Abstract
Obesity is a risk factor for stroke, but the determinants of increased stroke risk in obesity are unknown. We have previously reported that obese Zucker rats (OZRs) have a worse stroke outcome and display evidence of remodeling of the middle cerebral artery (MCA), in parallel with hypertension, compared with lean controls. This study tested the hypothesis that hypertension is an essential determinant of cerebral vascular remodeling and increased stroke damage in OZRs. Blood pressure was measured by telemetery in lean and obese rats with and without hydrochlorthiazide (HCT; 2 mg·kg−1·day−1) from 8 to 15 wk of age. A separate group of rats was also chronically fed a low-sodium (LS) diet. Vessel structure was assessed in isolated, pressurized MCAs. Cerebral ischemia was induced for 60 min using an intralumenal suture technique, followed by 24 h of reperfusion. HCT treatment effectively prevented the increase in blood pressure in obese rats; however, the LS diet did not lower pressure. Importantly, infarct size was normalized by HCT after ischemia-reperfusion injury. Additionally, HCT improved the changes in MCA structure observed in untreated OZRs. There were no benefits of the LS diet on stroke injury or vessel structure. These results indicate that increased pressure is essential for driving the changes in infarct size in OZRs.
Keywords: hypertension, vascular remodeling, hydrochlorothiazide
stroke is the third leading cause of death in the United States and the major cause of debilitation among adults. Obesity is also an escalating health problem and is considered a risk factor for cardiovascular disease, including stroke (15). Hypertension develops in many obese individuals and is considered the greatest modifiable risk factor for stroke (27). Although evidence suggests a relationship among obesity, hypertension, and end-organ damage, the extent to which hypertension contributes to stroke risk in obesity and the mechanisms thereof are largely unknown.
A previous study (22) from our laboratory demonstrated that adult obese Zucker rats (OZRs), a model of obesity, insulin resistance, and moderate hypertension (∼15-mmHg increase), suffer worse damage after cerebral ischemia compared with lean Zucker rats (LZRs). OZRs also show evidence of inward remodeling of the middle cerebral artery (MCA). Furthermore, these pathological differences were not observed in young OZRs, which are normotensive but already insulin resistant. These results suggest an association between increased blood pressure and cerebrovascular complications in OZRs.
A clear link between blood pressure and stroke outcome has been established in experimental models of severe hypertension. However, a direct relationship between pressure and stroke injury has not been determined in an obese model having a moderate, more clinically relevant increase in pressure similar to what is observed in obese humans. The purpose of this study was to determine the cerebrovascular effects of chronic maintenance of normal blood pressure in OZRs. Furthermore, this study tested the hypothesis that preventing the increase in blood pressure in OZRs would improve the response to cerebral ischemia and prevent inward remodeling of the MCA.
METHODS
Animals.
Male LZRs and OZRs were purchased from Harlan (Indiapolis, IN) at the age of 6 wk and were maintained on a 12:12-h light-dark cycle with access to food and water ad libitum. Rats were housed in an American Association for Accreditation of Laboratory Animal Care-accredited facility, and all protocols were approved by the Institutional Animal Care and Use Committee. LZRs and OZRs were randomly assigned to one of the following treatment groups: control or hydrochlorothiazide (HCT; Sigma, St. Louis, MO) treatment (2 mg·kg−1·day−1 in drinking water with 0.1% KCl supplementation). HCT treatment was initiated at 8 wk of age, before the onset of increased arterial pressure in OZR, and was maintained until 15 wk of age. HCT was replaced with regular drinking water 72 h before experimentation to allow for the drug to wash out, ruling out acute effects of HCT treatment. In a second protocol, the effects of salt intake on blood pressure, stroke injury, and cerebral vascular mechanics were assessed. LZRs and OZRs were fed sodium-restricted chow (0.02% sodium, CA.170950, Harlan Teklad) and were compared with rats receiving regular chow (0.2% sodium). Low sodium (LS) diet-fed rats were maintained on the diet from 8 to 15 wk of age.
Telemetry.
Six-week-old rats were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) in the abdominal aorta as previously described (11, 22). Rats were anesthetized using isoflurane in oxygen, and the abdominal aorta was exposed by a midline incision. The aorta was briefly occluded for the insertion of the transmitter catheter. The body of the transmitter was then sutured to the abdominal wall along the incision line as the incision was closed. The skin was closed with staples, which were removed 7 days after the procedure. After recovery, rats were placed on top of the telemetry receivers in individual cages, and arterial pressure waveforms were measured for 10 s every 10 min throughout the study. Average pressure was determined in 3-day increments. During the final week of recording, the number of times that systolic pressure exceeded a specified threshold (130 or 140 mmHg) was counted to determine the frequency of spikes in blood pressure.
