Abstract
The mechanisms responsible for anti-arrhythmic protection during ischemia-reperfusion (IR) in exercised hearts are not fully understood. The purpose of this investigation was to examine whether the ATP-sensitive potassium channels in the mitochondria (mito KATP) and sarcolemma (sarc KATP) provide anti-arrhythmic protection in exercised hearts during IR. Male Sprague-Dawley rats were randomly assigned to cardioprotective treadmill exercise or sedentary conditions before IR (I = 20 min, R = 30 min) in vivo. Subsets of exercised animals received pharmacological inhibitors for mito KATP (5-hydroxydecanoate) or sarc KATP (HMR1098) before IR. Blinded analysis of digital ECG tracings revealed that mito KATP inhibition blunted the anti-arrhythmic effects of exercise, while sarc KATP inhibition did not. Endogenous antioxidant enzyme activities for total, CuZn, and Mn superoxide dismutase, catalase, and glutathione peroxidase from ischemic and perfused ventricular tissue were not mitigated by IR, although oxidative stress was elevated in sedentary and mito KATP-inhibited hearts from exercised animals. These findings suggest that the mito KATP channel provides anti-arrhythmic protection as part of exercise-mediated cardioprotection against IR. Furthermore, these data suggest that the observed anti-arrhythmic protection may be associated with preservation of redox balance in exercised hearts.
Keywords: cardioprotection, ischemia-reperfusion, myocardial, oxidative stress, preconditioning
cardiovascular disease (CVD) is the leading cause of morbidity and mortality in industrialized countries (3). Injurious processes related to both ischemia and reperfusion, collectively termed ischemia-reperfusion (IR) injury, are among the most prevalent manifestations of CVD (8). IR injury reflects the ever-changing bioenergetic environment of the myocardium and involves progressive levels of damage, beginning with ventricular arrhythmias in the moments following coronary occlusion. In accordance, the scientific and medical communities have sought for decades to uncover endogenous mechanisms of protection as a means of countermeasure development against IR injury, including arrhythmia. As such, preemptive activation of known, and yet to be identified, protective mechanisms precondition the myocardium against acute IR (8). Cardiac preconditioning via exercise is among the most potent and consistent stimuli for eliciting resistance to IR injury (reviewed in Ref. 42). To date, the cellular mechanisms responsible for cardiac preconditioning in exercised hearts are not fully understood.
Early work to uncover mechanisms of exercise-mediated cardioprotection revealed an essential role for the endogenous antioxidant manganese superoxide dismutase (MnSOD) against lethal and nonlethal arrhythmias generated during IR insults (25). That elevated MnSOD enzyme activity plays an essential protective role in exercised hearts is logical in light of the fact that oxidative stress is a cornerstone of IR-mediated arrhythmia generation. Findings to implicate MnSOD as a protective mediator, however, do not completely account for the IR-resistant phenotype observed in exercise hearts (22, 25). Indeed, the notion that multiple protective factors are at work in exercise preconditioning is consistent with previous research on other forms of cardiac preconditioning, where myriad mechanisms exert cellular protection, both independently and in synergistic fashion (7, 24).
The ATP-sensitive potassium (KATP) channel is a potential protective mechanism at work in exercised hearts. The functional link between exercise and ischemic protection likely pertains to the energy-sensing capacity of this ion channel, which is closed in the presence of abundant ATP and opened by stress-related signals and falling cellular ATP levels (reviewed in Ref. 31). While the protection afforded by the KATP channel opening is not fully understood, recent evidence indicates that KATP channel subtypes on the inner mitochondrial membrane (mito KATP channel) and the sarcolemma (sarc KATP channel) provide significant protection through independent and interrelated means (35, 40). Preemptive opening of KATP channels with pharmacological or physiological stimuli elicits protection against IR-generated arrhythmias (35, 45). In accordance, we undertook the present investigation to test the hypothesis that the mito KATP channel is essential for exercise-mediated protection against IR-generated arrhythmias. Results from previous investigations of exercised hearts indicate the sarc KATP channel is essential for myocardial infarct sparing effects of exercise (12, 15, 17). Thus we also tested the rival hypothesis that the sarc KATP channel was responsible for the anti-arrhythmic protection provided by exercise. These hypotheses were carried out using a rat model of surgically induced IR following a short-duration (days) exercise preconditioning stimulus that was previously shown to be cardioprotective (22, 25, 43). Pharmacological inhibitors selective for both the mito and sarc KATP channels were used on an individual basis to test the contribution of the respective mito and sarc KATP channel mediation of anti-arrhythmic protection in exercised hearts. Finally, we explored whether mito and sarc KATP channel-mediated protection in exercised hearts was associated with preservation of endogenous antioxidant enzymes and attenuation of oxidative stress in the myocardium during IR.
