Abstract
Increased matrix metalloproteinase (MMP) abundance occurs with adverse left ventricular (LV) remodeling in a number of cardiac disease states, including those induced by long-standing arrhythmias. However, whether regionally contained aberrant electrical activation of the LV, with consequent dyskinesia, alters interstitial MMP activation remained unknown. Electrical activation of the LV of pigs (n = 10, 30–35 kg) was achieved by pacing (150 beats/min) at left atrial and LV sites such that normal atrioventricular activation (60 min) was followed by regional early LV activation for 60 min within 1.5 cm of the paced site and restoration of normal atrioventricular pacing for 120 min. Regional shortening (piezoelectric crystals) and interstitial MMP activity (microdialysis with MMP fluorogenic substrate) at the LV pacing site and a remote LV site were monitored at 30-min intervals. During aberrant electrical stimulation, interstitial MMP activity at the paced site was increased (122 ± 4%) compared with the remote region (100%, P < 0.05). Restoration of atrioventricular pacing after the 60-min period of aberrant electrical activation normalized segmental shortening (8.5 ± 0.4%), but MMP activity remained elevated (121 ± 6%, P < 0.05). This study demonstrates that despite the restoration of mechanical function, disturbances in electrical conduction, in and of itself, can cause acute increases in regional in vivo MMP activation and, therefore, contribute to myocardial remodeling.
Keywords: myocardium, interstitium
acute as well as chronic activation of matrix metalloproteinases (MMPs) within the myocardium can disrupt the integrity of the extracellular matrix (ECM) and, consequently, affect interstitial protein content, stability, and bioactive signaling pathways (9, 14, 19). Mechanical stimuli, such as abnormal myocardial stress and strain patterns, can also result in MMP induction (8, 12, 20). In this context, Garcia et al. (11) demonstrated that acute arrhythmias can disrupt electrical activation pathways and, therefore, induce alterations in the stretch/contraction patterns of the myocardium and serve as a primary impetus for MMP activation and a potential nidus for stimuli to propagate a remodeling process. For example, previous studies (1, 22) have shown that short-duration disruption of electrical conduction induced in the atria can progress to the development of a sustained arrhythmia with associated remodeling of the myocardium. However, whether and to what degree an increase in MMP induction as a result of acute changes in electrical activation persist after the restoration of normal conduction patterns remain unknown. Therefore, the present study was designed to test the hypothesis that short-term (i.e., acute) alterations in the electrical activation of the myocardium would cause in vivo MMP activation and that the restoration of electrical conduction would normalize interstitial MMP activity.
METHODS
Instrumentation.
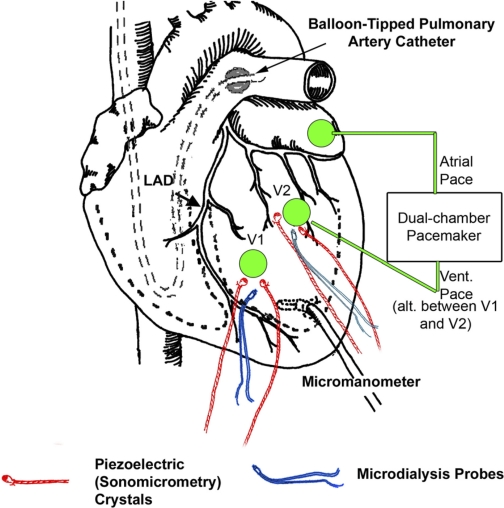
Animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Medical University of South Carolina Animal Care and Use Committee. Yorkshire pigs (n = 10, 30–35 kg, Hambone Farms, Orangeburg, SC) were instrumented with pacemaker leads, catheters for hemodynamic measurements of left ventricular (LV) pressure, arterial pressure, pulmonary artery pressure, and cardiac output, and microdialysis probes for measurements of interstitial membrane type 1 MMP (MT1-MMP) activity. Briefly, a thoracotomy was performed through a midline sternotomy in the anesthetized pigs, and a shielded pacemaker lead (Medtronic) was sutured onto the left atrial appendage. Two pairs of piezoelectric crystals (Sonometrics) were placed in the LV midmyocardium at two discrete locations. One pair of crystals, spaced 1.0–1.5 cm apart (to avoid surface blood vessels) and designated as site V1, was placed on the anterior free wall. The other pair of crystals, also spaced at 1.0–1.5 cm between crystals, was placed on the lateral LV wall between the second and third obtuse marginal coronary arteries emanating from the circumflex artery; this site was designated as site V2. The distance between the two pairs of crystals was between 2.0 and 2.5 cm and located approximately at half the distance between the apex and base of the LV. The anatomic location for the placement of the sonomicrometry crystal pairs at approximately the same distance between the apex and base of the LV was based on previous findings demonstrating regional differences with respect to contraction patterns based on the location of crystal pairs relative to the LV long axis (17). Microdialysis probes (CMA/20, CMA/Microdialysis) for interstitial MMP activity measurements were placed at each of the LV sites such that the tips of the probes were between the crystals at each site. To minimize any potential confounding influences from intrinsic regional variability in myocardial MMP activity measurements, the placement of the LV epicardial pacing lead was alternated between sites V1 and V2 on a pig-to-pig basis. A microtipped transducer (7.5 Fr, Millar Instruments, Houston, TX) was placed in the LV through a small apical stab wound. A schematic of this instrumentation protocol is shown in Fig. 1.