MCA occlusion.
The intralumenal suture model developed by Longa et al. (18) was used to induce cerebral ischemia. Rats were initially anesthetized with isoflurane in an induction chamber; anesthesia was maintained with 2% isoflurane in oxygen. Body temperature was maintained at 37°C by a heating pad. A small incision was made to expose the skull for the attachment of a laser-Doppler flow probe (Perimed, Stockholm, Sweden) to monitor cerebral blood flow. The common carotid artery was exposed by a midline incision. The lingual and thyroid arteries were cauterized, and the external carotid and pterygopalatine arteries were ligated with 6-0 suture. A 3-0 nylon monofilament with a rounded end was inserted into the common carotid artery and was advanced through the internal carotid artery to block blood flow at the origin of the MCA. A drop in laser-Doppler flow was used to verify MCA occlusion. Ischemia was induced for 1 h, and blood flow was then restored by pulling back the filament. Importantly, after the incision was closed, rats were allowed to wake up from anesthesia during the ischemic period, minimizing the exposure to isoflurane. After 24 h of reperfusion, rats were anesthetized and decapitated for removal of the brain. Brains were sliced into 2-mm sections and stained with 2% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO) to assess ischemic damage. Brains were then fixed in 2% paraformaldehyde (Sigma-Aldrich). Using digital images of brain slices, the percentage of infarction was determined by the following equation: Percent hemisphere infarcted = [(VL − VC)/VC] × 100, where VL is the volume of the lesioned hemisphere and VC is the volume of the control hemisphere (31).
Plasma measurements during stroke.
Separate groups of LZRs and OZRs with and without HCT treatment were used for the determination of arterial blood gases and glycemia during stroke. Rats were fasted before experimentation. Contrary to the rats used for infarct determination, these rats remained anesthetized during ischemia to allow for blood samples to be taken. The femoral artery was catheterized before the initiation of the MCA occlusion procedure. A baseline blood sample was taken. Blood samples were also taken 10 min after MCA occlusion (early ischemia), just before reperfusion (late ischemia), and 10 min after reperfusion. Blood gases, pH, and glycemia were analyzed using an iSTAT CG8+ cartridge (Heska, Loveland, CO).
Other plasma and urine measurements.
Trunk blood was collected from rats for the measurement of plasma aldosterone (Siemens, Los Angeles, CA), insulin (Alpco, Salem, NH), triglycerides (Wako Diagnostics, Richmond, VA), and cholesterol (Wako Diagnostics). Control and LS diet-fed rats were individually housed in metabolic cages before the initiation of the LS diet and then every 2 wk during the remainder of the study in 3-day increments. The first day was considered an equilibration day, and measurements were taken on the remaining 2 days. Food and water consumption were monitored, and urine was collected. Urinary albumin excretion was measured with an SPI Bio Rat Albumin EIA kit (Cayman Chemical, Ann Arbor, MI). Urinary and plasma electrolytes were measured with an EasyElectrolytes Analyzer (Medica, Bedford, MA).
MCA structure assessment.
After decapitation, brains were removed and MCAs were isolated. Vessels were cannulated on glass micropipettes of a pressure myograph (Living Systems Instrumentation, Burlington, VT) and equilibrated at 80 mmHg in Krebs buffer [containing (in mmol/l) 118 NaCl, 23.8 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.18 KH2PO4, and 11.1 glucose] gassed with 21% O2-5% CO2-balance N2 and maintained at 37°C. The intralumenal pressure of MCAs was controlled by a pressure servo control via a transducer connected to the pressure myograph. Vessels were visualized on a monitor, and lumen diameter and wall thickness were measured using a video dimension analyzer. Pressure-response curves were conducted in the absence of calcium to determine passive lumen diameter over a range of intralumenal pressures (0–140 mmHg). Measurements were made in naïve vessels that had not been exposed to ischemia.
Statistics.
Physiological parameters and cerebral infarct size were compared between LZRs, OZRs, LZRs + HCT treatment, and OZRs + HCT treatment using two-way ANOVA. Two-way ANOVA was also used to compare blood pressure, glycemia during stroke, and lumen diameters between these groups. t-Tests were used to compare physiological parameters and infarct size in rats fed a LS diet; blood pressure and pressure-response curves were compared by two-way ANOVA. P values of <0.05 were considered statistically significant. Values are presented as means ± SE.