METHODS
Animals and experimental design.
The experimental protocol was approved by the Appalachian State University Animal Care and Use committee and followed guidelines established by the American Physiological Society for the use of animals in research. Adult male Sprague-Dawley rats (∼4 mo old) were randomly assigned to cardioprotective exercise or sedentary (Sed) treatments. During the experimental period, all animals were housed on a 12:12-h light-dark cycle and provided rat chow (AIN93) and water ad libitum.
Cardioprotective exercise protocol.
Rats assigned to the exercise treatment received, over 10 days, an exercise regimen previously shown to elicit a cardioprotected phenotype against IR injury (22, 25, 43). The exercise regimen began with a habituation period of 5 consecutive days of treadmill exercise. Treadmill habituation involved a gradual increase in running time began on day 1 with 10 min of exercise. Successive days introduced time increases of 10 min/day, concluding with 50 min of total exercise on the 5th and final day of treadmill habituation. At the conclusion of the treadmill habituation period, animals received 2 days of rest, followed by 3 consecutive days of 60-min exercise bouts. Treadmill speed and grade were fixed at 30 m/min and a 0% grade, respectively, for both treadmill habituation and exercise training periods.
Exercise and Sed group study treatments.
Rats assigned to the exercise treatment were further randomized into groups to receive one of three treatments; either saline placebo (Ex), 5-hydroxydecanoate (5HD, 10 mg/kg), a selective pharmacological inhibitor to the mito KATP channel (Ex 5HD), or HMR1098 (10 mg/kg), a selective pharmacological inhibitor to the sarc KATP channel (Ex HMR1098). HMR1098 was a generous gift of Dr. Heinz Golgelein, Adventis Pharma, Germany. Placebo and pharmacological doses were administered via intraperitoneal injection 45 min before anesthesia for IR experimentation in vivo, based on previous work (23). Rats assigned to the Sed treatment were further randomized into either sham (no ischemia) or Sed treatments.
In vivo IR protocol.
Twenty-four hours following the final day of the exercise protocol, rats were exposed to a nonsurvival IR protocol in vivo, involving coronary artery ligation, as described previously (25, 43). Before surgery, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (65 mg/kg). After a surgical plane of anesthesia was reached, a tracheotomy was performed, and animals were mechanically ventilated (Kent Scientific, Torrington, CT) with room air. A catheter was placed in the jugular vein for supplemental delivery of pentobarbital sodium (10 mg/kg) as needed to maintain the surgical plane of anesthesia. An additional catheter attached to a pressure transducer was placed in the carotid artery for real-time assessment of arterial blood pressure. Simultaneous ECG recordings were monitored from limb leads. ECG and blood pressure data were interfaced to a personal computer using a physiological data-acquisition system (Biopac, Santa Barbara, CA). Following a left thoracotomy, a ligature was placed around the left anterior descending coronary artery close to its origin. The ligature was threaded through a small flexible piece of tubing, which was pressed against the coronary artery with a small surgical clamp to induce ischemia. The heart was observed to have both cyanotic and perfused regions. The appearance of ventricular ectopy was used to confirm induction of ischemia. Ligation of the coronary was maintained for 20 min, after which the surgical clamp was removed for a 30-min reperfusion period. Sed rats received an IR challenge identical to Ex rats. Sham rats received an identical surgery, save for induction of ischemia, for a 50-min time period. At the end of IR and sham protocols, ligatures were tightened, and a small volume of 1% Evans blue die was infused into the arterial blood for differentiation of perfused and nonperfused cardiac regions. Hearts were rapidly excised and dissected into perfused and nonperfused regions for quantification of the respective tissue masses. Ventricular tissue was rinsed in cold buffer, flash frozen, and stored at −80°C for subsequent biochemical analysis.
Arrhythmia scoring.
Electrocardiographic tracings were analyzed for ventricular arrhythmias to assess the magnitude of ventricular ectopy for the various treatments. Digital ECG files were read in a treatment-blinded fashion by three trained reviewers, who coded for preventricular contractions (PVCs), ventricular tachycardia (VT), and ventricular fibrillation (VF). An established arrhythmia scoring system was applied to digital arrhythmia tracings of ischemia and reperfusions, in accordance with Lambeth Convention guidelines previously used to assess clinical ECG responses to an ischemic insult (18–19, 46).
Analysis of antioxidant enzyme activity.
To assess the effects of exercise training and IR challenge on myocardial antioxidant enzyme capacity, perfused and nonperfused sections of left ventricular sections were homogenized in ice-cold 100 mM phosphate buffer (1:20 wt/vol, pH 7.4). Homogenates were centrifuged at 400 g for 10 min at 4°C. The supernatant was assayed for total protein content (11), along with the activities of SOD and its respective CuZn (CuZnSOD) and Mn (MnSOD) isoforms (36), glutathione peroxidase (GPx) (21), and catalase (CAT) (1) activities. All analyses were performed, at minimum, in quadruplicate at 25°C and were assayed on the same day to avoid interassay variation. The coefficients of variation for SOD, GPx, and CAT assays ranged between 2 and 5%.
