurysms (TAAs) carry significant morbidity and mortality (5, 7, 9) and form through dynamic remodeling of the aortic extracellular matrix (ECM), resulting in the disruption of elastin filaments and disordered collagen deposition (7). The primary mediators of this structural degradation and reorganization belong to a single family of zinc-dependent endopeptidases: matrix metalloproteinases (MMPs) (3). Numerous studies (10, 13, 24, 34) have demonstrated with both human specimens and animal models that the gelatinase subclass (MMP-2 and MMP-9) displays elevated production and activity in both abdominal and thoracic aneurysm disease. In a-well described animal model of abdominal aortic aneurysm (AAA), macrophage-derived MMP-9 was found to work in concert with mesenchymal cell-derived MMP-2 to induce aortic dilatation (24). Using a similar model in the thoracic aorta, a previous study (19) from this laboratory reported elevated MMP-9 levels localized to mesenchymal cells in the absence of inflammatory cell infiltration. These data suggested that there may be inherent differences between the thoracic and abdominal aorta that contribute to the unique mechanisms that drive aortic dilatation (31).
Studies (1, 15, 28, 40) examining human aneurysm specimens from both AAAs and TAAs have demonstrated increased abundance of a unique membrane-bound MMP, membrane-type 1 MMP (MT1-MMP), suggesting that this enzyme may play a significant role in aneurysm development. MT1-MMP is a class I transmembrane protein that has the potential to contribute to aneurysm development in three ways (18). First, MT1-MMP plays an essential role in the activation of MMP-2. Pro-MMP-2 is secreted into the extracellular space as an inactive propeptide, where it is activated by removal of the pro-domain through proteolytic cleavage mediated by an activational complex consisting of pro-MMP-2, tissue inhibitor of metalloproteinase (TIMP)-2, and MT1-MMP (16, 29). It has been recently reported that AAA growth is attenuated in TIMP-2 knockout mice, suggesting that MT1-MMP-dependent activation of pro-MMP-2 is essential during aneurysm development (39). Second, like other extracellular proteases, MT1-MMP possesses gelatinolytic and collagenolytic activity that can directly degrade the structural ECM (18). Finally, in addition to structural ECM proteins, MT1-MMP can also release ECM-sequestered matrikines, cytokines, and growth factors that can further act to drive ECM remodeling (18, 27). Thus, MT1-MMP sits at the crossroads of multiple pathways capable of modifying the aortic ECM and, therefore, may provide an important target for therapeutic intervention. The present study tested the hypothesis that MT1-MMP expression, abundance, and activity would be elevated during TAA development and that this protease would be produced primarily by mesenchymal cells within the thoracic aorta.
METHODS
Experimental design.
TAAs were induced in approximately equal numbers of male and female (8–12 wk old) C57BL/6J mice, which were then randomly assigned for terminal study at 2, 4, 8, or 16 wk post-TAA induction (n = 15 mice at 2 and 8 wk postinduction and 18 mice at 4 and 16 wk postinduction). Aortic diameter was measured at the time of TAA induction as well as at terminal surgery, and aneurysm tissue was harvested for quantitative real-time PCR, immunoblot/activity assays, or immunohistochemistry. Results from aneurysm-induced animals were compared with results obtained from unoperated reference control mice (n = 18), which were processed in an identical fashion. No differences in aortic diameter were observed between unoperated control animals and saline-induced sham-operated TAA animals (102 ± 4%, 4 wk after saline exposure vs. unoperated controls, P = 0.5710). All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Surgical TAA induction procedure.
Murine TAAs were induced as previously described (14). Briefly, after anesthetic induction, mice were intubated, and a surgical plane of anesthesia was maintained by nose cone with a 2% isoflurane-oxygen mixture. The descending thoracic aorta was exposed through a left thoracotomy, and a sponge soaked in 0.5 M CaCl2 was placed in direct contact with the periadventitial surface for 15 min. The chest was irrigated with normal saline and closed in layers, and the mice were then allowed to recover. Operative mortality for this procedure was ∼20%.
Aortic diameter measurements.
Aortic diameter was measured for each mouse at the time of induction (baseline) and terminal surgery. Aortic dimensions were captured by video micrometry using a 3-megapixel color charge-coupled device camera (PAXcam3, MIS, Villa Park, IL) linked to a laptop computer running PAX-it image management software with the live measurement module (MIS). A digital caliper was used to measure the aortic outer diameter. Terminal aortic size was expressed as the percent change from the respective baseline measurement.
Terminal surgery and tissue harvest.
At time of terminal surgery, the descending thoracic aorta was reexposed, and terminal aortic outer diameter measurements were recorded. The isoflurane mixture was then increased to 5%, and mice were euthanized under deep anesthesia by exsanguination induced by a right atriotomy. The descending thoracic aorta was then excised. Tissue designated for quantitative real-time PCR was stored in RNAlater for 24–48 h, subsequently snap frozen, and stored at −80°C (n = 5 for each time point). Tissue used for immunoblot analysis was immediately snap frozen and stored at −80°C (n = 10 for each time point). Additionally, tissue designated for immunohistochemistry was harvested from control and 16-wk TAA-induced mice after perfusion fixation as previously described (20). The descending thoracic aorta was then carefully excised and embedded in paraffin. Every 10th section was stained with hematoxylin and eosin to verify the site of TAA induction. All subsequent sections used for immunohistochemical analysis were located within the CaCl2-treated region of the thoracic aorta.
Quantitative real-time PCR.
Aortic specimens were homogenized in Buffer RLT (QIAGEN, Valencia, CA) using a Qiagen Tissuelyser (Qiagen) bead-mill homogenizer. Total RNA was extracted using the RNeasy Fibrous Tissue MiniKit (Qiagen) and reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR was performed using RT2 Real Time SYBR green/fluorescein PCR master mix (catalog no. PA-011, SuperArray Biosciences, Fredrick, MD) with primers for murine MT1-MMP, MMP-2, and TIMP-2 (catalog nos. PPM03642B, PPM03614B, and PPM03617C, respectively, SuperArray Biosciences). Primers for glucuronidase-β, hypoxanthine phosphoribosyltransferase 1, and heat shock protein 1 (Hsp27) were used as internal standards (catalog nos. PPM05490B, PPM03559E, and PPM02936B, respectively, SuperArray Biosciences). The initial denaturing cycle consisted of 10 min at 95°C followed by 45 cycles of 30 s at 95°C and 1 min at 60°C. Cycle threshold (Ct) values were recorded, and the fold expression of the target genes was calculated by the ΔΔCt method using mean Ct values of the internal standards and expression of the reference control aortas as baseline.
Immunoblot analysis.