RESULTS
Physiological parameters for LZRs and OZRs with and without HCT treatment are shown in Table 1. Compared with LZRs, OZRs had increased body mass and evidence of cardiac and renal hypertrophy. While there was an effect of treatment on body and heart and kidney masses (P < 0.05 by two-way ANOVA), body mass was not statistically different between OZRs and OZRs + HCT treatment; conversely, HCT treatment resulted in improvements in cardiac and renal hypertrophy. Untreated OZRs also had increased plasma levels of insulin, cholesterol, and triglycerides. Importantly, HCT treatment did not improve these indexes of metabolic dysfunction in OZRs.
Table 1.
Physiological parameters and plasma measurements in control and HCT-treated rats
| Parameter | LZRs | OZRs | HCT-Treated LZRs | HCT-Treated OZRs | Strain Effect? | Drug Effect? |
|---|---|---|---|---|---|---|
| Body mass, g | 401 ± 10 | 595 ± 15* | 340 ± 6† | 560 ± 11* | Yes | Yes |
| Heart mass, g | 1.18 ± 0.05 | 1.33 ± 0.04* | 0.97 ± 0.02† | 1.17 ± 0.03*† | Yes | Yes |
| Kidney mass, g | 1.34 ± 0.05 | 1.75 ± 0.18* | 1.07 ± 0.03† | 1.45 ± 0.04*† | Yes | Yes |
| Insulin, μg/l | 1.2 ± 0.5 | 5.4 ± 0.9* | 0.3 ± 0.1 | 5.4 ± 0.8* | Yes | No |
| Triglycerides, mg/dl | 72.6 ± 10.4 | 277.3 ± 56.0* | 32.0 ± 11.0 | 316.5 ± 49.9* | Yes | No |
| Cholesterol, mg/dl | 70.6 ± 6.7 | 121.5 ± 16.7* | 55.1 ± 5.2 | 115.7 ± 8.3* | Yes | No |
Values are means ± SE. LZRs, lean Zucker rats; OZRs, obese Zucker rats; HCT, hydrochlorthiazide.
P < 0.05 vs. treatment-matched LZRs;
P < 0.05 vs. strain-matched untreated rats.
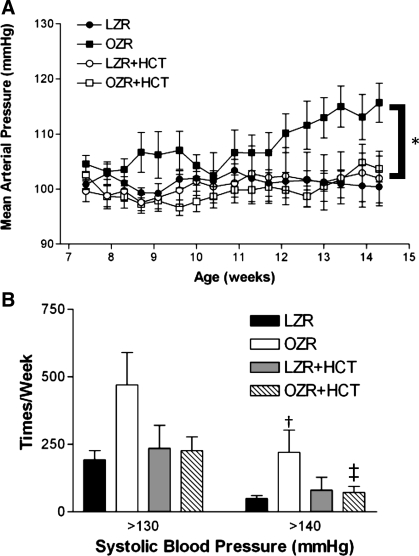
As previously reported by our laboratory and others (1, 9, 11, 22, 23, 28), blood pressure progressively increased in adult OZRs compared with LZRs. Treatment with HCT was sufficient to prevent this increase in blood pressure in OZRs and had no effect on pressure in LZRs (Fig. 1A). The frequency of spikes in blood pressure was also evaluated in the final week of treatment. Specifically, the occurrence of systolic blood pressures of >130 and 140 mmHg was counted. When the groups were compared, the elevated pressure counts at 130 mmHg did not reach statistical significance (P = 0.0749 by two-way ANOVA); however, OZRs demonstrated significantly more frequent bouts of periodic hypertension than other groups at 140 mmHg (Fig. 1B). This increased count was corrected by treatment with HCT in OZRs.
Fig. 1.
A: mean arterial blood pressures (MAP) measured by telemetry in lean Zucker rats (LZRs) and obese Zucker rats (OZRs) with and without hydrochlorothiazide (HCT) treatment. Values are 3-day averages. B: frequency of elevated systolic blood pressure during the final week of the study. Values are means ± SE. *P < 0.05 vs. LZRs, HCT-treated LZRs (LZR + HCT), and HCT-treated OZRs (OZR + HCT); †P < 0.05 vs. treatment-matched LZRs; ‡P < 0.05 vs. strain-matched untreated rats.
