Abstract
In decerebrated rats, we determined the pressor and cardioaccelerator reflex responses to static contraction of hindlimb muscles whose femoral arteries were either occluded 72 h before contraction, occluded 3 min before contraction, or freely perfused. We found that the pressor reflex arising from the limb whose femoral artery was occluded for 72 h before contraction (32 ± 5 mmHg, n = 16) was significantly higher than the pressor reflex arising from the contralateral freely perfused limb (15 ± 3 mmHg, n = 16, P < 0.001) or than that arising from the contralateral limb whose femoral artery was occluded for only 3 min (17 ± 4 mmHg, n = 16, P < 0.001). Moreover, the pressor reflex arising from the limb whose femoral artery was occluded for 3 min before the start of contraction was not significantly different than that arising from the contralateral freely perfused limb (n = 16, P = 0.819). The pressor component of the reflex arising from the limb whose femoral artery was occluded for 72 h was not changed by transient receptor potential vanilloid (TRPV) 1 receptor blockade with iodo-resiniferatoxin (n = 15, P = 0.272), although the cardioaccelerator component was significantly reduced (P = 0.005). In addition, the pressor response evoked by capsaicin injection in the femoral artery of the 72-h occluded limb was more than double that evoked from the freely perfused limb (P = 0.026). We conclude that chronic (i.e., 72 h) but not acute (3 min), femoral arterial occlusion augments pressor reflex arising from contraction of hindlimb muscles and that TRPV1 receptors play little role in this augmentation.
Keywords: static contraction, thin fiber muscle afferents, ischemia, neural control of circulation
peripheral artery disease is a pathophysiological condition that is frequently caused by atherosclerosis-induced narrowing of the vessels perfusing the muscles of the legs. Patients having peripheral artery disease experience intermittent claudication, which consists of exercise-induced leg pain and which is attributed to insufficient blood flow to the extremities. This condition does not evoke pain at rest when the metabolic demand of the leg muscles is low but does evoke pain during exercise when metabolic demand of the muscles is high. Consistent with reports of pain, the pressor and cardioaccelerator responses to exercise are higher in patients with intermittent claudication than they are in control subjects whose lower extremities are adequately perfused (3).
Baccelli et al. (2) have speculated that the exercise pressor reflex (20) is responsible for the exaggerated pressor response to exercise seen in patients with peripheral arterial disease. This reflex is believed to play an important role in evoking the cardiovascular responses to exercise in both physiological and pathophysiological states (4, 16, 20). The afferent arm of this reflex is comprised of group III and IV afferents (16, 35), the endings of which are stimulated by both mechanical and metabolic stimuli arising in contracting muscles (11, 12).
The speculation that the exaggerated pressor response to walking is caused by the exercise pressor reflex needs to be tested empirically. Prior et al. (22) and Yang et al. (38) have developed a well-characterized model of peripheral artery disease in rats in which blood flow to the hindlimb is compromised by ligation of the femoral artery. In this model of simulated peripheral artery disease, blood flow through collateral vessels is adequate at rest but is not adequate to satisfy metabolic demand during exercise. Moreover, blood flow reserve capacity to the hindlimb whose femoral artery has been ligated is only about 10–20% of normal (22, 38).
This rat model of simulated peripheral artery disease enabled us to determine if the exercise pressor reflex was greater when evoked from hindlimb muscles whose arterial supply was ligated than when it was evoked from hindlimb muscles that were freely perfused. In addition, recent evidence has suggested that ligation of a femoral artery in rats increased the number of transient receptor potential vanilloid (TRPV) 1 receptors on dorsal root ganglion cells whose axons innervated the hindlimb muscles (37). TRPV1 receptors are widely believed to play an important role in nociception, and their stimulation in skeletal muscle by capsaicin is well known to evoke powerful reflex pressor responses (5). The increase in the number of TRPV1 receptors caused by femoral arterial ligation in rats further prompted us to determine if the contraction-induced stimulation of TRPV1 receptors was responsible for the exaggerated exercise pressor reflex that is known to occur during peripheral artery disease.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center. A total of 49 adult male Sprague-Dawley rats (weighing 340–472 g) were used in three protocols, of which the first two involved the use of static contraction of the hindlimb muscles. In the first protocol, 24 of the rats were divided into three groups (n = 8 in each group) as follows: 1) 72 h occluded vs. 3 min occluded, 2) 72 h occluded vs. freely perfused, and 3) 3 min occluded vs. freely perfused. Before an experiment (72 h), 16 of the 24 rats had one femoral artery occluded according to the procedure described in detail elsewhere (22, 38). Briefly, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen; one femoral artery, randomly selected, was isolated and then tightly ligated just distal to the inguinal ligament. This procedure reduces blood flow reserve capacity to ∼10–20% of normal but allows sufficient blood flow to meet resting requirements (22, 38). The rats were allowed to recover 72 h before the experiments were started. Femoral artery occlusion has been reported to have no effect on normal cage activity (34).
