Abstract
We recently observed the enhanced serine and matrix metalloproteinase (MMP) activity in the spontaneously hypertensive rat (SHR) compared with its normotensive Wistar-Kyoto (WKY) rat and the cleavage of membrane receptors in the SHR by MMPs. We demonstrate in vivo that MMP-7 and MMP-9 injection leads to a vasoconstrictor response in microvessels of rats that is blocked by a specific MMP inhibitor (GM-6001, 1 μM). Multiple pathways may be responsible. Since the β2-adrenergic receptor (β2-AR) is susceptible to the action of endogenous MMPs, we hypothesize that MMPs in the plasma of SHRs are able to cleave the extracellular domain of the β2-AR. SHR arterioles respond in an attenuated fashion to β2-AR agonists and antagonists. Aorta and heart muscle of control Wistar rats were exposed for 24 h (37°C) to fresh plasma of male Wistar and WKY rats and SHRs with and without doxycycline (30 μM) and EDTA (10 mM) to reduce MMP activity. The density of extracellular and intracellular domains of β2-AR was determined by immunohistochemistry. The density of the extracellular domain of β2-AR is reduced in aortic endothelial cells and cardiac microvessels of SHRs compared with that of WKY or Wistar rats. Treatment of the aorta and the heart of control Wistar rats with plasma from SHRs, but not from WKY rats, reduced the number of extracellular domains, but not intracellular domains, of β2-AR in aortic endothelial cells and cardiac microvessels. MMP inhibitors (EDTA and doxycycline) prevented the cleavage of the extracellular domain. Thus MMPs may contribute to the reduced density of the extracellular domain of β2-AR in blood vessels and to the increased arteriolar tone of SHRs compared with normotensive rats.
Keywords: receptor cleavage, cardiac microvessels, aorta, arterioles
spontaneously hypertensive rats (SHRs) have elevated levels of proteolytic activity in their plasma and on endothelial cells. Matrix metalloproteinases (MMPs) cause cleavage of vasoactive substances and membrane receptors. Cleavage of the extracellular domain of membrane receptors such as the insulin receptor, the membrane integrin CD18, and the vascular endothelial growth factor receptor 2 has been observed in the SHRs associated with insulin resistance, diminished ability of leukocyte to adhere to the endothelium, and capillary apoptosis and rarefaction, respectively (7, 32).
The SHR has elevated levels of arteriolar contraction, a process that contributes to its central blood pressure elevation (27). The β2-adrenergic receptor (β2-AR) mediates vascular smooth muscle relaxation, reducing peripheral resistance. There are few studies about the expression of β2-AR in either aorta or cardiac microvessels in the SHR. A reduced vasodilatation to β2-AR agonist has been observed in the aorta of SHRs (5), as well as in forearm blood vessels in patients with established hypertension (22) or in borderline hypertension (28). No molecular mechanism has been proposed that may be responsible for the attenuated vasodilation to the β2-AR agonist in patients with hypertension. Stiles et al. (29) demonstrated that the β2-AR is susceptible to the action of endogenous MMPs. DeLano et al. (7) demonstrated elevated MMP activity in the SHR compared with the normotensive Wistar-Kyoto (WKY) rat. Thus we hypothesize that the β2-AR may be cleaved by the elevated levels of MMPs in the SHR, leading to the reduction of vasodilatation.
In the present study, we demonstrate that an acute injection of MMP-7 and MMP-9 causes diameter reductions of arterioles in Wistar rats. As one of several possible contractile mechanisms affected by the MMPs, we used an immunohistochemical approach to determine the expression of β2-AR in the cardiac microcirculation and aorta of WKY and the SHR and the degree to which its extracellular domain is cleaved. The level of the β2-AR proteolytic cleavage in the extra- and intracellular domain on naïve aortic endothelium of Wistar rats after acute exposure to plasma of WKY rat and the SHR was measured with and without MMP inhibition.
MATERIALS AND METHODS
Animals
The experimental protocol was reviewed and approved by the University of California San Diego Animal Subjects Committee. Age-matched male SHRs and normotensive WKY rats (Charles River, Wilmington, MA) at 12–18 wk of age (280–350 g) were used to study the acute action of β2-adrenergic agonist and antagonist on the mesenteric microcirculation and the central blood pressure. Wistar rats were used to study the acute action of MMPs on the mesenteric microcirculation. After general anesthesia with pentobarbital sodium (Nembutal, 50 mg/kg body wt im, Abbott, North Chicago, IL), polyethylene catheters (PE50; inner diameter of 0.5 mm, and outer diameter of 0.956 mm; Becton Dickinson Primary Care Diagnostics, Sparks, MD) were inserted into a femoral artery and a femoral vein before the start of abdominal surgery. The mean arterial pressure and heart rate were digitally recorded (MacLab with Power Macintosh G3). Supplemental doses of anesthesia were administered intravenously at a dose of 5 mg/kg as needed after reflex testing. Body temperature was maintained at 37°C by a water-heated animal stage. At the end of the study, the animals were euthanized with pentobarbital sodium (Nembutal, 120 mg/kg body wt iv).
In another group of rats, blood was collected from the abdominal aorta (∼8 ml) after general anesthesia, and the heart and thoracic aorta were collected right after euthanasia.
Acute Effect of β2-Adrenergic Agonist and Antagonist
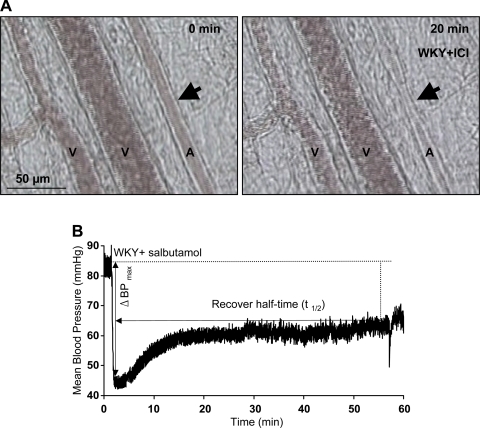
The acute effect of salbutamol (a nonselective β-adrenergic agonist, more potent at β2- than β1-receptors, 4 μg/kg body wt iv, Tocris Bioscience, Ellisville, MO) and ICI-118551 (a selective β2-adrenergic antagonist, 0.1 mg/kg body wt iv, Tocris Bioscience) on arterial pressure was studied on SHRs and WKY rats. An intravenous injection of 1 ml normal saline was used as a control. The maximum blood pressure change and recovery half-time were calculated base on the mean arterial pressure (Fig. 1). The acute effect of salbutamol and ICI-118551 was studied in the mesenteric microcirculation.