At the end of the 7-wk HCT treatment, rats were subjected to cerebral ischemia-reperfusion injury. After 1 h of ischemia and 24 h of reperfusion, control OZRs suffered more stroke damage than LZRs, consistent with our previous findings (22). HCT treatment normalized this increase in infarct size in OZRs but was without effect in LZRs (Fig. 2). Importantly, the degree of MCA occlusion was similar in all groups, as confirmed by a similar drop in laser-Doppler blood flow. Also, the improvement in infarct size in HCT-treated OZRs appears to be due to chronic normalization of blood pressure rather than acute changes in blood pressure regulation during stroke. Lean and obese rats with and without thiazide treatment had similar blood pressure responses to stroke. Namely, all rats demonstrated a similar blood pressure response to cerebral ischemia (Fig. 3); pressure was statistically increased in the face of ischemia and remained elevated during the reperfusion period. Therefore, differences in the acute blood pressure response to stroke are not an explanation for the worse stroke outcome in OZRs or the improved outcome in HCT-treated OZRs.
Fig. 2.
Bottom: outcome of cerebral ischemia in LZRs and OZRs with and without HCT treatment quantified as the percentage of the hemisphere infarcted after 1 h of ischemia and 24 h of reperfusion. Representative brain slices are also shown (top). Values are means ± SE. *P < 0.05 vs. LZRs; †P < 0.05 vs. strain-matched untreated rats.
Fig. 3.
Blood pressure responses to ischemia-reperfusion injury. Pressure was monitored via telemetry during the ischemia and reperfusion periods after middle cerebral artery occlusion in LZRs and OZRs with and without HCT treatment. Values are means ± SE.
Blood gases and pH were not different between groups at baseline or at any time point measured during the ischemia protocol. Baseline and late ischemia levels are shown in Table 2. Blood glucose levels were elevated in OZRs before the occlusion of the MCA (Fig. 4), likely due to anesthesia because OZRs are normoglycemic at the age used in the present study (11, 22). Interestingly, the glycemic response to stroke was different between LZRs and OZRs. LZRs maintained a relatively constant glycemic status throughout ischemia, and this was not affected by HCT treatment. OZRs, on the other hand, demonstrated progressively increased blood glucose levels throughout the duration of the stroke; in OZRs, glycemia was statistically higher in the early stages of ischemia than at baseline and was significantly elevated at the end of ischemia compared with the beginning of ischemia. The increased glycemic load, however, was not affected by treatment with HCT, suggesting that the elevation in blood glucose levels in OZRs during stroke is not driving the worse stroke damage as HCT-treated OZRs have an impaired glycemic response to stroke but an improvement in injury.
Table 2.
Arterial pH and Pco2 before and during stroke
| Parameter | LZRs | OZRs | HCT-Treated LZRs | HCT-Treated OZRs | Strain Effect? | Drug Effect? |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| pH | 7.44 ± 0.02 | 7.42 ± 0.01 | 7.42 ± 0.005 | 7.45 ± 0.01 | No | No |
| Pco2 | 49.4 ± 2.6 | 52.3 ± 0.8 | 48.3 ± 1.3 | 55.8 ± 1.3 | No | No |
| Ischemia | ||||||
| pH | 7.44 ± 0.02 | 7.40 ± 0.02 | 7.43 ± 0.01 | 7.45 ± 0.02 | No | No |
| Pco2 | 48.9 ± 1.5 | 50.7 ± 3.1 | 48.6 ± 2.2 | 49.8 ± 1.0 | No | No |
Values are means ± SE.
Fig. 4.
Glucose profile during stroke. Blood samples were taken from LZRs and OZRs with and without HCT treatment during baseline, early ischemia, late ischemia, and reperfusion time points. Values are means ± SE. *P < 0.05 vs. treatment-matched LZRs within the time point; †P < 0.05 vs. the previous time point.
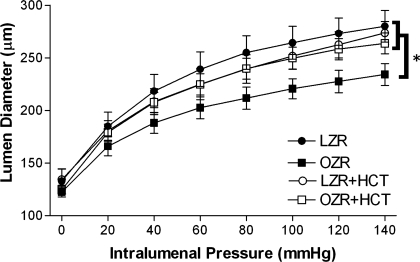
As there is a direct relationship between vessel lumen diameter and flow, MCA lumen diameter was measured in each group of rats over a range of pressures under passive conditions. As expected from previous findings, OZRs exhibited evidence of inward remodeling of the MCA (Fig. 5), providing a possible mechanism of worse stroke injury. Chronic treatment with HCT significantly improved inward remodeling of the MCA in OZRs, with a maximum lumen diameter similar to that of LZRs.