Fig. 1.
Schematic showing the instrumentation to implement early electrical activation of a portion of the left ventricle (LV) with concomitant collection of interstitial dialysate samples. Two pairs of piezoelectric crystals were placed in the LV midmyocardium at two discrete locations, designated as sites V1 and V2. Microdialysis probes were placed at each of the LV sites such that the tips of the probes were centered between the crystals at each site. A microtipped transducer was placed in the LV through a small apical stab wound, and a balloon-tipped catheter was placed in the pulmonary artery. An external dual chamber pacemaker was used for pacing of the atrial and ventricular sites. LAD, left anterior descending coronary artery.
An arterial line (8 Fr) was placed in the right carotid artery to continuously monitor systemic pressures, and a multilumened thermodilution catheter (7.5 Fr, Baxter Healthcare, Irvine, CA) was positioned in the pulmonary artery via the left external jugular vein. After instrumentation and a 30-min equilibration period, baseline measurements of heart rate, cardiac output, LV pressure, aortic pressure, pulmonary artery pressure, and pulmonary capillary wedge pressure were recorded.
Microdialysis.
Microdialysis probes with a molecular weight cutoff of 20 kDa and an outer diameter of 0.5 mm were placed in each of the two LV regions (2 probes/pig). A previously validated fluorogenic substrate specific for MMP-1, MMP-2, MMP-3, MMP-7, and MMP-9 (Calbiochem, La Jolla, CA) at a concentration of 60 μmol/l was infused at a rate of 5 μl/min through the two probes (8). The microdialysis system, with the substrate flowing through the connecting tubing, was allowed to equilibrate for 30 min before the beginning of the protocol. Throughout the experimental protocol detailed below, hemodynamic measurements and interstitial dialysate samples returning from both probes were collected into amber microcentrifuge tubes at 30-min intervals. On completion of the experiments, 100 μl of each dialysate sample were read at an excitation wavelength of 280 nm and an emission wavelength of 360 nm on a fluorescent microplate reader (FLUOstar Galaxy, BMG Technologies, Durham, NC).
Experimental timeline.
Baseline hemodynamics measurements and interstitial dialysates from both probes were collected with the pig in intrinsic normal sinus rhythm. After these measurements, pacing from the atrial site only was initiated at 150 beats/min and maintained for 60 min. Early electrical activation of the LV site was accomplished by a sequential pacing algorithm from a dual-chamber external pacemaker (Medtronic) such that the paced LV site would be depolarized earlier than the rest of the LV. Preliminary experiments (n = 2 pigs) were performed to determine the optimal atrioventricular (A-V) delay to affect early electrical activation. When paced from the atrial site at 150 beats/min, the P-R interval for these pigs was 124 ± 6 ms. Therefore, the A-V delay to the paced LV site was varied between 20 and 110 ms in 10-ms intervals. At A-V delays of <60 ms, the stimulation pulse captured the ventricular rhythm, resulting in the depolarization of the entire LV. At A-V delays of >100 ms, the pattern of shortening at the site of early depolarization remained similar to those recorded before pacing of the LV site was initiated, suggesting that normal LV depolarization through the His-Purkinje system captured the activation of the paced LV site. A-V delays of between 70 and 90 ms resulted in early activation of the paced LV site, and a clear difference in the contraction patterns of the stimulated and unstimulated LV sites was observed between 80 and 90 ms. Accordingly, an A-V delay of 80 ms was chosen as the optimal interval to achieve early electrical activation of the paced LV site. Electrical activation and mechanical contraction of the stimulated LV site was confirmed from the electrocardiogram recording (Fig. 2) as well as an earlier deflection of the sonomicrometry trace compared with the nonstimulated LV region. This atrial-ventricular pacing protocol was maintained for 120 min. For the final 120 min, the stimulus pulse to the LV site was turned off, thereby restoring pacing from only the atrial site. At the end of the pacing protocol, the LV was quickly harvested and placed in iced Krebs solution. Full thickness 1.0 × 1.0-cm sections from each of the two LV regions were obtained and flash frozen in liquid nitrogen for subsequent biochemical assays.
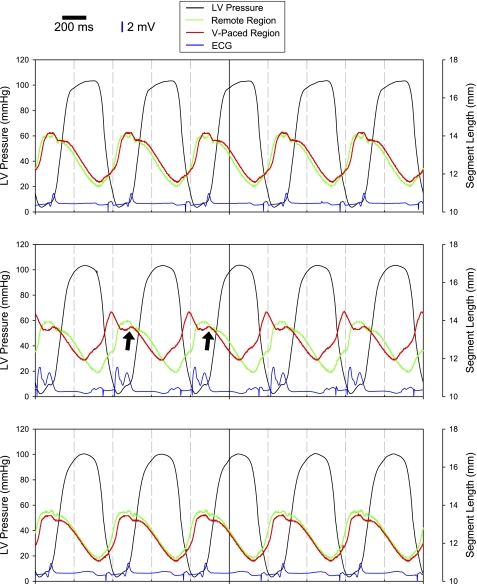
Fig. 2.