Aortic tissue was thawed and transferred to cold extraction/homogenization buffer [buffer volume: 1:6 (wt/vol)] containing 10 mM cacodylic acid (pH 5.0), 0.15 M NaCl, 10 mM ZnCl2, 1.5 mM NaN3, and 0.01% Triton X-100 (vol/vol) and homogenized using the Qiagen Tissuelyser (Qiagen). The homogenate was centrifuged (800 g, 10 min, 4°C), and 10 μg of the supernatant were loaded on a 4–12% bis-Tris gradient gel and fractionated by electrophoresis. Proteins were then transferred to nitrocellulose membranes (0.45 μm, Bio-Rad) and incubated in antiserum corresponding to MMP-2 [0.4 μg/ml, catalog no. IM33, EMD Biosciences (Calbiochem), La Jolla, CA], MT1-MMP (0.4 μg/ml, catalog no. AB815, Millipore Biosciences, Temecula, CA), and TIMP-2 (0.5 μg/ml, catalog no. AB38973, Abcam, Cambridge, MA). Antisera were diluted in 5% nonfat dry milk-PBS. The secondary peroxidase-conjugated antibody was then applied (1:5,000, 5% nonfat dry milk-PBS), and signals were detected with a chemiluminescent substrate (Western Lighting Chemiluminescence Reagent Plus, Perkin-Elmer). The film was developed in a Konica SRX-101A medical film processor, and band intensity was quantified using Gel-Pro Analyzer software (version 3.1.14, Media Cybernetics, Silver Spring, MD) and reported as the percent change from the unoperated reference control homogenates.
MT1-MMP quenched fluorogenic peptide activity assay.
MT1-MMP activity was determined using an MT1-MMP-specific quenched fluorogenic peptide assay. Briefly, equal amounts of each aortic homogenate (15 μg) from normal and TAA-induced mice were incubated for 60 min at 37°C with a quenched fluorogenic peptide that contains an MT1-MMP-specific cleavage site [MCA-Pro-Leu-Ala-Cys(p-OMeBz)-Trp-Ala-Arg(Dpa)-NH2, EMD Biosciences]. Upon cleavage of the peptide by active MT1-MMP, the quenching group becomes sufficiently separated from the fluorescent group, allowing a fluorescent signal to be emitted. Fluorescence was measured and recorded on a fluorescent microplate reader (Fluorostar Galaxy BMG Labtechnologies, Cary, NC) and compared with a standard curve using active recombinant MT1-MMP. Results were normalized to the amount of GAPDH present in each sample, as determined by ELISA assay (catalog no. 3401, Bioo Scientific, Austin, TX).
Immunohistochemistry and cellular colocalization.
Three independent tissue sections (3 μm), ∼50 μm apart, collected from control or TAA-induced animals were analyzed by immunohistochemical staining for MT1-MMP and one cell type-specific marker for fibroblasts, discoidin domain receptor 2 (DDR2). Aortic tissue sections were deparaffinized and rehydrated with deionized water. Dako citrate antigen retrieval (catalog no. S1699; Dako North America, Carpinteria, CA) was performed for 30 min at 99°C, the tissue was cooled to room temperature, and the slides were subsequently washed with water followed by 0.025% Triton X-100 in Tris-buffered saline (TBS). BSA (3%)-TBS was applied for 2 h to block the tissue, and it was then incubated with rabbit polyclonal antibody to MT1-MMP (1:250, catalog no. ab38971, Abcam) or goat polyclonal antibody to DDR2 (1:100, catalog no. sc-7555, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. After incubation, tissue sections were washed with 0.025% Triton X-100 in TBS and then incubated in 0.3% H2O2 in TBS for 30 min to block endogenous peroxidase activity. Tissue sections were then incubated with peroxidase-conjugated goat anti-rabbit antibody (1:250, catalog no. 6101, Vector Labs, Burlingame, CA) or peroxidase-conjugated rabbit anti-goat antibody (1:250, catalog no. BA-5000, Vector Labs) to identify positive MT1-MMP and DDR2 antibody binding, respectively. Sections were washed again with 0.025% Triton X-100 in TBS and then incubated with DAB reagent for 30 s (catalog no. SK-4100, Vector Labs). Sections were then dehydrated, cleared, coverslipped, and analyzed by light microscopy.
To determine the colocalization of MT1-MMP and DDR2, tissue sections were blocked with 3% BSA-TBS and first incubated overnight with the goat polyclonal antibody to DDR2 (1:100, catalog no. SC7555, Santa Cruz Biotechnology). Sections were then washed and incubated with peroxidase-conjugated rabbit anti-goat antibody (1:250, catalog no. BA-5000, Vector Labs) as described above. After a 30-min incubation, slides were developed in DAB reagent for 90 s. Tissue sections were then blocked again in 3% BSA-TBS for 1 h followed by a second overnight incubation with the rabbit polyclonal antibody for MT1-MMP (1:250, catalog no. AB815, Millipore Biosciences). After being washed as described above, tissue sections were incubated with alkaline phosphatase-conjugated goat anti-rabbit antibody (1:250, ABC-AP reagent, catalog no. AK-5001, Vector Labs) for 30 min and visualized using the Vector blue chromagen (catalog no. SK-5300, Vector Labs). Tissue sections were then dehydrated, cleared, coverslipped, and analyzed by light microscopy.
Multiple tissue sections from control and 16-wk TAA-induced mice (8 sections each, separated by at least 50 μm) were immunostained with MT1-MMP antibody. Digital images were recorded with a Zeiss AxioCam MRc mounted on a Zeiss Axioskop2 using a ×63 oil objective. Multiple high-power fields (control: n = 27 and 16 wk post-TAA induction: n = 51) were subjected to analysis by two methods: 1) cells were counted and expressed per square millimeter of tissue area and 2) positive MT1-MMP staining was analyzed using classical stereological techniques.
MT1-MMP-targeted microbubble contrast imaging.
Targeted microbubble contrast imaging was performed to assess MT1-MMP protein binding in control (n = 6) and TAA-induced (8 wk postinduction, n = 3) mice in real time by high-resolution microultrasound. Mice were anesthetized with 3–5% isoflurane vapor in a closed chamber. Mice were then placed ventrally on a thermostatic platform (37°C), and anesthesia was maintained by delivering 1.5–2.0% isoflurane through a nose cone. The hair over the thorax was depilated, and a layer of ultrasound gel was applied to facilitate imaging. The descending thoracic aorta was identified by two-dimensional ultrasound using a 40-MHz scanning head with a spatial resolution of 30 μm (Vevo 770 System, VisualSonics, Toronto, ON, Canada). Biotinylated MT1-MMP antibody (custom rabbit polyclonal, Thermo Scientific Open Biosystems, Huntsville, AL) was conjugated to the streptavidin-coated microbubble contrast agent (Vevo MicroMarker Target-Ready Contrast Agent Kit, VisualSonics). A 27-gauge butterfly needle attached to polyethylene-10 tubing was then inserted in the lateral tail vein and used to inject 70 μl of the MT1-MMP antibody-conjugated microbubbles over 2 s. This was immediately followed by 50 μl of saline to flush the catheter. In each animal, baseline cineloops were captured before the injection, during the injection (including the first pass of the targeted microbubbles through the descending thoracic aorta), and then at 5-min intervals through 15 min after the injection. Using Vevo 770 software, the baseline (preinjection) cineloop from each animal was processed as a reference to which all other cineloops from the same animal were compared by digital subtraction to identify targeted microbubble contrast binding and localization. MT1-MMP-targeted contrast microbubble binding was then quantified within a region of interest drawn at the site of TAA induction. Results were corrected for region volume between animals and normalized for the total injected microbubble dose, as determined by contrast microbubble binding within a region of interest drawn at a nontreated site of the aorta.