Analysis of tissue oxidative stress.
To determine the effects of exercise training and IR on tissue oxidative stress, two oxidative stress biomarkers and the reduced and oxidized forms of glutathione, GSH and GSSG, respectively, were assessed in both perfused and nonperfused tissue from all treatments. Myocardial protein carbonyl formation was assessed per manufacturer's instructions, using commercially available ELISA kits (Northwest Lifesciences, Vancouver, WA). Myocardial 4-hydroxynonenal content was evaluated per manufacturer's instructions via commercially available ELISA kits (Cell Biolaboratories, San Diego, CA). Myocardial GSH and GSSG content were assayed per manufacturer's instructions using commercially available assay kits (Cayman Chemical, Ann Arbor, MI).
Data analysis.
One-way ANOVA was used to evaluate perfused and nonperfused tissue areas, arrhythmia scoring data, and ventricular ectopy data. Two-way ANOVA was used to evaluate antioxidant enzyme data and oxidative stress measures. Significant group differences were determined via Tukey post hoc analysis. Kruskal-Wallis nonparametic analyses were used to assess differences in the incidence of ventricular ectopy. Significance was established a priori at P < 0.05.
RESULTS
Animal characteristics.
For this investigation, 52 animals were used. Animal attrition due to complications during the experimental surgery, such that the experimental protocol was incomplete, occurred once or twice for each of the respective IR treatments. Data from the remaining 45 animals, including animal numbers, body weights, and heart weights, are presented in Table 1. Statistical analysis revealed that body weights and heart weights were similar for all treatments. Analysis of the nonperfused tissue weight-to-total heart weight ratio (Fig. 1) indicates a similar involvement of ischemic tissue for all treatments receiving IR. Furthermore, the nonperfused tissue weight-to-total heart weight ratio from the four IR treatments was statistically similar to the nonperfused (nonischemic) tissue weight-to-total heart weight ratio from sham hearts.
Table 1.
Animal body and heart weights
| Group | n | Body Weight, g | Heart Weight, g |
|---|---|---|---|
| Sham | 8 | 345 ± 16.9 | 1.25 ± 0.06 |
| Sed | 9 | 346 ± 13.1 | 1.28 ± 0.05 |
| Ex | 10 | 322 ± 14.0 | 1.32 ± 0.04 |
| Ex 5HD | 9 | 324 ± 13.2 | 1.27 ± 0.04 |
| Ex HMR1098 | 9 | 318 ± 11.2 | 1.22 ± 0.04 |
Values are means ± SE; n, no. of animals. Sham, sham surgery without ischemia where nonperfused tissue equates to the ischemic region in all other treatments; Sed, sedentary; Ex, exercised placebo; Ex 5HD, exercised, 5-hydroxydecanoate mitochondrial ATP-sensitive potassium (KATP) channel inhibitor; Ex HMR1098, exercised, sarcolemmal KATP channel inhibitor.
Fig. 1.
Quantification of ischemic and perfused cardiac tissue in hearts exposed to ischemic and sham treatments. Values are mean percentages ± SE. Sham, sham surgery without ischemia where nonperfused tissue equates to the ischemic region in all other treatments; Sed, sedentary; Ex, exercised placebo; Ex 5HD, exercised, 5-hydroxydecanoate mitochondrial ATP-sensitive potassium (KATP) channel inhibitor; Ex HMR1098, exercised, sarcolemmal KATP channel inhibitor. P < 0.05.
Electrocardiographic activity during IR and sham treatments.
Ventricular arrhythmias were observed within minutes of surgically induced ischemia in all animals, with the majority of ectopic events occurring during ischemia (Fig. 2B). Episodes of VT and VF were observed in animals from all treatments receiving IR, although not all Ex animals experienced episodes of VT or VF. Notably, the majority of ventricular ectopy was observed during ischemia rather than reperfusion (Table 2). Statistical analyses of PVCs and episodes of VT and VF (data not shown) experienced during ischemia and reperfusion failed to produce a significant treatment by time effect (PVC, P = 0.240; VT, P = 0.216; VF, P = 0.156). Only three animals from the sham group experienced PVCs, presumably in response to passing the surgical ligature through excitable ventricular tissue. No sham animals experienced VT or VF.
Fig. 2.
A: representative images of digital ECG tracings from sham and ischemic conditions. B: ventricular arrhythmia scores. Values are means ± SE. Significantly different from *sham and #Ex, P < 0.05.
Table 2.