Tracings of LV pressure (black lines), ECGs (blue lines), and segmental distances in the remote region (green lines) and ventricular paced (V-paced) region (red lines). With pacing from the atrial site only (top), segmental shortening in the remote and V-paced region were similar in amplitude and phase. Pacemaker spikes before atrial and ventricular depolarization are clearly visible on the ECG tracings. With the initiation of atrioventricular (A-V) pacing (middle) with a shortened A-V interval, which caused the V-paced region to depolarize earlier than the rest of the LV, shortening in the V-paced region occurred earlier than in the remote region. The morphology of the QRS complex clearly shows early ventricular activation due to the introduction of the earlier stimulus followed by a second peak associated with normal ventricular depolarization. However, there was a secondary stretch in the V-paced region as contraction in the remote region was initiated (arrows). Restoration of pacing from the atrial site only (bottom) restored mechanical shortening of the V-paced region Scale bars = 200 ms along the time axis; vertical blue bars = 2 mV for the ECG recordings.
LV MMP and tissue inhibitors of metalloproteinase levels.
The abundance of representative MMP species from separate classes of this proteolytic enzyme family was determined in LV samples obtained from the remote region as well as the region of early electrical activation. Specifically, the abundance of MMP-13 (interstitial collagenase), MMP-2 and MMP-9 (gelatinases), and MT1-MMP was examined by immunoblot analysis or zymography. In addition, the regional myocardial abundance of the endogenous tissue inhibitors of MMP [tissue inhibitor of metalloproteinase (TIMP)-4] was examined by immunoblot analysis.
Relative LV MMP-2 and MMP-9 levels were examined using gelatin zymography (14). Purified human MMP-2 and MMP-9 (CC073, Chemicon) were included to provide internal standards. The relative abundance of MMP-13, MT1-MMP, and TIMP-4 were determined by immunoblot analysis (8, 10, 14). Briefly, tissue homogenates were standardized to a protein concentration of 1 μg/μl, loaded into a stacking gel, and electrophoretically separated. Gels were transferred onto nitrocellulose membranes (Bio-Rad semidry transfer blotter) and, after an overnight block with 5% milk, incubated with primary antisera [1 μg/ml anti-MMP-13 (AB8114), 0.1 μg/ml anti-MT-MMP-1 (AB38971) and 0.2 μg/ml anti-TIMP-4 (AB816), AbCam or Chemicon] at room temperature for 1 h. Membranes were washed, incubated for 1 h in peroxidase-conjugated secondary antibody (1:10,000 dilutions for MMP-13 and MT1-MMP and 1:5,000 dilution for TIMP-4) and then subjected to chemiluminescent activation (Renaissance chemiluminescence reagent plus, NEN). The luminescent signal was detected by an exposure to X-ray film (Eastman Kodak, Rochester, NY). Positive controls consisting of recombinant MMP-13 (CC068, Chemicon), MT-MMP1 proenzyme (CC1043, Chemicon), or TIMP-4 (CC1066, Chemicon) were included as appropriate. The zymograms and immunoblots were digitized, and relative MMP/TIMP levels were quantitated (Gel Pro Analyzer, Media Cybernetics) by two-dimensional integrated optical density.
COOH-terminus of collagen I telopeptide.
COOH-terminus of collagen I telopeptide (ICTP) levels were determined using a competitive enzyme immunoassay technique (catalog no. 06099, Orion Diagnostica, Espoo, Finland). Briefly, 50 μl of the interstitial microdialysate collected at each measurement time point from the stimulated and unstimulated LV regions were incubated with a known concentration of peroxidase conjugated ICTP protein and an ICTP primary antibody. Therefore, the amount of peroxidase-conjugated ICTP that would bind to the primary antibody would be inversely proportional to the amount of ICTP in the microdialysate samples. The solution was then incubated for 2 h at room temperature in a 96-well plate that was immobilized with a secondary antibody directed against the primary antibody. Microdialysate ICTP concentrations were calculated from a competitive semi-log calibration curve. The minimum detection limit for this ICTP immunoassay was 0.3 μg/l.
Data analysis.
Data were collected in a blinded fashion and remained coded until the end of the study. Hemodynamic parameters at the different time points were compared using repeated-measures ANOVA. The time course of changes in interstitial MMP activity and ICTP levels at the remote and paced regions was compared using a two-way ANOVA, where the LV region and time were considered the two main effects. Measured interstitial MMP activity and ICTP levels at the paced region were normalized to those recorded in the remote region and expressed as a percentage. A similar two-way ANOVA was performed to determine region- and time-dependent effects on the normalized interstitial MMP activity with the exception that the interaction term was omitted from the model since the normalization procedure causes the values of interstitial MMP activity and ICTP levels in the remote region to be 100%. For the biochemical measurements of MMP and TIMP levels, comparisons between the remote and paced regions were performed using a paired t-test. All statistical analyses were performed using the STATA statistical software package (Statacorp, College Station, TX). Values of P < 0.05 were considered to be statistically significant.
RESULTS
Hemodynamics.
Complete datasets were obtained for all 10 pigs included in this study. Upon instrumentation, and before the initiation of atrial pacing, the average heart rate was 109 ± 5 beats/min, with a range of 96–122 beats/min. Thus, there was no pig with an intrinsic heart rate of >150 beats/min. Consequently, none of the pigs needed pharmacological control of heart rate before the initiation of atrial pacing.