Fig. 5.
Passive pressure-diameter relationships. Lumen diameter was measured in isolated, pressurized vessels from LZRs and OZRs with and without HCT treatment under passive conditions. Values are means ± SE. *P < 0.05 vs. LZRs, HCT-treated LZRs, and HCT-treated OZRs.
Physiological parameters of rats fed a LS diet are shown in Table 3. LS diet-fed OZRs did not gain as much weight as control OZRs but were still significantly obese compared with LS diet-fed LZRs. Rats were placed in metabolic cages to determine food consumption as an attempt to explain the decreased body mass in LS diet-fed rats. At the end of the experimental period, rats fed with a sodium-deficient diet ate less than those fed regular chow. This difference in food consumption likely explains the difference in body mass between control and LS diet-fed rats. Urinary electrolytes were also measured to verify the decreased sodium excretion in LS diet-fed rats. As shown in Table 3, plasma sodium content was normal in all groups; however, sodium excretion was below detection (1 meq) in LS diet-fed rats. Obese rats with and without sodium restriction demonstrated evidence of renal injury, as indicated by increased urinary albumin excretion; this was not affected by diet. As expected during sodium restriction, LZRs and OZRs fed a LS diet had a marked increase in plasma aldosterone concentrations, but there were no differences between groups.
Table 3.
Physiological parameters in rats fed a LS diet
| Parameter | LZRs | OZRs | LS Diet-Fed LZRs | LS Diet-Fed OZRs | Strain Effect? | Diet Effect? |
|---|---|---|---|---|---|---|
| Body mass, g | 374 ± 15 | 565 ± 21* | 299 ± 8† | 450 ± 11*† | Yes | Yes |
| Food consumption, g/day | 21 ± 2 | 29 ± 5* | 15 ± 1 | 21 ± 1† | Yes | Yes |
| Plasma Na+, mM | 139 ± 3 | 138 ± 2 | 141 ± 1 | 139 ± 2 | No | No |
| Urinary Na+ excretion, mEq | 1.4 ± 0.1 | 2.3 ± 0.3* | <1 | <1 | ||
| Urinary albumin excretion, mg/day | 0.26 ± 0.02 | 14.04 ± 5.03 | 0.23 ± 0.05 | 13.16 ± 8.46 | Yes | No |
| Plasma aldosterone, pg/ml | 222 ± 47 | 91 ± 15 | 8895 ± 1364† | 8708 ± 1363† | No | Yes |
Values are means ± SE. LS diet, low-sodium diet.
P < 0.05 vs. diet-matched LZRs;
P < 0.05 vs. strain-matched control rats.
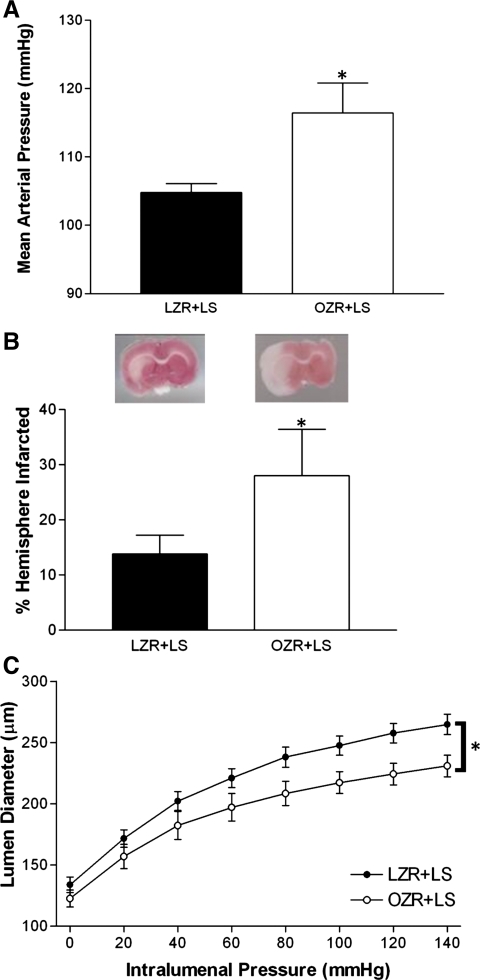
Blood pressure was monitored throughout the diet manipulation and is shown in Fig. 6A. Restricting sodium consumption in OZRs did not affect mean arterial pressure (MAP); by adulthood, pressure was significantly elevated in LS diet-fed OZRs compared with LS diet-fed LZRs, much like what was observed in control OZRs. LS diet-fed rats were subjected to cerebral ischemia at the end of the diet protocol. MCA occlusion was induced for 1 h, followed by 24 h of reperfusion. Stroke damage was worse in LS diet-fed OZRs compared with LS diet-fed LZRs (Fig. 6B), indicating that there was no effect of diet on infarct size. Sodium restriction also failed to influence passive MCA diameter (Fig. 6C).