Fig. 1.
A: an example of microvessel diameter change in response to ICI-118551 (ICI). Arrows indicate location of arteriolar constriction with ICI-118551. B: an example of blood pressure in response to salbutamol. V, venule; A, arteriole; WKY, Wistar-Kyoto rats; ΔBPmax, maximum blood pressure change.
Intravital microscopic observation of mesentery microcirculation.
The abdominal cavity was exposed via a midline incision. The intestinal mesentery was withdrawn and loosely draped over a heated observation window (37°C) by carefully manipulating the intestine with cotton-tipped applicators. To avoid drying and hyperosmotic injury, the exposed intestine and adjacent mesenteric sections were covered with wet gauze and continuously superfused with Krebs-Henseleit solution (pH 7.4, 305 mosmol/l, 37°C).
The mesenteric microcirculation was viewed with a ×10 water-immersion lens (Zeiss, Oberkochen, Germany) on an intravital microscope (Leitz, Wetzlar, Germany). Bright-field images were recorded with a color charge-coupled device camera (model VI-470, Optronics, Goleta, CA) and stored with a DVD recorder (Sony DVO 1000MD). Selected tissue areas were videotaped for the duration of the experiment. The intravital microscopic observation readily permits detection of the vascular diameter and other events in the blood vessels and tissue parenchyma.
Experimental protocol.
Animals were subdivided into treatment groups (n = 3). After general anesthesia and mesentery preparation, images of selected mesenteric arterioles and venules were continuously recorded before and over 2 h after the infusion of normal saline, salbutamol, or ICI-118551 in 1 ml (Fig. 1A). The videos were then analyzed off-line.
Acute Effect of MMPs on the Vascular Diameter
Wistar rats were subdivided into four groups (n = 3): control (saline), the purified enzyme MMP-7 (200 μM, Calbiochem, Gibbstown, NJ) treatment, the purified enzyme MMP-9 (200 μM, Calbiochem) treatment, and a mixture of the purified MMP-7 and MMP-9 (100 μM for each in the mixture) treatment without and with a specific MMP inhibitor (GM-6001, 1 μM, Ilomastat, Chemicon Millipore, Billerica, MA). The mesentery microcirculation as described in Intravital microscopic observation of mesentery microcirculation was viewed with a ×40 water-immersion lens (numerical aperture, 0.75; Zeiss). At start time, t = 0, the enzymes were infused into a microvascular network via the superior mesenteric artery. Images of selected mesenteric arterioles and venules were continuously recorded before and over 1 h of infusion.
Determination of the Expression of β2-AR
Reagents.
The following chemicals were obtained commercially: purified rabbit polyclonal antibodies against the extracellular domain of the β2-AR (against a peptide at the NH2-terminus, for immunohistochemistry, NLS2662, Novus Biologicals, Littleton, CO; and for Western blot analysis, AP7263a, Abgent, San Diego, CA) and antibody against the intracellular domain of the β2-AR (against a peptide at the COOH-terminus, M-20, sc-570, Santa Cruz Biotechnology, San Diego, CA), which were used to label the receptor. As a metal chelating agent, ethylenediaminetetraacetic acid (EDTA, 10 mM, Fisher Scientific) was used as a positive control to block MMP activity (26a) and doxycycline (West-Ward Pharmaceutical, Eatontown, NJ) as a clinically approved MMP inhibitor. Doxycycline is a tetracycline class antibiotic that has MMP inhibitory activity separate from its antimicrobial activity (11). Specifically, doxycycline has been shown to interact with structural ions of MMPs, including Ca2+ and Zn2+ (10). The doxycycline dose was 30 μM at which it inhibits MMP activity (17).
Tissue processing.
BASAL CONTROLS.
To determine the β2-AR expression at control levels, the heart and thoracic aorta of SHRs and Wistar and WKY rats were washed in 0.01 M cold phosphate-buffered saline solution (Sigma-Aldrich, St. Louis, MO) and then were either frozen in liquid nitrogen and stored (−80°C) or incubated in paraformaldehyde solution (4%, Fisher Scientific, Pittsburg, PA) for 24 h and in sucrose solution (30%) for 24 h at 4°C. The vessels were embedded in a tissue-freezing medium (Optimal Cutting Temperature, Fisher Scientific) in specimen molds (Electron Microscopy Sciences, Hatfield, PA), frozen in liquid nitrogen, and stored (−80°C). Sections (6 μm for aorta, and 8 μm for hearts) were cut on a cryostat (at −20°C, Leica CM 3050 S, Heidelberg, Germany), collected on microscopy slides (Fisher Scientific), and stored (−80°C) for later use.
EXPOSURE TO SHR PLASMA.
To determine whether proteases in the plasma of SHRs could cleave the extracellular domain of the β2-AR on the surface of the endothelium of thoracic aorta and cardiac microvessels in Wistar rats, heparinized blood from SHRs and WKY and Wistar rats was centrifuged (1,000 g for 20 min), and the plasma was collected and stored (−20°C). Thoracic aorta rings (2 mm thickness) and heart muscle sections (2 mm thickness) of Wistar rats were suspended in plasma of Wistar, WKY, and SHRs with or without doxycycline (30 μM) or EDTA (10 mM) as a positive control (24 h at 37°C). Tissues were then sectioned (in the midplane of the tissue blocks) and labeled the same way as described in basal controls.