Effects of exercise and mitochondria and sarcolemma KATP channel inhibition on arrhythmia incidence, duration, and severity during ischemia and reperfusion
| Arrhythmia Incidence, no./% |
Arrhythmia Duration, s |
||||||
|---|---|---|---|---|---|---|---|
| Group | n | Period | PVC | VT | VF | VT | VF |
| Sham | 8 | Perfused | 3/38 | 0/0 | 0/0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Sed | 9 | Ischemia-reperfusion | 9/100* | 8/89* | 7/78* | 185.6 ± 117.4 | 160.4 ± 94.5 |
| Ischemia | 9/100* | 8/89* | 7/78* | 151.0 ± 92.3 | 156.1 ± 91.5 | ||
| Reperfusion | 9/100* | 6/67* | 3/33 | 34.6 ± 25.1 | 4.3 ± 3.3 | ||
| Ex | 10 | Ischemia-reperfusion | 8/80 | 7/70* | 3/30 | 45.0 ± 19.1 | 3.4 ± 2.1 |
| Ischemia | 8/80 | 7/70* | 3/30 | 40.4 ± 18.7 | 3.4 ± 2.1 | ||
| Reperfusion | 8/80 | 5/50* | 0/0 | 4.6 ± 2.2 | 0.0 ± 0.0 | ||
| Ex 5HD | 9 | Ischemia-reperfusion | 8/89* | 8/89* | 5/56* | 89.5 ± 21.7 | 62.6 ± 23.0 |
| Ischemia | 8/89* | 8/89* | 5/56* | 62.7 ± 16.2 | 59.1 ± 20.6 | ||
| Reperfusion | 8/89* | 7/78* | 2/22 | 26.8 ± 11.4 | 3.5 ± 2.4 | ||
| Ex HMR1098 | 9 | Ischemia-reperfusion | 7/78 | 7/78* | 2/22 | 31.6 ± 10.5 | 2.0 ± 1.3 |
| Ischemia | 7/78 | 7/78* | 2/22 | 24.1 ± 10.6 | 1.0 ± 0.8 | ||
| Reperfusion | 7/78 | 4/44 | 1/11 | 7.6 ± 4.2 | 1.0 ± 0.5 | ||
Arrhythmia incidence values are no. of incidence/group percentage. Arrhythmia duration values are means ± SE; n, no. of animals. PVC, preventricular contraction; VT, ventricular tachycardia; VF, ventricular fibrillation.
Significantly different from sham, P < 0.05.
Analysis of arrhythmia scoring-system-derived values of the collective ventricular ectopy experienced during IR reveal that, compared with sham, IR elicited a significant rise in ventricular arrhythmias (Fig. 2B). In contrast, exercise resulted in significant, albeit partial, attenuation of IR-induced arrhythmias. In contrast, the mean arrhythmia scores for Ex 5HD animals were statistically identical to those for Sed, indicating that the mito KATP channel contributes to exercise-mediated protection against arrhythmias during IR. Ex HMR1098 arrhythmia scores, alternately, were similar to those of Ex, indicating that the sarc KATP channel is not essential for anti-arrhythmic protection against IR in exercised hearts. Individual evaluation of PVCs between the five treatments (Fig. 3A) revealed mean response, which was similar to the arrhythmia scoring data. Due to large variations in PVCs identified between animals, however, statistical significance was only present between sham animals and Sed, Ex 5HD, and Ex HMR1098 treatments. Similarly, findings for VT (Fig. 3B) and VF (Fig. 3C) revealed nonsignificant differences between the four IR treatments, with the exception of a significant difference in the number of VF episodes between Ex 5HD and Ex HMR1098 treatments.
Fig. 3.
A: preventricular contractions/ischemia-reperfusion experiment. B: ventricular tachycardia episodes/ischemia-reperfusion experiment. C: ventricular fibrillation episodes/ischemia-reperfusion experiment. Values are means ± SE. Significantly different from *sham and ♦Ex 5HD, P < 0.05.
Myocardial antioxidant enzyme activity in perfused and ischemic tissue.
To determine the impact of antioxidant enzyme activity on the preservation of myocardial redox status, several important endogenous antioxidant enzymes were evaluated in both ischemic and perfused tissue exposed to IR. Evaluation of SOD activities demonstrated that total SOD activities (Fig. 4A) were not impacted significantly between ischemic and perfused tissue for the four IR treatments. Similar findings were present for CuZnSOD (Fig. 4B). In contrast, MnSOD enzyme activity levels (Fig. 4C) were elevated significantly in both perfused and ischemic tissues of all three exercised treatments undergoing IR compared with sham and Sed IR treatments. Elevated MnSOD activity was consistent between ischemic and perfused tissue for all three exercised treatments. Evaluation of myocardial CAT activity (Fig. 5A) indicated no differences between treatments exposed to IR for either perfused or ischemic tissue. Similarly, perfused and ischemic myocardial GPx enzyme activities (Fig. 5B) were similar between sham and the four IR treatments.