In the second protocol, performed on 15 rats, we examined the effect of TRPV1 receptor blockade on the reflex pressor and cardioaccelerator responses to static contraction in rats whose femoral arteries were occluded for 72 h before the experiment. In 10 of these 15 rats, we cannulated (PE-10, polyethylene tubing) the contralateral right femoral artery in a retrograde direction and advanced the tip to the bifurcation of the abdominal aorta. This allowed us to administer drugs in the arterial supply of the left hindlimb. In the remaining five rats, we cannulated the ipsilateral left femoral artery peripheral to the previously ligated site and advanced the tip of the cannula so that it was located just before the popliteal artery. These procedures allowed the injection of substances directly in the circulation of the left hindlimb via either the left common iliac or femoral artery. For both types of cannulations, a reversible vascular occluder was placed around the abdominal aorta and the inferior vena cava just above the aortic bifurcation. When tightened, this occluder helped to keep the injectate within the circulation of the left hindlimb. No attempt was made to determine the maximal level of tension developed during static contraction in any of the rats serving in the first two protocols. Consequently, the relationship between tension development and the pressor response to contraction was not determined because this assessment must be made when tension development is “normalized” across rats (29).
In the third protocol performed on 10 rats, we compared the pressor and cardioaccelerator responses to capsaicin (0.5 μg in 100 μl) injected in the femoral artery of rats whose hindlimb muscles were freely perfused with the pressor and cardioaccelerator responses to capsaicin of rats (n = 5) whose femoral arteries were occluded 72 h previously. All injections were made in the femoral artery below the point of occlusion.
Surgical preparation.
On the day of the experiment, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL; Statham). Heart rate (HR) was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood-gas analyzer (model ABL-700; Radiometer). Arterial Pco2 and pH were maintained within the normal range by either adjusting ventilation or by intravenous administration of sodium bicarbonate (8.5%). A rectal temperature probe was inserted, and the core body temperature of the animal was maintained at 37–38°C by a water-perfused heating pad and a lamp. In the experiments in which we occluded the femoral artery for 3 min, we placed a snare around the femoral artery.
The rat was placed in a Kopf stereotaxic frame. Dexamethasone (0.2 mg) was injected intravenously just before the decerebration procedure to minimize brain stem edema. The left common carotid artery was tied off, and a precollicular decerebration was performed. All neural tissue rostral to the section was removed. To minimize cerebral hemorrhage, small pieces of oxidized regenerated cellulose (Ethicon; Johnson & Johnson) were placed on the internal skull surface, and the cranial cavity was packed with cotton.
In the rats in which the hindlimb muscles were statically contracted, a laminectomy exposing the lower lumbar and sacral portions of the spinal cord (L1–L5) was performed. The rat was secured in a customized spinal frame by clamps placed on rostral lumbar vertebrae and the pelvis. Using the skin on the back, a pool was formed that was filled with warm (37°C) mineral oil. The dura of the cord was cut and reflected, allowing visual identification of the spinal roots. In 24 rats, both the left and the right L4 and L5 ventral roots were identified and cut close to their exits from the spinal cord. In the 15 rats that were given iodo-resiniferatoxin (IRTX), only the left L4 and L5 ventral roots were cut, and the calcaneal bone of each contracted limb was severed. Once the surgeries were completed, the anesthesia was withdrawn, and the lungs were ventilated with room air. A minimum recovery period of 90 min was employed after decerebration before beginning any experimental protocol.
The L4 and L5 ventral roots were placed on shielded stimulating electrodes. Each calcaneal tendon was attached to a force transducer (model FT 03; Grass) that, in turn, was attached to a rack-and-pinion. The tendon was stretched so that baseline tension was set between 50 and 100 g. Static contraction was evoked by electrically stimulating (40 Hz, 0.1 ms, >2 times motor threshold) the cut peripheral ends of the L4 and L5 ventral roots. All contractions lasted for 60 s. The order of the muscle contractions was chosen at random. Acute occlusion was performed by clamping the femoral artery 3 min before the start of the static contraction. This occlusion was maintained for the 60-s contraction period and for 30 s after the end of contraction.