Immunohistochemistry.
The density of the extracellular and intracellular domains of β2-AR on the surface of the endothelium and smooth muscle cells of thoracic aorta and cardiac microvessels in SHRs and WKY as well as Wistar rats before and after exposure to the plasma of the SHRs and WKY rats was determined by an immunolabeling of the extra- and intracellular domains of β2-AR.
The aorta and heart sections were initially incubated for 30 min in a 0.3% hydrogen peroxide solution to block the endogenous peroxidase and for 1 h in a solution containing 0.03% Tween-20, 5% bovine serum albumin, and either the purified rabbit polyclonal antibody against the extracellular domain of the β2-AR (50 mg/l) or the purified rabbit polyclonal antibody against the intracellular domain of the β2-AR (10 mg/l). Negative control sections were treated in the same manner as the experimental slides except that the primary antibodies were replaced with phosphate-buffered saline. They showed no detectable labeling density (results not shown). Slides were then incubated with a biotin-conjugated secondary antibody against rabbit IgG (Impress anti-rabbit Ig-peroxidase-kit, Vector) for 1 h. A peroxidase enzyme substrate (NovaRED, Vector) was used to visualize both β2-AR domains on tissue specimens after secondary antibody labeling. No counterstain was applied to facilitate quantitative measurements of the immunolabel intensity by optical density measurements.
Digital image analysis.
Under standardized microscope light conditions and fixed substage condenser settings, monochromatic images of the immunolabeling density were digitally recorded (digital units between 0 and 256 from white to black; Fujifilm FinePix S1Pro, Fuji Photo Film, Tokyo, Japan) and quantitatively analyzed (ImageJ, v. 1.38×, Wayne Rasband, National Institutes of Health).
The density of the immune substrate label (NovaRed, Vector) was determined on the endothelium of selected segments of the aorta and on cardiac microvessels. Averages of at least five selected segments of the aorta and over 10 heart microvessels per rat were used to compute the means ± SE for all animals (indicated in the bar graphs) in each group.
Western blot analysis.
The aortic specimen (including endothelium and smooth muscle cells) and heart muscle (with capillaries and arterioles and venules) were homogenized in ice-cold buffer containing 50 mM Tris (pH to 7.4, Sigma), 150 mM NaCl, 3.5 mM SDS, 12 mM sodium deoxycholate, 16.5 mM Nonidet P-40, 2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 1 μM phosphoramidon, 130 μM bestatin, 14 μM E-64, 1 μM leupeptin, 0.2 μM aprotinin, and 10 μM pepstatin A and were centrifuged (10,000 g for 10 min at 4°C). The pellet was dispersed in 100 μl of the original buffer for aortas and 500 μl for hearts. The protein concentration was determined (DC protein assay kit, Bio-Rad, Hercules, CA) using lyophilized bovine serum albumin as a standard. The protein sample (40 μg) was separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes. After an incubation in blocking solution (5% non-fat milk, overnight at 4°C), the membranes were incubated with either 1:300 diluted primary antibody against the extracellular domain of the β2-AR or 1:200 diluted primary antibody against the intracellular domain of the β2-AR (1 h at room temperature). The membranes were then washed three times for 15 min in Tris-buffered saline solution. A horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (1:1,000 dilution) was used as a secondary antibody and incubated for 1 h at room temperature followed by three 15-min washes in Tris-buffered saline solution. The membranes were incubated with enhanced chemiluminescence reagent and exposed to X-ray films. The films were then scanned, and the relative intensities of signals were analyzed (ImageJ 3.0, National Institutes of Health). The membranes stained with Ponceau (Sigma-Aldrich) were used as a control to correct for sample loading.
Statistics
Measurements are presented as means ± SE. One-way ANOVA followed by Student-Newman-Keuls multiple comparisons test was used as appropriate. A probability of P < 0.05 was considered significant.
RESULTS
Acute Effect of β2-Adrenergic Agonist and Antagonist on the SHR
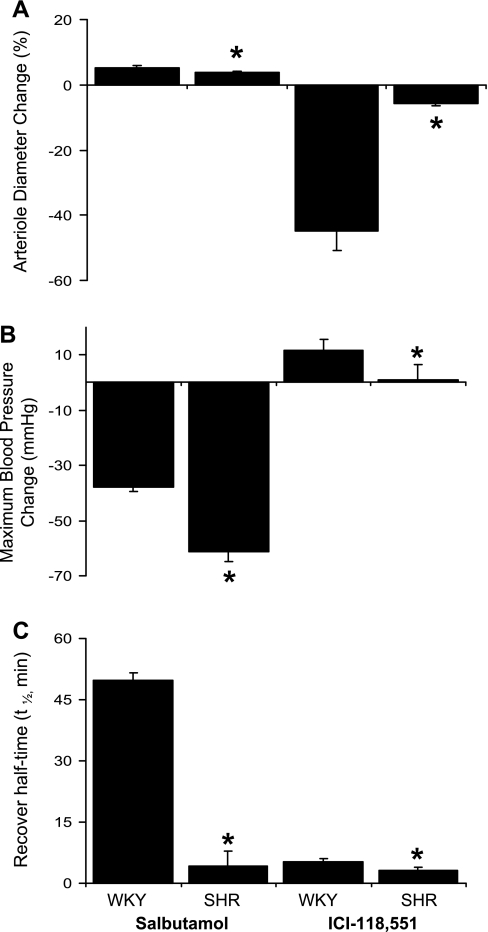
When compared with that of WKY microvessels, the SHR arteriolar diameter change is significantly lower in response to both salbutamol and ICI-118551 (Fig. 2A). In the SHR, the maximum blood pressure change is larger with salbutamol infusion, whereas it is smaller with ICI-118551 infusion (Fig. 2B). However, recovery half-times in response to both salbutamol and ICI-118551 are significantly shorter in the SHR (Fig. 2C). An infusion of saline did not make any change in microvessel diameter or on blood pressure.
