Abstract
Responses to the Rho kinase inhibitor Y-27632 were investigated in the anesthetized rat. Under baseline conditions intravenous injections of Y-27632 decreased pulmonary and systemic arterial pressures and increased cardiac output. The decreases in pulmonary arterial pressures were enhanced when baseline tone was increased with U-46619, and under elevated tone conditions Y-27632 produced similar percent decreases in pulmonary and systemic arterial pressures. Injections of Y-27632 prevented and reversed the hypoxic pulmonary vasoconstrictor response. The increase in pulmonary arterial pressure in response to ventilation with a 10% O2-90% N2 gas mixture was not well maintained during the period of hypoxic exposure. Treatment with the nitric oxide (NO) synthase (NOS) inhibitor nitro-l-arginine methyl ester (l-NAME) increased pulmonary arterial pressure and prevented the decline or fade in the hypoxic pulmonary vasoconstrictor response. The hypoxic pulmonary vasoconstrictor response was reversed by Y-27632 in control and in l-NAME-treated animals. The Rho kinase inhibitor attenuated increases in pulmonary arterial pressures in response to intravenous injections of serotonin, angiotensin II, and Bay K 8644. Y-27632, sodium nitrite, and BAY 41-8543, a guanylate cyclase stimulator, decreased pulmonary and systemic arterial pressures and vascular resistances in monocrotaline-treated rats. These data suggest that Rho kinase is involved in the regulation of baseline tone and in the mediation of pulmonary vasoconstrictor responses. The present data suggest that the hypoxic pulmonary vasoconstrictor response is modulated by the release of NO that mediates the nonsustained component of the response in the anesthetized rat. These data suggest that Rho kinase and NOS play important roles in the regulation of vasoconstrictor tone in physiological and pathophysiological states and that monocrotaline-induced pulmonary hypertension can be reversed by agents that inhibit Rho kinase, generate NO, or stimulate soluble guanylate cyclase.
Keywords: pulmonary vasodilation, systemic vasodilation, hypoxic pulmonary vasoconstriction, nitric oxide release, BAY 41-8543
the small GTPase Rho A and its downstream effector Rho kinase have been shown to play an important role in the regulation of vascular smooth muscle contraction (2, 22–25, 28–31). Smooth muscle contraction is mediated by the phosphorylation of myosin light chains, and the extent of myosin light chain phosphorylation is regulated by the opposing activities of myosin light chain kinase and phosphatase. The activity of myosin light chain phosphatase is regulated by Rho kinase, and inhibition of Rho kinase results in myosin light chain dephosphorylation and vasodilation (2, 22–25, 30, 31). Studies on the role of Rho kinase in the regulation of vasoconstrictor tone in vivo have been assisted by the development of selective Rho kinase inhibitors (32, 34). Y-27632 is one of the best-characterized Rho kinase inhibitors that selectively targets P160-Rho kinase from the family of Rho-associated protein kinases (32, 34). Much of our knowledge about the vascular effects of Y-27632 have come from studies in in vitro models, and an early study reported that Y-27632 had no effect on systemic arterial pressure in normotensive rats (32, 34). Although Y-27632 has been shown to attenuate the hypoxic pulmonary vasoconstrictor response in the intact rat and isolated perfused rat lung, the effects of Y-27632 on vasoconstrictor responses induced by other mechanisms have not been examined in the intact rat and responses have not been compared in the pulmonary and systemic vascular beds (14, 25, 29). Y-27632 has been used in the treatment of pulmonary hypertension in rodents (1, 14, 17a). An important side effect of vasodilator agents such as PGI2 when used in the treatment of pulmonary hypertension is a decrease in systemic arterial pressure, and it has been hypothesized that Rho kinase inhibitors may have selective vasodilator activity in the pulmonary vascular bed (17, 20, 21). The purpose of the present study was to test the hypothesis that Y-27632 has selective pulmonary vasodilator activity and to investigate responses to Y-27632 when vasoconstrictor tone was increased by diverse mechanisms in the pulmonary vascular bed. The effect of the nitric oxide (NO) synthase (NOS) inhibitor nitro-l-arginine methyl ester (l-NAME) and of Y-27632 on the hypoxic pulmonary vasoconstrictor response was investigated, and vasodilator responses to Y-27632, BAY 41-8543 (a stimulator of soluble guanylate cyclase), and sodium nitrite, an agent that generates NO, were investigated in rats with monocrotaline-induced pulmonary hypertension. The results of these studies show that Y-27632 has potent vasodilator activity in the pulmonary and systemic vascular beds and that pulmonary vasoconstrictor responses mediated by diverse mechanisms are attenuated by Y-27632. The present results indicate that the hypoxic pulmonary vasoconstrictor response is modulated by Rho kinase and the release of NO from the endothelium that mediates the fade in the response in the anesthetized rat.