In 10 of 15 rats whose femoral arteries had been occluded 72 h previously, we blocked TRPV1 receptors by injecting IRTX (10 μg) retrogradely in the contralateral femoral artery catheter. Because of the possibility that the amount of IRTX reaching the lower limb by this method of injection was limited by the ligation of the femoral artery, the remaining five rats received IRTX anterogradely in the ipsilateral femoral artery. Immediately before injection, the vascular occluder was inflated and maintained for 5 min to trap IRTX in the circulation of the experimental limb. The occluder was then deflated, and, after waiting an additional 10 min, we challenged the effectiveness of the TRPV1 receptor blockade with capsaicin (0.5–1 μg in 100 μl), which was injected before and after administration of IRTX in the same manner as that used for administration of IRTX. After verifying blockade of TRPV1 receptors, we waited 10 min and contracted the hindlimb muscles again. At the end of each experiment in which we injected IRTX and capsaicin, we injected blue dye retrogradely in the contralateral or anterogradely in the ipsilateral femoral arterial catheter. In each case, the triceps surae muscles were stained blue, verifying that IRTX and capsaicin had access to this muscle group.
Data analysis.
Arterial blood pressure, HR, muscle tension, and electrocardiogram were recorded with a Spike 2 data acquisition system (CED) and stored on a computer hard drive (Dell). Mean arterial pressure (MAP) is expressed in millimeters of mercury and HR in beats per minute. The initial 60-s values were used to compare the differences between baseline and the response to each maneuver. In addition, the time course of the MAP and HR responses to static contraction were analyzed every second following the onset of each maneuver until the maneuver ended. The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level (Spike 2) and is expressed in kilograms seconds.
All values are expressed as means ± SE. Either a one- or two-way repeated-measures ANOVA followed by Tukey's post hoc tests were used to determine statistical significance. The criterion for statistical significance was set at P < 0.05.
RESULTS
In the first protocol, we measured the pressor and cardioaccelerator responses to static contraction of the hindlimb muscles in three groups of decerebrated rats. In the first group (n = 8), we compared the pressor-cardioaccelerator responses to contraction between the limb whose femoral artery was occluded for 72 h with the contralateral limb whose femoral artery was occluded for 3 min. In the second group (n = 8), we compared the pressor-cardioaccelerator responses to contraction between the limb whose femoral artery was occluded for 72 h with the contralateral limb whose femoral artery was freely perfused. In the third group (n = 8), we compared the pressor-cardioaccelerator responses to contraction between the limb whose femoral artery was occluded for 3 min with the contralateral limb whose femoral artery was freely perfused. To compare peak effects, we combined the pressor and cardioacclerator responses to static contraction across the three groups; this yielded 16 data points for each of the three conditions.
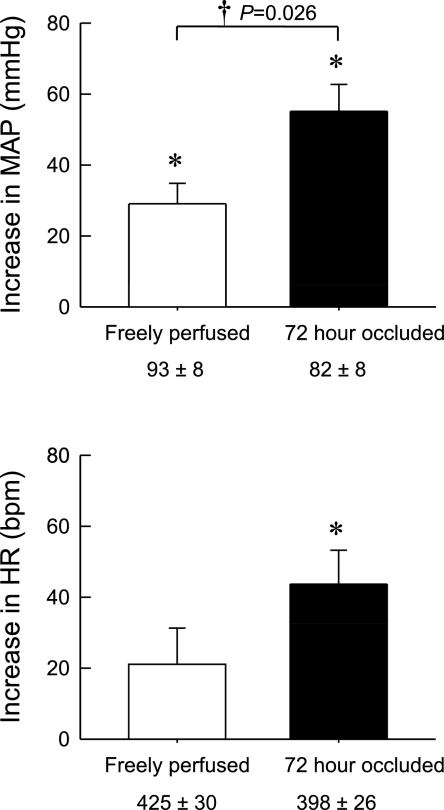
Overall, static contraction of the hindlimb, regardless of whether its circulation was occluded or not, rapidly increased both MAP and HR, effects that reached their peak levels around 10 s from the onset of tension development. Subsequently, these increases decreased from their peak levels even though the hindlimb muscles continued to contract (Figs. 1–3). Surprisingly, the increases in arterial pressure were modest in the postcontraction period in both the 72-h occluded and the 3-min occluded groups (Figs. 1–3). On average, the peak pressor responses to static contraction of the hindlimb whose femoral artery was occluded 72 h before the start of the experiment were almost double those in either the freely perfused (P < 0.001) or the 3-min occluded (P < 0.001) groups (Fig. 4). There were no significant differences in peak increases in MAP between freely perfused and 3-min occluded groups (P = 0.819). Likewise, there were no significant differences in the cardioaccelerator responses to static contraction among the freely perfused, the 3-min occluded, and the 72-h occluded groups (P = 0.503; Fig. 4).