Fig. 4.
A: total myocardial superoxide dismutase activity. B: myocardial CuZn superoxide dismutase activity. C: myocardial Mn superoxide dismutase activity. Values are means ± SE. Open bars represent perfused tissue. Solid bars represent ischemic or nonperfused (sham) tissue. Significantly different from *sham and ΨSed, P < 0.05.
Fig. 5.
A: myocardial catalase activity. B: myocardial glutathione peroxidase activity. Values are means ± SE. Open bars represent perfused tissue. Solid bars represent ischemic or nonperfused (sham) tissue. P < 0.05.
Myocardial oxidative stress following IR.
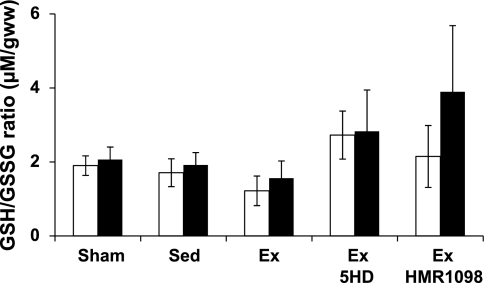
Several biomarkers of myocardial redox status were evaluated to determine the magnitude of oxidative stress imposed by the experimental IR protocol. Analysis of total myocardial protein carbonyl content (Fig. 6A) revealed that IR resulted in a significant rise in the ischemic tissue of Sed and Ex 5HD hearts. In contrast, myocardial 4-hydroxynonenal content (Fig. 6B) was not impacted by IR in either perfused or ischemic tissue from any treatment group. Finally, the short-duration IR employed in this investigation did not impact the GSH/GSSG levels in the perfused and ischemic myocardium (Fig. 7).
Fig. 6.
A: myocardial protein carbonyl content. B: myocardial 4-hydroxynonenal content. Values are means ± SE. Open bars represent perfused tissue. Solid bars represent ischemic or nonperfused (sham) tissue. Significantly different from *sham, ΨSed, ♦Ex 5HD, and Ωperfused: P < 0.05.
Fig. 7.
Myocardial GSH/GSSG (μmol/g wet wt) content. Values are means ± SE. Open bars represent perfused tissue. Solid bars represent ischemic or nonperfused (sham) tissue.
DISCUSSION
Summary of principal findings.
This is the first study to investigate whether the mito and sarc KATP channels are essential for exercise-mediated attenuation of arrhythmia generation during IR. Results confirm previous study findings, which indicate that exercised hearts exposed to experimental IR suffer fewer and less severe arrhythmias compared with Sed counterparts (6, 25, 27). The current finding that the exercised heart is 5HD sensitive extends on existing knowledge, demonstrating that the mito KATP channel may promote anti-arrhythmic protection during IR, while the sarc KATP channel does not. Findings from the current investigation suggest that the anti-arrhythmic effects of mito KATP channels in exercised hearts may be associated with improved myocardial redox status.
Exercise and cardiac preconditioning against IR-induced arrhythmias.
Hull et al. (27) demonstrated that a 6-wk exercise regimen completely abolished the incidence of VF in dogs identified to be at high risk for ventricular ectopy. Exercise elicited numerous anti-arrhythmic cardiovascular adaptations, including improvements in heart rate variability and vagal tone (27). A more recent study in dogs demonstrated that a single bout of treadmill exercise completely prevented VF during IR and raised survival by 7.5-fold over Sed controls (6). The first mechanistic examination of exercise and arrhythmia prevention was performed by Hamilton et al. (25), where 3 days of exercise before IR resulted in significantly lower incidence and severity of ventricular arrhythmias. The endogenous antioxidant enzyme MnSOD was partially responsible for this protection, as demonstrated by the fact that anti-arrhythmic protection was mitigated in Ex animals that received antisense oligonucleotides directed against MnSOD transcripts (25). Data from the present study, which utilized identical species, sex, exercise preconditioning, and IR protocols, builds on the existing knowledge by demonstrating that the mito KATP channels in exercised myocardium may also contribute to anti-arrhythmic protection against IR. That both MnSOD and the mito KATP channel protect against IR-generated arrhythmias suggests myriad protection occurs in exercised hearts. In contrast, the sarc KATP channels were not essential for exercise-induced protection against IR-generated arrhythmias. This latter finding, although not universally supported, may indicate that preemptive opening of the sarc KATP channel could promote arrhythmias through heterogenous repolarization of the myocardium (41, 47).
KATP channels and arrhythmia prevention during IR.