Hemodynamics recorded during the entire experimental timeline beginning with the initiation of atrial pacing at 150 beats/min are shown in Table 1. There were no significant differences in any of the measured hemodynamic parameters during the progression from atrial-only pacing to early ventricular pacing or when reverting to atrial-only pacing.
Table 1.
Hemodynamics with atrial-only pacing and early activation of the LV site with atrioventricular pacing
| Atrial-Only Pacing |
Atrioventricular Pacing |
Restoration of Atrial-Only Pacing |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | 210 min | 240 min | |
| LV pressures | |||||||||
| Peak pressure, mmHg | 112 ± 6 | 108 ± 5 | 110 ± 6 | 109 ± 6 | 107 ± 5 | 110 ± 3 | 112 ± 3 | 110 ± 2 | 110 ± 3 |
| LV end-diastolic pressure, mmHg | 9 ± 1 | 8 ± 1 | 8 ± 1 | 9 ± 2 | 10 ± 2 | 9 ± 2 | 9 ± 3 | 9 ± 2 | 10 ± 2 |
| Peak positive dP/dt, mmHg/s | 1,383 ± 105 | 1,285 ± 121 | 1,294 ± 113 | 1,275 ± 151 | 1,312 ± 138 | 1,344 ± 111 | 1,308 ± 119 | 1,296 ± 108 | 1,321 ± 103 |
| Peak negative dP/dt, mmHg/s | 1,924 ± 124 | 1,954 ± 131 | 1,908 ± 138 | 1,868 ± 153 | 1,906 ± 144 | 1,874 ± 133 | 1,860 ± 142 | 1,902 ± 120 | 1,881 ± 132 |
| Arterial pressures, mmHg | |||||||||
| Systolic pressure | 112 ± 7 | 106 ± 6 | 109 ± 6 | 109 ± 6 | 108 ± 7 | 112 ± 4 | 112 ± 3 | 108 ± 3 | 108 ± 4 |
| Diastolic pressure | 81 ± 7 | 78 ± 6 | 82 ± 5 | 80 ± 8 | 82 ± 7 | 83 ± 7 | 84 ± 6 | 84 ± 7 | 81 ± 5 |
| Mean pressure | 94 ± 6 | 90 ± 8 | 92 ± 7 | 90 ± 8 | 90 ± 7 | 95 ± 6 | 94 ± 6 | 92 ± 7 | 89 ± 6 |
| Pulmonary arterial pressures, mmHg | |||||||||
| Systolic pressure | 17 ± 2 | 16 ± 3 | 18 ± 1 | 16 ± 2 | 17 ± 3 | 18 ± 3 | 17 ± 3 | 18 ± 3 | 18 ± 2 |
| Diastolic pressure | 13 ± 3 | 13 ± 2 | 13 ± 2 | 13 ± 1 | 11 ± 4 | 13 ± 2 | 13 ± 2 | 12 ± 3 | 13 ± 3 |
| Mean pressure | 15 ± 3 | 14 ± 2 | 16 ± 2 | 14 ± 2 | 13 ± 3 | 14 ± 3 | 14 ± 3 | 14 ± 3 | 15 ± 2 |
| Pulmonary capillary wedge pressure | 10 ± 2 | 9 ± 1 | 7 ± 1 | 8 ± 1 | 9 ± 2 | 9 ± 2 | 10 ± 2 | 10 ± 2 | 9 ± 1 |
| Cardiac output, l/min | 3.1 ± 0.5 | 2.9 ± 0.4 | 3.0 ± 0.6 | 2.9 ± 0.6 | 2.9 ± 0.8 | 3.2 ± 0.6 | 2.9 ± 0.5 | 3.1 ± 0.5 | 3.0 ± 0.6 |
Values are presented as means ± SE. LV, left ventricular.
Segmental shortening.
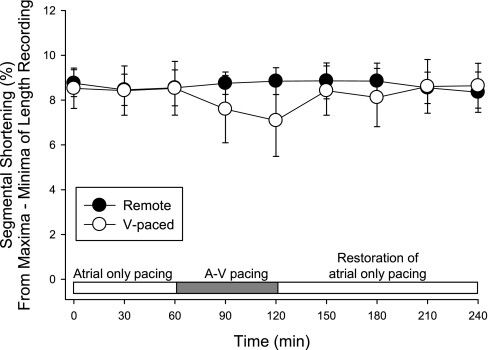
Before the initiation of A-V pacing, segmental shortening values at the sites V1 and V2 were 8.8 ± 0.6% and 8.1 ± 0.9%, respectively, with no differences with respect to the site of pacing (P = 0.81). With A-V pacing, segmental shortening patterns in the ventricular paced region reflected the earlier activation, regardless of whether sites V1 or V2 were stimulated. As shown in the representative series of tracings of LV pressure and segmental shortening in Fig. 2, atrial-only pacing (top) produced a normal pattern of segmental shortening at both LV sites. Initiation of early regional LV stimulation altered the phase of segmental shortening at the paced LV site such that regional shortening occurred earlier than segmental shortening in the unstimulated region (Fig. 2). Thus, based on this pattern of activation, fractional segmental shortening at the stimulated LV region was computed based on the pattern of early segmental shortening of the paced LV region, where fractional shortening was determined as the percent difference between maximum and minimum recording of the sonomicrometry trace. The results from these computations are shown in Fig. 3. There were no significant differences in the time course of changes in fractional shortening values between the two regions (Fig. 3). Restoration of atrial-only pacing resulted in the pattern of segmental shortening of the previously paced LV site to return to that recorded before ventricular pacing (Fig. 2).