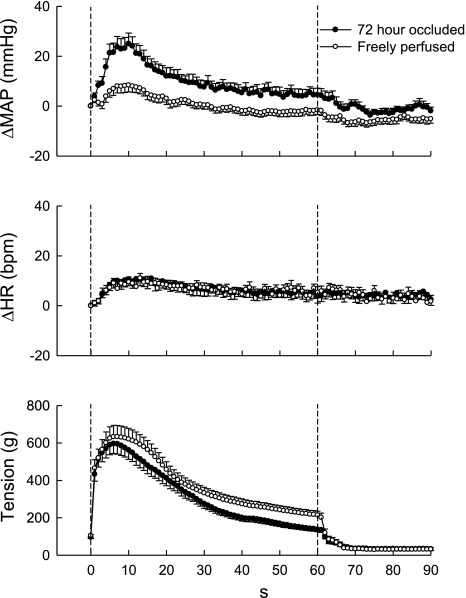
Fig. 1.
Time courses of the average changes in mean arterial pressure (MAP), heart rate (HR), and the triceps surae muscle tension during static contraction: 72-h occluded vs. freely perfused limb. The overall pressor response to static contraction was larger in the 72-h occluded limb than in the freely perfused limb. The time course of the changes in HR was similar irrespective of the limb conditions. Values belonging to the same treatments of the femoral artery were pooled. Values are means ± SE (n = 16 rats) and plotted at 1-s intervals.
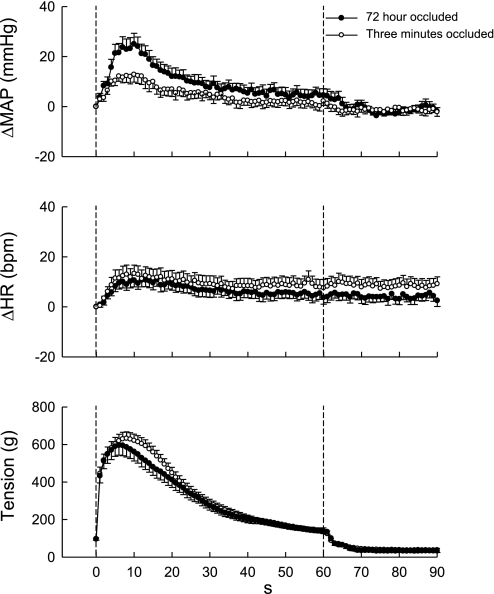
Fig. 2.
Time courses of the average changes in MAP, HR, and the triceps surae muscle tension during static contraction: 72-h occluded vs. 3-min occluded limb. The overall pressor response to static contraction was larger in the 72-h occluded limb than in the 3-min occluded limb. The time course of the changes in HR was similar irrespective of the limb conditions. Values belonging to the same treatments of the femoral artery were pooled. Values are means ± SE (n = 16) and plotted at 1-s intervals.
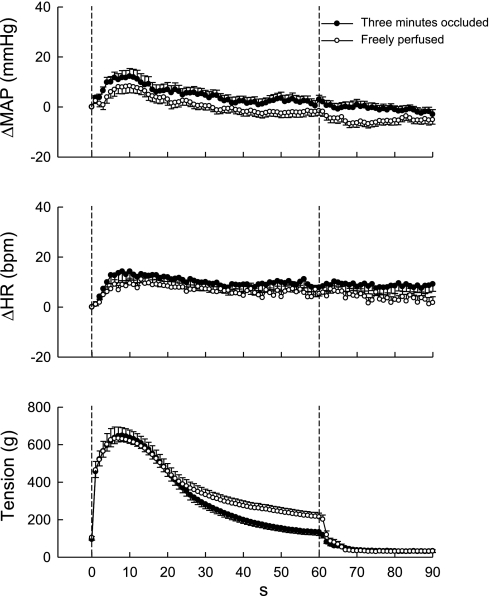
Fig. 3.