The constitutively expressed, energy-sensing KATP channels support metabolic function by opening in response to a host of stimuli, including a drop in cytosolic ATP levels or elevation in bioenergetic metabolites and stress-related signals (31). While IR resistance afforded by the KATP channel opening is not fully understood, recent evidence indicates that channel subtypes on the inner mitochondrial membrane and on the sarcolemma provide significant protection through independent and interrelated means (35, 40). Although some debate persists regarding the anti-arrhythmic potential of KATP channel opening, preemptive mito and sarc KATP channel activation for improved cardiac health and performance in the face of acute IR is established (34). Protection is partially attributed to prevention of reentrant arrhythmias by mitigating phase 3 of the cardiac action potential during early ischemia and low-flow perfusion (39). The precise roles of these ion channels across biochemically diverse physiological scenarios related to IR, however, are not fully understood. Early studies in myocytes from exercised hearts revealed that cardiac KATP channel opening elicited resistance to anoxic stress, while the nonselective KATP channel inhibitor, glibenclamide, abolished this protection (28). Follow-up study indicated that exercise stimulated overexpression of the sulfonylurea receptor portion of the sarc KATP channel promoted protection against IR injury (15). Selective pharmacological blockade of the sarc KATP channel demonstrated an essential role for this channel against long-duration ischemic insults, although protection against IR-generated arrhythmias was not evaluated (12). Collectively, previous and present findings may indicate the sarc KATP channel mediates tissue preservation during IR, while the mito KATP channel protects against arrhythmias (20). The protective contribution of the mito and sarc KATP channels does not, however, appear to be consistent across all experimental paradigms and requires further investigation (12–13, 17). Accordingly, we employed a relatively short-duration ischemia (20 min) and proportionate reperfusion period (30 min) to evaluate the impact of these ion channels as potential mechanisms of anti-arrhythmic protection in the exercised heart and investigated biochemical outcomes in perfused and ischemic myocardium before the overt onset of tissue death occurred.
Exercise, KATP channels, and myocardial redox balance.
How mito KATP channels cardioprotect against short-duration IR in exercised hearts is not currently known. Since adaptations to exercise include cellular ATP preservation during IR, it is plausible that ATP-independent stimuli are responsible for mito KATP channel opening in the exercised heart (9–10, 28–30). Mito KATP channels, for instance, can open in response to a variety of stimuli, independent of a drop in cellular ATP, including PKCϵ (33, 38). In support, application of a short-term exercise regimen similar to the current training protocol resulted in PKCϵ overexpression in the heart (16). Once opened, a prevailing rationale supporting anti-arrhythmic effects of the mito KATP channels during IR postulates that preconditioned hearts maintain a favorable redox status due to preserved ATP availability. This notion is supported by the finding that cytosolic ATP levels are maintained at 40% of baseline after 30 min of ischemia (2). This rationale may explain why exercise prevented an IR-induced rise in myocardial protein carbonyls, while pre-IR administration of the mito KATP channel blocker 5HD resulted in protein carbonyl elevations similar to that of Sed animals. Although cause and effect are not confirmed, it is plausible that exercise-mediated mito KATP channel opening maintained the mitochondrial inner membrane potential. As such, preserved state 3 respiration would be associated with attenuated mitochondrial superoxide release and prevention of ventricular arrhythmias due to regional irregularities in early after-depolarization (4, 44).
A novel aspect of this investigation was evaluation of important endogenous antioxidant enzymes in both perfused and ischemic tissue. Current findings from perfused exercised heart tissue exhibited a significant rise in MnSOD enzyme activity and agree with previously observed elevations in MnSOD activity from unstressed “exercise sham” ventricular tissue (25, 32). The present data extend on this understanding by demonstrating that elevated MnSOD enzyme activity measured in post-IR ischemic tissue of exercised heart was unaffected by short-duration IR (25). The IR challenge of this study also did not influence CAT, GPx, total SOD, or CuZnSOD enzyme activities for any treatment group. This outcome may reflect the tissue condition at the time of sampling, as it is plausible that MnSOD enzyme activity was diminished at the unobserved conclusion of ischemia, but recovered to basal levels during the relatively short reperfusion period. The enzyme activities from sedentary ischemic and perfused tissue were statistically similar and support this rationale. In continuation with this rationale, the IR duration from the present study was not sufficient to produce elevations in lipid peroxidation, as measured by 4-hydroxynonenal concentrations, to inactivate endogenous antioxidant enzymes or to impact myocardial glutathione levels. Unlike many previous investigations that document antioxidant and enzymatic activities from unstressed tissues, cardiac GSH/GSSG levels in the present study were examined in the ischemic and perfused myocardium. Moreover, the extended experimental duration in vivo may have facilitated glutathione fluxuations not typically observed in isolated cell preparations. Collectively, the present findings emphasize that redox alterations to physiological stressors, such as ischemia, are dynamic and can be complex.