Data analysis.
Target gene fold expression versus baseline unoperated control aortas was determined by the ΔΔCt method, and significant differences were identified by a twofold increase in gene expression or detected by a two-tailed Student's t-test comparing ΔCt values. Stata statistical software (Intercooled version 8.2, College Station, TX) was used for all calculations, and significance was defined as P < 0.05. To assess statistical changes in data generated as percent changes from baseline or control (analysis of aortic diameter and quantitative immunoblot analysis), one-sample mean comparison tests were used to compare the percent change versus a fixed value of 100%. Comparisons between treatment groups were done using one-way ANOVA with a post hoc Tukey's wholly significant difference test for separation of means. All values are reported as means ± SE. The time-dependent change in aortic diameter was determined by calculating the difference in aortic diameter in each mouse from the mean aortic diameter determined at the previous time point. Pairwise regression analysis with a linear model was then used to compare MT1-MMP activity to the time-dependent change in aortic diameter. In addition, a linear mixed model was used to verify significant predictors of MT1-MMP activity. MT1-MMP-targeted microbubble contrast binding was expressed as the percent change in binding from the initial 5-min postinjection time point, and statistical significance was determined by Mann-Whitney rank sum analysis between groups at each time point. The relationship between MT1-MMP-targeted microbubble contrast binding and the change in aortic diameter was compared using Spearman's rank correlation. The amount of MT1-MMP cellular immunostaining was quantitated from high-powered microscopic fields in control (n = 27) and 16-wk TAA-induced (n = 51) tissue sections. The number of positively stained cells on each section was determined by direct counting. The area (in μm2) of tissue analyzed was determined using SigmaScan Pro version 5.0 software, and the number of cells per square millimeter was reported. In addition, as a second measure of MT1-MMP-specific staining, positive staining on each tissue section was determined by established stereological methods (36, 38). Briefly, a 10 × 10 grid was placed over a high-powered microscopic image and scored positive when an intersection point fell on a region of increased cellular staining. MT1-MMP immunostaining was then expressed as the percent change from control, and statistical significance was determined by Mann-Whitney rank sum analysis.
RESULTS
Aortic dilatation and TAA formation.
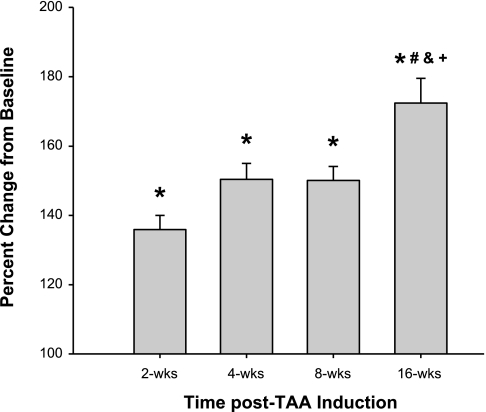
After the periadventitial application of CaCl2, the descending thoracic aortic diameter was increased from baseline at all time points after the TAA induction surgery. At 4 wk post-TAA induction, aortic diameter increased 50 ± 5% over baseline, reaching a true aneurysm by definition. Aortic dilation appeared to plateau between 4 and 8 wk post-TAA induction and then continued to dilate between 8 and 16 wk post-TAA induction, reaching a maximum diameter increase of 72 ± 7% with no mortality due to aneurysm rupture (Fig. 1).
Fig. 1.
Change in aortic diameter over time after thoracic aortic aneurysm (TAA) induction. Aortic diameter was determined at baseline and terminal surgery in each animal using digital micrometry. Aortic diameter values were expressed as percent changes from baseline (100%) in each animal. After TAA induction, aortic diameter was elevated at all time points (*P < 0.001 vs. reference control), and 16-wk values were significantly greater than those at the other time points (#P < 0.05 vs. 2 wk, &P < 0.05 vs. 4 wk, and +P < 0.05 vs. 8 wk).
TAA-dependent changes in gene expression.
To establish the expression levels MT1-MMP during TAA development, aortic tissue was harvested at each time point after TAA induction, and the gene expression was measured by quantitative real-time PCR. MT1-MMP mRNA levels were elevated by approximately fivefold at 2 wk post-TAA induction but were not different from control at the other time points. Additionally, mRNA expression levels of the pro-MMP-2 activational complex members (MMP-2 and TIMP-2) were also determined. The data revealed elevated MMP-2 mRNA at 2 and 4 wk post-TAA induction, whereas the levels of TIMP-2 mRNA were not significantly altered at any time point (Table 1).
Table 1.
Fold increase in aortic gene expression in TAA induction compared with reference control
| Time After TAA Induction |
||||
|---|---|---|---|---|
| Gene | 2 wk | 4 wk | 8 wk | 16 wk |
| Mmp14 | 5.53* | 1.30 | 1.38 | 1.07 |
| Mmp2 | 4.80* | 1.66* | 1.58 | 1.40 |
| Timp2 | 1.47 | 1.11 | 1.09 | 1.14 |
Quantitative real-time PCR was used to measure the fold gene expression of matrix metalloproteinase 14, matrix metalloproteinase 2, and tissue inhibitor of metalloproteinase 2 at each time point in thoracic aortic aneurysm (TAA) progression.
P < 0.05 vs. reference control.
TAA-dependent changes in protein levels.