METHODS
The Institutional Animal Care and Use Committee of the Tulane University School of Medicine approved the experimental protocol used in these experiments, and all procedures were conducted in accordance with institutional guidelines. In these experiments adult male Sprague-Dawley rats (Charles River) weighing 297–424 g were anesthetized with Inactin (100 mg/kg ip; Sigma-Aldrich) and were placed in the supine position on an operating table. Supplemental doses of Inactin were administered intravenously to maintain a uniform level of anesthesia. Body temperature was maintained with a heating lamp. The trachea was cannulated with a short segment of polyethylene (PE)-240 tubing to maintain a patent airway, and the animals spontaneously breathed room air or in experiments with hypoxia a 10% O2-90% N2 gas mixture from a plastic hood over the end of the endotracheal tube. A femoral artery was catheterized with PE-50 tubing for the measurement of systemic arterial pressure. The left jugular and femoral veins were catheterized with PE-50 tubing for intravenous injections and infusions, respectively. For measurement of pulmonary arterial pressure a specially designed 3F simple lumen catheter with a radio-opaque marker and curved tip was passed from the right jugular vein into the main pulmonary artery under fluoroscopic guidance (Picker-Surveyor Fluoroscope) as described previously (3, 7, 9, 10). Another 3F radio-opaque catheter was passed into the left ventricle from the right carotid artery in some experiments to measure left ventricular end-diastolic pressure as a measure of left atrial pressure. Pulmonary and systemic arterial pressures and left ventricular end-diastolic pressure were measured with Namic Preceptor DT transducers, digitized by a Biopac MP-100 data acquisition system, and stored on a Dell computer. Cardiac output was measured by the thermodilution technique with a Cardiomax III Thermodilution Cardiac Ouput Computer (Columbus Instruments). A known volume (0.2 ml) of room temperature 0.9% NaCl solution was injected into the jugular vein catheter with its tip near the right atrium, and changes in blood temperature were detected by a 1.5F thermistor microprobe (Columbus Instruments) positioned in the aortic arch from the left carotid artery.
In the first set of experiments changes in pulmonary and systemic arterial pressures and cardiac output in response to intravenous injections of graded doses of Y-27632 (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexane carboxamide hydrochloride (Tocris Bioscience) dissolved in 0.9% NaCl were investigated under baseline conditions. The order of injection of the various doses of Y-27632 was randomized, and sufficient time (10–60 min) was permitted between injections for parameters to return toward base line value.
In the second set of experiments responses to Y-27632 were measured when pulmonary arterial pressure was increased to ∼30 mmHg by continuous intravenous infusion of U-46619. After starting at a high priming rate, the U-46619 infusion was adjusted (80–320 ng/min) to maintain pulmonary arterial pressure at ∼30 mmHg. U-46619 (Cayman Chemical Comp) was dissolved in 95% ethanol and diluted with 0.9% NaCl solution and infused with a Harvard infusion pump.
In the next set of experiments responses to Y-27632 were investigated when pulmonary arterial pressure was increased by ventilation with a 10% O2-90% N2 gas mixture. The 10% O2-90% N2 gas mixture was delivered by way of a plastic funnel over the opening of the endotracheal tube. A time course for the hypoxic pulmonary vasoconstrictor response was established, and Y-27632 was injected at the peak of the response. The effect of injection of Y-27632, 5 min before the hypoxic response was initiated, was also investigated. Arterial Po2, Pco2, and pH were measured with a Radiometer NPT analyzer using a 0.2-ml blood sample from the femoral artery. Working drug solutions were prepared on a frequent basis, and the saline vehicle had no significant effect on baseline hemodynamic values or on the responses to hypoxia and the vasoactive agonists. The effect of the NOS inhibitor l-NAME on the hypoxic pulmonary vasoconstrictor response was investigated in the next set of experiments. The effect of Y-27632 on the increase in pulmonary arterial pressure in response to intravenous injections of Bay K 8644 and angiotensin II and serotonin (Sigma-Aldrich) was investigated in the next set of experiments. The effect of Y-27632, BAY 41-8543, and sodium nitrite on pulmonary and systemic arterial pressures was investigated in animals treated with monocrotaline (60 mg/kg iv) 28 days after the administration of the plant alkaloid. Sodium nitrite (Sigma-Aldrich) was dissolved in 0.9% NaCl solution, and BAY 41-8543 (Bayer Health Care) was dissolved in Transcutol/Cremophor EL/0.9% NaCl solution in a ratio of (10/10/80).
The data are presented as means ± SE and analyzed statistically using paired and unpaired t-tests and ANOVA with Dunnett's post hoc test. The criteria for significance is P < 0.05. Pulmonary vascular resistance was calculated by dividing pulmonary arterial pressure by the cardiac output after determining that left ventricular end-diastolic pressure was not changed. Systemic vascular resistance is calculated by dividing systemic arterial pressure by the cardiac output.
RESULTS
Responses to Y-27632.