Time courses of the average changes in MAP, HR, and the triceps surae muscle tension during static contraction: 3-min occluded vs. freely perfused limb. The overall pressor and cardioaccelerator responses to static contraction were similar irrespective of the limb conditions. Values belonging to the same treatments of the femoral artery were pooled. Values are means ± SE (n = 16) and plotted at 1-s intervals.
Fig. 4.
Peak increases in MAP and HR during static contraction. The peak increases of MAP during static contraction in 72-h occluded limb were almost two times as large as that in freely perfused or 3-min occluded limb. Three minutes of acute femoral artery occlusion did not augment the pressor response compared with freely perfused limb during static contraction. The differences of the peak increases in HR during static contraction were not statistically significant among these limbs. Values are means ± SE (n = 16). The pressor responses to contraction in each of the three conditions were significantly increased from their respective baselines (*P < 0.05). Horizontal brackets signify that the increases are significantly different from each other (†P < 0.05).
In each of four rats tested, the pressor response to contraction of the hindlimb whose femoral artery was occluded for 72 h was abolished by paralysis induced by vecuronium bromide. After paralysis, passive stretch of the triceps surae muscles in hindlimb whose femoral artery was occluded 72 h before the experiment rapidly increased MAP. In each of four rats tested, transection of the dorsal roots below L2 abolished the pressor response to static contraction. There were no significant differences in baseline MAP (P = 0.100) and HR (P = 0.289) immediately before the static contraction in freely perfused, 3-min occluded, or 72-h occluded hindlimbs (Table 1). Likewise, there were also no significant differences in the TTIs among freely perfused, 3-min occluded, or 72-h occluded hindlimbs (P = 0.377; Table 2).
Table 1.
Baseline MAP and HR
| MAP, mmHg | HR, beats/min | |
|---|---|---|
| Freely perfused | 79 ± 3 | 433 ± 24 |
| Occluded (3 min) | 88 ± 6 | 407 ± 26 |
| Occluded (72 h) | 86 ± 7 | 397 ± 35 |
Values are means ± SE; n = 16 rats. MAP, mean arterial pressure; HR, heart rate. There were no significant differences in MAP (P = 0.100) or HR (P = 0.289) among these limbs.
Table 2.
The TTI during static contraction
| TTI, kg·s | |
|---|---|
| Freely perfused | 16.3 ± 2.4 |
| Occluded (3 min) | 13.6 ± 1.6 |
| Occluded (72 h) | 14.3 ± 1.9 |
Values are means ± SE; n = 16 rats. TTI, tension time index. There were no significant differences among these limbs (P = 0.377).
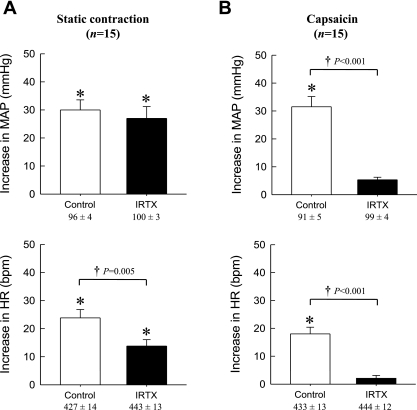
In the second protocol, we determined in 15 rats the effect of IRTX (10 μg) on the exercise pressor reflex evoked from the 72-h occluded limb. IRTX was injected retrogradely in the contralateral femoral artery in 10 rats and anterogradely in the ipsilateral femoral artery in the remaining 5 rats. Static contraction significantly increased (P < 0.05) both MAP and HR over baseline levels both before and after injection of IRTX (Fig. 5). The pressor responses to static contraction were not attenuated by IRTX, irrespective of the routes of administration (contralateral, P = 0.220; ipsilateral, P = 0.931). On the other hand, the cardioaccelerator responses to static contraction were significantly decreased (P = 0.007) when IRTX was injected in the contralateral cannula but not when it was injected in the ipsilateral cannula (P = 0.381) (Fig. 5).
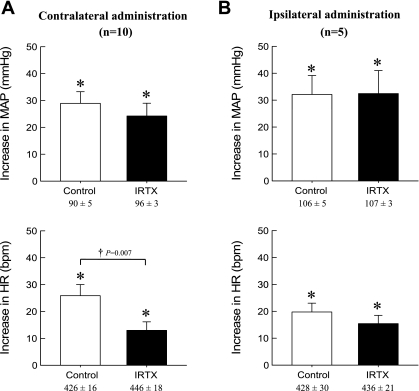
Fig. 5.