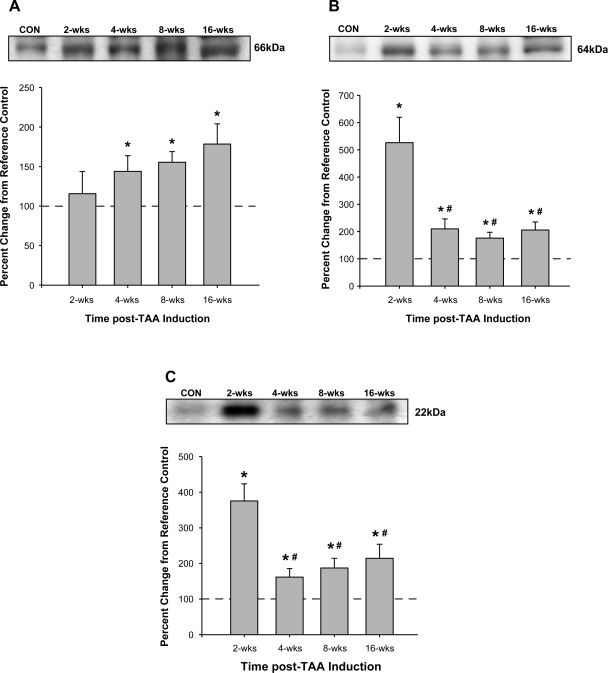
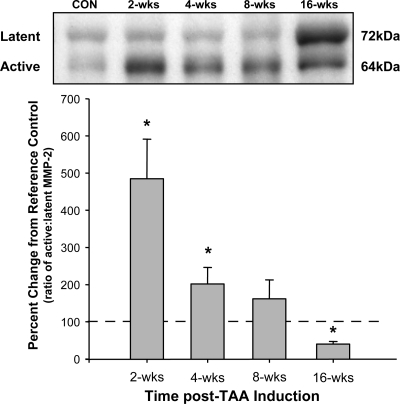
Aortic homogenates from control and TAA-induced mice were subjected to fractionation by electrophoresis, and the protein levels of MT1-MMP and pro-MMP-2 activational complex members were determined. Compared with control animals, MT1-MMP protein abundance increased progressively over the 16-wk time course and was significantly different from control at each time point from 4 to 16 wk post-TAA induction (Fig. 2A). Interestingly, both MMP-2 and TIMP-2 protein levels peaked 2 wk post-TAA induction and then reduced but remained elevated compared with control levels at each subsequent time point (Fig. 2, B and C, respectively). The ratio of active to latent MMP-2 displayed a sharp fivefold increase at 2 wk post-TAA induction, remained elevated at 4 wk post-TAA induction (2-fold), and decreased below baseline at 16 wk post-TAA induction (∼48% of control; Fig. 3).
Fig. 2.
Quantitative immunoblot analysis was used to measure membrane type-1 (MT1) matrix metalloproteinase (MMP), MMP-2, and tissue inhibitor of metalloproteinase (TIMP)-2 protein abundance. Values are expressed as percent changes from reference control (CON) animals. A: MT1-MMP was elevated at 4 wk post-TAA induction and progressively increased through the 16-wk time course (*P < 0.05 vs. reference control animals). B and C: MMP-2 (B) and TIMP-2 (C) protein levels were increased at all time points after TAA induction (*P < 0.05 vs. reference control animals). Values of the 4-, 8-, and 16-wk time points were significantly different from peak protein abundance observed at 2 wk (#P < 0.05 vs. 2 wk).
Fig. 3.
Ratio of active to latent MMP-2 as determined by quantitative immunoblot analysis. The percent change in the ratio of the active (64 kDa) to latent (72 kDa) MMP-2-immunoreactive bands was elevated at 2 and 4 wk post-TAA induction. No change was observed at 8 wk post-TAA induction, and a decrease in the ratio was observed at 16 wk post-TAA induction (*P < 0.05 vs. reference control animals).
Quantitation of MT1-MMP activity in aortic tissue.
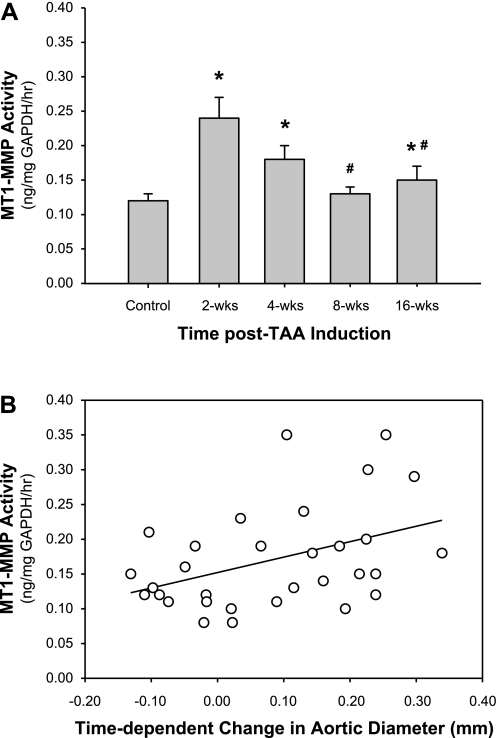
MT1-MMP-specific activity was determined in aortic homogenates from control and TAA-induced mice using a quenched fluorogenic peptide cleavage assay. MT1-MMP activity was increased at 2 wk post-TAA induction, remained elevated at 4 wk post-TAA induction, returned to baseline at 8 wk post-TAA induction, and increased again at 16 wk post-TAA induction (Fig. 4A). To determine whether a relationship existed between MT1-MMP activity and aortic dilatation, pairwise linear regression analysis was performed. The results revealed a significant correlation (r = 0.4142, P = 0.0205) between MT1-MMP activity and the time-dependent change in aortic diameter (Fig. 4B). In addition, linear mixed model analysis was performed to determine the relationships among time, aortic diameter, and MT1-MMP activity. The test of fixed effects demonstrated that significant predictors of MT1-MMP activity included aortic diameter, time, and interactions between aortic diameter and time (F = 16.85, P = 0.0004; F = 6.09, P = 0.0205; and F = 7.62, P = 0.0104, respectively).
Fig. 4.
MT1-MMP activity and functional significance. A: activity of MT1-MMP was measured in aortic homogenates from normal and TAA-induced mice using an MT1-MMP-specific quenched fluorogenic peptide activity assay. The results demonstrated elevated MT1-MMP activity (in ng·mg GAPDH−1·h−1) at 2, 4, and 16 wk post-TAA induction (*P < 0.05 vs. reference control animals; #P < 0.05 vs. 2 wk). B: pairwise regression analysis was performed between MT1-MMP activity (in ng·mg GAPDH−1·h−1) and the time-dependent change in aortic diameter (in mm). The results demonstrated a significant correlation (r = 0.4142, P = 0.0205), suggesting that MT1-MMP activity is required for aortic dilatation during TAA development. Linear mixed model analysis demonstrated that significant predictors of MT1-MMP activity included aortic diameter (F = 16.85, P = 0.0004), time (F = 6.09, P = 0.0205), and the interaction between aortic diameter and time (F = 7.62, P = 0.0104).
MT1-MMP-targeted microbubble contrast imaging in live animals.