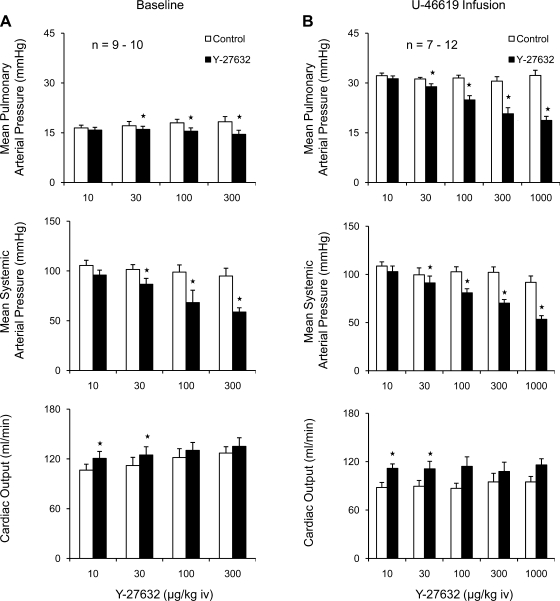
Under baseline conditions intravenous injections of Y-27632 in doses of 10–300 μg/kg iv produced small decreases in pulmonary arterial pressure, larger dose-dependent decreases in systemic arterial pressure, and increases in cardiac output (Fig. 1A). When tone in the pulmonary vascular bed was increased by infusion of U-46619, the intravenous injection of Y-27632 produced larger dose-dependent decreases in pulmonary arterial pressure, similar decreases in systemic arterial pressure, and increases in cardiac output (Fig. 1B).
Fig. 1.
Bar graphs comparing changes in pulmonary and systemic arterial pressure and cardiac output in response to intravenous injections of the Rho-kinase inhibitor Y-27632 under baseline conditions (A) and during intravenous infusion of U-46619 to increase pulmonary arterial pressure to ∼30 mmHg (B). n, number of experiments. *P < 0.05 when compared with baseline.
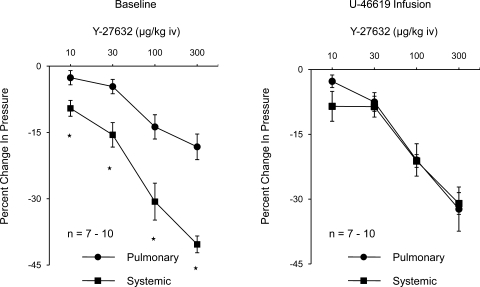
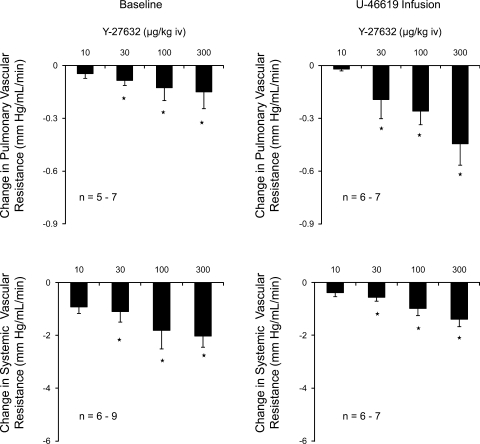
The percent decreases in systemic arterial pressure in response to Y-27632 were significantly greater than the percent decreases in pulmonary arterial pressure under baseline tone conditions (Fig. 2). The percent decreases in pulmonary and systemic arterial pressure were similar in U-46619-infused animals (Fig. 2). These data indicate that Y-27632 has similar vasodilator activity in the pulmonary and systemic vascular beds under physiological conditions. The decreases in pulmonary and systemic vascular resistances in response to intravenous injections of Y-27632 under baseline and elevated tone conditions are summarized in Fig. 3, and intravenous injections of the Rho kinase inhibitor produced significant decreases in pulmonary and systemic vascular resistances.
Fig. 2.
Dose response curves comparing percent decrease in pulmonary and systemic arterial pressure in response to intravenous injections of Y-27632 in the control period (left) and during intravenous infusion of U-46619 (right). n, number of experiments. *P < 0.05 when compared with baseline.
Fig. 3.
Decreases in pulmonary and systemic vascular resistances in response to intravenous injections of Y-27632 under baseline conductions and when pulmonary arterial pressure is increased to ∼30 mmHg with U-46619. n, number of experiments. *P < 0.05, compared with baseline.
Effect of Y-27632 on the response to hypoxia.