Effects of administration of iodo-resiniferatoxin (IRTX) from two different routes on peak MAP and HR responses during static contraction. IRTX was administered either retrogradely in contralateral (A, n = 10) or anterogradely in ipsilateral (B, n = 5) femoral artery. Values under the x-axis labels are their baseline values. The pressor and HR responses to contraction in each condition were significantly increased from their respective baselines (*P < 0.05). A: retrograde administration of IRTX did not affect the peak increases of MAP but decreased the peak HR responses during static contraction in 72-h occluded limb. B: anterograde administration of IRTX did not affect either peak MAP or HR responses to static contraction. Values are means ± SE.
When the data from the 15 rats injected with IRTX were combined and analyzed statistically, IRTX had no significant effect on the peak pressor response to static contraction (P = 0.272), whereas the antagonist significantly decreased the peak cardioaccelerator responses to contraction (P = 0.007) (Fig. 6A). The average TTIs in these 15 rats were not affected by IRTX (Table 3). Injection of capsaicin in the femoral artery significantly increased (P < 0.05) both MAP and HR over baseline levels before injection of IRTX (Fig. 6B). IRTX almost eliminated the pressor (P < 0.001) and cardioaccelerator (P < 0.001) responses to capsaicin injection in the femoral artery.
Fig. 6.
Effects of IRTX on peak MAP and HR responses during static contraction (A) and during intra-arterial injection of capsaicin (B). The data from the 15 rats injected with IRTX were combined. Values under the x-axis labels are their baseline values. A: the peak MAP and HR values during static contraction were significantly increased from their respective baselines (*P < 0.05). IRTX (10 μg) had no significant effect on the peak pressor response to static contraction (P = 0.272), whereas the antagonist significantly decreased the peak cardioaccelerator responses to contraction (P = 0.007). B: the peak MAP and HR responses to capsaicin injection in femoral circulation were significantly attenuated by pretreatment of IRTX. Horizontal brackets signify that the increases are significantly different from each other (†P < 0.05). Values are means ± SE (n = 15).
Table 3.
The TTI during static contraction before and after administration of IRTX
| TTI, kg/s |
|||
|---|---|---|---|
| Routes of Administration | n | Before | After |
| Contralateral | 10 | 28.0 ± 2.8 | 24.5 ± 1.7 |
| Ipsilateral | 5 | 25.9 ± 3.8 | 26.1 ± 3.4 |
| Overall average | 15 | 27.3 ± 2.2 | 25.0 ± 1.6 |
Values are means ± SE; n, no. of rats. Overall average is for contralateral plus ipsilateral. There were no significant differences in TTIs before and after administration of iodo-resiniferatoxin (IRTX) among these groups.
In the third protocol, we compared the pressor and cardioaccelerator responses to capsaicin injection (0.5 μg in 100 μl) in rats (n = 5) whose femoral arteries were occluded for 72 h with the responses to capsaicin injection in rats whose femoral arteries were freely perfused (n = 5). We found that the pressor responses to femoral artery injection of this TRPV1 agonist in the 72-h occluded rats were significantly greater (P < 0.026) than the pressor responses to injection of the agonist in the freely perfused rats. The cardioaccelerator responses were not significantly different from each other (Fig. 7).
Fig. 7.
Effects of capsaicin (0.5 μg) injected in the femoral artery on MAP and HR. Nos. under the x-axis are baseline values. *Significant increase in either MAP or HR over its respective baseline value. Horizontal bracket represents significant difference between pressor response to capsaicin in rats whose femoral artery was freely perfused and rats whose femoral artery was occluded for 72 h.
DISCUSSION
In decerebrate unanesthetized rats, we found that the exercise pressor reflex evoked from hindlimb muscles whose femoral artery had been occluded for 72 h was significantly larger than the reflex evoked from freely perfused hindlimb muscles or from hindlimb muscles whose femoral artery had been occluded for only 3 min. Moreover, the exercise pressor reflex evoked from hindlimb muscles whose femoral artery had been occluded for 3 min was not different from that evoked from hindlimb muscles that were freely perfused. We also found that blockade of TRPV1 receptors had no effect on the contraction-induced pressor reflex evoked from the hindlimb whose femoral artery was occluded 72 h before the start of the experiment. In contrast to the pressor reflex, blockade of TRPV1 receptors significantly decreased the contraction-induced cardioaccelerator reflex by about half.