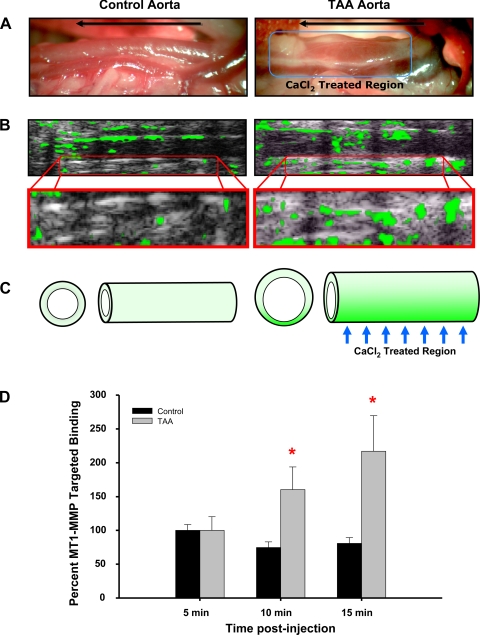
To establish that MT1-MMP was elevated in the descending thoracic aorta after TAA induction, control and 8-wk TAA-induced mice were subjected to high-resolution microultrasound in the presence of MT1-MMP antibody-conjugated microbubble contrast agent. Quantitation of targeted contrast binding revealed elevated MT1-MMP within the descending thoracic aorta (at the site of TAA induction) in 8-wk TAA-induced mice versus control animals. MT1-MMP binding was significantly elevated at 10 min (160 ± 33% in TAA-induced mice vs. 75 ± 8% in control mice) and 15 min (217 ± 53% in TAA-induced mice vs. 81 ± 8% in control mice) after the microbubble injection (Fig. 5). Correlation analysis reveal a significant relationship between aortic diameter and MT1-MMP-targeted microbubble binding (Spearman's rho coefficient = 0.6667, P < 0.05).
Fig. 5.
MT1-MMP-targeted microbubble contrast imaging. MT1-MMP antibody-conjugated microbubble contrast agent was injected into the tail vein and used to identify elevated MT1-MMP protein levels in the descending thoracic aorta of control and TAA-induced (8 wk) mice. A: intraoperative images of the descending thoracic aorta at terminal surgery showing increased aortic diameter at 8 wk post-TAA induction (right) versus a control animal (left). The direction of blood flow is shown by the arrow in each image, and the CaCl2 treatment region is shown on the right. B: high-resolution ultrasound imaging (equivalent region as shown in A) after the injection of MT1-MMP antibody-conjugated microbubble contrast agent (top). The green overlay indicates areas of enhanced MT1-MMP-targeted contrast binding. Specific binding was quantitated within a region of interest equivalent to the CaCl2-treated region in both control and TAA-induced animals. The bottom images show enlargement of the quantitated region of interest at 15 min after the contrast injection. C: pictogram summarizing the study results showing enhanced aortic dilatation accompanied by increased MT1-MMP-targeted microbubble contrast binding within the CaCl2-treated region. D: quantitative results of MT1-MMP-targeted microbubble contrast imaging demonstrating elevated binding at 10 and 15 min after the contrast injection. *P < 0.05 vs. 5 min. Representative images are shown.
Cellular localization of MT1-MMP.
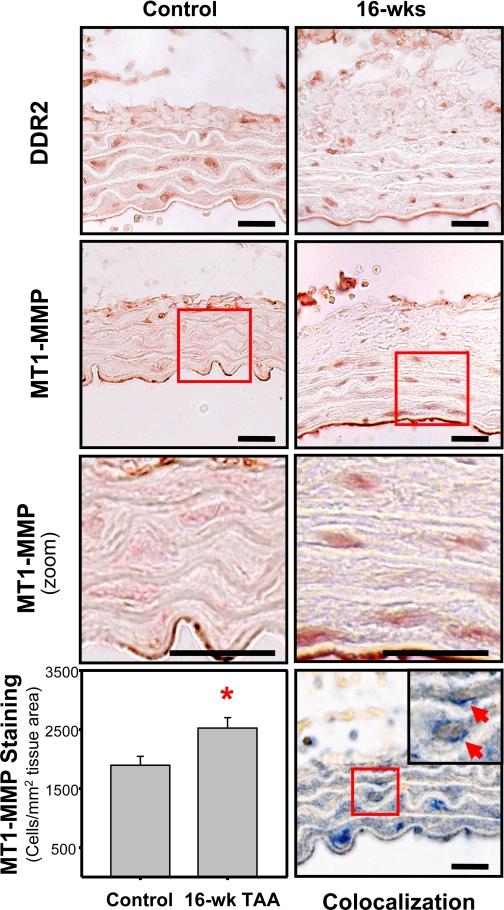
To identify a cellular source for MT1-MMP production within the aorta during TAA development, aortic tissue sections from control and TAA-induced mice (16 wk postinduction) were immunostained with antibodies specific for MT1-MMP or DDR2, one cell type-specific marker for fibroblasts (12). The immunostaining results revealed that MT1-MMP was localized in a cellular pattern similar to what was seen and previously reported with DDR2 (Fig. 6, top and middle, respectively) (19, 20). MT1-MMP-positive staining was examined using stereological techniques and displayed an elevated number of positively stained cells in 16-wk TAA-induced sections (20 ± 6% increase vs. control, P < 0.05). When the number of cells was expressed per square millimeter of tissue area, 16-wk TAA-induced sections demonstrated a 33.3% increase in the number of positively stained cells [control (n = 27): 1,895 ± 153 cells/mm2 tissue vs. TAA induction (n = 51): 2,526 ± 175 cells/mm2 tissue; Fig. 6, bottom left]. The sequential staining of tissue sections with DDR2 followed by MT1-MMP demonstrated significant colocalization of MT1-MMP and DDR2 (Fig. 6, bottom right).
Fig. 6.
Immunohistochemical localization of discoidin domain receptor 2 (DDR2) and MT1-MMP. Immunostaining of 3-μm aortic tissue sections from control and TAA-induced mice (16 wk post-TAA induction) demonstrated the presence of DDR2-positive cells (top) and MT1-MMP-positive cells (middle top and middle bottom) within the aortic wall. Bottom left: quantitation of MT1-MMP immunostaining revealed an increased number MT1-MMP-positive cells in the 16-wk TAA-induced sections (33.3% increase vs. control, *P < 0.05). Bottom right: dual sequential immunostaining demonstrated the colocalization of MT1-MMP (blue) with DDR2 (brown) in aortic fibroblasts. Representative images are shown. Bars = 20 μm.
DISCUSSION
Despite surgical intervention, TAAs carry considerable morbidity and mortality. Understanding the molecular pathogenesis of this insidious disease could therefore significantly influence patient survival as well as quality of life. In the present study, a murine model of TAA was used to examine the coordinate changes in MT1-MMP, MMP-2, and TIMP-2 abundance during aortic dilatation in TAA development. It was hypothesized that the induction of TAA would alter the expression, abundance, and activity of MT1-MMP and that this protease would be produced by endogenous mesenchymal cells within the thoracic aortic wall. The unique findings of this study were threefold. First, the distinct proteolytic profiles of MT1-MMP, MMP-2, and TIMP-2 suggest a multifunctional contribution of MT1-MMP toward TAA formation defined by the activation of MMP-2 early in aortic dilatation and through the degradation/release of other ECM substrates such as latent ECM-bound growth factors at later time points. Second, MT1-MMP was localized to endogenous fibroblasts or fibroblast-like cells within the aortic wall at all time points during TAA formation, further implicating this cell type as a major mediator of TAA development. Third, high-resolution microultrasound imaging with an MT1-MMP-targeted microbubble contrast agent revealed elevated MT1-MMP protein binding at the site of TAA induction in live animals, suggesting that MT1-MMP may serve as a trackable marker for TAA progression. Taken together, this study suggests that MT1-MMP plays a multifunctional role TAA development and identifies MT1-MMP as a critical target that could be exploited for therapeutic or diagnostic benefits.