Fig. 3.
Changes in segmental shortening with early ventricular activation. Segmental shortening was computed as the percent difference between minima and maxima recorded for each cardiac cycle. Segmental shortening in the V-paced region remained similar to that of the remote region during the period of A-V pacing. *P < 0.05 vs. the remote region.
Interstitial MMP activity and ICTP levels.
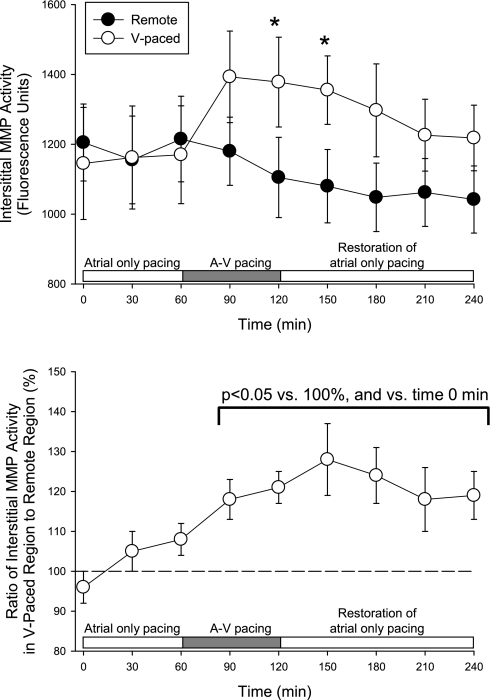
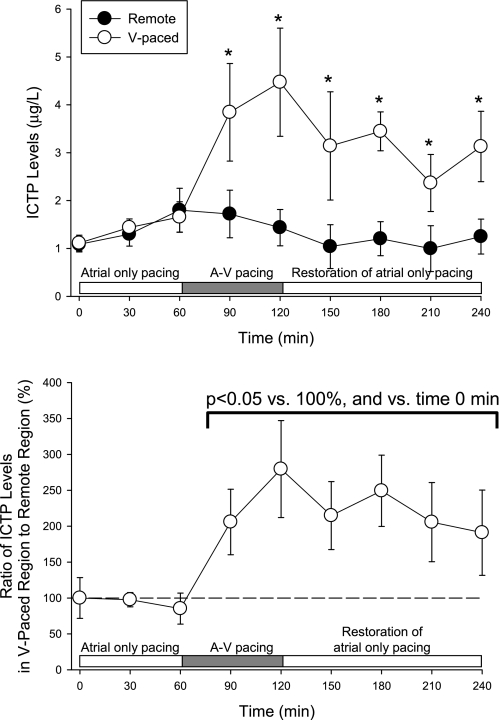
At baseline, before the initiation of atrial-only pacing, interstitial MMP activity was similar at the two LV sites (1,223 ± 81 vs. 1,133 ± 70 fluorescence units, P = 0.47). The time course of changes in interstitial MMP activity is shown in Fig. 4. With pacing at 150 beats/min from the atrial site only, interstitial MMP activity levels were similar in sites V1 and V2 (Fig. 4). With the initiation of A-V pacing, interstitial MMP activity in the ventricular paced region was higher than the corresponding unstimulated region, regardless of which pacing site (site V1 or V2) was used. In terms of fluorescence units, interstitial MMP activity in the paced region was significantly higher than in the remote region during the latter portion of the ventricular pacing period. There was a significant interactive effect between the regional rise in interstitial MMP activity and the time course of the pacing protocol (F ratio: 2.65, P = 0.009). When normalized to values recorded in the remote region (Fig. 4, bottom), interstitial MMP activity at the paced LV site was higher than the remote region during pacing of the LV site. With the restoration of atrial-only pacing, interstitial MMP activity remained elevated at the paced LV site compared with the remote LV site. Before the initiation of A-V pacing, ICTP levels (Fig. 5) were similar at the two LV sites. With the initiation of A-V pacing, ICTP levels in the region subjected to early electrical activation were higher than values in the corresponding unstimulated region. With the restoration of atrial-only pacing, ICTP levels in the previously paced region remained higher than in the unstimulated region (Fig. 5).
Fig. 4.
Before the initiation of A-V pacing, interstitial matrix metalloproteinase (MMP) activity was similar between the remote region and the region at which ventricular pacing would be applied. With A-V pacing, interstitial MMP activity was higher in the V-paced region by 120 min, and this effect was not observed in the remote region (top). Interstitial MMP activity within the V-paced region was normalized for simultaneous changes in the interstitial MMP activity in the remote region (bottom) to account for systematic alterations in MMP activity during the protocol. A significant increase in interstitial MMP activity was observed during A-V pacing, which persisted with the restoration of atrial-only pacing. *P < 0.05 vs. the remote region.