Blockade of these receptors, however, greatly attenuated the pressor and cardioaccelerator responses to capsaicin injection in the arterial supply of the same limb as the one that was statically contracted. We interpret the latter finding as evidence that our blockade of TRPV1 receptors with IRTX was effective and that it reached the endings of afferents with TRPV1 receptors. In rats, it is not clear whether both group III and IV muscle afferents are stimulated by TRPV1 agonists. In cats and dogs, about 75% of the group IV, but only 25% of the group III, afferents are stimulated by capsaicin (10, 11), a potent TRPV1 agonist. Capsaicin, however, is capable of evoking a pressor response when injected in the arterial supply of the hindlimb of rats (15, 37), although both unmyelinated afferents from both skin and muscle may contribute to this response (7).
Xing et al. (37) reported that the expression of TRPV1 receptors on dorsal root ganglia cell bodies, which innervated the triceps surae muscles, was increased after 24 h of femoral artery occlusion in rats. They also found that the pressor response to femoral arterial injection of capsaicin was augmented by 24 h of occlusion. We have extended their latter finding by showing that this augmented pressor response to capsaicin injection is also found in rats whose femoral arteries were occluded for 72 h. More importantly, our findings suggest that TRPV1 receptors play little role in causing the augmented pressor responses to static contraction. Even though TRPV1 blockade with IRTX decreased the cardioaccelerator response to contraction in rats whose femoral arteries were occluded for 72 h, TRPV1 blockade did not significantly change the magnitude of the pressor responses to contraction. Consequently, the participation of TRPV1 receptors on group III and IV afferents in causing the augmented pressor response to contraction in rats whose femoral arteries were occluded for 72 h is questionable. In any event, an increase in receptor number, determined immunocytochemically as done by Xing et al. (37), cannot be interpreted as evidence that these receptors have a functional effect. Moreover, femoral arterial occlusion in the experiments performed by Xing et al. (37) may have upregulated other receptors on thin fiber muscle afferents, and these may have been responsible for the exaggerated exercise pressor reflex seen in our experiments.
We were surprised to find that the exercise pressor reflex in rats in which the femoral artery was occluded for 3 min before and during the period of static contraction was not significantly greater than the exercise pressor reflex in which the hindlimb was freely perfused. Moreover, the pressor responses to postcontraction arterial occlusion in either the 3-min or 72-h occluded groups were modest, if they were present at all. At first glance, these findings contrast markedly with those in the cat and the human (1, 4, 8, 16, 21, 33). One must realize, however, that, after femoral arterial ligation in our experiments, the residual blood flow capacity to the hindlimb muscles of the rat was still three times greater than the blood flow measured when these muscles were inactive (34). Consequently, the mismatch between blood supply and demand in either the 72-h or the 3-min occluded groups may not have been severe enough to evoke a pressor response during the postcontraction period. Likewise, this mismatch probably does not explain the exaggerated pressor response during contraction in the 72-h occluded group. The residual blood flow capacity in the 72-h occluded rats may explain the contrast between our study in rats and those in humans and cats in which circulatory occlusion was induced by placing a cuff or ligature around the outside of the thigh and was then tightened to suprasystolic pressures. We speculate that, in these studies in humans and cats, there was little, if any, residual blood flow capacity in the contracting muscles.
We would also like to offer two alternative explanations for why femoral arterial occlusion had only a modest effect on the postcontraction pressor response that is frequently found in both cats and humans. The first is that the rat possesses fewer group IV metaboceptive afferents than does either the cat or the human. Indeed, comparisons of counts between myelinated and unmyelinated fibers in cats and rats can be viewed as consistent with this possibility (13, 26). The second alternative explanation is that dexamethasone prevented a postcontraction pressor response to occlusion. If this second explanation is the case, then the effect of this steroid, which was given to prevent swelling of the brain, is different from that in cats, which has a robust pressor response to postcontraction occlusion (17).
In several respects, the exercise pressor reflex in the rat is different from that in the cat, a species frequently used to study the reflex cardiovascular responses to contraction of hindlimb muscles. First, the magnitude of the pressor reflex response in the decerebrate rat is less than half that in the decerebrate cat (9, 29). Second, the threshold level of tension development by the statically contracting hindlimb muscles required to evoke a reflex pressor response in the rat is often near its maximal level (29), whereas the threshold level of tension development by the statically contracting hindlimb muscles required to evoke a reflex pressor response in the cat is frequently a quarter of its maximal level (9). Third, anesthesia prevents the exercise pressor reflex in the rat, whereas it modestly attenuates it in the cat (9, 29, 36).