Fig. 2.
Acute effect of β2-adrenergic agonist and antagonist on the spontaneously hypertensive rat (SHR). A: when compared with that of WKY microvessels, the SHR arteriole diameter change is significantly lower in response to both salbutamol and ICI-118551. B: in the SHR, the maximum blood pressure change is larger with salbutamol infusion, whereas it is smaller with ICI-118551 infusion. C: recovery half-times in response to both salbutamol and ICI-118551 are significantly shorter in the SHR. *P < 0.05, SHR vs. WKY.
The Acute Effect of MMPs on the Vascular Diameter of Arterioles and Venules in Mesentery of Wistar Rats
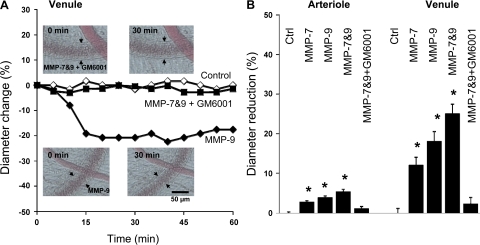
Our current evidence indicates that both MMP-7 and MMP-9 activities are elevated in the SHR plasma (32). Therefore, we tested the acute response in microvessels to these enzymes. The diameter of both arterioles and venules in mesentery of control Wistar rats were significantly reduced after an acute injection of MMP-7, MMP-9, or a combination of both MMPs (Fig. 3). This arteriolar constriction was blocked in the presence of the MMP inhibitor GM-6001, whereas GM-6001 by itself had no effect under these experimental conditions.
Fig. 3.
A: representative trace of the time course of the contractile response of a selected mesenteric venule of Wistar rat with matrix metalloproteinase (MMP)-9 treatment without and with the MMP inhibitor GM-6001. Arrows indicate location of venular endothelium. B: diameter reduction after MMP exposure relative to the resting diameter (before exposure) of mesenteric arterioles and venules in Wistar rats. The microvessels were intraluminally exposed either to MMP-7, MMP-9, or a combination of both MMPs. *P < 0.05 vs. control (Ctrl).
Basal Density of Extracellular and Intracellular Domains of the β2-AR
Immunohistochemistry analysis.
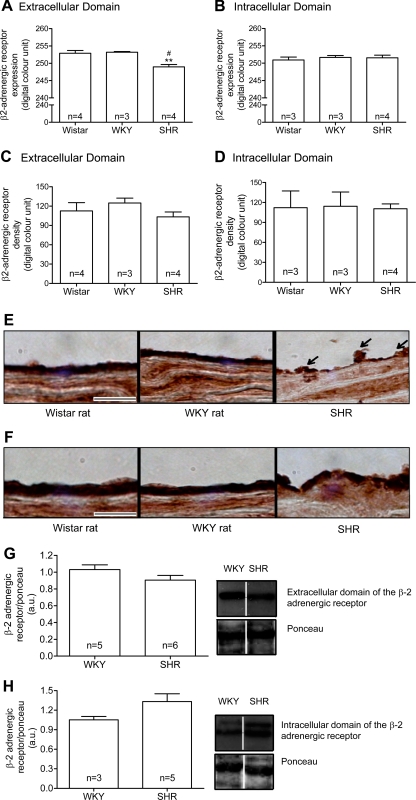
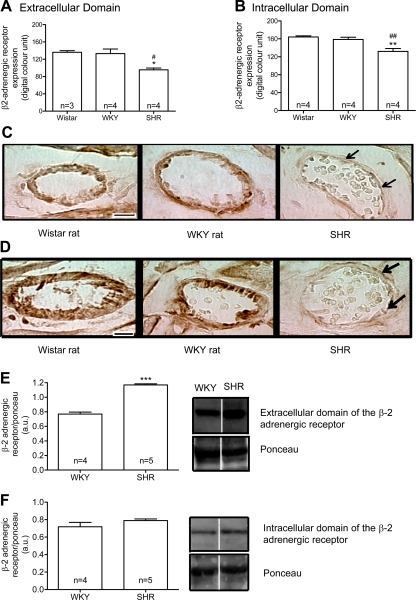
The density of the β2-AR extracellular domains (Fig. 4, A and E), but not of the β2-AR intracellular domains (Fig. 4, B and F), in the thoracic aorta endothelial cells layer was significantly reduced in SHRs compared with Wistar and WKY rats. There was no significant difference in the density of both β2-AR domains in the smooth muscle cell layer (Fig. 4, C–F). In cardiac microvessels, the labeling density of both extracellular (Fig. 5, A and C) and intracellular (Fig. 5, B and D) domains of the β2-AR was significantly reduced in SHRs compared with Wistar and WKY rats.
Fig. 4.
Means ± SE of the density values (in digital optical units) of the extracellular and intracellular domains of the β2-adrenergic receptor (β2-AR) at basal level in the thoracic aorta endothelium layer (A and B) and smooth muscle cells layer (C and D) from control Wistar and WKY rats and SHRs are shown. Representative micrographs show the intensity of the avidin-biotin-diaminobenzidine immunocomplexes for both the extracellular (E) and intracellular (F) domains of the β2-AR at basal level in thoracic aorta endothelial cells and smooth muscle cell layers for the same strains. Arrows point toward SHR endothelium with reduced density of the extracellular domain receptor label. Representative Western blots show the expression of the β2-AR extracellular (G) and intracellular (H) domains and corresponding Ponceau at basal level in thoracic aorta from control WKY and SHRs. AU, arbitrary units. The blots were selected as the closest to the measured mean values from a single Western gel for all WKY and SHR samples and are separated by white space. For this, 40 μg of proteins were used. Numbers in the bars indicate rat group sizes. **P < 0.01 vs. Wistar; #P < 0.05 vs. WKY. Bar = 20 μm.
Fig. 5.