Study limitations.
Short-duration exercise as a model of cardioprotection has several important advantages, including the fact that the cellular mechanisms responsible for acute IR resistance are not confounded by effects related to cardiac remodeling. It should be noted that short-duration exercise exposure may impose a stress response not directly attributable to exercise (14). Whether long-duration exercise preconditioning would have altered anti-arrhythmic protection mediated by either the mito or sarc KATP channels cannot not be determined currently.
To investigate ventricular arrhythmias following IR, we employed an established scoring system for collective analysis of PVCs, VT, and VF. These clinically derived scoring systems have been applied more recently to medically relevant research paradigms like the present investigation (18–19). Collective analysis of ventricular arrhythmias is advantageous for identifying physiologically meaningful outcomes, which may be masked statistically due to high variability in PVC, VT, or VF alone. Case in point, the ECG arrhythmia scoring system utilized in the present study yielded robust group-dependent outcomes compared with categorical analysis, as demonstrated by the nonsignificant “trends” observed between Sed and Ex animals (Table 2, Fig. 3). In this regard, retrospective power analyses indicate fourfold increases in sample size would be needed delineate treatment differences for arrhythmias experienced during ischemia or reperfusion alone. Whether KATP channels contribute more protection to exercised hearts against arrhythmias generated during ischemia or reperfusion is uncertain at this time.
The popular pharmacological inhibitors 5HD and HMR-1098 were used to inhibit the respective mito KATP and sarc KATP channels. It is now generally held that pharmacological blockade of the mito KATP channel in the absence of a preconditioning stimulus does not impact post-IR outcomes (reviewed in Ref. 37). Characteristic of experimentation with pharmacological inhibitors, however, we cannot rule out the possibility of unknown pharmacological effects in the present study, including 5HD-specific effects on substrate availability (5, 26) and sex specific effects (17). Despite the potential role of 5HD metabolism within the heart, the resulting implications for outcomes related to preconditioning remain unknown. Finally, we cannot reconcile in the present study whether simultaneous pharmacological inhibition of the mito and sarc KATP channels of exercised hearts would have altered arrhythmia generation and oxidative stress.
Conclusions.
Data collected for this investigation indicate that the mito KATP channel plays an important role in preventing short-duration IR-mediated arrhythmias in the exercised heart, while the sarc KATP channel does not. These findings highlight the complex, multifaceted nature of both IR injury and exercise-induced cardiac preconditioning. The emerging data from this line of study further highlight the unique nature of exercise preconditioning compared with ischemic and pharmacological stimuli (reviewed in Ref. 42). In light of the sustainable and cost-effective nature of exercise in clinical populations, exercise-based research into mediators against IR damage may play a growing role in the search for therapeutic treatments against IR injury (8). Future investigations may further elucidate the role of both the mito and sarc KATP channels as mediators of exercise-generated cardioprotection against other forms of IR injury, including myocardial stunning and apoptotic cell death.
GRANTS
These experiments were supported by grants from the Appalachian State University Research Council (J. C. Quindry) and the National Heart, Lung, and Blood Institute (J. C. Quindry, HL087256). HMR1098 was a generous gift of Dr. Heinz Golgelein, Adventis Pharma, Germany.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Aebi H. Catalyase in vitro. Methods Enzymol 105: 121–126, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, Flaherty JT. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993 [PubMed] [Google Scholar]
- 3.American Heart Association Heart disease and stroke statistics-2004 update. Dallas, TX: American Heart Association, 2004, p. 1–52 [Google Scholar]
- 4.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A 101: 11880–11885, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babai L, Szigeti Z, Parratt JR, Vegh A. Delayed cardioprotective effects of exercise in dogs are aminoguanidine sensitive: possible involvement of nitric oxide. Clin Sci (Lond) 102: 435–445, 2002 [PubMed] [Google Scholar]
- 7.Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292: H19–H27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bowles DK, Farrar RP, Starnes JW. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am J Physiol Heart Circ Physiol 263: H804–H809, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Bowles DK, Starnes JW. Exercise training improves metabolic response after ischemia in isolated working rat heart. J Appl Physiol 76: 1608–1614, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 12.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol 569: 913–924, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol 95: 2510–2518, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol 103: 1979–1985, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, Moore RL. Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol 564: 619–630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson LD, Korzick DH. Dose-dependent effects of acute exercise on PKC levels in rat heart: is PKC the heart's prophylactic? Acta Physiol Scand 178: 97–106, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Chicco AJ, Johnson MS, Armstrong CJ, Lynch JM, Gardner RT, Fasen GS, Gillenwater CP, Moore RL. Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am J Physiol Heart Circ Physiol 292: H2432–H2437, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Curtis MJ, Macleod BA, Walker MJ. Models for the study of arrhythmias in myocardial ischaemia and infarction: the use of the rat. J Mol Cell Cardiol 19: 399–419, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res 22: 656–665, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Flagg TP, Nichols CG. Sarcolemmal K(ATP) channels: what do we really know? J Mol Cell Cardiol 39: 61–70, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol 105: 114–121, 1984 [DOI] [PubMed] [Google Scholar]