The specific metabolite that is responsible for the augmented exercise pressor reflex remains to be identified. Possible candidates include cyclooxygenase metabolites of arachidonic acid, bradykinin, and lactic acid, each of which has been shown to stimulate thin fiber muscle afferents (18, 19, 23, 27). Moreover, both bradykinin and cyclooxygenase metabolites have been shown to sensitize group III muscle afferents to contraction (19, 25). Furthermore, circulatory occlusion is known to increase the production of both bradykinin and arachidonic acid by contracting muscles (24, 32). Alternatively, or in combination with increased concentrations of metabolites, receptor number may be increased by circulatory occlusion. The finding that hindlimb ischemia increases the production of nerve growth factor within hindlimb muscles may be the mechanism whereby the number or affinity of receptors on sensory endings is increased (6).
We note with interest that other pathophysiological states, namely congestive heart failure (28) and hypertension (31), have been shown to augment the exercise pressor reflex in rats. In hypertension, for example, the augmented reflex was attributed to an increase in the sensitivity to contraction by both mechano- and metaboreceptive thin fiber muscle afferents (14). In heart failure, the augmented reflex was attributed solely to an increase in the sensitivity of mechanosensitive thin fiber afferents (28, 30). Much less is known about the discharge properties of thin fiber muscle afferents in rats than in cats. In particular, their responses to static contraction in either healthy rats or in those with an underlying pathology remain to be described. Likewise, the mechanical and metabolic sensitivity of thin fiber muscle afferents in both healthy rats and those with peripheral vascular disease remain to be investigated.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-30710.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Jennifer Probst for technical assistance.
REFERENCES
- 1.Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crayton SC, Mitchell JH, Payne FC., III Reflex cardiovascular response during the injection of capsaicin into skeletal muscle. Am J Physiol Heart Circ Physiol 240: H315–H319, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106: 2257–2262, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Foster RW, Ramage AG. The action of some chemical irritants on somatosensory receptors of the cat. Neuropharmacology 20: 191–198, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Freund PR, Hobbs SF, Rowell LB. Cardiovascular responses to muscle ischemia in man: dependency on muscle mass. J Appl Physiol 45: 762–767, 1978 [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto GA, Waldrop TG, Kaufman MP, Botterman BR, Rybicki KJ, Mitchell JH. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. J Appl Physiol 59: 459–467, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50: 133–139, 1982 [DOI] [PubMed] [Google Scholar]
- 11.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 12.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Langford LA. Unmyelinated axon ratios in cat motor, cutaneous and articular nerves. Neurosci Lett 40: 19–22, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Maile MD, Sinoway A, Sinoway LI. The muscle pressor reflex: the potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol 97: 1709–1714, 2004 [DOI] [PubMed] [Google Scholar]
- 16.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCord JL, Hayes SG, Kaufman MP. Acid sensing ion and epithelial sodium channels do not contribute to the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1017–H1024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E-2. Brain Res 225: 95–105, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Mense S, Meyer H. Bradykinin-induced modulation of the response behavour of different types of feline group III and IV muscle receptors. J Physiol 398: 49–63, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol 417: 13–24, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Rotto DM, Massey KD, Burton KP, Kaufman MP. Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol 66: 2721–2724, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol 68: 861–867, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec 215: 71–81, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol 69: 1053–1059, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108: 1126–1132, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Smith SA, Mitchell GS, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC. Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol 69: 1225–1230, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Stebbins CL, Longhurst JC. Potentiation of the exercise pressor reflex by muscle ischemia. J Appl Physiol 66: 1046–1053, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586: 1649–1667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tibes U. Reflex inputs to the cardiovascular and respiratory centers from dynamically working canine muscles: some evidence for involvement of group III or IV nerve fibers. Circ Res 41: 332–341, 1977 [DOI] [PubMed] [Google Scholar]
- 36.Vissing J, Wilson LB, Mitchell JH, Victor RG. Static muscle contraction reflexly increases adrenal sympathetic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 261: R1307–R1312, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral Artery Occlusion Augments TRPV1-Mediated Sympathetic Responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]