Fig. 6.
A: MAP measured by telemetry in LZRs and OZRs fed a low-sodium (LS) diet. Values are 3-day averages. B, bottom: responses to 1 h of ischemia and 24 h of reperfusion in LZRs and OZRs fed a LS diet. Representative brains slices are also shown (top). C: passive pressure-diameter relationship of the middle cerebral artery from LZRs and OZRs fed a LS diet. Values are means ± SE. *P < 0.05 vs. LS diet-fed LZRs.
DISCUSSION
The present study demonstrates that the moderate increase in blood pressure observed in OZRs is a major risk factor for stroke injury in this model of obesity. The key findings relevant to this observation are that 1) control of blood pressure with a diuretic prevents stroke injury and vascular remodeling; 2) these improvements were independent of changes in metabolic or endocrine status; and 3) these changes were not accounted for by salt intake, as hypertension and stroke injury are resistant to a LS diet in OZRs.
Experimental and clinical evidence have established a clear relationship between blood pressure and stroke occurrence and outcome. Hypertension is considered the greatest modifiable risk factor for stroke, and several clinical trials have indicated beneficial effects of lowering blood pressure on the occurrence of stroke. Specifically, the Heart Outcomes Prevention Evaluation Study and Perindopril Protection Against Recurrent Stroke Study showed that antihypertensive therapy improves the occurrence of new-onset and recurrent strokes, respectively, compared with placebo (23a, 33). Similar results are true for animal studies. For example, the spontaneously hypertensive rat, a model of malignant hypertension, has a worse outcome after cerebral ischemia than normotensive counterparts (10); chronic treatment with the antihypertensive agent hydralazine resulted in improvement in stroke injury (30). The novelty of the present study is that OZRs demonstrate a moderate increase in blood pressure, yet we report improvement in stroke injury when this increase in blood pressure is prevented.
Beyond elevations in MAP, the present study also demonstrates that the frequency of pressure spikes is increased in OZRs compared with lean counterparts. While average blood pressure is only moderately elevated in OZRs, these findings suggest that they may suffer greater damage to perfused tissues with more frequent exposure to spikes in pressure. Treatment with HCT not only prevented the increased MAP in OZRs but also normalized the increased frequency of pressure elevations, perhaps buffering pressure-induced end-organ damage.
Of note, there were no differences between groups in pH or arterial Pco2 before or during ischemia. Therefore, differential control of these parameters does not explain the increase in infarct size in OZRs. We (11, 22) have previously demonstrated that OZR are normoglycemic at the age used in the present study, but, interestingly, OZRs demonstrate evidence of altered glycemic status during ischemia, consistent with a previous report (3) of glucose intolerance in OZRs. Before the initiation of the MCA occlusion procedure, blood glucose concentrations measured in anesthetized rats were elevated in all obese rats, and OZRs with and without HCT treatment exhibited a dramatic increase in glycemic load during ischemia and just after reperfusion; glycemic status remained relatively unchanged in both groups of LZRs.
Siemkowicz and colleagues (29) showed that increasing blood glucose levels before 10 min of complete brain ischemia led to increased mortality rates in rats. Also, a recent study by Martín et al. (19) demonstrated that the induction of acute hyperglycemia in nonobese rats is associated with worse stroke injury after transient MCA occlusion. Furthermore, a meta-analysis conducted by Capes et al. (8) indicated that hyperglycemia is associated with worse functional outcome and increased mortality in humans. Although stroke-induced hyperglycemia is a potential mechanism by which stroke injury is increased in OZRs, the finding that HCT-treated OZRs have a comparable increase in blood glucose levels during stroke but have decreased stroke injury suggests that the hyperglycemia is not sufficient to explain the increased infarct in OZRs.