Fig. 5.
Levels of the COOH-terminal telopeptide of collagen type I (ICTP) in the remote and V-paced region. Before the initiation of A-V pacing, ICTP levels were similar between the remote region and the region at which ventricular pacing would be applied. With A-V pacing, ICTP levels increased in the V-paced region by 90 min, and this effect was not observed in the remote region (top). When normalized to time-matched values in the remote region, ICTP levels within the V-paced region was elevated with the initiation of A-V pacing and remained elevated with the restoration of atrial-only pacing. *P < 0.05 vs. the remote region.
MMP and TIMP biochemistry.
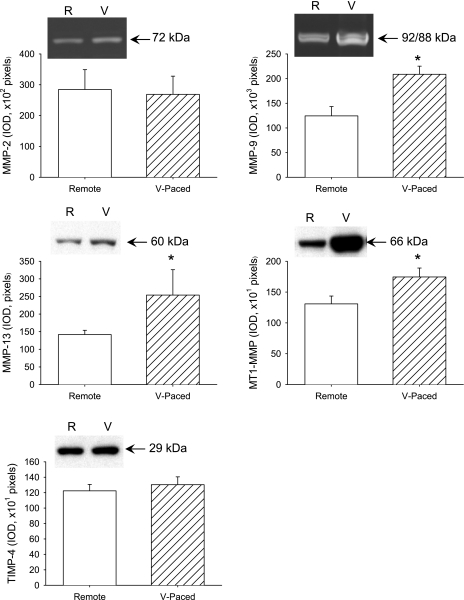
Representative zymograms and a summary of MMP-2 and MMP-9 findings are shown in Fig. 6. Whereas MMP-2 zymographic levels were similar in the remote and paced LV regions, MMP-9 levels were increased in the paced LV region compared with the remote region. Representative immunoblots for MMP-13, MT1-MMP, and TIMP-4 are shown in Fig. 6. Whereas MMP-13 and MT1-MMP levels were increased in the paced LV region compared with the remote region, TIMP-4 levels were similar between the two regions.
Fig. 6.
At the end of the pacing protocol, myocardium from the remote (R) and V-paced (V) regions were obtained and assayed for MMP-2, MMP-9 (gelatin zymography), MMP-13, membrane type 1 MMP (MT1-MMP), and tissue inhibitor of metalloproteinase (TIMP)-4 (immunoblot analysis). Representative zymograms or immunoblots for each analyte are shown. Positive controls were included for each assay. Bars represent the integrated optical density (IOD). MMP-9, MMP-13, and MT1-MMP levels were higher in the V-paced region compared with the remote region. *P < 0.05 vs. the remote region.
DISCUSSION
MMPs and endogenous TIMPs play critical roles in the normal and pathological remodeling of the myocardium (6, 7). MMPs are generally synthesized and released in a “on demand” basis due to the induction of neurohormonal and/or cytokine-mediated signaling pathways (9, 15). In addition, mechanical perturbation, including stretching of myocardial preparations, can induce MMP release. In this context, aberrant electrical activation can disrupt the normal contraction patterns within the LV and result in abnormal in vivo stretch/stress patterns in the myocardial region affected by the arrhythmia (11). However, an increase in MMP abundance may not necessarily translate to increased in vivo MMP activity since synthesized MMPs may not get activated and/or compensatory mechanisms, such as TIMPs, could be invoked simultaneously (9). Therefore, the effects of early regional activation on regional in vivo MMP activity remain unknown. In the present study, the time course of changes in interstitial MMP activity was determined with early electrical activation of a portion of the LV and after cessation of the aberrant stimulation pattern. The main findings of this study were that localized early electrical activation altered the pattern of mechanical functioning and increased interstitial MMP activity in the LV region with early electrical activation. Moreover, despite the restoration of mechanical function with the cessation of early electrical activation, interstitial MMP activity remained elevated and was associated with directional changes in the levels of several MMP types. These findings suggest that acute regional disruption of electromechanical functioning of the myocardium may serve as a sufficient impetus to initiate processes critical to myocardial remodeling.
To induce a localized arrhythmia, the present study used a pacing algorithm previously published by Garcia et al. (11). Specifically, using a dual-chamber pacemaker, a portion of the LV was “precontracted” by controlling the interval between stimulation pulses delivered to the atrial and ventricular stimulation sites. In the present study, the A-V delay for the early activation LV site was set at 80 ms, which was ∼75% of the intrinsic P-R delay when the atria were stimulated at a rate of 150 beats/min. Shuros et al. (18), in the context of inducing early electrical activation of the border region of a myocardial infarct (MI), demonstrated that the reduction in regional strain with early electrical activation was inversely related to the A-V delay relative to the intrinsic P-R interval. Specifically, at an A-V delay of 10% of the intrinsic P-R interval, Shuros et al. (18) observed a significant reduction in the shortening (i.e., strain) in the early activation region. At longer A-V delays (25%, 50%, and 75% of the P-R interval), the attenuation of segmental shortening in the early activation region was progressively reduced. Concomitantly, stroke work and cardiac output were reduced at A-V delays of 10% and 25% of the P-R interval. However, at A-V delays of 50% or greater of the P-R interval, stroke work and cardiac output were similar to values without early electrical activation. Consistent with the findings of Shuros et al. (18), cardiac output and developed LV pressures at the A-V delay used in the present study were not altered during the period of early electrical activation. A potential explanation of this finding is that with A-V delays that are close to the intrinsic P-R interval, the spread of depolarization in the region of myocardium that is activated early is relatively small and that LV pressure development is driven by depolarization of the LV through the normal His-Purkinje system. Since changes in LV loading conditions have been previously demonstrated to be associated with changes in myocardial MMP levels (8, 12, 20), the preservation of systemic hemodynamics in the model of early electrical activation used in the present study was an important consideration to determine whether and to what degree electromechanical activation of a portion of the myocardium altered regional MMP activation.