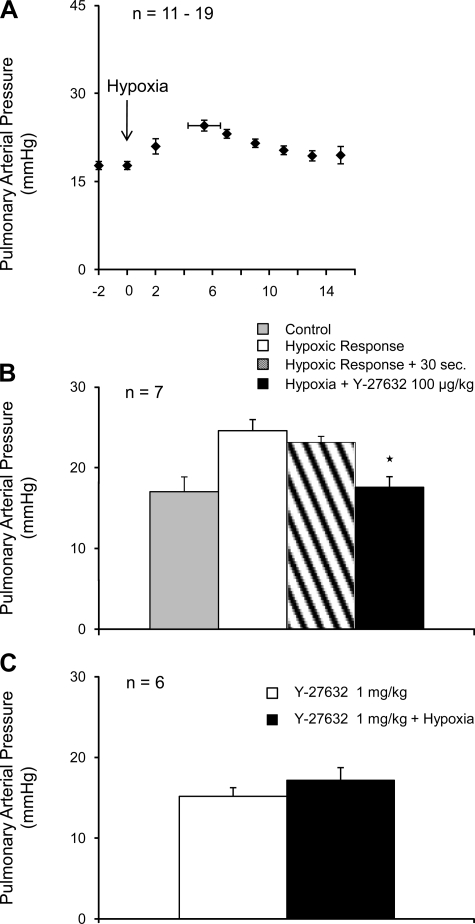
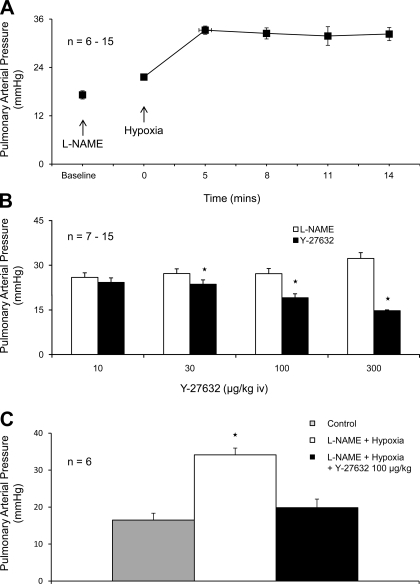
Ventilation with a 10% O2-90% N2 gas mixture decreased arterial Po2 and increased pulmonary arterial pressure (Fig. 4A and Table 1). The peak increase in pressure occurred at ∼5 min after onset of ventilation with the 10% O2-90% N2 gas mixture (Fig. 4A). However, following administration of an intravenous injection of Y-27632, 100 μg/kg, at the peak of the hypoxic pulmonary pressor response, a significant reduction in pulmonary arterial pressure was observed (Fig. 4B). The spontaneous decline in pulmonary arterial pressure 20–30 s after the peak is reached is shown by the cross-hatched bar in Fig. 4B. The intravenous injection of Y-27632, 1 mg/kg 5 min before ventilation with the 10% O2-90% N2 gas mixture prevented the increase in pulmonary arterial pressure in response to ventilation with the hypoxic gas mixture (Fig. 4C).
Fig. 4.
A: time course of the increase in the pulmonary arterial pressure in response to ventilation with a 10% O2-90% N2 gas mixture. Effect of injection of Y-27632 on the response to hypoxia when the Rho-kinase inhibitor was injected at the peak of the hypoxic pulmonary vasoconstrictor response. The hatched bar indicates the spontaneous level of fade in the response at 20–30 s (B). C: effect of pretreatment with Y-27632 5 min before onset of ventilation with the 10% O2 gas mixture on the hypoxic pulmonary vasoconstrictor response. n, number of experiments. *P < 0.05.
Table 1.
Effect of ventilation with a 10% O2 gas mixture on arterial blood gases and pH
| Room Air | 90% N2-10% O2 Gas | |
|---|---|---|
| Po2, mmHg | 81 ± 1 | 37 ± 1* |
| Pco2, mmHg | 51 ± 2 | 37 ± 2* |
| pH | 7.37 ± 0.01 | 7.46 ± 0.01* |
Values are means ± SE; n = 6-11.
P < 0.05 compared with control.
The hypoxic pulmonary vasoconstrictor response was not well maintained, and pulmonary arterial pressure declined back toward baseline value after the peak increase in pulmonary arterial pressure was attained (Fig. 4A).
Effect of l-NAME.
The role of endothelial NOS (eNOS) in mediating the fade or decline of the hypoxic pulmonary vasoconstrictor response was investigated, and after intravenous injection of l-NAME in doses of 10 and 50 mg/kg, the increase in pulmonary arterial pressure was well maintained after the peak increase in pressure was reached and very little fade was observed when the animals continued to breathe the 10% O2-90% N2 gas mixture (Fig. 5A). Inasmuch as the effect of NOS inhibition with l-NAME on the pulmonary vascular bed is uncertain, the effect of a wide range of doses of l-NAME was investigated. The administration of l-NAME in doses of 1–100 mg/kg iv produced significant increases in pulmonary and systemic arterial pressures and decreases in cardiac output in the rat (Table 2). The increases in pulmonary vascular resistance in response to l-NAME are summarized in Table 2. The intravenous injections of Y-27632 in l-NAME-treated rats produced dose-related decreases in pulmonary arterial pressure (Fig. 5B). In addition, the increase in pulmonary arterial pressure in response to ventilation with 10% O2-90% N2 gas mixture in l-NAME-treated rats was reversed by intravenous injections of Y-27632 at the peak of the hypoxic pressor response (Fig. 5C).
Fig. 5.
A: effect of pretreatment with nitro-l-arginine methyl ester (l-NAME; 10–50 mg/kg iv) on hypoxic pulmonary vasoconstrictor response. B: effect of intravenous injections of Y-27632 on pulmonary arterial pressure in l-NAME-treated animals. C: effect of injection of Y-27632 (100 μg/kg iv) on the increase in pulmonary arterial pressure in response to ventilation with the 10% O2 gas mixture in l-NAME-treated animals. n, number of experiments. *Response is significantly different than control.
Table 2.