Means ± SE of the optical density values (in digital units) of the extracellular (A) and intracellular (B) domains of the β2-AR at basal level in heart microvessels from control Wistar and WKY rats and SHRs are shown. Numbers in the bars indicate rat group sizes. *P < 0.05 and **P < 0.01 vs. Wistar; #P < 0.05 and ##P < 0.01 vs. WKY. Micrographs show the intensity of the avidin-biotin-diaminobenzidine immunocomplexes for both the extracellular (C) and intracellular (D) domains of the β2-AR at basal level in heart microvessels from the same strains. Arrows point toward SHR endothelium with reduced density of the extracellular domain receptor label. Representative Western blots show the expression of the β2-AR extracellular (E) and intracellular (F) domains and corresponding Ponceau at basal level in heart from control WKY and SHRs. The blots were selected as the closest to the measured mean values from a single Western gel for all WKY and SHR samples and are separated by white space. For this, 40 μg of proteins were used. ***P < 0.001 vs. WKY. Bar = 10 μm.
Western blot analysis.
No significant difference was observed in the density of β2-AR extracellular (Fig. 4G) and intracellular domains (Fig. 4H) in the thoracic aorta of SHRs compared with WKY rats, although there was a tendency in SHRs for a lower level with the antibody against the extracellular binding site (Fig. 4G). In heart muscle, the density of the extracellular domain (Fig. 5E), but not of the intracellular domain (Fig. 5F), was increased in SHRs compared with WKY rats.
The Effect of SHR Plasma on Extracellular and Intracellular Domain Density of the β2-AR on Endothelium of Naïve Wistar Rats
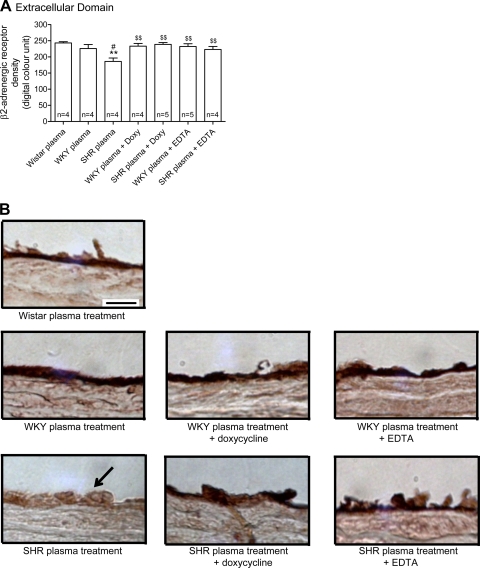
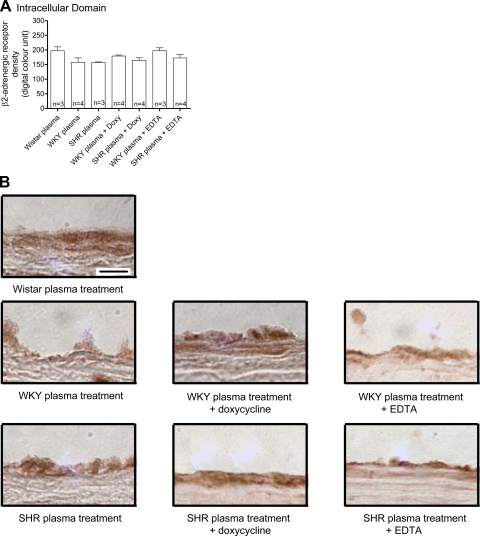
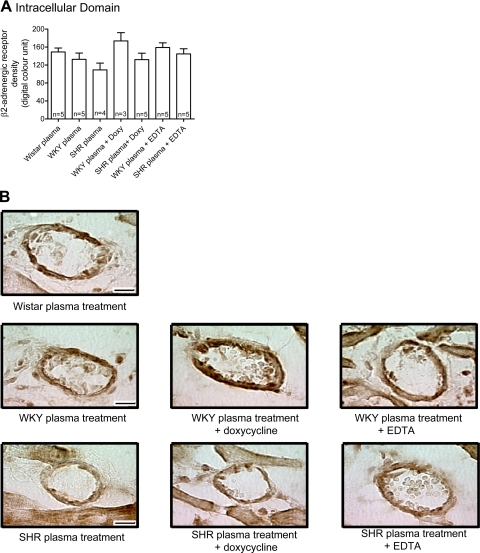
The labeling density of the extracellular domain of β2-AR (Fig. 6), but not of the intracellular domain (Fig. 7), in the thoracic aorta endothelium from Wistar rats was reduced after treatment with plasma of SHRs, indicating cleavage of the receptor by SHR plasma. No significant reduction was observed with WKY plasma, although the average value tends to be reduced compared with the values in Wistar controls. The addition of doxycycline to the SHR but not WKY plasma attenuated the reduction of the extracellular domain of β2-AR to a degree similar to that after of the addition of EDTA (Fig. 6). Neither addition of EDTA or doxycycline to SHR plasma affected the density of the intracellular domain of β2-AR (Fig. 6). This indicates that the plasma of SHRs cleaves the extracellular domain of the β2-AR, and MMPs seem to play a role in this reaction.
Fig. 6.
A: means ± SE of the density (in digital optical units) of the extracellular domain of the β2-AR in thoracic aorta endothelium from Wistar rats after 1 h treatment with plasma from control Wistar and from both WKY rats and SHRs with or without either doxycycline (Doxy, 30 μM) or 10 mM EDTA addition. Numbers in the bars indicate rat group sizes. **P < 0.01 vs. Wistar; #P < 0.05 vs. WKY; $$P < 0.01 vs. SHR. B: micrographs showing the intensity of the avidin-biotin-diaminobenzidine immunocomplex for the extracellular domain of the β2-AR in thoracic aorta endothelial cells layer from Wistar rats after different plasma treatments. Arrow points toward SHR endothelium with reduced density of the extracellular domain receptor label. Bar = 20 μm.
Fig. 7.