Advantages of the murine TAA model.
Many investigators have studied aneurysm wall specimens collected at the time of surgical repair to examine structural and biochemical alterations. While informative, this tissue can only provide a snapshot of the histologic transformations and enzymatic activity occurring at that current stage of the disease. Alternatively, murine aneurysm models have allowed the examination of the rate of aortic dilation, progression of matrix remodeling, and temporal pattern of gene expression during aneurysm growth (3, 19, 37). The present study used a CaCl2-induced murine aneurysm model that has been well established in both the abdominal and thoracic aorta (6, 11, 14, 24). Previous work from Basalyga and coworkers (4) demonstrated that the calcification of vascular elastic fibers induced MMP production by an as-yet-undescribed mechanism, suggesting that the early MMP induction initiates aortic dilatation and aneurysm development. The present study followed mice for 16 wk after CaCl2 treatment and documented a 72% increase in aortic diameter over the 16-wk time course in the absence of atherosclerosis and a significant inflammatory component. Demonstrating that this model system results in a true TAA lends credibility to the subsequent biochemical and imaging experiments carried out at intermediate time points and, therefore, elevates the potential to identify therapeutic targets that could influence aneurysm progression.
MT1-MMP in TAA progression.
The potential role for MT1-MMP in TAA development has become increasingly apparent due to evidence of enhanced activity in several human and animal studies of aneurysm disease (8, 15, 28, 35, 40). Based on this enzyme's known substrates and activity, it has been suggested to contribute to TAA development through enhanced activation of MMP-2 (8). Hence, this study investigated the temporal pattern of production for all three components of the pro-MMP-2 activational complex. Consistent with work from another group (32), MMP-2 mRNA and protein levels increased concordantly in this model during early TAA development, as evidenced by the near fivefold increase in the ratio of active to latent MMP-2 during early aortic dilatation. These data suggested that components of the pro-MMP-2 activational complex should likewise be elevated during that time period. Interestingly, mRNA levels of MT1-MMP and TIMP2 did not mirror that of MMP-2 throughout the 16-wk time course. At 2 wk post-TAA induction, MT1-MMP mRNA levels were increased but returned to baseline at subsequent time points. MT1-MMP protein levels, however, remained unchanged from control at 2 wk post-TAA induction but then progressively increased through 16 wk post-TAA induction. TIMP-2 mRNA levels, on the other hand, remained unchanged at all time points, whereas protein levels were markedly elevated 2 wk post-TAA induction and remained elevated, albeit at a lower level, for the remainder of the study. These observed disparities between mRNA and protein levels suggest that a posttranscriptional regulatory mechanism may play a significant role in mediating MMP-2 activation (25, 30, 41). For example, the TIMP-2 mRNA half-life may be very short, and, upon mRNA structural modification, stability may be significantly increased, allowing for rapid protein production under the appropriate conditions. In similar fashion, translational regulatory mechanisms may inhibit MT1-MMP protein production at 2 wk despite elevated mRNA levels. This may also suggest that the baseline levels of MT1-MMP are sufficient to activate pro-MMP-2. Moreover, it may indicate that MT1-MMP naturally localizes to cellular sites where pro-MMP-2 is processed and is perhaps preloaded in the activational complex. Together, these data suggest that the formation of the pro-MMP-2 activational complex is the likely source of increased active MMP-2 observed in the initial weeks after TAA induction, thereby implicating MT1-MMP in early thoracic aneurysm development.
At later time points (4–16 wk post-TAA induction), MT1-MMP protein levels were elevated from baseline and continued to increase through 16 wk post-TAA induction. Although some MT1-MMP activity was likely required for the continued activation of pro-MMP-2, the temporal association of elevated MT1-MMP with continued aneurysm growth between 8 and 16 wk post-TAA induction would suggest that MT1-MMP contributes to TAA progression through alternate activities. These may include the activation of other MMPs, such as pro-MMP-13 (22, 33), the degradation of the local ECM, and the release of ECM-bound growth factors. This is further supported by the observation that the relative ratio of active to latent MMP-2 decreases at the later time points, when MT1-MMP protein levels are elevated. Together, this suggests that posttranslational regulatory mechanisms may alter the activity and/or cellular localization of MT1-MMP and thereby alter its substrate access or specificity. Recent work by Moss et al. (26) demonstrated that human recombinant MT1-MMP could be phosphorylated by PKC-δ on Thr567 within its short cytoplasmic domain. MT1-MMP phosphorylation has been shown to induce tumor cell migration and proliferation, possibly due to enhanced proteolytic activity. Interestingly, a recent study (23) has revealed a role for increased transforming growth factor (TGF)-β signaling in genetic aneurysm syndromes. Bound in a large latent complex, TGF-β is typically found associated with fibrillin-1 (17), a vital component of elastin microfibrils and a known substrate for MT1-MMP (2). Enhanced MT1-MMP production during continued aneurysm growth, may therefore function to release latent TGF-β, which could then further drive TAA progression. Elucidating the role of MT1-MMP throughout TAA development may therefore identify multiple unique therapeutic strategies focused on this single protease.
To examine the temporal changes in MT1-MMP activity during TAA development, an MT1-MMP-specific activity assay was performed. The results revealed two primary implications. First, discordance between MT1-MMP activity and protein content suggest that MT1-MMP activity is regulated independently of its protein abundance. This observation is consistent with the findings of Moss et al. (26) and suggests that the posttranslational modification of MT1-MMP may play an important role in the regulation of its activity and/or cellular localization. Second, the temporal changes in MT1-MMP activity observed in the present study were not dissimilar to the time-dependent change in aortic diameter. Aortic diameter was increased at 2 wk post-TAA induction, was further dilated at 4 wk post-TAA induction, plateaued at 8 wk post-TAA induction, and increased further at 16 wk post-TAA induction. Accordingly, regression analysis revealed a significant correlation between MT1-MMP activity and the time-dependent change in aortic diameter. To verify the relationship among time, aortic diameter, and MT1-MMP activity, a linear mixed model analysis was used and confirmed the significant predictors of MT1-MMP activity. Thus, these results suggest that elevated MT1-MMP activity may be essential during aortic dilatation.