Effect of l-NAME on systemic and pulmonary arterial pressure and on cardiac output
| Systemic Arterial Pressure, mmHg |
Pulmonary Arterial Pressure, mmHg |
Normalized Cardiac Output, ml·min−1·100 gms−1 |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Control | l-NAME | Control | l-NAME | Control | l-NAME | Increase in Pulmonary Vascular Resistance, mmHg·ml−1·min−1 | |
| 1 mg/kg iv | 6 | 109 ± 7 | 133 ± 8* | 19 ± 1 | 25 ± 1* | 36 ± 3 | 33 ± 4 | 0.11 ± 0.05* |
| 5 mg/kg iv | 15 | 97 ± 4 | 123 ± 4* | 18 ± 3 | 24 ± 1* | 32 ± 1 | 23 ± 1 | 0.15 ± 0.03* |
| 10 mg/kg iv | 11 | 101 ± 4 | 134 ± 6* | 17 ± 1 | 28 ± 1* | 32 ± 2 | 24 ± 2 | 0.23 ± 0.02* |
| 50 mg/kg iv | 20 | 88 ± 4 | 128 ± 6* | 16 ± 1 | 27 ± 1* | 33 ± 1 | 21 ± 1 | 0.25 ± 0.02* |
| 100 mg/kg iv | 24 | 96 ± 2 | 141 ± 3* | 18 ± 1 | 28 ± 1* | 27 ± 1 | 16 ± 1 | 0.32 ± 0.02* |
Values are means ± SE.
P < 0.05 compared with control. l-NAME, nitro-l-arginine methyl ester.
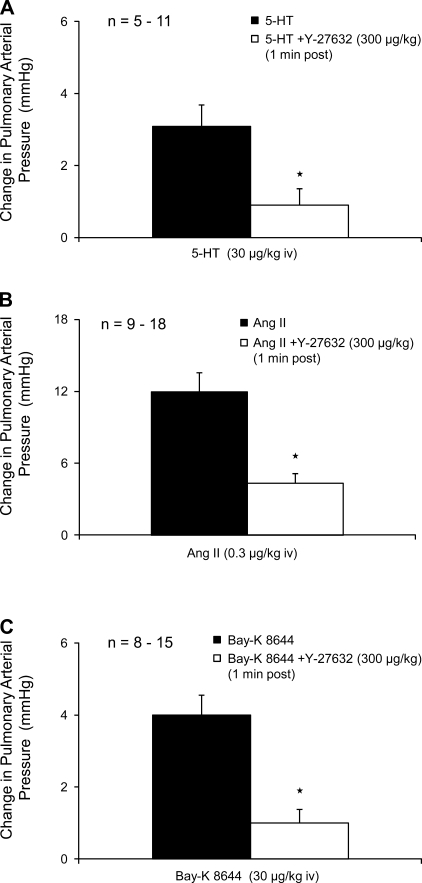
Effect of Y-27632 on responses to intravenous injections of angiotensin II, Bay K 8644, and serotonin.
The intravenous injections of angiotensin II, Bay K 8644, and serotonin increased pulmonary arterial pressure, and the increases in pulmonary arterial pressure were significantly attenuated when the vasoconstrictor agents were injected 1 min after intravenous injection of 300 μg/kg Y-27632 (Fig. 6). Responses to the three vasoconstrictor agonists were not attenuated by injection of Y-27632 when the vasoconstrictor agents were injected 5–10 min after injection of the Rho kinase inhibitor (data not shown).
Fig. 6.
Bar graph comparing increases in pulmonary arterial pressure in response to intravenous injections of serotonin (A), angiotensin II (B), and Bay K 8644 (C) in experiments in which the agonists were given before and after Y-27632 (300 μg/kg). n, number of experiments. *Response is significantly different than control.
Effect of Y-27632 in monocrotaline-treated rats.
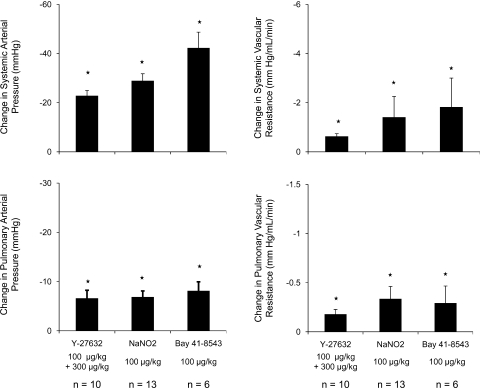
Responses to Y-27632 in monocrotaline-treated rats were investigated, and these data are summarized in Fig. 7. The intravenous injection of monocrotaline in a dose of 60 mg/kg produced a significant increase in pulmonary arterial pressure when pressures were measured 28 days after administration of the plant alkaloid (Table 3). The intravenous injection of 100 μg/kg or 300 μg/kg iv Y-27632 produced a significant decrease in pulmonary and systemic arterial pressures and an increase in cardiac output in monocrotaline-treated animals. The intravenous injection of BAY 41-8543 and sodium nitrite also produced significant decreases in pulmonary and systemic arterial pressures and vascular resistances in monocrotaline-treated rats (Fig. 7). The percent decreases in pulmonary and systemic arterial pressures in response to BAY 41-8543 and sodium nitrite were similar in monocrotaline-treated animals.