A: means ± SE of the intracellular domain of the β2-AR (in digital optical units) in thoracic aorta endothelial cells from Wistar rats after 1 h treatment with plasma from control Wistar and from both WKY and SHRs with or without 30 μM doxycycline or 10 mM EDTA. Numbers in the bars indicate rat group sizes. B: micrographs showing the density of the avidin-biotin-diaminobenzidine immunocomplex for the β2-AR intracellular domain in the thoracic aorta endothelial layer of Wistar rats after different plasma treatments. Bar = 20 μm.
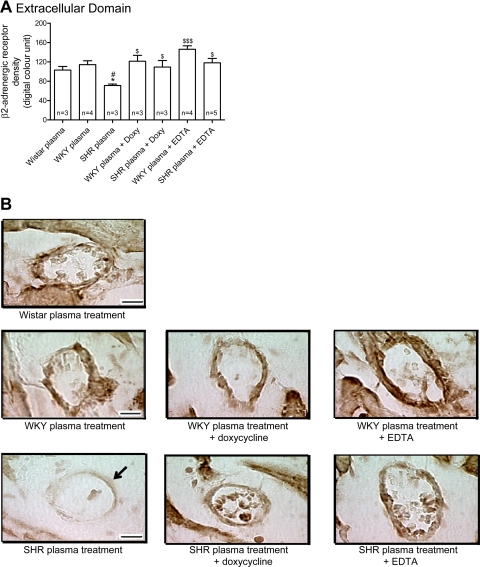
The density of the extracellular domain of β2-AR (Fig. 8), but not of the intracellular domain (Fig. 8), in cardiac microvessels from Wistar rats was reduced after treatment with plasma from SHRs. Doxycycline blocked the reduction of the extracellular domain of β2-AR in a way similar to that observed after the addition of EDTA to the SHR plasma (Fig. 7). Just like in the aorta, doxycycline and EDTA did not affect the density of the intracellular domain of β2-AR (Fig. 9). This evidence indicates that SHR plasma cleaves the β2-AR extracellular domain not only in large vessels but also in small vessels, and MMPs seem to cause the receptor cleavage in both tissues.
Fig. 8.
A: means ± SE of the density of the extracellular domain of the β2-AR in heart microvessels from Wistar rats after 1 h treatment with plasma from control Wistar and from both WKY and SHRs with or without doxycycline (30 μM) or EDTA (10 mM). Numbers in the bars indicate rat group sizes. *P < 0.05 vs. Wistar; #P < 0.05 vs. WKY; $P < 0.05 and $$$P < 0.001 vs. SHR. B: micrographs showing the intensity of the avidin-biotin-diaminobenzidine immunocomplex for the extracellular domain of the β2-AR in heart microvessels from Wistar rats without and with different plasma treatments. Arrow points toward SHR endothelium with reduced density of the extracellular domain receptor label. Bar = 10 μm.
Fig. 9.
A: means ± SE of the density of the intracellular domain of the β2-AR in heart microvessels from Wistar rats after 1 h treatment with plasma from control Wistar and from both WKY and SHRs with or without doxycycline (30 μM) or EDTA (10 mM). Numbers in the bars indicate rat group sizes. B: micrographs showing the intensity of the avidin-biotin-diaminobenzidine immunocomplex for the intracellular domain of the β2-AR in heart microvessels from Wistar rats without and with different plasma treatments. Bar = 10 μm.
DISCUSSION
Receptor Cleavage in SHRs
The cleavage of the β2-AR in the SHR observed in the current study is in line with the cleavage of its insulin receptor and its CD18 membrane adhesion receptor (7). The cleavage affects the extracellular domain of each of these receptors and, in the case of the insulin receptor, causes insulin resistance and hyperglycemia (7) and, in the case of CD18, causes a reduced adhesion between leukocytes and endothelium together with an increased leukocyte count in the circulation (1, 7). Both of these defects are significantly attenuated by a chronic treatment with an MMP inhibitor. The receptor cleavage is also in line with recently observed cleavage of the extracellular domain of the vascular endothelial growth factor receptor 2 in the SHR (32), an event that causes an enhanced endothelial apoptosis and capillary rarefaction and can also be attenuated by chronic MMP inhibition.
With the exception of the microvessels of the heart muscle, we consistently saw that in the presence of SHR plasma, the density of the extracellular domain of the β2-AR was lower than the intracellular domain (Fig. 4). Furthermore, in light of the significantly enhanced proteolytic activity in SHR plasma (7), the cleavage of the extracellular, but less with the intracellular, domain is to be expected after acute exposure of the receptor to SHR plasma in vitro (Figs. 6–9).
The reduction of the intracellular domain density in the SHR heart muscle microvessels in vivo (Fig. 5, B and D) is in some contrast to the lack of a reduction in the intracellular receptor labeling density in the SHR thoracic aorta (Fig. 4, B and D). Besides individual tissue properties, this difference may be due to the internalization kinetics of the intracellular domain of the β2-AR and its protein synthesis patterns after cleavage. In addition, it is likely that other receptors, e.g., β1-AR, and other vasoconstrictor peptides (8), may also be subject to enzymatic cleavage by MMPs. These issues require further investigation.
The absence of a reduction in expression of the β2-AR extracellular domain in SHR aorta by Western blot analysis of whole tissue homogenates may reflect the larger contribution of the β2-AR localized in smooth muscle cells compared with those in the thin layer of endothelial cells. Sufficient amounts of protein for Western blot analysis require the use of complete aortas, and therefore the domain density is determined by both the receptors on the endothelium and in the smooth muscle layers. The latter tends to dominate, and therefore a reduction of the receptor density on the endothelium, as seen with immunohistochemistry, is not readily detectable with homogenates of the entire aortic wall. We saw a slight but nonsignificant reduction of the β2-AR extracellular (but not the intracellular) domain receptor density (Fig. 4G), in line with this possibility. The same issue seems to occur in hearts where the number of β2-AR present in other cell types, e.g., myocytes, can readily overshadow in a tissue homogenate the reduced expression observed in microvessels. The measurement of receptor density by Western blot analysis using whole tissue homogenates is also influenced by the different levels of cell apoptosis in endothelial and parenchymal cells in SHRs and their controls (13, 19). Apoptosis reduces the number of viable cells in the tissue and may change their size, so that the reference volume of tissue may become different in WKY and SHRs. It is evident that immunohistochemistry may be a requirement to detect local cleavage of receptors in the microcirculation.