In the present study, early electrical activation caused an earlier initiation of shortening of the paced region, which was followed by a transient lengthening when the remainder of the myocardium was depolarized subsequent to normal activation through the His-Purkinje system. Therefore, aberrant early stimulation resulted in altered strain patterns of the paced region. Previous in vitro studies (12, 20) have demonstrated that stretching of myocardial preparations can induce MMP release. Consistent with previous results in this in vivo model (11), the present study demonstrated that acute changes in myocardial strain were associated with higher MMP-9 levels in the region subjected to early electrical activation. Moreover, the present study builds on these previous findings (11) by demonstrating that levels of collagenase, MMP-13, and MT1-MMP were increased in the region of early electrical activation. Since these MMP types can be activated through signaling pathways unrelated to direct stimulation by proinflammatory mediators (9), it is possible that other factors, such as mechanical stretch and associated changes in intracellular signaling cascades, also contributed to the changes in MMP levels. In addition, the present study demonstrated that the cumulative effects of the increase in the levels of these MMP types, with the concomitant preservation of TIMP-4 levels, was to increase in vivo interstitial MMP activity in the region of early electrical activation. Taken together, the findings of this short-term study suggest that even an acute disruption in the electrical and mechanical functioning of a portion of the myocardium can initiate processes that contribute to myocardial remodeling.
The observed increase in regional interstitial MMP activity during–and after–the period of abnormal ventricular activation suggests an increased potential for proteolysis of the ECM. Indeed, Garcia et al. (11) demonstrated that collagen degradation was higher in the region subjected to early electrical activation. The present study builds on these previous results by demonstrating that the increase in interstitial levels of ICTP, which is a marker for ECM proteolysis (3, 21), observed with regional early electrical activation persisted after the restoration of normal electrical activation. An overall loss of ECM scaffolding has been shown to cause functional deficits in terms of mechanical performance of ex vivo myocardial preparations (2). In the present study, the concomitant increases in interstitial MMP activity and ICTP levels during the period of early electrical activation suggest proteolytic damage to the ECM at the paced region. Moreover, the elevation in interstitial MMP activity and ICTP levels persisted after the cessation of aberrant electrical activation. However, there was a complete recovery of mechanical function with the cessation of early electrical activation. Taken together, these findings suggest that the elevation of interstitial MMP activity and ICTP levels that occurred during the short duration of early electrical activation may have been insufficient to cause substantial ECM degradation and consequent deterioration of mechanical function. Moreover, it is possible that abnormal regional myocardial function with early electrical activation initiated processes, such as cytokines, neurohormonal factors, and bioactive molecules, that then contributed to the hysteresis with respect to the elevation of interstitial MMP activity over the short followup duration. Whether and to what degree the persistent increase in interstitial MMP activity may alter regional function over longer durations remain unknown and warrant further investigation.
Limitations and conclusions.
Electrical activation of myocytes initiates a number of ionic processes, which then culminate in mechanical contraction (13, 16). Therefore, changes in the electrical activation pattern of the myocardium can alter the intracellular milieu of myocytes as well as the mechanical functioning of the affected myocardium (16). Since a number of intracellular ions, e.g., Ca2+, play crucial roles as mediators/activators in signaling cascades, and mechanical stretch can induce stretch-activated receptors, abnormalities in electrical activation may alter intracellular signaling pathways. However, given that the myocardium functions as a electromechanical syncytium, it remains problematic with respect to the determination of whether the effects on interstitial MMP activity that were observed in the present study were due to the aberrant timing of electrical activation or the subsequent change in regional mechanical function. Moreover, induction and activation of the MMPs may have occurred as a function of the elaboration of cytokines and other bioactive molecules, e.g., endothelin, caused by the alterations in regional shortening during the period of early electrical stimulation. However, the mechanism(s) that contributed to the increase in interstitial MMP activity during and after the period of aberrant electrical activation was not determined in the present study. Future studies that target specific signaling cascades and/or bioactive molecules would be needed to address this issue.