There is much evidence of hypertension-induced alterations in the structural properties of cerebral arteries, and several models of hypertensive rats display evidence of cerebrovascular remodeling (4–7, 13). Alterations in the structural properties of cerebral blood vessels may affect the perfusion of brain tissue. As vessel size is directly proportional to flow, decreased lumen diameter leads to flow reductions, especially in the face of ischemia. The present study demonstrates that OZRs have a decrease in lumen diameter of the MCA, suggesting impaired perfusion capability during stroke, which was normalized by preventing the increased blood pressure in OZRs. Therefore, these results demonstrate that lumen diameter is associated with blood pressure and stroke outcome in OZRs.
It has also been demonstrated that other rodent models of obesity suffer more damage after cerebral ischemia. For example, the ob/ob mouse has an increase in infarct size after ischemia-reperfusion injury compared with lean controls (20, 32). Also, Nagai et al. (21) demonstrated that both ob/ob mice and high-fat-diet fed mice have greater stroke injury than lean counterparts after photochemically induced thrombosis of the MCA. Rats fed a high-fat diet also show evidence of worse stroke outcome and vascular remodeling (12). The link between obesity and stroke extends into clinical studies as well, including a study by Kurth et al. (17) demonstrating an increased risk of both ischemic and hemorrhagic stroke in men with a body mass index over 30.
In addition to OZRs, LZRs were also treated with HCT to determine whether the reported improvements in cerebrovascular complications in OZRs were due to nonspecific effects of treatment with HCT. The administration of HCT to LZRs did not lower blood pressure, suggesting that any differences observed between control LZR and HCT-treated LZRs would be attributable to pressure-independent effects of the drug. However, the response to cerebral ischemia was not improved by HCT in LZRs, and the functional and structural characteristics of the MCA were similar in control and HCT-treated LZRs. These findings suggest that improvements in stroke injury and passive lumen diameter in HCT-treated OZRs are due to maintenance of normal blood pressure in these rats.
The thiazide diuretic HCT was used as an antihypertensive agent in this study in attempts to avoid directly manipulating the renin-angiotensin system (RAS) or directly affecting smooth muscle tone with agents such as hydralazine. Evidence suggests that RAS blockade has beneficial effects on the response to cerebral ischemia beyond blood pressure reduction (14). Therefore, attempting to lower blood pressure in OZRs by blocking a component of this system could confound interpretation of the results regarding stroke injury. Whereas it has been suggested that there are indirect vasodilatory effects of HCT, evidence is lacking. A study by Zhou et al. (34) demonstrated that chronic HCT treatment lowers blood pressure in Dahl salt-sensitive rats on a high-salt diet; however, there was no improvement in endothelium-dependent vasodilation in the aorta. Furthermore, the dose of HCT used Zhou and colleagues (75 mg·kg−1·day−1) was higher than that of the present study (2 mg·kg−1·day−1), further negating the potential for nonspecific vasodilatory effects of HCT in the present study.
The second component of this study was to examine the effect of salt consumption on blood pressure and cerebrovascular function in OZRs. Since food intake is increased by ∼30% in OZRs (26), it is conceivable that increased sodium intake explains both the increased pressure and worse stroke outcome, as evidence suggests that increased sodium consumption increases blood pressure in OZRs (24). Contrary to our expectations, the LS diet did not lower pressure in OZRs. Blood pressure was elevated in LS diet-fed OZRs to a similar degree as what was observed in control OZRs. This is consistent with a previous report (16) of shorter regimen of LS feeding. A previous study by Riazi et al. (25) may shed light on the ineffectiveness of sodium restriction to decrease blood pressure in OZRs. LZRs and OZRs were switched from a normal salt diet to 0.02% NaCl. Blood pressure fell in OZRs when the diet was switched; however, it began to return to baseline after a few days. The study was not carried out beyond 8 days, so there is no information about the blood pressure effects of the LS diet after that point. In addition to failing to elicit an effect on blood pressure, the LS diet did not improve stroke outcome or MCA lumen diameter in OZRs. These results provide new evidence that blood pressure is not salt sensitive in OZRs, even over extended periods of time, and stroke outcome is not determined by sodium intake per se.
Taken together, the results from the present study demonstrate that moderate hypertension is required for stroke damage in the context of obesity in OZRs. The hypertensive damage that explains stroke may be the chronic effects of moderate hypertension or the periodic systolic hypertension that is more evident in obese animals. Moreover, while metabolic dysfunction is insufficient to cause stroke injury alone, it may alter the relationship among blood pressure, vascular function, and stroke risk. Unraveling the next generation of these mechanisms is worthy of future study.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant R01-HL-076533 and an American Heart Association Grant-In-Aid (to D. W. Stepp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. William Rainey and Tony Cohen for assistance with the aldosterone measurements.