Fig. 7.
Bar graph showing the effect of intravenous injection of Y-27632, sodium nitrite, and BAY 41-8543 on pulmonary and systemic arterial pressures and vascular resistances in monocrotaline-treated rats. n, number of experiments *P < 0.05.
Table 3.
Effect of monocrotaline on systemic and pulmonary arterial pressure and cardiac output
| n | Systemic Arterial Pressure, mmHg | Pulmonary Arterial Pressure, mmHg | Cardiac Output, ml/min | |
|---|---|---|---|---|
| Control | 9–10 | 106 ± 4 | 16 ± 1 | 106 ± 7 |
| Monocrotaline, 28 days | 8–11 | 100 ± 5 | 43 ± 3* | 110 ± 14 |
Values are mean ± SE.
P < 0.05 when compared with control.
DISCUSSION
Although it is known that Y-27632 decreases pulmonary arterial pressure in rodents with monocrotaline and hypoxia induced pulmonary hypertension, less is known about responses to the Rho kinase inhibitor when tone is increased by other mechanisms and responses have not been compared in the pulmonary and systemic vascular beds (14, 25, 26). The results of the present study show that intravenous injections of Y-27632 decrease pulmonary and systemic arterial pressures under baseline conditions and that decreases in pulmonary arterial pressure are enhanced when baseline tone in the pulmonary vascular bed is increased by diverse mechanisms. When tone in the pulmonary vascular bed is increased by infusion of the thromboxane (TP) receptor agonist U-46619, hypoxia, or the NOS inhibitor l-NAME, the intravenous injections of Y-27632 produced dose-related decreases in pulmonary and systemic arterial pressures, and both pulmonary and systemic arterial pressures were decreased in a similar manner. Inasmuch as cardiac output was increased and left ventricular end-diastolic pressure was not changed, the decreases in pressures indicate that pulmonary and systemic vascular resistances were decreased. These data indicate that Y-27632 has significant vasodilator activity in the pulmonary and systemic vascular beds. These data are not in agreement with an early study reporting that the Y-27632 did not decrease systemic arterial pressure in normotensive rats (34). The results showing that Y-27632 decreases baseline pressure in the pulmonary vascular bed are consistent with results in conscious chronically instrumented rats and provide additional evidence that Y-27632 reduces basal pulmonary arterial pressure (25).
In addition to reversing the pulmonary hypertensive response to U-46619, Y-27632 prevented and reversed the increase in pulmonary arterial pressure when the rats breathed a 10% O2-90% N2 gas mixture. These data are consistent with previous results in the conscious and anesthetized rat and the isolated perfused rat lung (14, 25, 26). It has been hypothesized that Rho kinase-mediated calcium sensitization plays an important role in mediating the hypoxic pulmonary vasoconstrictor response (25, 28–30, 35, 36). The results showing that the response to hypoxia can be prevented or reversed by Y-27632 are consistent with this hypothesis. However, the observation that the pulmonary hypertensive response to U-46619 and the NOS inhibitor l-NAME are blunted or reversed suggests that the inhibitory effects of Y-27632 are not selective for hypoxia in the anesthetized rat. The effect of intravenous injections of Y-27632 on increases in pulmonary arterial pressure in response to intravenous bolus injections of serotonin, angiotensin II, or Bay K 8644 was investigated, and responses to the vasoconstrictor agonists were attenuated when Y-27632 was injected 1 min but not 5 or 10 min before injection of the vasoconstrictor agent. These data indicate that Y-27632 is not as effective as fasudil or SB-770277-B in blunting responses to the vasoconstrictor agonists (3, 9). These data provide support for the hypothesis that Rho kinase inhibition can blunt vasoconstrictor responses mediated by a variety of mechanisms including G coupled receptor activation, Bay K 8644 enhanced calcium entry, and l-NAME-induced endothelial dysfunction.
The hypoxic pulmonary vasoconstrictor response in anesthetized rats and dogs is not well maintained or sustained during the period of hypoxic exposure, and pulmonary arterial pressure declines after the peak increase in pressure is attained (18, 33). The mechanism of this decline or fade in pulmonary arterial pressure has not been determined (18, 33). The hypothesis that the decline or fade in the hypoxic pulmonary vasoconstrictor response is mediated by the release of NO was investigated, and after treatment with the NOS inhibitor l-NAME, the decline in pulmonary arterial pressure was significantly attenuated. These data provide evidence in support of the role of endothelial NO release in mediating the nonsustained component or fade of the hypoxic pulmonary vasoconstrictor response in the anesthetized rat.