Chronically Elevated MMP Activity in SHRs
The SHR has elevated plasma protease activity, which involves serine and MMP, and was confirmed by immunohistochemistry, microzymography with specific fluorescently quenched substrates, gel zymography, and the use of effective MMP inhibitors (GM-6001, EDTA, and doxycycline) (7, 32). Measurements with fluorescently quenched substrates calibrated by purified enzymes indicate that the plasma concentrations are on average 68 nM for MMP-9 and 135 nM for MMP-7 (32). The activity can be documented on fresh plasma; on various microvascular cells, including the endothelium; and on circulating leukocytes. The SHR has elevated elastinolytic (MMP-2) activity in cardiac muscle (21) that degrades extracellular proteins and decreases cardiac tissue strength. This is in addition to elevated chymase activity (12). As alternative hypertensive model, the Dahl salt-sensitive rat has elevated levels of MMP-9 in heart muscle and is associated with decreased ejection fraction (26). MMP activity is observed in many models of cardiovascular disease (6, 24, 25, 31, 35) despite inhibitors in plasma, e.g., tissue inhibitors of metalloproteinases and α2-macroglobulin (2, 33).
Vasoconstrictor Effect of MMPs
The vasoconstriction of both arterioles and venules caused by an injection of MMP-7 and MMP-9 in the rat circulatory system in this study (Fig. 3) is in accordance with observations by Hao et al. (14). These investigators reported that the activation of MMP-7 promotes the vasoconstriction of mesenteric arteries in Sprague-Dawley rats through a mechanism dependent on the epidermal growth factor receptor. Moreover, they demonstrated that the effect is important for the maintenance of the vascular tone. The constriction we saw at concentrations of 100 μM is rapid. At a lower physiological concentration, we saw a much slower response that required a long-term exposure of the tissue which interferes with vasoconstrictor activities (e.g., ambient room oxygen). The vasoconstriction after an acute MMP-7 and MMP-9 application in the SHR is abolished in the presence of a specific MMP inhibitor (GM-6001). Mice lacking the gene encoding MMP-9 demonstrate increased flow-dependent vasodilatory responses and increased endothelial nitric oxide synthase levels (30).
A reduced vasodilation in response to β2-AR agonists has been observed in both large and small vessels during hypertension (5, 22). The current evidence suggests that MMPs cause cleavage of the extracellular domain of this receptor, and MMPs may play a role in the control of microvascular diameter in arterioles that in turn contribute to the regulation of arterial blood pressure (27, 36). Thus enzymatic cleavage of the extracellular receptor domain by MMPs followed by the disruption of the signaling cascade between endothelium and smooth muscle cell may reduce vascular relaxation signals. Consequently, an enzymatic destruction of the vasodilatory mechanisms, such as the β2-AR in the SHR, may become a treatment target, e.g., by the intervention against MMP in plasma and on the endothelium. Indeed, a chronic MMP inhibition serves to normalize the blood pressure of the SHR (7).
The β2-Adrenegic Receptor as Vasodilator in the SHR
β2-Adrenergic-specific agonists mediate an endothelium-dependent relaxation of rat aorta (9, 16). An increase in the β2-adrenergic expression of the SHR carotid artery leads to an improved vasodilatation to a level similar to that observed in normotensive WKY rats (15). Chang (3, 4) demonstrated that a β2-AR agonist causes arteriolar relaxation in the diaphragmatic microcirculation. Linderoth et al. (20) studied the effect of β2-adrenoceptors in the skin and muscle microcirculation. Vleeming et al. (34) demonstrated vasodilatation caused by the β2-AR agonist in hearts. Thus several studies demonstrated vasodilatation after β2-AR stimulation and a reduction of vasodilatation after β2-AR-specific blockage in large and small vessels.
The results in this study confirm that the SHR exhibits an attenuated response to β2-adrenergic agonist and antagonist, indicating an abnormal expression or structure of the β2-AR compared with its normotensive WKY control. The lack of the ability to dilate to an agonist makes a potential contribution to the elevated tone in the SHR arterioles (27) and, consequently, to the elevated arteriolar resistance and central blood pressure elevation (36). A chronic blockade of MMPs in this model leads to a normalization of the central blood pressure (7).
Significance
The possibility that hypertension is associated with the proteolytic receptor cleavage may have a number of implications. We highlight one issue here.
Angiotensin-converting enzyme (ACE) inhibitors to treat hypertension and diabetes, drugs that also reduce the blood pressure of the SHR (23), have in the past been predominantly looked at from the point of view of renin-angiotensin pathways. It needs to be recognized that several ACE inhibitors also have significant MMP inhibitory activity (18). It needs to be investigated to what degree ACE inhibitors block MMP activity and possible receptor cleavage, so that a variety of complications that accompany hypertension may be blocked by a common pathway that involves receptor cleavage. There is the possibility that many receptors are subject to enzymatic cleavage (e.g., grow factor receptors and adhesion receptors).
Conclusion
The current evidence suggests that the enhanced MMP activity in the SHR, which causes cleavage of the extracellular domain of membrane receptors such as the insulin receptor and the membrane integrin CD18, seems to affect also adrenergic receptors that control vasodilation, the peripheral vascular resistance, and the blood pressure levels.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-10881 and in part by an unrestricted academic gift from Leading Ventures. S. F. Rodrigues is the recipient of a scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. J. Steven Alexander (from Louisiana State University Health Sciences Center, Shreveport, LA) for kindly helping us with the Western blot assays.