- 22.French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J 22: 2862–2871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol 278: H305–H312, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Gross ER, Gross GJ. Pharmacologic therapeutics for cardiac reperfusion injury. Expert Opin Emerg Drugs 12: 367–388, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hamilton KL, Quindry JC, French JP, Staib J, Hughes J, Mehta JL, Powers SK. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med 37: 1360–1368, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Hanley PJ, Drose S, Brandt U, Lareau RA, Banerjee AL, Srivastava DK, Banaszak LJ, Barycki JJ, Van Veldhoven PP, Daut J. 5-Hydroxydecanoate is metabolised in mitochondria and creates a rate-limiting bottleneck for beta-oxidation of fatty acids. J Physiol 562: 307–318, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hull SS, Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation 89: 548–552, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Jew KN, Moore RL. Exercise training alters an anoxia-induced, glibenclamide-sensitive current in rat ventricular cardiocytes. J Appl Physiol 92: 1473–1479, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Jew KN, Moore RL. Glibenclamide improves postischemic recovery of myocardial contractile function in trained and sedentary rats. J Appl Physiol 91: 1545–1554, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Jew KN, Olsson MC, Mokelke EA, Palmer BM, Moore RL. Endurance training alters outward K+ current characteristics in rat cardiocytes. J Appl Physiol 90: 1327–1333, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol 38: 937–943, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lennon SL, Quindry JC, Hamilton KL, French JP, Hughes J, Mehta JL, Powers SK. Elevated MnSOD is not required for exercise-induced cardioprotection against myocardial stunning. Am J Physiol Heart Circ Physiol 287: H975–H980, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Light PE, Kanji HD, Fox JE, French RJ. Distinct myoprotective roles of cardiac sarcolemmal and mitochondrial KATP channels during metabolic inhibition and recovery. FASEB J 15: 2586–2594, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Reiter MJ, Xu Y, Chicco A, Greyson CR, Schwartz GG. Thiazolidinedione drugs block cardiac KATP channels and may increase propensity for ischaemic ventricular fibrillation in pigs. Diabetologia 51: 675–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matejikova J, Kucharska J, Pinterova M, Pancza D, Ravingerova T. Protection against ischemia-induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: involvement of mitochondrial K(ATP) channels and reactive oxygen species. Physiol Res 58: 9–19, 2009 [DOI] [PubMed] [Google Scholar]
- 36.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055, 1969 [PubMed] [Google Scholar]
- 37.O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res 94: 420–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol 294: C169–C177, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Pasnani JS, Ferrier GR. Differential effects of glyburide on premature beats and ventricular tachycardia in an isolated tissue model of ischemia and reperfusion. J Pharmacol Exp Ther 262: 1076–1084, 1992 [PubMed] [Google Scholar]
- 40.Patel HH, Gross ER, Peart JN, Hsu AK, Gross GJ. Sarcolemmal KATP channel triggers delayed ischemic preconditioning in rats. Am J Physiol Heart Circ Physiol 288: H445–H447, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Picard S, Rouet R, Ducouret P, Puddu PE, Flais F, Criniti A, Monti F, Gerard JL. KATP channels and “border zone” arrhythmias: role of the repolarization dispersion between normal and ischaemic ventricular regions. Br J Pharmacol 127: 1687–1695, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quindry J, Hamilton K. Exercise induced cardioprotection: an overview and critical comparison with ischemic preconditioning. Curr Cardiol Revi 3: 193–201, 2007 [Google Scholar]
- 43.Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J Appl Physiol 103: 1056–1062, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, Karagueuzian H, Garfinkel A, Weiss JN, Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc Natl Acad Sci U S A 106: 2983–2988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato T, Costa AD, Saito T, Ogura T, Ishida H, Garlid KD, Nakaya H. Bepridil, an antiarrhythmic drug, opens mitochondrial KATP channels, blocks sarcolemmal KATP channels, and confers cardioprotection. J Pharmacol Exp Ther 316: 182–188, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, Cobbe SM, Coker SJ, Harness JB, Harron DW, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction and reperfusion. Cardiovasc Res 22: 447–455, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Wilde AA, Veldkamp MW, van Ginneken AC, Opthof T. Phentolamine blocks ATP sensitive potassium channels in cardiac ventricular cells. Cardiovasc Res 28: 847–850, 1994 [DOI] [PubMed] [Google Scholar]