There is an emerging interest in using electrical stimulation of the myocardium as a means to attenuate and/or prevent adverse LV remodeling after MI (4, 5, 18). For example, in a clinically relevant porcine MI model, Shuros et al. (18) demonstrated that early electrical activation of the border region attenuated the increase in LV dimensions 8 wk after MI induction. In a group of MI patients with narrow QRS complexes implanted with cardiac resynchronization devices, Chung et al. (5) reported that early electrical activation of the MI border region was associated with a reduction in LV end-diastolic volumes 12 mo after the initiation of pacing. These results formed the basis for the ongoing Prevention of Myocardial Enlargement and Dilatation Post-MI clinical trial (4). In the present study, regional early electrical activation of a portion of the non-MI myocardium, using an A-V delay similar to that used by Shuros et al. (18) and Chung et al. (5), resulted in MMP activation. Whether regional early electrical activation maintained for longer durations would alter MMP induction and/or the balance between MMPs and TIMPs remains unknown and warrants further investigation. These issues notwithstanding, the results of the present short-term study demonstrate that even acute changes in the electromechanical functioning of the myocardium can initiate processes critical to remodeling of the myocardium.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-45024, HL-97012, and PO1-48788 and by a Veterans Affairs Merit Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Allessie M. The “second factor”: a first step toward diagnosing the substrate of atrial fibrillation? J Am Coll Cardiol 53: 1192–1193, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol 284: H122–H132, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Cavallari LH, Groo VL, Momary KM, Stamos TD, Vaitkus PT. Markers of cardiac collagen turnover are similar in patients with mild and more severe symptoms of heart failure. Congest Heart Fail 13: 275–279, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chung ES, Mazur W, Menon SG, Schloss EJ, Chow T, Kereiakes DJ. Peri-infarct pacing with CRT in the early postinfarct phase to attenuate long-term remodeling. J Cardiovasc Trans Res 2: 126–129, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Chung ES, Menon SG, Weiss R, Schloss EJ, Chow T, Kereiakes DJ, Mazur W, Salo RW, Galle E, Pastore JM. Feasibility of biventricular pacing in patients with recent myocardial infarction: impact on ventricular remodeling. Congest Heart Fail 13: 9–15, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cleutjens JP, Creemers EE. Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. J Card Fail 8: S344–S348, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 284: H364–H371, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Deschamps AM, Apple KA, Leonardi AH, McLean JE, Yarbrough WM, Stroud RE, Clark LL, Sample JA, Spinale FG. Myocardial interstitial matrix metalloproteinase activity is altered by mechanical changes in LV load: interaction with the angiotensin type 1 receptor. Circ Res 96: 1110–1118, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res 69: 666–676, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Deschamps AM, Yarbrough WM, Squires CE, Allen RA, McClister DM, Dowdy KB, McLean JE, Mingoia JT, Sample JA, Mukherjee R, Spinale FG. Trafficking of the membrane type-1 matrix metalloproteinase in ischemia and reperfusion: relation to interstitial membrane type-1 matrix metalloproteinase activity. Circulation 111: 1166–1174, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Garcia RA, Brown KL, Pavelec RS, Go KV, Covell JW, Villarreal FJ. Abnormal cardiac wall motion and early matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol 288: H1080–H1087, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Husse B, Briest W, Homagk L, Isenberg G, Gekle M. Cyclical mechanical stretch modulates expression of collagen I and collagen III by PKC and tyrosine kinase in cardiac fibroblasts. Am J Physiol Regul Integr Comp Physiol 293: R1898–R1907, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Niederer S, Nordsletten D, Le Grice I, Smail B, Kay D, Smith N. Coupling contraction, excitation, ventricular and coronary blood flow across scale and physics in the heart. Philos Transact A Math Phys Eng Sci 367: 2311–2331, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 107: 618–625, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee R, Mingoia JT, Bruce JA, Austin JS, Stroud RE, Escobar GP, McClister DM, Jr, Allen CM, Alfonso-Jaume MA, Fini ME, Lovett DH, Spinale FG. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol Heart Circ Physiol 291: H2216–H2228, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Rudy Y, Ackerman MJ, Bers DM, Clancy CE, Houser SR, London B, McCulloch AD, Przywara DA, Rasmusson RL, Solaro RJ, Trayanova NA, Van Wagoner DR, Varro A, Weiss JN, Lathrop DA. Systems approach to understanding electromechanical activity in the human heart: a national heart, lung, and blood institute workshop summary. Circulation 118: 1202–1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta PP, Khandheria BK, Korinek J, Wang J, Jahangir A, Seward JB, Belohlavek M. Apex-to-base dispersion in regional timing of left ventricular shortening and lengthening. J Am Coll Cardiol 47: 163–172, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Shuros AC, Salo RW, Florea VG, Pastore J, Kuskowski MA, Chandrashekhar Y, Anand IS. Ventricular preexcitation modulates strain and attenuates cardiac remodeling in a swine model of myocardial infarction. Circulation 116: 1162–1169, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Spinale FG, Coker ML, Bond BR, Zellner JL. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc Res 46: 225–238, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Tyagi SC, Lewis K, Pikes D, Marcello A, Mujumdar VS, Smiley LM, Moore CK. Stretch-induced membrane type matrix metalloproteinase and tissue plasminogen activator in cardiac fibroblast cells. J Cell Physiol 176: 374–382, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Umar S, Bax JJ, Klok M, van Bommel RJ, Hessel MH, den Adel B, Bleeker GB, Henneman MM, Atsma DE, van der Wall EE, Schalij MJ, van der Laarse A. Myocardial collagen metabolism in failing hearts before and during cardiac resynchronization therapy. Eur J Heart Fail 10: 878–883, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92: 1954–1968, 1995 [DOI] [PubMed] [Google Scholar]