In addition to attenuating the fade in the hypoxic pulmonary vasoconstrictor response, the NOS inhibitor increased the magnitude of the hypoxic pulmonary vasoconstrictor response and increased baseline pulmonary arterial pressure in the rat. The role of NO release in regulating baseline tone in the pulmonary vascular bed is controversial (12, 13). Although it has been reported that NO does not have a role in maintaining baseline tone in the pulmonary vascular bed in the rat, the results of the present study show that l-NAME administration in doses of 1–100 mg/kg iv increased pulmonary and systemic arterial pressures and decreased cardiac output. The results of the present study and previous studies from this and other laboratories are consistent with the hypothesis that the formation of NO by eNOS plays an important role in modulating the hypoxic pulmonary vasoconstrictor response and in maintaining baseline tone at a low level in the pulmonary vascular bed of the anesthetized rat (3, 4, 6, 9, 19, 11). The effect of the NOS inhibitor on baseline tone in the rat is consistent with studies in a variety of species including the human showing that NO plays an important role in maintaining baseline tone at a low level in the pulmonary vascular bed (3–5, 9, 17). The reason for the difference in results is uncertain but may involve differences in pulmonary blood flow and shear stress or differences in the experimental model or preparation used (4, 12, 13, 16). It has been hypothesized that the effect of l-NAME on the pulmonary vascular bed may represent an adverse effect of NOS inhibitor (12, 13). The results showing that the pulmonary vasoconstrictor response to l-NAME is reversed by Y-27632 and previous studies showing reversal with NO donors and sodium nitrite are consistent with the hypothesis that the pulmonary vasoconstrictor response to l-NAME results from a decrease in NO release from the endothelium (3–7, 9, 16, 27).
Recent attention has been focused on the role of Rho kinase in the regulation of vasoconstrictor tone (2, 3, 9, 10, 22–25, 30, 31). The inhibition of Rho kinase results in the activation of myosin phosphatase, the dephosphorylation of myosin light chains, and vasodilation (2, 22–25, 30, 31, 35, 36). The observation that Y-27632 decreases pulmonary and systemic arterial pressures under baseline conditions is consistent with the hypothesis that Rho kinase is tonically active and plays an important role in the regulation of baseline tone in the pulmonary and systemic vascular beds. The results of studies with hypoxia, U-46619, l-NAME, Bay K 8644, serotonin, and angiotensin II suggest that the pulmonary vasodilator response to Y-27632 is independent of the physiological mechanism used to increase tone. The finding that Y-27632 produced similar percent decreases in pulmonary and systemic arterial pressure when baseline tone is increased suggests that Y-27632 does not have selective pulmonary vasodilator activity in this model. These results suggest that there is no fundamental difference in the role of Rho kinase in regulating vasoconstrictor tone in the pulmonary and systemic vascular beds and are consistent with the results of previous studies (3, 9, 10).
Responses to Y-27632 were also investigated in monocrotaline-treated rats, and the Rho kinase inhibitor produced similar percent decreases in pulmonary and systemic arterial pressures when responses were measured 28 days after administration of the plant alkaloid. These data are consistent with results with SB-772077-B and fasudil and provide support for the hypothesis that inhibition of Rho kinase does not produce a selective vasodilator effect in the pulmonary vascular bed (3, 9, 10).
Responses to sodium nitrite and BAY 41-8543 were investigated in monocrotaline-treated rats, and these data show that both agents decrease pulmonary and systemic arterial pressures. Sodium nitrite is not a conventional nitro vasodilator. It has been reported that sodium nitrite has vasodilator activity in experimental animals and humans and that vasorelaxant responses are associated with an increase in cGMP levels (7, 8, 19). It has been reported that vasodilator responses to sodium nitrite are attenuated by allopurinol, suggesting that xanthine oxidoreductase reduces nitrite to vasoactive NO in the rat (7). The present data suggest that NO formed from the reduction of nitrite and the stimulation of soluble guanylate cyclase can promote vasodilation in animals with pulmonary hypertension. However, both agents were not selective for the pulmonary vascular bed. These data suggest that soluble guanylate cyclase is responsive to NO and that the heme iron of the enzyme is not oxidized or inactivated in monocrotaline-treated animals 28 days after administration of the plant alkaloid.