To establish the functional significance of MT1-MMP in TAA development, MT1-MMP abundance was quantitated in the descending thoracic aorta in live TAA-induced mice using a novel imaging approach. High-resolution microultrasound was used to quantitate the specific binding of an MT1-MMP antibody-targeted microbubble contrast agent at the site of TAA induction. Streptavidin-conjugated lipid vesicles (microbubbles) were incubated in the presence of biotinylated MT1-MMP rabbit polyclonal antibody. The subsequent MT1-MMP-targeted contrast agent was then injected into the tail vein of normal and TAA-induced mice. Cineloops of microultrasound images were analyzed by digital subtraction to reveal areas of MT1-MMP microbubble binding within the aortic wall. The results demonstrated increased MT1-MMP-targeted contrast binding within the CaCl2-treated region of the aorta in TAA-induced animals, whereas minimal binding was observed in the same region in control animals. These unique data demonstrate, for the first time, that elevated MT1-MMP protein levels coincide with increased aortic dilatation in developing aneurysms in live mice and further support the hypothesis that TAA induction results in elevated MT1-MMP expression and abundance. This noninvasive imaging modality could be further developed to track MT1-MMP-dependent TAA progression while simultaneously providing high-resolution aortic structural information.
MT1-MMP localization to fibroblasts.
The wall of an aortic aneurysm is populated by multiple cell types, including fibroblasts, smooth muscle cells, and inflammatory cells. Because each of these cell types is capable of producing MT1-MMP (18), identifying the source of the protease in this model was imperative. Previous work from this laboratory (19) has demonstrated that endogenous fibroblasts produce MMP-9 during early aortic dilatation. In addition, degeneration of the elastic architecture was found to occur concomitantly with the emergence of a population of fibroblast-derived myofibroblasts (20). Moreover, upon isolation of aortic fibroblasts from control and TAA-induced mice, it was determined that the TAA-induced fibroblasts had undergone a significant phenotypic change during TAA formation, resulting in an enhanced proteolytic gene expression profile, including a twofold increase in MT1-MMP expression (21). Accordingly, the present study assessed the localization of MT1-MMP in aortic sections from control and TAA-induced mice and determined whether MT1-MMP, like MMP-9, would localize to mesenchymal fibroblasts. Indeed, MT1-MMP demonstrated significant colocalization with DDR2-positive cells (fibroblasts) within the thoracic aortic wall. In a previous study by Nollendorfs et al. (28), MT1-MMP was shown to localize to both mesenchymal cells as well as infiltrating monocytes/macrophages during aneurysm development in the abdominal aorta. Moreover, in an elegant study by Xiong and coworkers (40), AAA formation was found to be highly dependent on macrophage-derived MT1-MMP. The authors clearly demonstrated that lethally irradiated wild-type mice reconstituted with bone marrow from MT1-MMP-deficient animals failed to form AAAs in response to periadventitial CaCl2 exposure. While the results of the present study are consistent with those of Nollendorfs et al. (28) in part, suggesting that MT1-MMP was expressed in mesenchymal cells during aneurysm development, previous studies (19, 20) from this laboratory have failed to show a significant accumulation of monocytes/macrophages in the aortic media during TAA formation. Taken together, these data suggest that exposure of the thoracic aorta to CaCl2 instigates aortic dilation in the thoracic aorta via a different mechanism than in the abdominal aorta. For example, CaCl2 may induce changes in gene expression in native mesenchymal cells, leading to enhanced MMP production and the initiation of aortic dilatation. These results further substantiate the hypothesis of regional heterogeneity between the thoracic and abdominal aorta, extending the evidence to include differential mechanisms regulating MT1-MMP expression and abundance (31). Interestingly, the present results, demonstrating a progressive elevation of MT1-MMP over the time course of TAA development, combined with recent results (20) reporting the emergence of a fibroblast-derived myofibroblast population, suggest that the elevated MT1-MMP levels observed may be due to an increased number of fibroblasts/myofibroblasts expressing MT1-MMP in the aorta. Accordingly, these results may also explain the discordance between MT1-MMP mRNA levels and protein abundance over the time course of TAA formation. When MT1-MMP was localized in control and 16-wk TAA-induced aortic tissue sections, a 33% increase in the number of cells staining positive for MT1-MMP was observed in 16-wk TAA-induced sections. While this technique does not account for changes in staining intensity, the measured increase in MT1-MMP protein abundance at 16 wk post-TAA induction (Fig. 2A) would suggest that not only is there an increase in the number of cell producing MT1-MMP but also an increase in the amount of MT1-MMP being produced by each cell.
Limitations.
While this study clearly demonstrated that the expression, abundance, and activity of MT1-MMP were temporally regulated throughout TAA development, this study is not without limitations. First, the mRNA and protein levels measured at each time point in this study were compared relative to baseline values; no absolute quantification was performed. Therefore, the baseline levels of MT1-MMP were assumed to be sufficient for pro-MMP-2 activation. Additional studies will be required to define the dynamic cellular localization and association with pro-MMP-2 activational complex members. Second, while this murine model of TAA recapitulates many of the hallmarks of human aneurysmal disease, some aspects, such as intraluminal thrombosis and atherosclerosis, are not evident at the time points studied. Thus, care should be taken in extrapolating these results to human TAAs. Third, while the present data suggests that MT1-MMP functions early in TAA development to activate pro-MMP-2, other MT1-MMP-dependent activities (such as the activation of pro-MMP-13 or the release latent ECM-bound growth factors) were not measured in this study and may significantly contribute to TAA development. Thus, future studies will be required to identify and examine other MT1-MMP-dependent targets during TAA formation. Fourth, immunohistochemical analysis with the cell marker DDR2 cannot distinguish between fibroblasts and fibroblast-derived myofibroblasts. Further experimentation addressing the cellular-specific changes in MT1-MMP production is necessary. Finally, because MT1-MMP homozygous-deficient animals die at approximately postnatal day 21, providing direct evidence that MT1-MMP is required for TAA development has been challenging. This issue is further complicated by the fact that MMP inhibitors lack sufficient specificity and induce a large number of side effects that could affect TAA development when used at effective concentrations. Nevertheless, these limitations do not nullify the implications of the present study. While the present study demonstrates that changes in MT1-MMP occur during TAA development, the biomolecular tools needed for advanced genetic experiments designed to demonstrate that MT1-MMP is necessary for TAA formation are lacking and their development is now warranted.
Summary and clinical relevance.
Characterizing potential targets for nonsurgical interventions to impede thoracic aortic aneurysm growth may significantly reduce the morbidity and mortality associated with this disease. Despite limitations, the novel findings of the present study are threefold. First, the results demonstrate that MT1-MMP protein levels are progressively elevated during TAA development. This was established through immunoblot analysis of aortic homogenates from control mice and TAA-induced mice as well as by high-resolution microultrasound, demonstrating, for the first time, elevated MT1-MMP protein levels in live 8-wk TAA-induced mice through enhanced MT1-MMP antibody-targeted microbubble contrast binding to the descending thoracic aorta at the site of TAA induction. Second, the present study demonstrated that MT1-MMP activity was correlated with the time-dependent change in aortic diameter, suggesting that MT1-MMP may be required for aortic dilatation. Finally, the present study demonstrated that MT1-MMP localizes to DDR2-positive cells within the aortic wall, suggesting that the structural enhancements that occur with TAA development may be mediated in part by MT1-MMP produced within endogenous fibroblasts or fibroblast-derived cells.