REFERENCES
- 1.Arndt H, Smith CW, Granger DN. Leukocyte-endothelial cell adhesion in spontaneously hypertensive and normotensive rats. Hypertension 21: 667–673, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Cawston TE, Mercer E. Preferential binding of collagenase to alpha 2-macroglobulin in the presence of the tissue inhibitor of metalloproteinases. FEBS Lett 209: 9–12, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Chang HY. Role of nitric oxide in vasodilator response induced by salbutamol in rat diaphragmatic microcirculation. Am J Physiol Heart Circ Physiol 272: H2173–H2179, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Chang HY. The involvement of ATP-sensitive potassium channels in beta 2-adrenoceptor agonist-induced vasodilatation on rat diaphragmatic microcirculation. Br J Pharmacol 121: 1024–1030, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YY, Doggrell SA. Responsiveness, affinity constants and beta-adrenoceptor reserves for isoprenaline on aortae from normo-, pre- and hypertensive rats. J Pharm Pharmacol 54: 515–522, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 50: 329–339, 2005 [DOI] [PubMed] [Google Scholar]
- 7.DeLano FA, Schmid-Schönbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension 52: 415–423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Patron C, Stewart KG, Zhang Y, Koivunen E, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ Res 87: 670–676, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Ferro A, Coash M, Yamamoto T, Rob J, Ji Y, Queen L. Nitric oxide-dependent beta2-adrenergic dilatation of rat aorta is mediated through activation of both protein kinase A and Akt. Br J Pharmacol 143: 397–403, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia RA, Pantazatos DP, Gessner CR, Go KV, Woods VL, Jr, Villarreal FJ. Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Mol Pharmacol 67: 1128–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 12: 12–26, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Guo C, Ju H, Leung D, Massaeli H, Shi M, Rabinovitch M. A novel vascular smooth muscle chymase is upregulated in hypertensive rats. J Clin Invest 107: 703–715, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamet P, Richard L, Dam TV, Teiger E, Orlov SN, Gaboury L, Gossard F, Tremblay J. Apoptosis in target organs of hypertension. Hypertension 26: 642–648, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res 94: 68–76, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, Koch WJ, Trimarco B. Beta(2)-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation 106: 349–355, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Isenovic E, Walsh MF, Muniyappa R, Bard M, Diglio CA, Sowers JR. Phosphatidylinositol 3-kinase may mediate isoproterenol-induced vascular relaxation in part through nitric oxide production. Metabolism 51: 380–386, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Lalu MM, Cena J, Chowdhury R, Lam A, Schulz R. Matrix metalloproteinases contribute to endotoxin and interleukin-1beta induced vascular dysfunction. Br J Pharmacol 149: 31–42, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebetrau M, Burggraf D, Wunderlich N, Jager G, Linz W, Hamann GF. ACE inhibition reduces activity of the plasminogen/plasmin and MMP systems in the brain of spontaneous hypertensive stroke-prone rats. Neurosci Lett 376: 205–209, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Lim HH, DeLano FA, Schmid-Schönbein GW. Life and death cell labeling in the microcirculation of the spontaneously hypertensive rat. J Vasc Res 38: 228–236, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery 35: 711–719, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Mujumdar VS, Smiley LM, Tyagi SC. Activation of matrix metalloproteinase dilates and decreases cardiac tensile strength. Int J Cardiol 79: 277–286, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Naslund T, Silberstein DJ, Merrell WJ, Nadeau JH, Wood AJ. Low sodium intake corrects abnormality in beta-receptor-mediated arterial vasodilation in patients with hypertension: correlation with beta-receptor function in vitro. Clin Pharmacol Ther 48: 87–95, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Nunez E, Hosoya K, Susic D, Frohlich ED. Enalapril and losartan reduced cardiac mass and improved coronary hemodynamics in SHR. Hypertension 29: 519–524, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Pearce WH, Shively VP. Abdominal aortic aneurysm as a complex multifactorial disease: interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann NY Acad Sci 1085: 117–132, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Peterson JT, Hallak H, Johnson L, Li H, O'Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation 103: 2303–2309, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez WE, Tyagi N, Deng AY, Adeagbo A, Joshua IG, Tyagi SC. Congenic expression of tissue inhibitor of metalloproteinase in Dahl-salt sensitive hypertensive rats is associated with reduced LV hypertrophy. Arch Physiol Biochem 114: 340–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Rosário HS, Waldo SW, Becker SA, Schmid-Schönbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol 164: 1707–1716, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid-Schönbein GW, Zweifach BW, DeLano FA, Chen P. Microvascular tone in a skeletal muscle of spontaneously hypertensive rats. Hypertension 9: 164–171, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Stein CM, Nelson R, Deegan R, He H, Wood M, Wood AJ. Forearm beta adrenergic receptor-mediated vasodilation is impaired, without alteration of forearm norepinephrine spillover, in borderline hypertension. J Clin Invest 96: 579–585, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiles GL, Strasser RH, Kilpatrick BF, Taylor SR, Lefkowitz RJ. Endogenous proteinases modulate the function of the beta-adrenergic receptor-adenylate cyclase system. Biochim Biophys Acta 802: 390–398, 1984 [DOI] [PubMed] [Google Scholar]
- 30.Su J, Palen DI, Lucchesi PA, Matrougui K. Mice lacking the gene encoding for MMP-9 and resistance artery reactivity. Biochem Biophys Res Commun 349: 1177–1181, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine 35: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran ED, Delano FA, Schmid-Schönbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res 47: 423–431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincenti MP. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol Biol 151: 121–148, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Vleeming W, Wemer J, Riezebos J, Van Amsterdam JG, De Wildt DJ, Porsius AJ. Modulation by pertussis toxin of salbutamol- and arecoline-induced effects in the isolated heart and aorta of the rat. Eur J Pharmacol 250: 415–422, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe N, Ikeda U. Matrix metalloproteinases and atherosclerosis. Curr Atheroscler Rep 6: 112–120, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Zweifach BW, Kovalcheck S, DeLano FA, Chen P. Micropressure-flow relationship in a skeletal muscle of spontaneously hypertensive rats. Hypertension 3: 601–614, 1981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.