REFERENCES
- 1.Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension 28: 1047–1054, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Apweiler R, Freund P. Development of glucose intolerance in obese (fa/fa) Zucker rats. Horm Metab Res 25: 521–524, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Arribas SM, Gonzalez C, Graham D, Dominiczak AF, McGrath JC. Cellular changes induced by chronic nitric oxide inhibition in intact rat basilar arteries revealed by confocal microscopy. J Hypertens 15: 1685–1693, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Arribas SM, Gordon JF, Daly CJ, Dominiczak AF, McGrath JC. Confocal microscopic characterization of a lesion in a cerebral vessel of the stroke-prone spontaneously hypertensive rat. Stroke 27: 1113–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension 21: 816–826, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13: 968–972, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32: 2426–2432, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35: 403–408, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Coyle P. Different susceptibilities to cerebral infarction in spontaneously hypertensive (SHR) and normotensive Sprague-Dawley rats. Stroke 17: 520–525, 1986 [DOI] [PubMed] [Google Scholar]
- 11.D'Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW. Exaggerated cardiovascular stress responses and impaired beta-adrenergic-mediated pressor recovery in obese Zucker rats. Hypertension 48: 1109–1115, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Deutsch C, Portik-Dobos V, Smith AD, Ergul A, Dorrance AM. Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc Res 78: 100–106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn WR, Wallis SJ, Gardiner SM. Remodelling and enhanced myogenic tone in cerebral resistance arteries isolated from genetically hypertensive Brattleboro rats. J Vasc Res 35: 18–26, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Fujii K, Weno BL, Baumbach GL, Heistad DD. Effect of antihypertensive treatment on focal cerebral infarction. Hypertension 19: 713–716, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67: 968–977, 1983 [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Snider T, Abel M, Zemel MB. Hypertension in young, healthy Zucker obese rats is not responsive to reduced salt intake. J Nutr 124: 713–716, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, Buring JE, Manson JE. Body mass index and the risk of stroke in men. Arch Intern Med 162: 2557–2562, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Martin A, Rojas S, Chamorro A, Falcon C, Bargallo N, Planas AM. Why does acute hyperglycemia worsen the outcome of transient focal cerebral ischemia? Role of corticosteroids, inflammation, and protein O-glycosylation. Stroke 37: 1288–1295, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Mayanagi K, Katakam PV, Gaspar T, Domoki F, Busija DW. Acute treatment with rosuvastatin protects insulin resistant (C57BL/6J ob/ob) mice against transient cerebral ischemia. J Cereb Blood Flow Metab 28: 1927–1935, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai N, Van Hoef B, Lijnen HR. Plasminogen activator inhibitor-1 contributes to the deleterious effect of obesity on the outcome of thrombotic ischemic stroke in mice. J Thromb Haemost 5: 1726–1731, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension 53: 381–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overton JM, Williams TD, Chambers JB, Rashotte ME. Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 280: R1007–R1015, 2001 [DOI] [PubMed] [Google Scholar]
- 23a.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358: 1033–1041, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Reddy SR, Kotchen TA. Dietary sodium chloride increases blood pressure in obese Zucker rats. Hypertension 20: 389–393, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Riazi S, Khan O, Hu X, Ecelbarger CA. Aldosterone infusion with high-NaCl diet increases blood pressure in obese but not lean Zucker rats. Am J Physiol Renal Physiol 291: F597–F605, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Romanko OP, Stepp DW. Reduced constrictor reactivity balances impaired vasodilation in the mesenteric circulation of the obese Zucker rat. Am J Physiol Heart Circ Physiol 289: H2097–H2102, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Schreihofer AM, Hair CD, Stepp DW. Reduced plasma volume and mesenteric vascular reactivity in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R253–R261, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Siemkowicz E, Hansen AJ. Clinical restitution following cerebral ischemia in hypo-, normo- and hyperglycemic rats. Acta Neurol Scand 58: 1–8, 1978 [PubMed] [Google Scholar]
- 30.Slivka A. Effect of antihypertensive therapy on focal stroke in spontaneously hypertensive rats. Stroke 22: 884–888, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10: 290–293, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke 39: 943–950, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Zhou MS, Schulman IH, Jaimes EA, Raij L. Thiazide diuretics, endothelial function, and vascular oxidative stress. J Hypertens 26: 494–500, 2008 [DOI] [PubMed] [Google Scholar]