In summary, the results of the present study show that Y-27632 decreases pulmonary and systemic arterial pressures and that when tone is increased in the pulmonary vascular bed similar decreases in pulmonary and systemic arterial pressures are observed. The hypoxic pulmonary vasoconstrictor response is not well maintained in the anesthetized rat, and treatment with l-NAME attenuated the fade or decline in the response to hypoxia. In addition the magnitude of the hypoxic vasoconstrictor response was increased by l-NAME and the response could be prevented or blunted by Y-27632. These data suggest that the hypoxic pulmonary vasoconstrictor response is modulated by a Y-27632 reversible mechanism and NO. The observation that Y-27632 can promote vasodilation when tone is increased by diverse mechanisms including hypoxia, activation of G-coupled receptors, enhanced calcium entry, endothelial dysfunction, or monocrotaline treatment may in part explain the beneficial effects of Rho kinase inhibitors in the treatment of pulmonary hypertensive disorders. The finding that sodium nitrite and BAY 41-8543 have significant pulmonary vasodilator activity in monocrotaline-treated rats suggests that soluble guanylate cyclase is not inactivated by treatment with the plant alkaloid.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-62000 and HL-77412.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshita A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94: 385–393, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Badejo AM, Jr, Dhaliwal JS, Casey DB, Gallen TB, Greco AJ, Kadowitz PJ. Analysis of pulmonary vasodilator responses to the Rho-kinase inhibitor fasudil in the anesthetized rat. Am J Physiol Lung Cell Mol Physiol 295: L828–L836, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Barnard JW, Wilson PS, Moore TM, Thompson WJ, Taylor AE. Effect of nitric oxide and cyclooxygenase products on vascular resistance in dog and rat lungs. J Appl Physiol 74: 2940–2948, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Blitzer ML, Loh E, Roddy MA, Stamler JS, Creager MA. Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans. J Am Coll Cardiol 28: 591–596, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Brashers VL, Peach MJ, Rose CE., Jr Augmentation of hypoxic pulmonary vasoconstriction in the isolated perfused rat lung by in vitro antagonists of endothelium-dependent relaxation. J Clin Invest 82: 1495–1502, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey DB, Badejo AM, Jr, Dhaliwal JS, Murthy SN, Hyman AL, Nossaman BD, Kadowitz PJ. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. Am J Physiol Heart Circ Physiol 296: H524–H533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116: 1821–1831, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dhaliwal JS, Casey DB, Greco AJ, Badejo AM, Jr, Gallen TB, Murthy SN, Nossaman BD, Hyman AL, Kadowitz PJ. Rho-kinase and Ca2+ entry mediate increased pulmonary and systemic vascular resistance in l-NAME-treated rats. Am J Physiol Lung Cell Mol Physiol 293: L1306–L1313, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Dhaliwal JS, Badejo AM, Jr, Casey DB, Murthy SN, Kadowitz PJ. Analysis of pulmonary vasodilator responses to SB-772077-B [4-(7-((3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1H-imidazo(4,5-c)pyridin-2-yl)-1,2,5-oxadiazol-3-amine], a novel aminofurazan-based Rho kinase inhibitor. J Pharmacol Exp Ther 330: 334–341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng C, Cheng D, Kaye A, Kadowitz P, Nossaman B. Influence of Nomega-nitro-l-arginine methyl ester, LY83583, glybenclamide and L158809 on pulmonary circulation. Eur J Pharmacol 261: 133–140, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Hampl V, Weir EK, Archer SL. Endothelium-derived nitric oxide is less important for basal tone regulation in the pulmonary than the renal vessels of adult rat. J Vasc Med Biol 5: 22–30, 1994 [Google Scholar]
- 13.Hampl V, Herget J. Role of nitric oxide in pathogenesis of chronic pulmonary hypertension. Physiol Rev 80: 1337–1372, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Homma N, Nagaoka T, Karoor V, Imamura M, Tarasevicience-Stewart L, Walker LA, Fagan KA, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell Mol Physiol 295: L71–L78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyman AL, Hao Q, Tower A, Kadowitz PJ, Champion HC, Gumusel B, Lippton H. Novel catheterization technique for the in vivo measurement of pulmonary vascular responses in rats. Am J Physiol Heart Circ Physiol 274: H1218–H1229, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hyman AL, Kadowitz PJ. Pulmonary vasodilator activity of prostacyclin (PGI2) in the cat. Circ Res 45: 404–409, 1979 [DOI] [PubMed] [Google Scholar]
- 17a.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Igari Tatsumi K, Sugito K, Kasahara Y, Saito M, Tani T, Kimura H, Kuriyama T. Role of EDRF in pulmonary circulation during sustained hypoxia. Role of EDRF in pulmonary circulation during sustained hypoxia. J Cardiovasc Pharmacol 31: 299–305, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther 218: 739–749, 1981 [PubMed] [Google Scholar]
- 20.Jiang B, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol 49: 85–89, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kadowitz PJ, Chapnick BM, Feigen LP, Hyman AL, Nelson PK, Spannhake EW. Pulmonary and systemic vasodilator effects of the newly discovered prostaglandin, PGI2. J Appl Physiol 45: 408–413, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 272: 12257–12260, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 270: 29051–29054, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Oka M, Homma N, Tarasevicience-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Parker T, Roe G, Grover T, Abman S. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Robertson T, Aaronson P, Ward J. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol Heart Circ Physiol 268: H301–H307, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Robertson T, Dipp M, Ward J, Aaronson P, Evans A. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol 131: 5–9, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson T, Hague D, Aaronson P, Ward J. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 525: 669–680, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Takahara A, Sugiyama A, Satoh Y, Yoneyama M, Hashimoto K. Cardiovascular effects of Y-27632, a selective Rho-associated kinase inhibitor, assessed in the halothane-anesthetized canine model. Eur J Pharmacol 460: 51–57, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Tucker A, Reeves JT. Nonsustained pulmonary vasoconstriction during acute hypoxia in anesthetized dogs. Am J Physiol 228: 756–761, 1975 [DOI] [PubMed] [Google Scholar]
- 34.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389: 990–994, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Weigand L, Foxson J, Shimoda L, Sylvester J. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 293: L674–L685, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Lanner M, Jin N, Swartz D, Li L, Rhoades R. Hypoxia inhibits myosin phosphatase in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol 29: 465–471, 2003 [DOI] [PubMed] [Google Scholar]