Together, these novel results suggest a multifunctional role for MT1-MMP in TAA development that is defined through two phases of expression and activity. The first phase defines a role for MT1-MMP early in aneurysm development, when its primary function involves the activation of MMP-2. The second phase defines a role for MT1-MMP at later time points, when the activation of other MMPs, pericellular degradation of matrix components, and potential release of latent bound growth factors are likely prominent functions of MT1-MMP. Thus, these results suggest that MT1-MMP may be a turnkey mediator of TAA formation and suggest that targeting this protease may have significant therapeutic implications.
GRANTS
This work was supported by Department of Veterans Affairs Career Development Award CDA-2 (to J. A. Jones) and a Merit Award (to F. G. Spinale) and by National Heart, Lung, and Blood Institute Grant HL-075488-05 (to J. S. Ikonomidis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Stacia M. DeSantis for the expert assistance with statistical modeling.
REFERENCES
- 1.Annabi B, Shedid D, Ghosn P, Kenigsberg RL, Desrosiers RR, Bojanowski MW, Beaulieu E, Nassif E, Moumdjian R, Beliveau R. Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J Vasc Surg 35: 539–546, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J 340: 171–181, 1999 [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res 139: 292–307, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110: 3480–3487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ, Cherry KJ, Joyce JW, Lie JT. Thoracic aortic aneurysms: a population-based study. Surgery 92: 1103–1108, 1982 [PubMed] [Google Scholar]
- 6.Chiou AC, Chiu B, Pearce WH. Murine aortic aneurysm produced by periarterial application of calcium chloride. J Surg Res 99: 371–376, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 17: 615–635, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Eagleton MJ, Ballard N, Lynch E, Srivastava SD, Upchurch GR, Jr, Stanley JC. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during angiotensin II induced aneurysm formation. J Surg Res 135: 345–351, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 74: S1877–S1892, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 15: 1145–1151, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Freestone T, Turner RJ, Higman DJ, Lever MJ, Powell JT. Influence of hypercholesterolemia and adventitial inflammation on the development of aortic aneurysm in rabbits. Arterioscler Thromb Vasc Biol 17: 10–17, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn 230: 787–794, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidis JS, Barbour JR, Amani Z, Stroud RE, Herron AR, McClister DM, Jr, Camens SE, Lindsey ML, Mukherjee R, Spinale FG. Effects of deletion of the matrix metalloproteinase 9 gene on development of murine thoracic aortic aneurysms. Circulation 112: I242–248, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, Spinale FG. A murine model of thoracic aortic aneurysms. J Surg Res 115: 157–163, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, 3rd, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation 114: I365–I370, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Ohuchi E, Aoki T, Nomura H, Fujii Y, Sato H, Seiki M, Okada Y. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinases 2. Cancer Res 56: 2707–2710, 1996 [PubMed] [Google Scholar]
- 17.Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 278: 2750–2757, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol 206: 1–8, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Jones JA, Barbour JR, Lowry AS, Bouges S, Beck C, McClister DM, Jr, Mukherjee R, Ikonomidis JS. Spatiotemporal expression and localization of matrix metalloproteinase-9 in a murine model of thoracic aortic aneurysm. J Vasc Surg 44: 1314–1321, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JA, Beck C, Barbour JR, Zavadzkas JA, Mukherjee R, Spinale FG, Ikonomidis JS. Alterations in aortic cellular constituents during thoracic aortic aneurysm development: myofibroblast-mediated vascular remodeling. Am J Pathol 175: 1746–1756, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones JA, Zavadzkas JA, Chang EI, Sheats N, Koval C, Stroud RE, Spinale FG, Ikonomidis JS. Cellular phenotype transformation occurs during thoracic aortic aneurysm development. J Thorac Cardiovasc Surg In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knauper V, Bailey L, Worley JR, Soloway P, Patterson ML, Murphy G. Cellular activation of proMMP-13 by MT1-MMP depends on the C-terminal domain of MMP-13. FEBS Lett 532: 127–130, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 355: 788–798, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 110: 625–632, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J 21: 3949–3959, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss NM, Wu YI, Liu Y, Munshi HG, Stack MS. Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices. J Biol Chem 284: 19791–19799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 157: 493–507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nollendorfs A, Greiner TC, Nagase H, Baxter BT. The expression and localization of membrane type-1 matrix metalloproteinase in human abdominal aortic aneurysms. J Vasc Surg 34: 316–322, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem 272: 2446–2451, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci 116: 3905–3916, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS. Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg 136: 1123–1130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmoker JD, McPartland KJ, Fellinger EK, Boyum J, Trombley L, Ittleman FP, Terrien C, 3rd, Stanley A, Howard A. Matrix metalloproteinase and tissue inhibitor expression in atherosclerotic and nonatherosclerotic thoracic aortic aneurysms. J Thorac Cardiovasc Surg 133: 155–161, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Schneider F, Sukhova GK, Aikawa M, Canner J, Gerdes N, Tang SM, Shi GP, Apte SS, Libby P. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation 117: 931–939, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Sinha I, Bethi S, Cronin P, Williams DM, Roelofs K, Ailawadi G, Henke PK, Eagleton MJ, Deeb GM, Patel HJ, Berguer R, Stanley JC, Upchurch GR., Jr A biologic basis for asymmetric growth in descending thoracic aortic aneurysms: a role for matrix metalloproteinase 9 and 2. J Vasc Surg 43: 342–348, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Sinha I, Hannawa KK, Eliason JL, Ailawadi G, Deogracias MP, Bethi S, Ford JW, Roelofs KJ, Grigoryants V, Henke PK, Stanley JC, Upchurch GR., Jr Early MT-1 MMP expression following elastase exposure is associated with increased cleaved MMP-2 activity in experimental rodent aortic aneurysms. Surgery 136: 176–182, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Spinale FG, Zellner JL, Tomita M, Crawford FA, Zile MR. Relation between ventricular and myocyte remodeling with the development and regression of supraventricular tachycardia-induced cardiomyopathy. Circ Res 69: 1058–1067, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Van Vickle-Chavez SJ, Tung WS, Absi TS, Ennis TL, Mao D, Cobb JP, Thompson RW. Temporal changes in mouse aortic wall gene expression during the development of elastase-induced abdominal aortic aneurysms. J Vasc Surg 43: 1010–1020, 2006 [DOI] [PubMed] [Google Scholar]
- 38.West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci 22: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Xiong W, Knispel R, Mactaggart J, Baxter BT. Effects of tissue inhibitor of metalloproteinase 2 deficiency on aneurysm formation. J Vasc Surg 44: 1061–1066, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Xiong W, Knispel R, MacTaggart J, Greiner TC, Weiss SJ, Baxter BT. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem 284: 1765–1771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211: 19–26, 2007 [DOI] [PubMed] [Google Scholar]